Artemisia annua Extract Improves the Cognitive Deficits and Reverses the Pathological Changes of Alzheimer’s Disease via Regulating YAP Signaling
Abstract
1. Introduction
2. Results
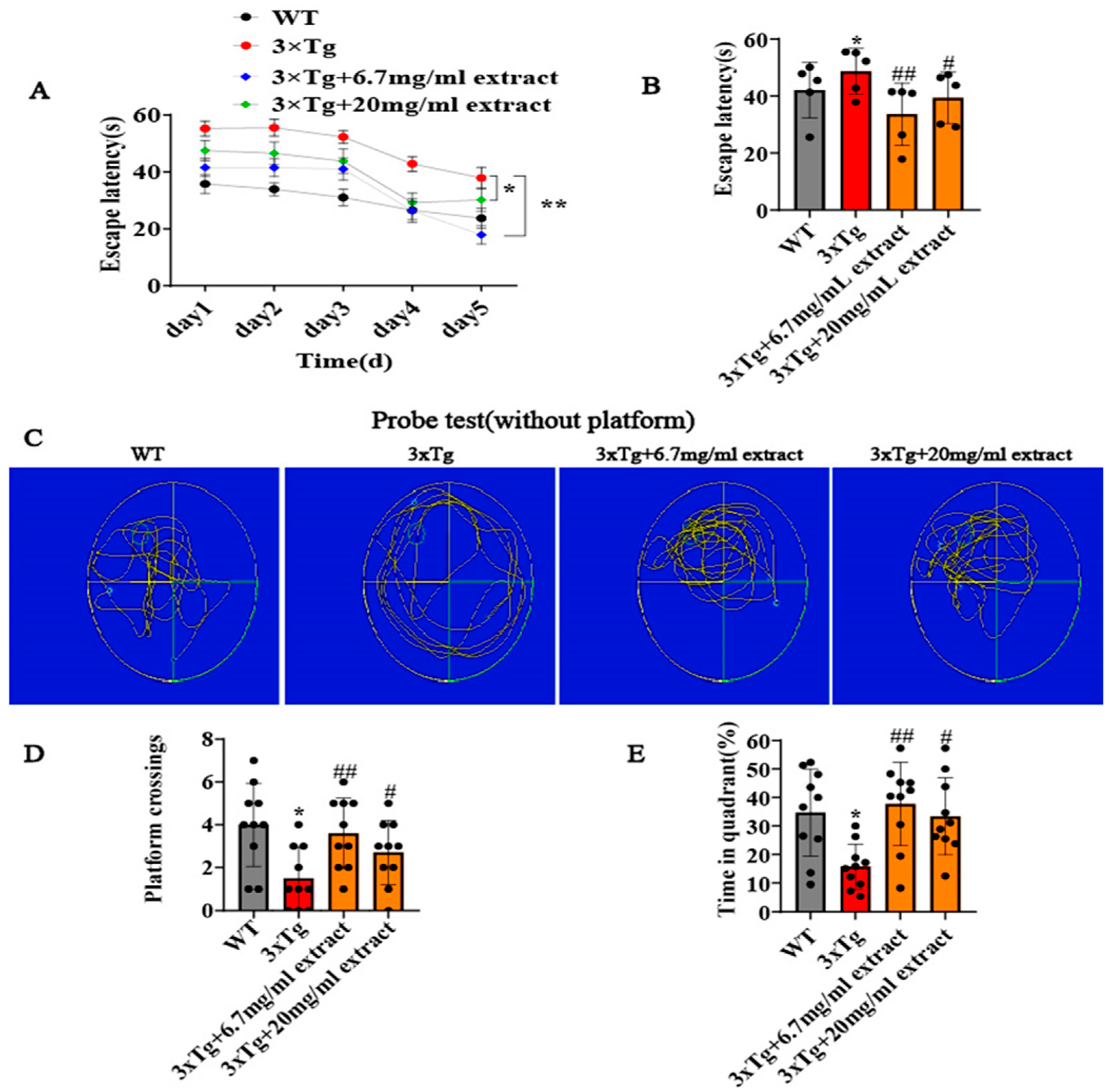
2.1. A. annua Extract Improved the Cognitive Deficits of 3xTg AD Mice
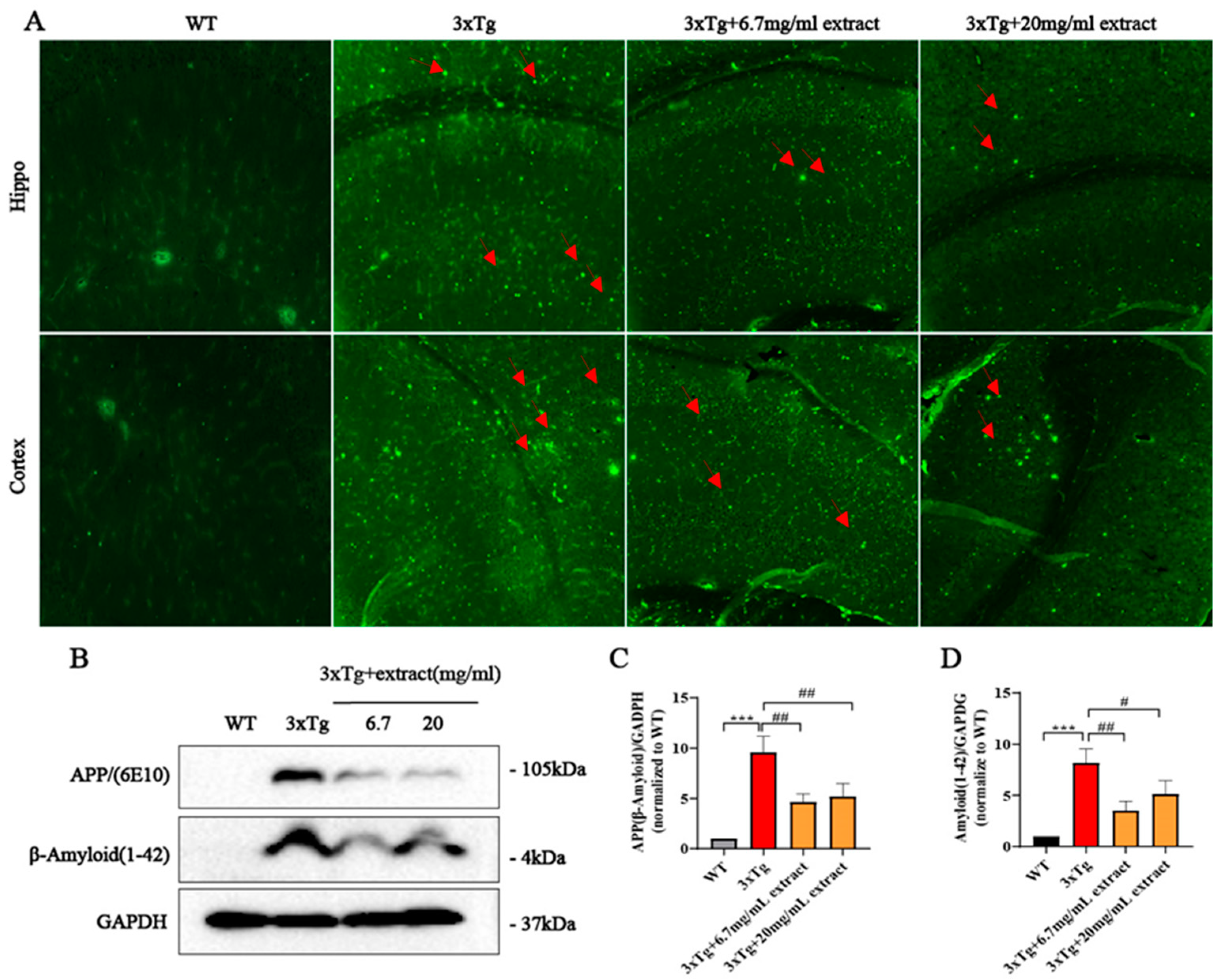
2.2. A. annua Extract Reduced Aβ Accumulation in 3xTg AD Mice
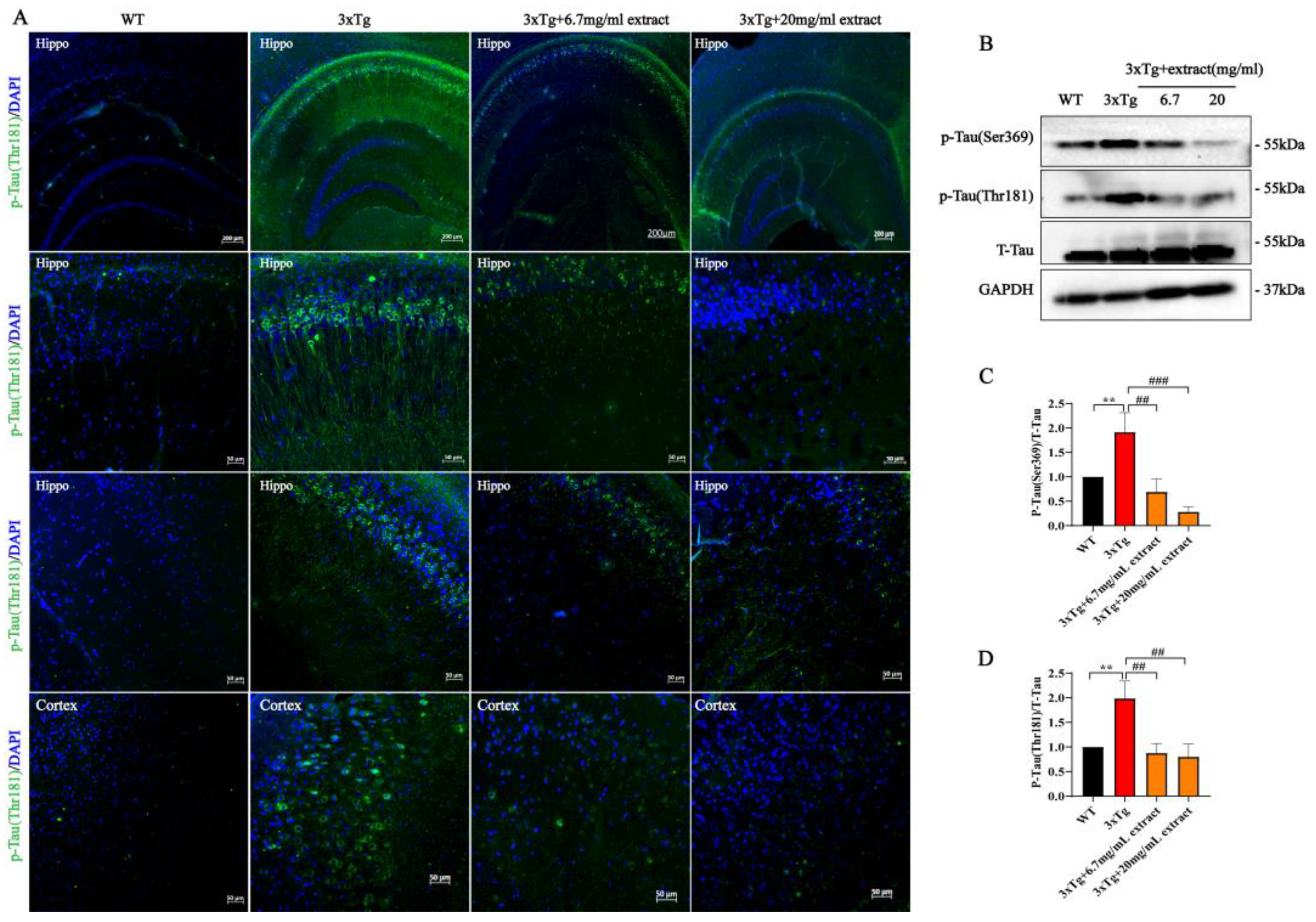
2.3. A. annua Extract Reduced Tau-Phosphorylation in 3xTg AD Mice
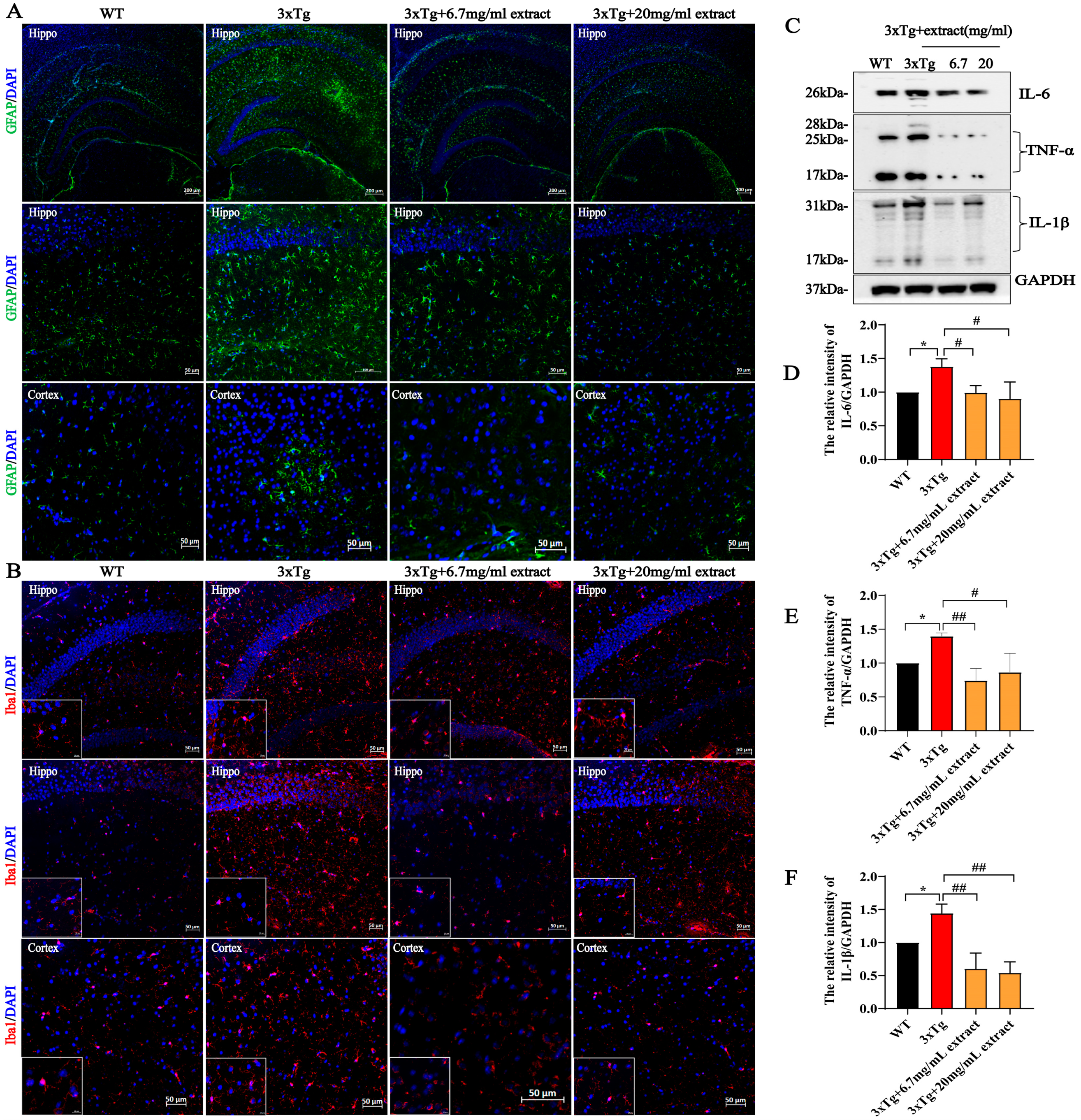
2.4. A. annua Extract Attenuated Neuroinflammation in 3xTg AD Mice
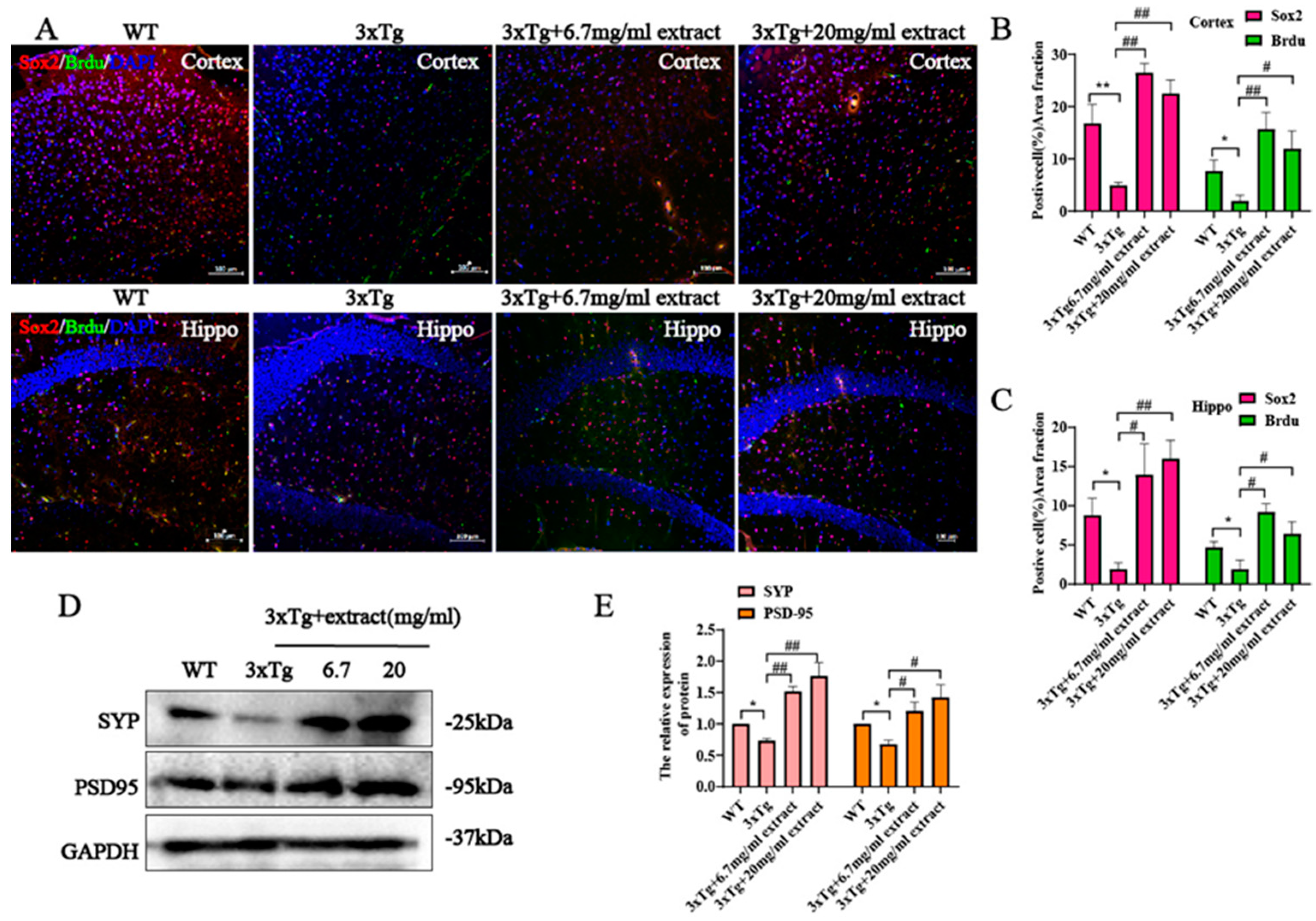
2.5. Extract Promoted the Proliferation of Neural Progenitor Cells and Increased the Expression Synaptic Proteins in 3xTg Mice
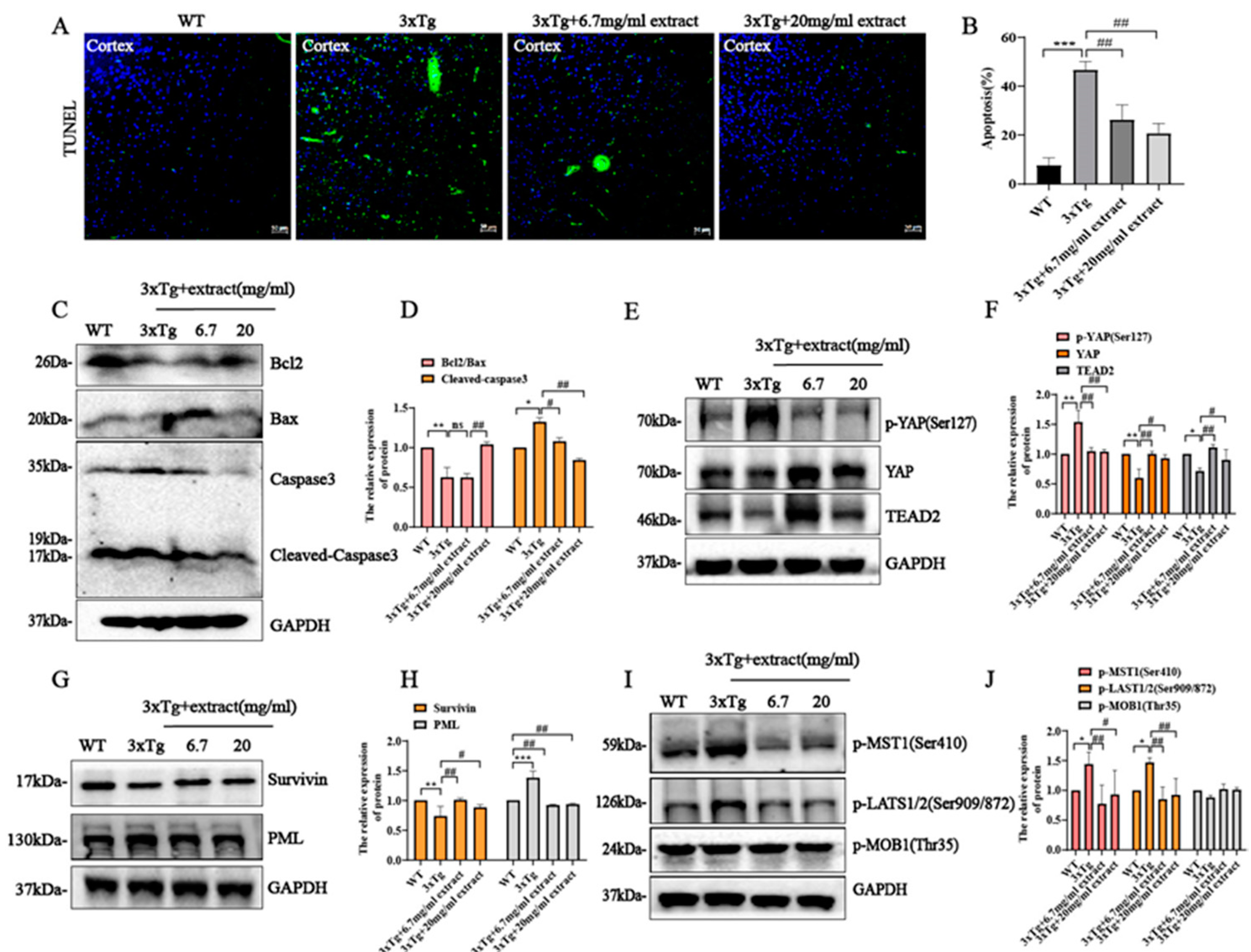
2.6. Extract Rescued Neuronal Cell Apoptosis in 3xTg AD Mice via Regulating Hippo/YAP Signaling
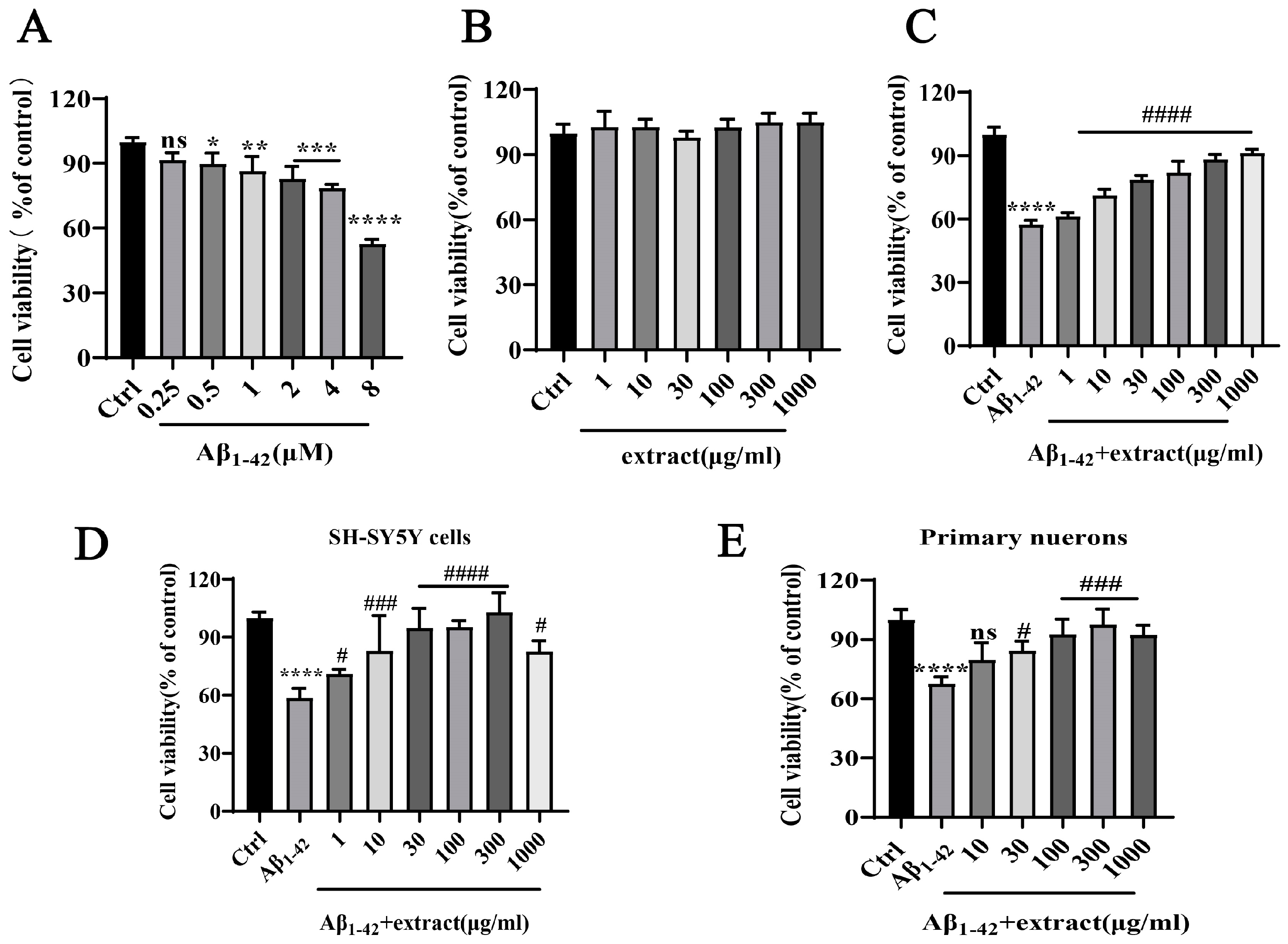
2.7. A. annua Extract Antagonized Aβ1–42-Induced Neurotoxicity and Increased the Viability of Neuronal Cells In Vitro
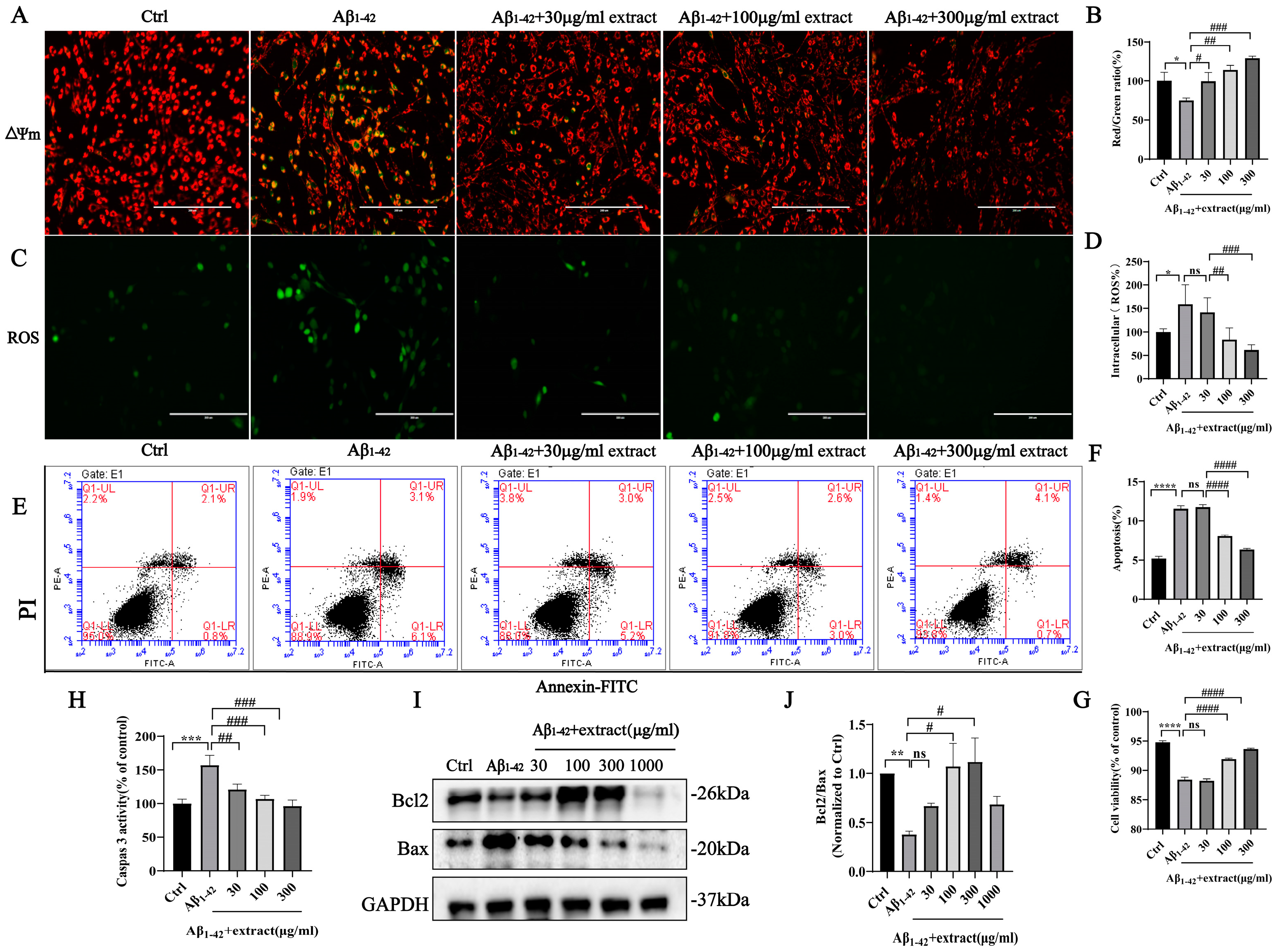
2.8. Extract Treatment Alleviated Aβ1–42-Induced Apoptosis by Aβ1–42 in PC12 Cells
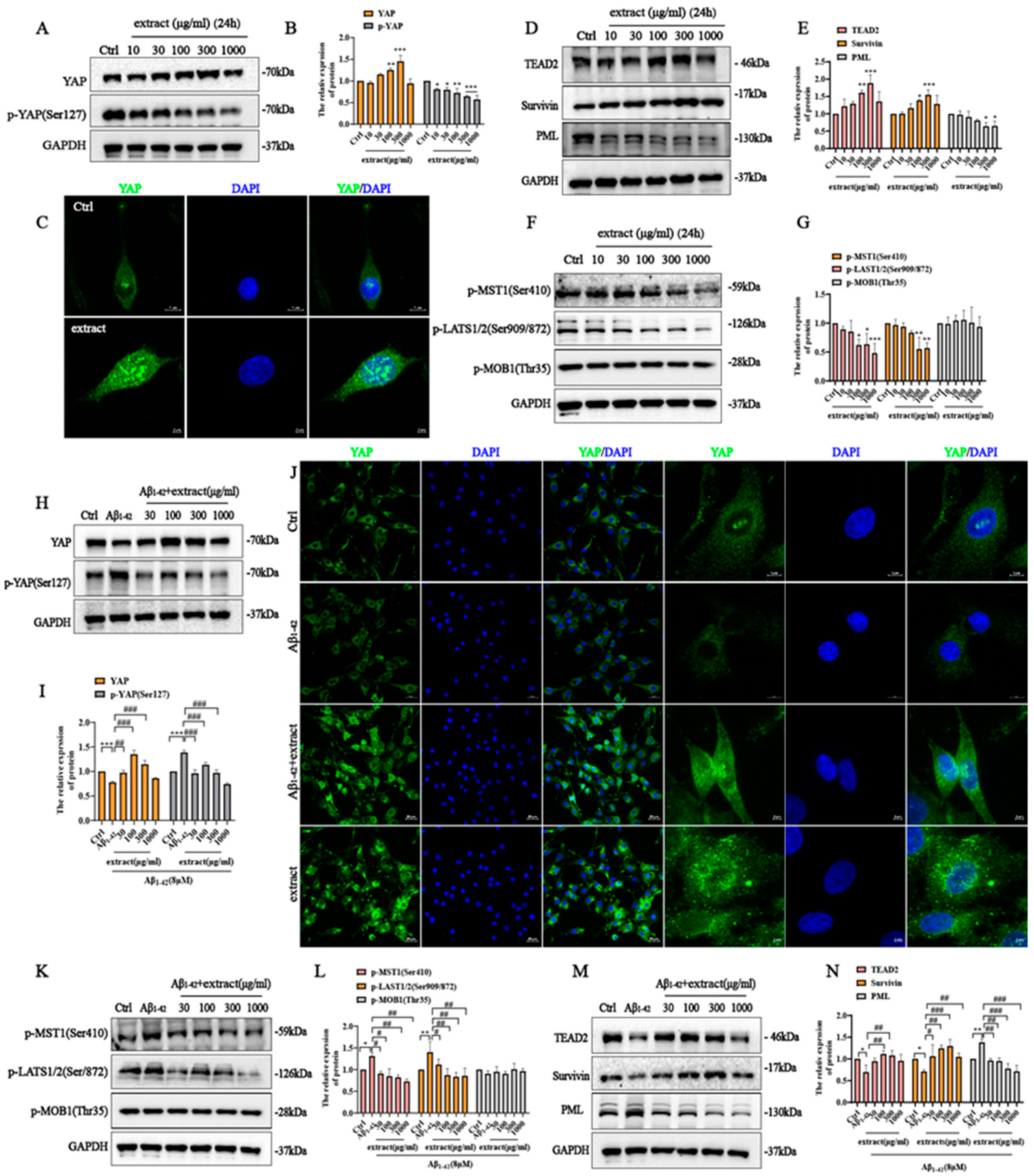
2.9. YAP Involved in the Protective Effect of A. annua Extract in PC12 Cells
2.10. HPLC Analysis of A. annua Extract Powder
3. Discussion
4. Materials and Methods
4.1. Extract from Artemisia annua Preparation
4.2. Component Analysis of Extract Powder with HPLC-MS
4.3. Animals and Treatment
4.4. Behavioral Tests
4.5. BrdU Labeling and Tissue Sample Preparation
4.6. Immunofluorescence Analysis
4.7. Cells Culture and Treatments
4.8. CRISPR/Cas9 Genome Editing
4.9. MTT Assay
4.10. Measurement of Reactive Oxygen Species (ROS)
4.11. Measurement of Mitochondrial Membrane Potential (Δψm)
4.12. Caspase 3 Activity Assay
4.13. TUNEL Assay
4.14. Flow Cytometry
4.15. Western Blotting
4.16. Statistical Analysis
5. Conclusions
6. Limitations of This Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic Approach to Alzheimer’s Disease: Current Treatments and New Perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Marques, B.L.; Maciel, G.F.; Júnior, M.R.B.; Dias, L.D.; Scalzo, S.; Santos, A.K.; Kihara, A.H.; da Costa Santiago, H.; Parreira, R.C.; Birbrair, A. Regulatory mechanisms of stem cell differentiation: Biotechnological applications for neurogenesis. