A Sulfur Containing Melanogenesis Substrate, N-Pr-4-S-CAP as a Potential Source for Selective Chemoimmunotherapy of Malignant Melanoma
Abstract
1. Introduction
2. Results
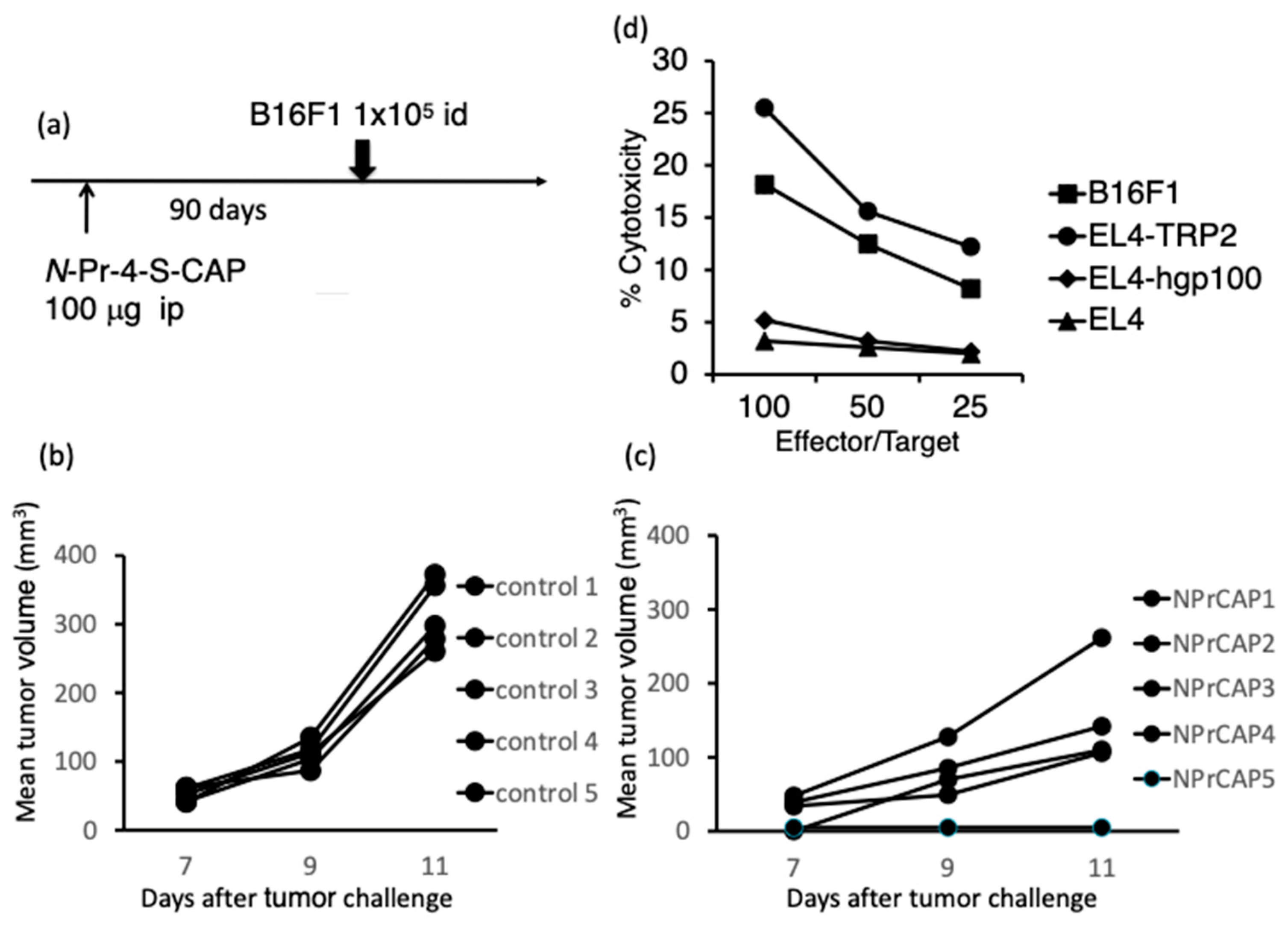
2.1. Administration of N-Pr-4-S-CAP Induced an Anti-Melanoma Immune Response in Naïve Mice
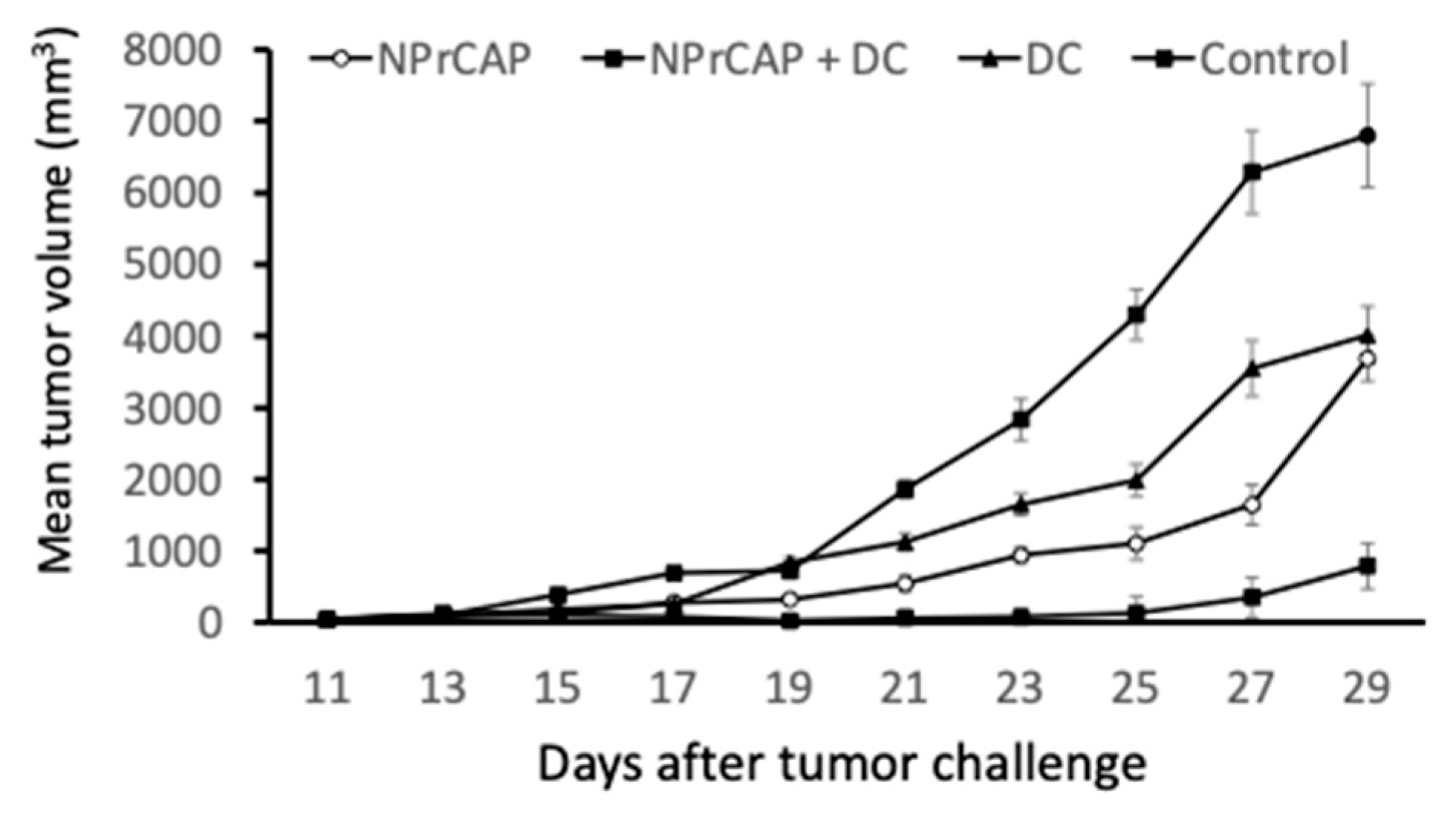
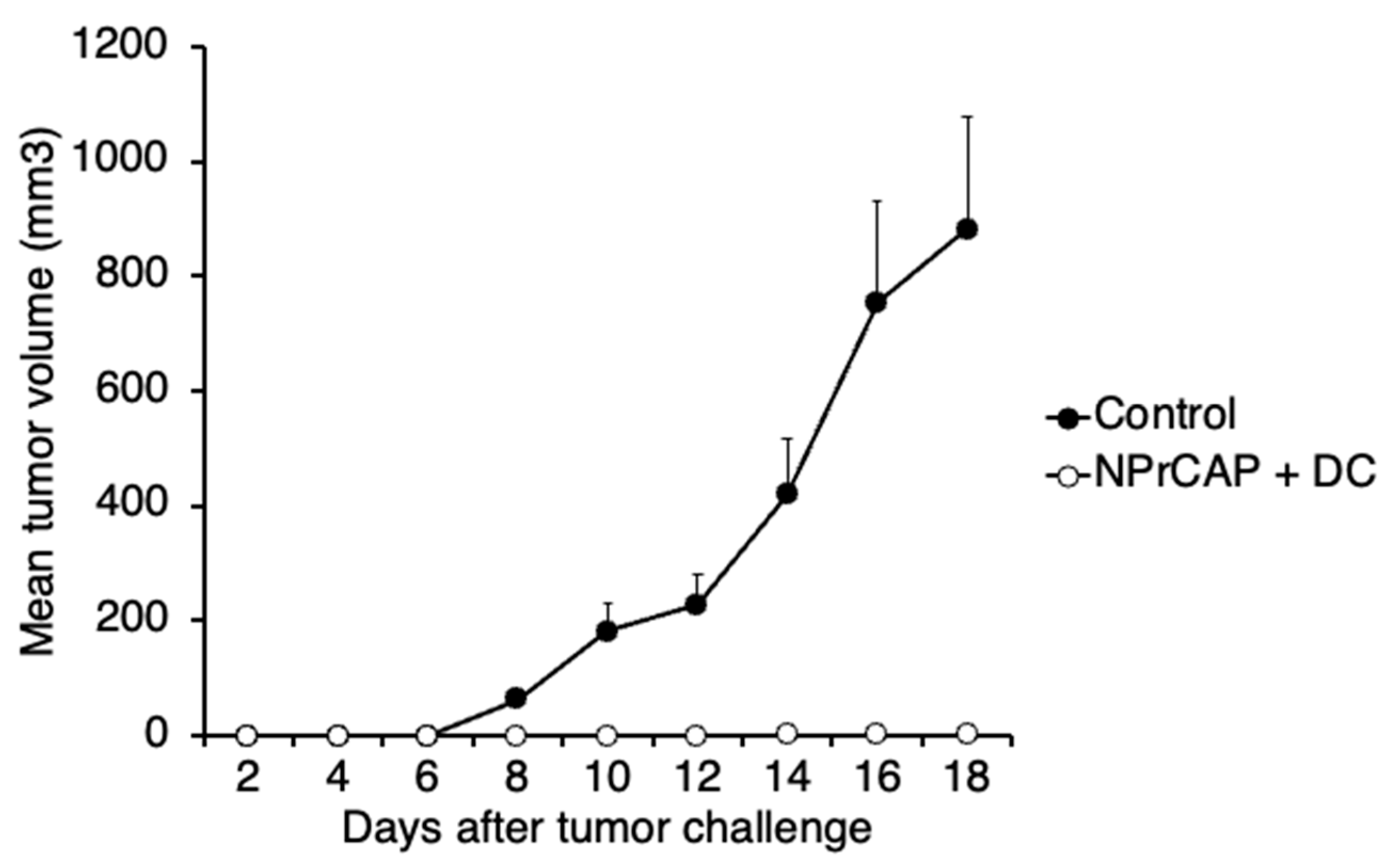
2.2. Intratumoral Injection of BMDCs in Combination with N-Pr-4-S-CAP Treatment Resulted in Marked Suppression of Melanoma Growth
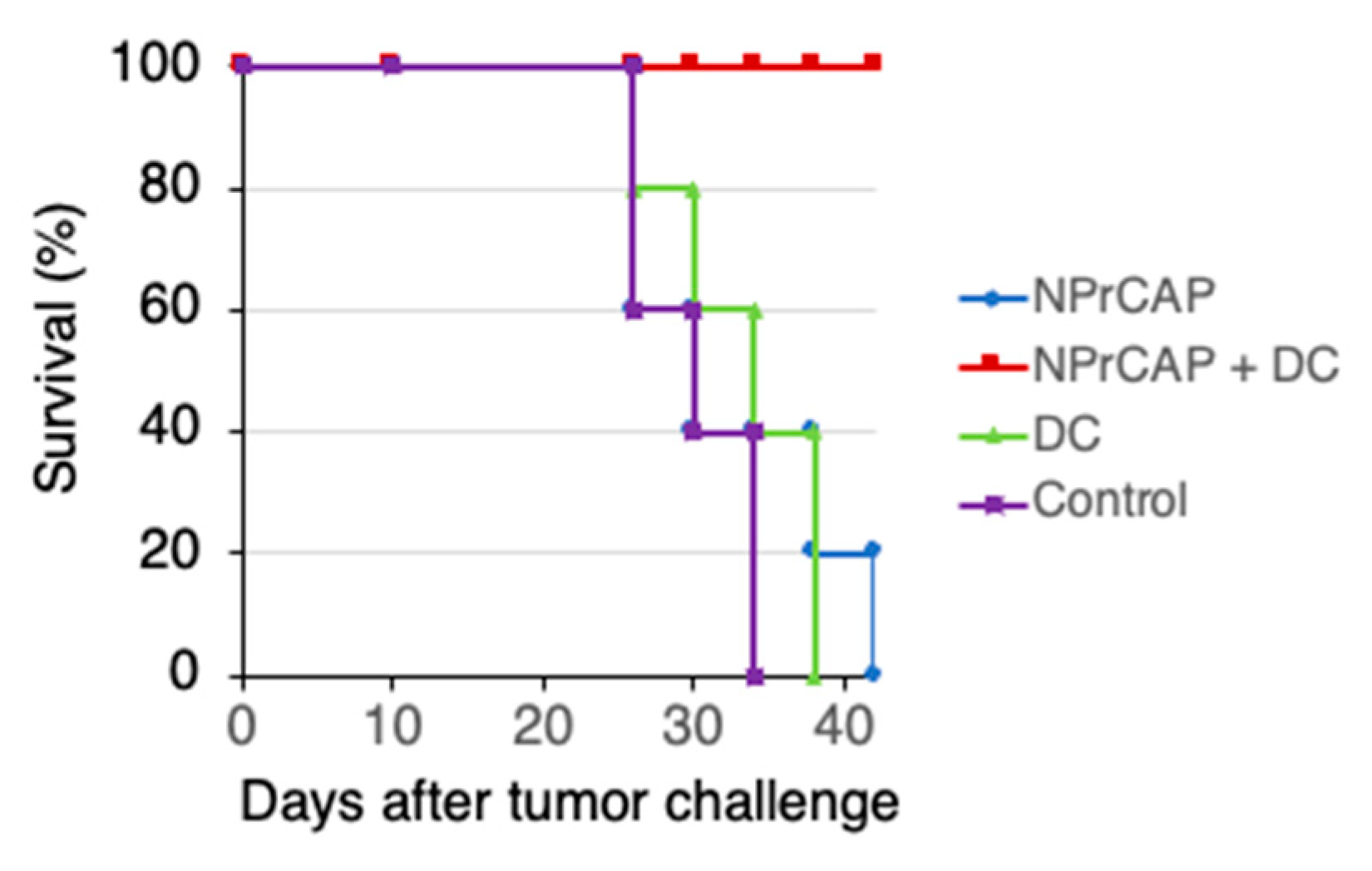
2.3. Combination Therapy Using N-Pr-4-S-CAP and DCs Elicited Anti-tumor Immunity
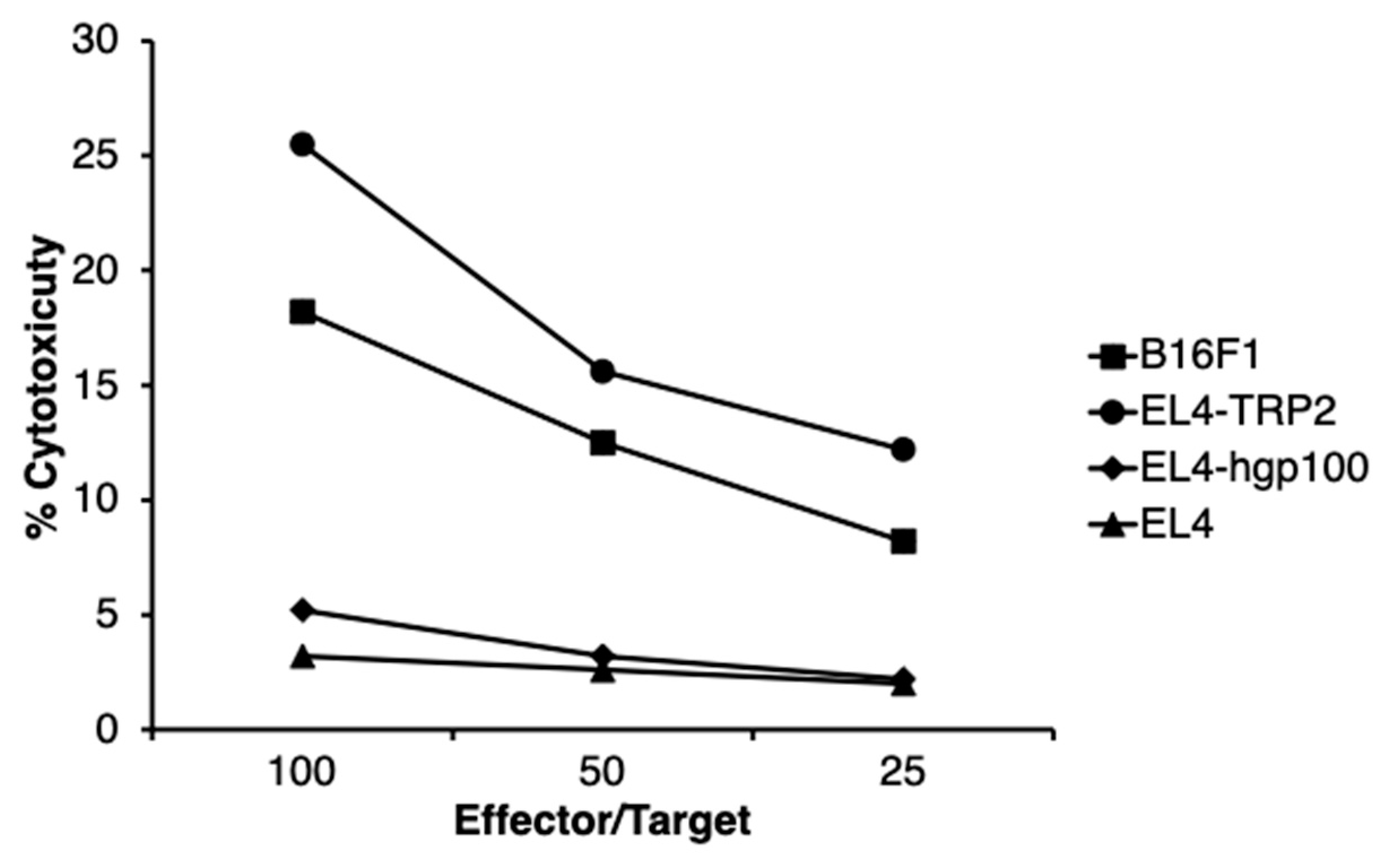
