Recognizing Minor Leukemic Populations with Monocytic Features in Mixed-Phenotype Acute Leukemia by Flow Cell Sorting Followed by Cytogenetic and Molecular Studies: Report of Five Exemplary Cases
Abstract
1. Introduction
2. Results
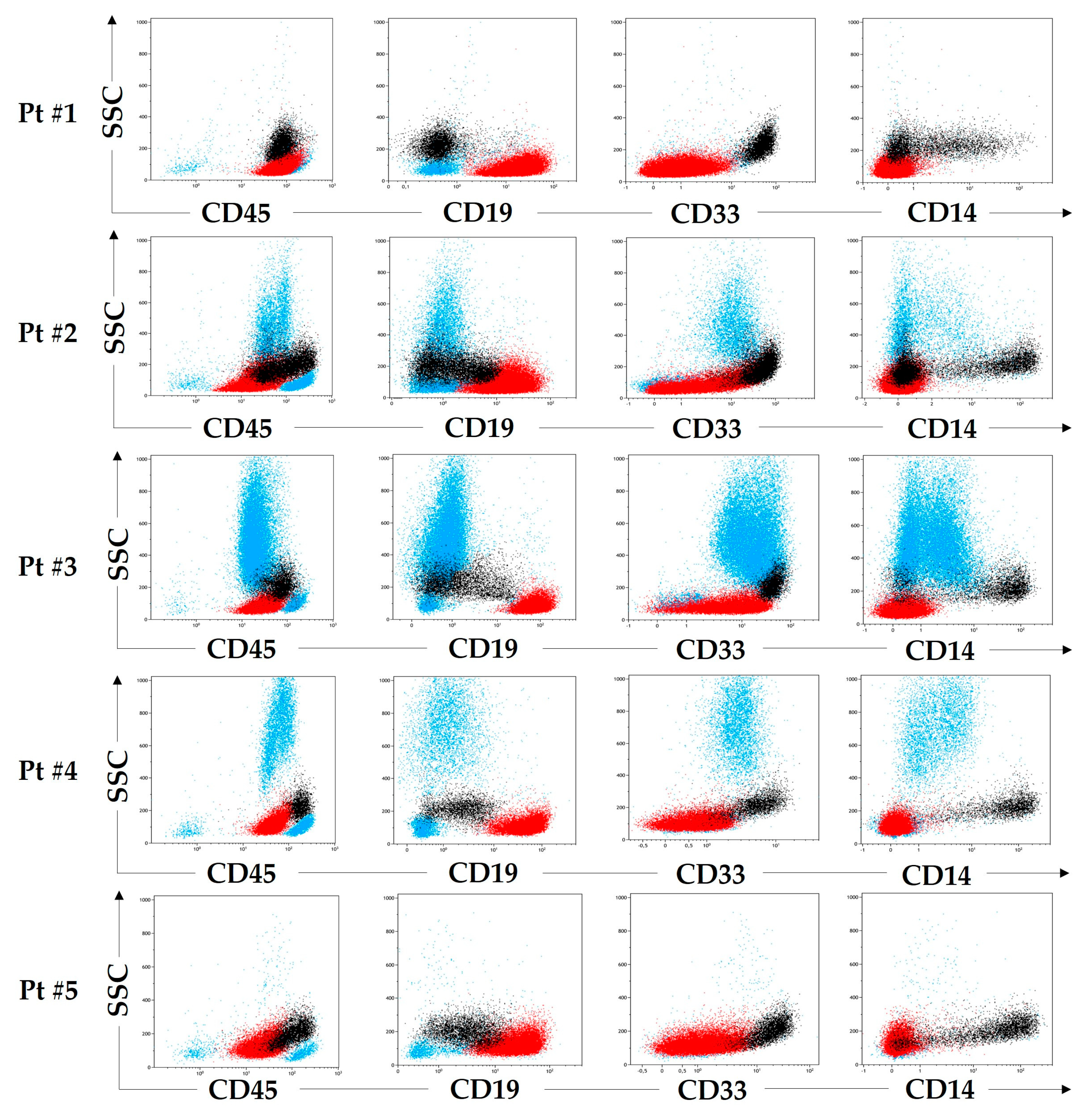
2.1. Patient #1
2.2. Patient #2
2.3. Patient #3
2.4. Patient #4
2.5. Patient #5
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Morphology and Cytochemistry
4.3. Flow Cytometry
4.4. Flow Cell Sorting
4.5. Fluorescence In Situ Hybridization
4.6. Detection of IG Gene Rearrangements by Multiplex PCR
4.7. Detection of Clonal Rearrangements of IG and TR Genes by Next-Generation Sequencing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinberg, O.K.; Arber, D.A. Mixed-phenotype acute leukemia: Historical overview and a new definition. Leukemia 2010, 24, 1844–1851. [Google Scholar] [CrossRef]
- Weir, E.G.; Ali Ansari-Lari, M.; Batista, D.A.; Griffin, C.A.; Fuller, S.; Smith, B.D.; Borowitz, M.J. Acute bilineal leukemia: A rare disease with poor outcome. Leukemia 2007, 21, 2264–2270. [Google Scholar] [CrossRef]
- Shi, R.; Munker, R. Survival of patients with mixed phenotype acute leukemias: A large population-based study. Leuk. Res. 2015, 39, 606–616. [Google Scholar] [CrossRef]
- Hrusak, O.; de Haas, V.; Stancikova, J.; Vakrmanova, B.; Janotova, I.; Mejstrikova, E.; Capek, V.; Trka, J.; Zalilova, M.; Luks, A.; et al. International cooperative study identifies treatment strategy in childhood ambiguous lineage leukemia. Blood 2018, 132, 264–276. [Google Scholar] [CrossRef]
- Matutes, E.; Pickl, W.F.; Van’t Veer, M.; Morilla, R.; Swansbury, J.; Strobl, H.; Attarbaschi, A.; Hopfinger, G.; Ashley, S.; Bene, M.C.; et al. Mixed-phenotype acute leukemia: Clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood 2011, 117, 3163–3171. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Charles, N.J.; Boyer, D.F. Mixed-phenotype acute leukemia: Diagnostic criteria and pitfalls. Arch. Pathol. Lab. Med. 2017, 141, 1462–1468. [Google Scholar] [CrossRef]
- Bene, M.C.; Porwit, A. Acute leukemias of ambiguous lineage. Semin. Diagn. Pathol. 2012, 29, 12–18. [Google Scholar] [CrossRef]
- Bene, M.C.; Castoldi, G.; Knapp, W.; Ludwig, W.D.; Matutes, E.; Orfao, A.; van’t Veer, M.B. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 1995, 9, 1783–1786. [Google Scholar]
- Bene, M.C.; Porwit, A. Mixed phenotype/lineage leukemia: Has anything changed for 2021 on diagnosis, classification, and treatment? Curr. Oncol. Rep. 2022, 24, 1015–1022. [Google Scholar] [CrossRef]
- Porwit, A.; Bene, M.C. Multiparameter flow cytometry applications in the diagnosis of mixed phenotype acute leukemia. Cytom. Part B Clin. Cytom. 2019, 96, 183–194. [Google Scholar] [CrossRef]