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in aging and age-related neurodegenerative diseases. Ageing Res. Rev. 2022, 78, 101636. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Wang, H.; Shang, Y.; Wang, E.; Xu, X.; Zhang, Q.; Qian, C.; Yang, Z.; Wu, S.; Zhang, T. MST1 mediates neuronal loss and cognitive deficits: A novel therapeutic target for Alzheimer’s disease. Prog. Neurobiol. 2022, 214, 102280. [Google Scholar] [CrossRef]
- Tang, W.; Li, M.; Yangzhong, X.; Zhang, X.; Zu, A.; Hou, Y.; Li, L.; Sun, S. Hippo signaling pathway and respiratory diseases. Cell Death Discov. 2022, 8, 213. [Google Scholar] [CrossRef]
- Sileo, P.; Simonin, C.; Melnyk, P.; Chartier-Harlin, M.-C.; Cotelle, P. Crosstalk between the Hippo Pathway and the Wnt Pathway in Huntington’s Disease and Other Neurodegenerative Disorders. Cells 2022, 11, 3631. [Google Scholar] [CrossRef]
- Luo, J.; Li, P. Context-dependent transcriptional regulations of YAP/TAZ in stem cell and differentiation. Stem. Cell Res. Ther. 2022, 13, 10. [Google Scholar] [CrossRef]
- Wu, Z.; Guan, K.-L. Hippo signaling in embryogenesis and development. Trends Biochem. Sci. 2021, 46, 51–63. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef]
- Sahu, M.R.; Mondal, A.C. Neuronal Hippo signaling: From development to diseases. Dev. Neurobiol. 2021, 81, 92–109. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, S.; Dong, Y.; Yuan, Z. The role and regulatory mechanism of hippo signaling components in the neuronal system. Front. Immunol. 2020, 11, 281. [Google Scholar] [CrossRef]
- Ho, W.E.; Peh, H.Y.; Chan, T.K.; Wong, W.S. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol. Ther. 2014, 142, 126–139. [Google Scholar] [CrossRef]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; Gaafary, M.E.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomed. Int. J. Phytother. Phytopharm. 2019, 62, 152962. [Google Scholar] [CrossRef]
- Alesaeidi, S.; Miraj, S. A Systematic Review of Anti-malarial Properties, Immunosuppressive Properties, Anti-inflammatory Properties, and Anti-cancer Properties of Artemisia annua. Electron. Physician 2016, 8, 3150–3155. [Google Scholar] [CrossRef]
- Bai, L.; Li, H.; Li, J.; Song, J.; Zhou, Y.; Liu, B.; Lu, R.; Zhang, P.; Chen, J.; Chen, D.; et al. Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int. Immunopharmacol. 2019, 70, 313–323. [Google Scholar] [CrossRef]