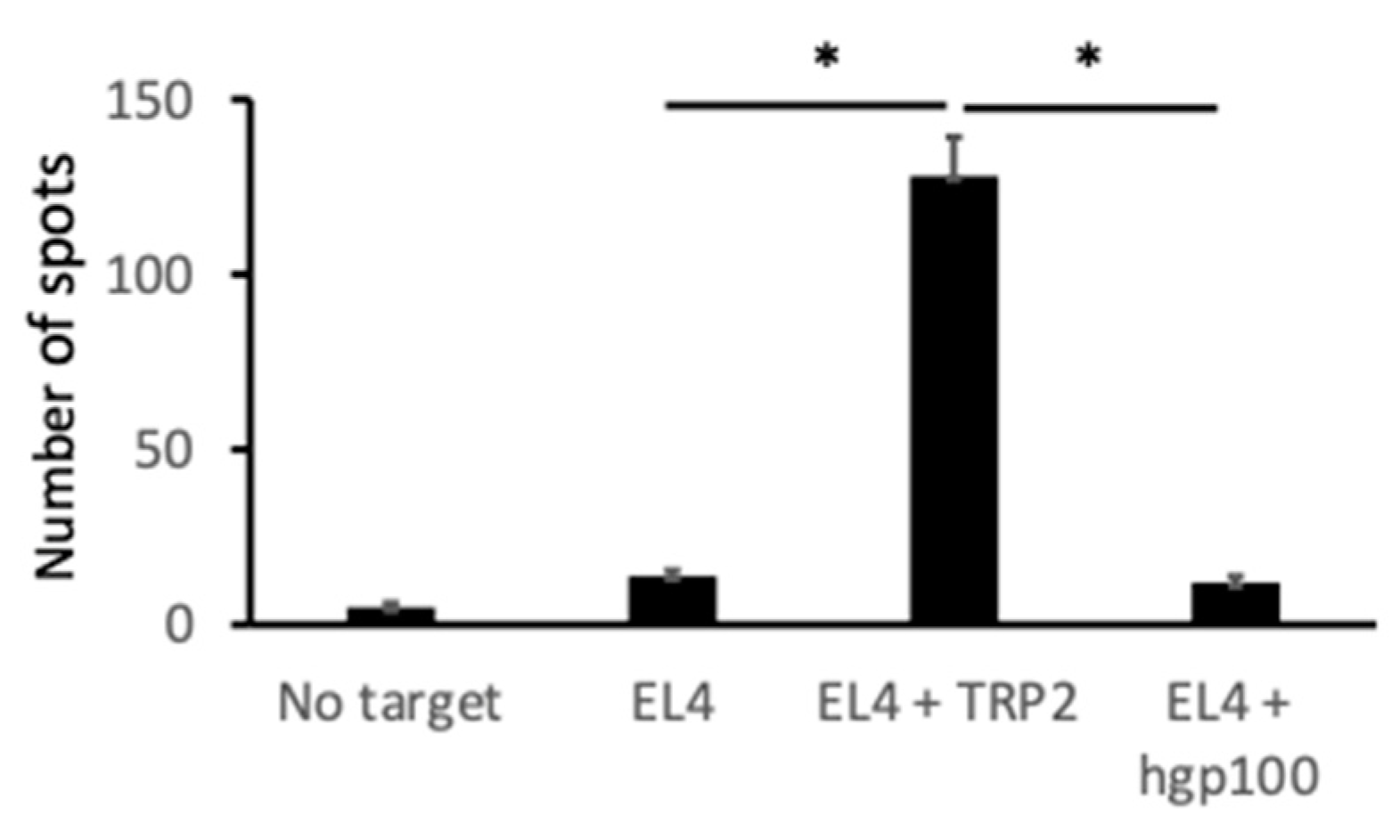
2.4. Melanoma-Specific Antigen TYRP2-Specific Cytotoxic T Cells Were Induced in Mice Treated with N-Pr-4-S-CAP and DCs
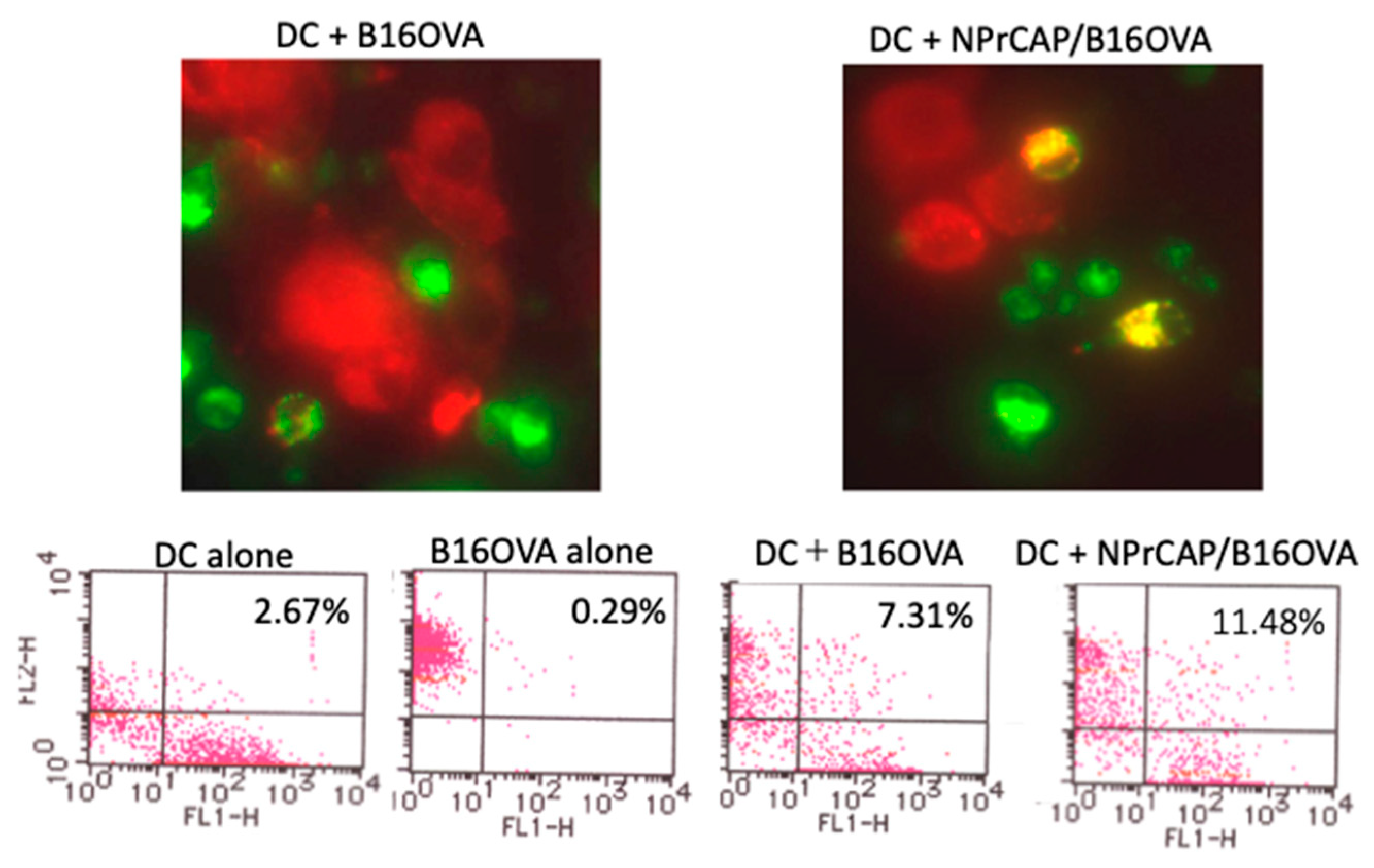
2.5. BMDCs Phagocytosed N-Pr-4-S-CAP-Treated Melanoma Cells and Cross-Presented Melanoma-Specific Antigen to CD8+ T Cells
3. Discussion
4. Materials and Methods
4.1. Mice and Cells
4.2. Peptides
4.3. Preparation of N-Pr-4-S-CAP (NPrCAP)
4.4. Animal Models for Tumor Formation
4.5. In Vivo T Cell Depletion Assay
4.6. In Vitro Cytotoxicity Assay
4.7. ELISPOT Assay
4.8. BMDC Phagocytosis Assay
4.9. In Vitro Cross-Presentation Assay
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Alternating magnetic field. |