- Porwit, A.; Bene, M.C. Acute leukemias of ambiguous origin. Am. J. Clin. Pathol. 2015, 144, 361–376. [Google Scholar] [CrossRef]
- Tandon, S.; Visser, R.; Astwood, E.; Payne, J.; Gray, J.; Wheeler, L.; Irving, J.; Virgo, P. Paediatric ambiguous lineage leukaemia with monocytic differentiation at diagnosis: Case series and review of literature. Br. J. Haematol. 2022, 196, e34–e39. [Google Scholar] [CrossRef]
- Monma, F.; Nishii, K.; Ezuki, S.; Miyazaki, T.; Yamamori, S.; Usui, E.; Sugimoto, Y.; Lorenzo, V.F.; Katayama, N.; Shiku, H. Molecular and phenotypic analysis of Philadelphia chromosome-positive bilineage leukemia: Possibility of a lineage switch from T-lymphoid leukemic progenitor to myeloid cells. Cancer Genet. Cytogenet. 2006, 164, 118–121. [Google Scholar] [CrossRef]
- Kotrova, M.; Musilova, A.; Stuchly, J.; Fiser, K.; Starkova, J.; Mejstrikova, E.; Stary, J.; Zuna, J.; Hrusak, O.; Trka, J.; et al. Distinct bilineal leukemia immunophenotypes are not genetically determined. Blood 2016, 128, 2263–2266. [Google Scholar] [CrossRef]
- Alexander, T.B.; Gu, Z.; Iacobucci, I.; Dickerson, K.; Choi, J.K.; Xu, B.; Payne-Turner, D.; Yoshihara, H.; Loh, M.L.; Horan, J.; et al. The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature 2018, 562, 373–379. [Google Scholar] [CrossRef]
- Orfao, A.; Matarraz, S.; Pérez-Andrés, M.; Almeida, J.; Teodosio, C.; Berkowska, M.A.; van Dongen, J.J.M.; EuroFlow. Immunophenotypic dissection of normal hematopoiesis. J. Immunol. Methods 2019, 475, 112684. [Google Scholar] [CrossRef]
- Matarraz, S.; Almeida, J.; Flores-Montero, J.; Lécrevisse, Q.; Guerri, V.; López, A.; Bárrena, S.; Van Der Velden, V.H.J.; Te Marvelde, J.G.; Van Dongen, J.J.M.; et al. Introduction to the diagnosis and classification of monocytic-lineage leukemias by flow cytometry. Cytom. Part B Clin. Cytom. 2017, 92, 218–227. [Google Scholar] [CrossRef]
- Wagner-Ballon, O.; Bettelheim, P.; Lauf, J.; Bellos, F.; Della Porta, M.; Travaglino, E.; Subira, D.; Lopez, I.N.; Tarfi, S.; Westers, T.M.; et al. ELN iMDS flow working group validation of the monocyte assay for chronic myelomonocytic leukemia diagnosis by flow cytometry. Cytom. Part B Clin. Cytom. 2023, 104, 66–76. [Google Scholar] [CrossRef]
- Bene, M.C. Biphenotypic, bilineal, ambiguous or mixed lineage: Strange leukemias! Haematologica 2009, 94, 891–893. [Google Scholar] [CrossRef]
- Borowitz, M.J. Mixed phenotype acute leukemia. Cytom. Part B Clin. Cytom. 2014, 86, 152–153. [Google Scholar] [CrossRef]