- Qin, D.P.; Li, H.B.; Pang, Q.Q.; Huang, Y.X.; Pan, D.B.; Su, Z.Z.; Yao, X.J.; Yao, X.S.; Xiao, W.; Yu, Y. Structurally diverse sesquiterpenoids from the aerial parts of Artemisia annua (Qinghao) and their striking systemically anti-inflammatory activities. Bioorganic Chem. 2020, 103, 104221. [Google Scholar] [CrossRef]
- Jiao, J.; Yang, Y.; Liu, M.; Li, J.; Cui, Y.; Yin, S.; Tao, J. Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Vet. Parasitol. 2018, 254, 172–177. [Google Scholar] [CrossRef]
- Qiang, W.; Cai, W.; Yang, Q.; Yang, L.; Dai, Y.; Zhao, Z.; Yin, J.; Li, Y.; Li, Q.; Wang, Y.; et al. Artemisinin B Improves Learning and Memory Impairment in AD Dementia Mice by Suppressing Neuroinflammation. Neuroscience 2018, 395, 1–12. [Google Scholar] [CrossRef]
- Kim, W.S.; Choi, W.J.; Lee, S.; Kim, W.J.; Lee, D.C.; Sohn, U.D.; Shin, H.S.; Kim, W. Anti-inflammatory, Antioxidant and Antimicrobial Effects of Artemisinin Extracts from Artemisia annua L. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2015, 19, 21–27. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. [Google Scholar] [CrossRef]
- Skowyra, M.; Gallego, M.G.; Segovia, F.; Almajano, M.P. Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions. Antioxidants 2014, 3, 116–128. [Google Scholar] [CrossRef]
- Li, S.; Chaudhary, S.C.; Zhao, X.; Gaur, U.; Fang, J.; Yan, F.; Zheng, W. Artemisinin Protects Human Retinal Pigmented Epithelial Cells Against Hydrogen Peroxide-induced Oxidative Damage by Enhancing the Activation of AMP-active Protein Kinase. Int. J. Biol. Sci. 2019, 15, 2016–2028. [Google Scholar] [CrossRef]
- Eteng, M.U.; Abolaji, A.O.; Ebong, P.E.; Brisibe, E.A.; Dar, A.; Kabir, N.; Iqbal Choudhary, M. Biochemical and haematological evaluation of repeated dose exposure of male Wistar rats to an ethanolic extract of Artemisia annua. Phytother. Res. PTR 2013, 27, 602–609. [Google Scholar] [CrossRef]
- Wan, X.; Ahmad, H.; Zhang, L.; Wang, Z.; Wang, T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agric. 2018, 98, 3715–3721. [Google Scholar] [CrossRef]
- Song, Z.H.; Cheng, K.; Zheng, X.C.; Ahmad, H.; Zhang, L.L.; Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Gaur, U.; Zheng, W. Artemisinin Improved Neuronal Functions in Alzheimer’s Disease Animal Model 3xtg Mice and Neuronal Cells via Stimulating the ERK/CREB Signaling Pathway. Aging Dis. 2020, 11, 801–819. [Google Scholar] [CrossRef]