| NAcCAP | N-acetyl-4-S-cysteaminylphenol. |
| BMDCs | Bone marrow-derived dendritic cells. |
| CTI | Chemo–thermo–immuno. |
| CTLs | Cytotoxic T cells. |
| 4-S-CAP | 4-S-cysteaminylphenol. |
| DMEM | Dulbecco’s modified Eagle’s medium. |
| HSPs | Heat shock proteins. |
| N-Pr-4-S-CAP (NPrCAP) | N-propionyl-4-S-cysteaminylphenol. |
| OVA | Ovalbumin. |
| ROS | Reactive oxygen species. |
References
- Shah, D.J.; Dronca, R.S. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin. Proc. 2014, 89, 504–519. [Google Scholar] [CrossRef]
- Uhara, H. Recent advances in therapeutic strategies for unresectable or metastatic melanoma and real-world data in Japan. Int. J. Clin. Oncol. 2019, 24, 1508–1514. [Google Scholar] [CrossRef]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of melanogenesis cascade for identifying pathophysiology and therapeutic approach of pigmentary disorders and melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef]
- Gili, A.; Thomas, P.D.; Ota, M.; Jimbow, K. Comparison of in vitro cytotoxicity of N-acetyl and N-propionyl derivatives of phenolic thioether amines in melanoma and neuroblastoma cells and the relationship to tyrosinase and tyrosine hydroxylase enzyme activity. Melanoma Res. 2000, 10, 9–15. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, A.; Tamura, Y.; Hida, T.; Kamiya, T.; Torigoe, T.; Honda, H.; Ito, S.; Jimbow, K. Molecular events in the melanogenesis cascade as novel melanoma-targeted small molecules: Principle and development. Cancers 2022, 14, 5588. [Google Scholar] [CrossRef]
- Jimbow, K. N-acetyl-4-S-cysteaminylphenol as a new type of depigmenting agent for the melanoderma of patients with melasma. Arch. Dermatol. 1991, 127, 1528–1534. [Google Scholar] [CrossRef]
- Jimbow, K.; Iwashina, T.; Alena, F.; Yamada, K.; Pankovich, J.; Umemura, T. Exploitation of pigment biosynthesis pathway as a selective chemotherapeutic approach for malignant melanoma. J. Investig. Dermatol. 1993, 100, 231S–238S. [Google Scholar] [CrossRef]
- Tandon, M.; Thomas, P.D.; Shokravi, M.; Singh, S.; Samra, S.; Chang, D.; Jimbow, K. Synthesis and antitumour effect of the melanogenesis-based antimelanoma agent N-propionyl-4-S-cysteaminylphenol. Biochem. Pharmacol. 1998, 55, 2023–2029. [Google Scholar] [CrossRef]
- Thomas, P.D.; Kishi, H.; Cao, H.; Ota, M.; Yamashita, T.; Singh, S.; Jimbow, K. Selective incorporation and specific cytocidal effect as the cellular basis for the antimelanoma action of sulphur containing tyrosine analogs. J. Investig. Dermatol. 1999, 113, 928–934. [Google Scholar] [CrossRef]
- Miura, S.; Ueda, T.; Jimbow, K.; Ito, S.; Fujita, K. Synthesis of cysteinylphenol, cysteaminylphenol, and related compounds, and in