- Semchenkova, A.; Mikhailova, E.; Komkov, A.; Gaskova, M.; Abasov, R.; Matveev, E.; Kazanov, M.; Mamedov, I.; Shmitko, A.; Belova, V.; et al. Lineage conversion in pediatric B-cell precursor acute leukemia under blinatumomab therapy. Int. J. Mol. Sci. 2022, 23, 4019. [Google Scholar] [CrossRef]
- Bartram, J.; Balasch-Carulla, M.; Bhojaraja, S.; Adams, S.; Cheng, D.; Inglott, S.; Kulkarni, N.; Mahendrayogam, A.; O’Connor, O.; Pavasovic, V.; et al. Blinatumomab for paediatric mixed phenotype acute leukaemia. Br. J. Haematol. 2021, 195, 289–292. [Google Scholar] [CrossRef]
- Øbro, N.F.; Ryder, L.P.; Madsen, H.O.; Andersen, M.K.; Lausen, B.; Hasle, H.; Schmiegelow, K.; Marquart, H.V. Identification of residual leukemic cells by flow cytometry in childhood B-cell precursor acute lymphoblastic leukemia: Verification of leukemic state by flow-sorting and molecular/cytogenetic methods. Haematologica 2012, 97, 137–141. [Google Scholar] [CrossRef]
- Øbro, N.F.; Madsen, H.O.; Ryder, L.P.; Andersen, M.K.; Schmiegelow, K.; Marquart, H.V. Approaches for cytogenetic and molecular analyses of small flow-sorted cell populations from childhood leukemia bone marrow samples. J. Immunol. Methods 2011, 369, 69–73. [Google Scholar] [CrossRef]
- Semchenkova, A.; Brilliantova, V.; Shelikhova, L.; Zhogov, V.; Illarionova, O.; Mikhailova, E.; Raykina, E.; Skorobogatova, E.; Novichkova, G.; Maschan, A.; et al. Chimerism evaluation in measurable residual disease-suspected cells isolated by flow cell sorting as a reliable tool for measurable residual disease verification in acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Cytom. Part B Clin. Cytom. 2020, 100, 568–573. [Google Scholar] [CrossRef]
- Mikhailova, E.; Semchenkova, A.; Illarionova, O.; Kashpor, S.; Brilliantova, V.; Zakharova, E.; Zerkalenkova, E.; Zangrando, A.; Bocharova, N.; Shelikhova, L.; et al. Relative expansion of CD19-negative very-early normal B-cell precursors in children with acute lymphoblastic leukaemia after CD19 targeting by blinatumomab and CAR-T cell therapy: Implications for flow cytometric detection of minimal residual disease. Br. J. Haematol. 2021, 193, 602–612. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef]
- Novikova, I.; Verzhbitskaya, T.; Movchan, L.; Tsaur, G.; Belevtsev, М.; Popov, A. Russian-Belarusian multicenter group standard guidelines for childhood acute lymphoblastic leukemia flow cytometric diagnostics. Oncohematology 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Kalina, T.; Flores-Montero, J.; Lecrevisse, Q.; Pedreira, C.E.; van der Velden, V.H.; Novakova, M.; Mejstrikova, E.; Hrusak, O.; Böttcher, S.; Karsch, D.; et al. Quality assessment program for EuroFlow protocols: Summary results of four-year (2010–2013) quality assurance rounds. Cytom. Part A 2015, 87, 145–156. [Google Scholar] [CrossRef]