- Zhao, Y.; Long, Z.; Ding, Y.; Jiang, T.; Liu, J.; Li, Y.; Liu, Y.; Peng, X.; Wang, K.; Feng, M.; et al. Dihydroartemisinin Ameliorates Learning and Memory in Alzheimer’s Disease Through Promoting Autophagosome-Lysosome Fusion and Autolysosomal Degradation for Aβ Clearance. Front. Aging Neurosci. 2020, 12, 47. [Google Scholar] [CrossRef]
- Fu, C.; Shi, H.; Chen, H.; Zhang, K.; Wang, M.; Qiu, F. Oral Bioavailability Comparison of Artemisinin, Deoxyartemisinin, and 10-Deoxoartemisinin Based on Computer Simulations and Pharmacokinetics in Rats. ACS Omega 2021, 6, 889–899. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s disease: Current progress in molecular signaling and therapeutics. Inflammation 2022, 1–17. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Bassani, T.B.; Bonato, J.M.; Machado, M.M.F.; Cóppola-Segovia, V.; Moura, E.L.R.; Zanata, S.M.; Oliveira, R.M.M.W.; Vital, M.A.B.F. Decrease in Adult Neurogenesis and Neuroinflammation Are Involved in Spatial Memory Impairment in the Streptozotocin-Induced Model of Sporadic Alzheimer’s Disease in Rats. Mol. Neurobiol. 2017, 55, 4280–4296. [Google Scholar] [CrossRef]
- Sahu, M.R.; Mondal, A.C. The emerging role of Hippo signaling in neurodegeneration. J. Neurosci. Res. 2020, 98, 796–814. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, D.F.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.L.; Bi, R.; Yao, Y.G. A systematic integrated analysis of brain expression profiles reveals YAP1 and other prioritized hub genes as important upstream regulators in Alzheimer’s disease. Alzheimers Dement 2018, 14, 215–229. [Google Scholar] [CrossRef]
- Felley-Bosco, E.; Stahel, R. Hippo/YAP pathway for targeted therapy. Transl. Lung Cancer Res. 2014, 3, 75–83. [Google Scholar]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, Y.; Li, F.; Tong, X.; Ren, Y.; Han, X.; Yao, S.; Long, F.; Yang, Z.; Fan, H.; et al. YAP promotes malignant progression of Lkb1-deficient lung adenocarcinoma through downstream regulation of survivin. Cancer Res. 2015, 75, 4450–4457. [Google Scholar] [CrossRef]
- Liu, W.-w.; Wang, F.; Li, C.; Song, X.-y.; Otkur, W.; Zhu, Y.-y.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H. Silibinin relieves UVB-induced apoptosis of human skin cells by inhibiting the YAP-p73 pathway. Acta Pharmacol. Sin. 2022, 43, 2156–2167. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Guerrero-Castilla, A.; Cano, M.; Muñoz, M.F.; Ayala, A.; Argüelles, S. Dysregulation of the Hippo pathway signaling in aging and cancer. Pharmacol. Res. 2019, 143, 151–165. [Google Scholar] [CrossRef]
- Leong, Y.Q.; Ng, K.Y.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab. Brain Dis. 2020, 35, 11–30. [Google Scholar] [CrossRef]
- Rajesh, Y.; Kanneganti, T.-D. Innate immune cell death in neuroinflammation and Alzheimer’s disease. Cells 2022, 11, 1885. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shimohama, S.; Kamoshima, W.; Ota, T.; Matsuoka, Y.; Nomura, Y.; Smith, M.A.; Perry, G.; Whitehouse, P.J.; Taniguchi, T. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 1998, 780, 260–269. [Google Scholar] [CrossRef]
- Hollville, E.; Romero, S.E.; Deshmukh, M. Apoptotic cell death regulation in neurons. FEBS J. 2019, 286, 3276–3298. [Google Scholar] [CrossRef]
- Deng, H.; Li, M.; Zheng, R.; Qiu, H.; Yuan, T.; Wang, W.; Yang, Q.; Long, Z.; Huang, X. YAP promotes cell proliferation and epithelium-derived cytokine expression via NF-κB pathway in nasal polyps. J. Asthma Allergy 2021, 14, 839. [Google Scholar] [CrossRef]
- Bhandari, R.; Paliwal, J.K.; Kuhad, A. Neuropsychopathology of autism spectrum disorder: Complex interplay of genetic, epigenetic, and environmental factors. Pers. Food Interv. Ther. Autism Spectr. Disord. Manag. 2020, 97–141. [Google Scholar]
- Li, S.; Zhao, X.; Lazarovici, P.; Zheng, W. Artemether Activation of AMPK/GSK3β(ser9)/Nrf2 Signaling Confers Neuroprotection towards β-Amyloid-Induced Neurotoxicity in 3xTg Alzheimer’s Mouse Model. Oxidative Med. Cell. Longev. 2019, 2019, 1862437. [Google Scholar] [CrossRef]
- Bignante, E.A.; Ponce, N.E.; Heredia, F.; Musso, J.; Krawczyk, M.C.; Millán, J.; Pigino, G.F.; Inestrosa, N.C.; Boccia, M.M.; Lorenzo, A. APP/Go protein Gβγ-complex signaling mediates Aβ degeneration and cognitive impairment in Alzheimer’s disease models. Neurobiol. Aging 2018, 64, 44–57. [Google Scholar] [CrossRef]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 2019, 18, e12873. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- De Sousa, R.A.L. Reactive gliosis in Alzheimer’s disease: A crucial role for cognitive impairment and memory loss. Metab. Brain Dis. 2022, 37, 851–857. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.; Wang, Z.; Xu, H.; Wu, T.; Marshall, C.; Gao, J.; Xiao, M. Microglia prevent beta-amyloid plaque formation in the early stage of an Alzheimer’s disease mouse model with suppression of glymphatic clearance. Alzheimer’s Res. Ther. 2020, 12, 125. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Long, H.-Z.; Zhou, Z.-W.; Cheng, Y.; Luo, H.-Y.; Li, F.-J.; Xu, S.-G.; Gao, L.-C. The role of microglia in Alzheimer’s disease from the perspective of immune inflammation and iron metabolism. Front. Aging Neurosci. 2022, 14, 888989. [Google Scholar] [CrossRef]
- Terzioglu-Usak, S.; Negis, Y.; Karabulut, D.S.; Zaim, M.; Isik, S. Cellular Model of Alzheimer’s Disease: Aβ1-42 Peptide Induces Amyloid Deposition and a Decrease in Topo Isomerase IIβ and Nurr1 Expression. Curr. Alzheimer Res. 2017, 14, 636–644. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila model of Alzheimer’s disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef]
- Zhang, T.; Song, C.; Li, H.; Zheng, Y.; Zhang, Y. Different Extracellular β-Amyloid (1-42) Aggregates Differentially Impair Neural Cell Adhesion and Neurite Outgrowth through Differential Induction of Scaffold Palladin. Biomolecules 2022, 12, 1808. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Huang, Z. YAP: A novel target for Alzheimer’s disease. Aging 2022, 14, 3724–3725. [Google Scholar] [CrossRef]
- Tanaka, H.; Homma, H.; Fujita, K.; Kondo, K.; Yamada, S.; Jin, X.; Waragai, M.; Ohtomo, G.; Iwata, A.; Tagawa, K.; et al. YAP-dependent necrosis occurs in early stages of Alzheimer’s disease and regulates mouse model pathology. Nat. Commun. 2020, 11, 507. [Google Scholar] [CrossRef]
- Cao, X.; Pfaff, S.L.; Gage, F.H. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008, 22, 3320–3334. [Google Scholar] [CrossRef]
- Berger, T.; Lee, H.; Young, A.H.; Aarsland, D.; Thuret, S. Adult hippocampal neurogenesis in major depressive disorder and Alzheimer’s disease. Trends Mol. Med. 2020, 26, 803–818. [Google Scholar] [CrossRef]
- Li Puma, D.D.; Piacentini, R.; Grassi, C. Does impairment of adult neurogenesis contribute to pathophysiology of alzheimer’s disease? A still open question. Front. Mol. Neurosci. 2021, 13, 578211. [Google Scholar] [CrossRef]