- den Nijs, J.I.; Gonggrijp, H.S.; Augustinus, E.; Leeksma, C.H. Hot bands: A simple G-banding method for leukemic metaphases. Cancer Genet. Cytogenet. 1985, 15, 373–374. [Google Scholar] [CrossRef]
- Langerak, A.W.; Groenen, P.J.; Brüggemann, M.; Beldjord, K.; Bellan, C.; Bonello, L.; Boone, E.; Carter, G.I.; Catherwood, M.; Davi, F.; et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 2012, 26, 2159–2171. [Google Scholar] [CrossRef]
- Komkov, A.; Miroshnichenkova, A.; Nugmanov, G.; Popov, A.; Pogorelyy, M.; Zapletalova, E.; Jelinkova, H.; Pospisilova, S.; Lebedev, Y.; Chudakov, D.; et al. High-throughput sequencing of T-cell receptor alpha chain clonal rearrangements at the DNA level in lymphoid malignancies. Br. J. Haematol. 2020, 188, 723–731. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semchenkova, A.; Zerkalenkova, E.; Demina, I.; Kashpor, S.; Volchkov, E.; Zakharova, E.; Larin, S.; Olshanskaya, Y.; Novichkova, G.; Maschan, A.; et al. Recognizing Minor Leukemic Populations with Monocytic Features in Mixed-Phenotype Acute Leukemia by Flow Cell Sorting Followed by Cytogenetic and Molecular Studies: Report of Five Exemplary Cases. Int. J. Mol. Sci. 2023, 24, 5260. https://doi.org/10.3390/ijms24065260
Semchenkova A, Zerkalenkova E, Demina I, Kashpor S, Volchkov E, Zakharova E, Larin S, Olshanskaya Y, Novichkova G, Maschan A, et al. Recognizing Minor Leukemic Populations with Monocytic Features in Mixed-Phenotype Acute Leukemia by Flow Cell Sorting Followed by Cytogenetic and Molecular Studies: Report of Five Exemplary Cases. International Journal of Molecular Sciences. 2023; 24(6):5260. https://doi.org/10.3390/ijms24065260
Chicago/Turabian StyleSemchenkova, Alexandra, Elena Zerkalenkova, Irina Demina, Svetlana Kashpor, Egor Volchkov, Elena Zakharova, Sergey Larin, Yulia Olshanskaya, Galina Novichkova, Alexey Maschan, and et al. 2023. "Recognizing Minor Leukemic Populations with Monocytic Features in Mixed-Phenotype Acute Leukemia by Flow Cell Sorting Followed by Cytogenetic and Molecular Studies: Report of Five Exemplary Cases" International Journal of Molecular Sciences 24, no. 6: 5260. https://doi.org/10.3390/ijms24065260
APA StyleSemchenkova, A., Zerkalenkova, E., Demina, I., Kashpor, S., Volchkov, E., Zakharova, E., Larin, S., Olshanskaya, Y., Novichkova, G., Maschan, A., Maschan, M., & Popov, A. (2023). Recognizing Minor Leukemic Populations with Monocytic Features in Mixed-Phenotype Acute Leukemia by Flow Cell Sorting Followed by Cytogenetic and Molecular Studies: Report of Five Exemplary Cases. International Journal of Molecular Sciences, 24(6), 5260. https://doi.org/10.3390/ijms24065260