- Sung, P.-S.; Lin, P.-Y.; Liu, C.-H.; Su, H.-C.; Tsai, K.-J. Neuroinflammation and neurogenesis in Alzheimer’s disease and potential therapeutic approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Giménez-Llort, L. Persistent hyperactivity and distinctive strategy features in the Morris water maze in 3xTg-AD mice at advanced stages of disease. Behav. Neurosci. 2015, 129, 129–137. [Google Scholar] [CrossRef]
- Tian, H.; Ding, N.; Guo, M.; Wang, S.; Wang, Z.; Liu, H.; Yang, J.; Li, Y.; Ren, J.; Jiang, J.; et al. Analysis of Learning and Memory Ability in an Alzheimer’s Disease Mouse Model using the Morris Water Maze. J. Vis. Exp. JoVE 2019, 152, e60055. [Google Scholar] [CrossRef]
- Hosseini, F.; Koohpar, Z.K.; Falahati, M. Induction of neural stem cell proliferation by iron oxide nanoparticles and magnetic field and Ki67 gene expression in rat hippocampus after ischemia/reperfusion. J. Shahrekord Univ. Med. Sci. 2019, 21, 64–69. [Google Scholar] [CrossRef]
- Giordano, G.; Costa, L.G. Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. Methods Mol. Biol. 2011, 758, 13–27. [Google Scholar] [CrossRef]
- Sahu, M.P.; Nikkilä, O.; Lågas, S.; Kolehmainen, S.; Castrén, E. Culturing primary neurons from rat hippocampus and cortex. Neuronal. Signal. 2019, 3, NS20180207. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Fu, L.; Hu, Y.; Song, M.; Liu, Z.; Zhang, W.; Yu, F.-X.; Wu, J.; Wang, S.; Izpisua Belmonte, J.C.; Chan, P. Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biol. 2019, 17, e3000201. [Google Scholar] [CrossRef]
- Zheng, W.; Chong, C.M.; Wang, H.; Zhou, X.; Zhang, L.; Wang, R.; Meng, Q.; Lazarovici, P.; Fang, J. Artemisinin conferred ERK mediated neuroprotection to PC12 cells and cortical neurons exposed to sodium nitroprusside-induced oxidative insult. Free Radic Biol. Med. 2016, 97, 158–167. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]


| Antibodies | Cat.No | Company |
|---|---|---|
| APP/β-Amyloid (NAB228) | 2450 | CST |
| β-Amyloid (1-42 Specific) (D3E10) | 12843 | CST |
| Phospho-Tau (Thr181) | D9F4G | CST |
| Anti-Tau (phospho S396) | ab109390 | Abcam |
| β-Amyloid, (6E10) | 803004 | BioLegend |
| Tau (Tau46) | 4019 | CST |
| Iba1 | GTX1004 | GeneTex |
| GFAP (GA5) | 3670 | CST |
| SOX2 | MAB5603 | Millipore |
| BrdU (Bu20a) mouse mAb | 5292 | CST |
| IL6 | 32064 | SAB |
| IL-1beta (3A6) | 12242s | CST |
| TNF-α (D2D4) XP® Rabbit mAb | 11948 | CST |
| Anti-SYP/Synaptophysin Antibody | BA3279 | Boster |
| PSD95 (D27E11) XP® Rabbit mAb | 3450 | CST |
| Bax Antibody | 2772s | CST |
| Bcl-2 (D17C4) Rabbit mAb | 3498s | CST |
| Cleaved Caspase-3 | 9661s | CST |
| Caspase-3 (8G10) Rabbit mAb | 9665 | CST |
| p-YAP(Ser127) | 4911s | CST |
| YAP antibody | SC-101199 | Santa Cruz |
| TEAD2 Antibody | 33900 | SAB |
| Survivin Antibody | 24092 | SAB |
| PML Antibody | 32211 | SAB |
| MST1 (Phospho-Thr183) Antibody | 12144 | SAB |
| LATS1/2(Phospho-Ser909/872) Antibody | 12514 | SAB |
| Phospho-MOB1 (Thr35) (D2F10) Rabbit mAb | 8699 | CST |
| MOB1 (E1N9D) Rabbit mAb | 13730 | CST |
| GAPDH | 21612 | SAB |
| Aβ1–42 | Detail Information |
|---|---|
| Sequence (Three-Letter Code) | H–Asp–Ala–Glu–Phe–Arg–His–Asp–Ser–Gly–Tyr–Glu -Val–His–His–Gln–Lys–Leu–Val–Phe–Phe–Ala–Glu–Asp -Val–Gly–Ser–Asn–Lys–Gly–Ala–Ile–Ile–Gly–Leu–Met -Val–Gly–Gly–Val–Val–Ile–Ala–OH |
| One Letter Code | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA |
| Molecular Formula | C203H311N55O60S |
| Relative Molecular Mass | 4514.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Lei, B.; Yang, C.; Silva, M.; Xing, X.; Yu, H.; Lu, J.; Zheng, W. Artemisia annua Extract Improves the Cognitive Deficits and Reverses the Pathological Changes of Alzheimer’s Disease via Regulating YAP Signaling. Int. J. Mol. Sci. 2023, 24, 5259. https://doi.org/10.3390/ijms24065259
Zhou W, Lei B, Yang C, Silva M, Xing X, Yu H, Lu J, Zheng W. Artemisia annua Extract Improves the Cognitive Deficits and Reverses the Pathological Changes of Alzheimer’s Disease via Regulating YAP Signaling. International Journal of Molecular Sciences. 2023; 24(6):5259. https://doi.org/10.3390/ijms24065259
Chicago/Turabian StyleZhou, Wenshu, Bingxi Lei, Chao Yang, Marta Silva, Xingan Xing, Hua Yu, Jiahong Lu, and Wenhua Zheng. 2023. "Artemisia annua Extract Improves the Cognitive Deficits and Reverses the Pathological Changes of Alzheimer’s Disease via Regulating YAP Signaling" International Journal of Molecular Sciences 24, no. 6: 5259. https://doi.org/10.3390/ijms24065259
APA StyleZhou, W., Lei, B., Yang, C., Silva, M., Xing, X., Yu, H., Lu, J., & Zheng, W. (2023). Artemisia annua Extract Improves the Cognitive Deficits and Reverses the Pathological Changes of Alzheimer’s Disease via Regulating YAP Signaling. International Journal of Molecular Sciences, 24(6), 5259. https://doi.org/10.3390/ijms24065259