vivo evaluation of antimelanoma effect. Arch. Dermatol. Res. 1987, 279, 219–225. [Google Scholar] [CrossRef]
- Yamada, I.; Seki, S.; Matsubara, O.; Ito, S.; Suzuki, S.; Kasuga, T. The cytotoxicity of cysteinylcatechols and related compounds to human melanoma cells in vitro. J. Investig. Dermatol. 1987, 88, 538–540. [Google Scholar] [CrossRef]
- Inoue, S.; Ito, S.; Wakamatsu, K.; Jimbow, K.; Fujita, K. Mechanism of growth inhibition of melanoma cells by 4-S-cysteaminylphenol and its analogues. Biochem. Pharmacol. 1990, 39, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Ishii-Osai, Y.; Yamashita, T.; Tamura, Y.; Sato, N.; Ito, A.; Honda, H.; Wakamatsu, K.; Ito, S.; Nakayama, E.; Okura, M.; et al. N-propionyl-4-S-cysteaminylphenol induces apoptosis in B16F1 cells and mediates tumor-specific T-cell immune responses in a mouse melanoma model. J. Dermatol. Sci. 2012, 67, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Jimbow, K. Selective cytotoxicity of 4-S-cysteaminylphenol on follicular melanocytes of the black mouse: Rational basis for its application to melanoma chemotherapy. Cancer Res. 1987, 47, 3278–3284. [Google Scholar]
- Pankovich, J.M.; Jimbow, K. Tyrosine transport in a human melanoma cell line as a basis for selective transport of cytotoxic analogues. Biochem. J. 1991, 280, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Pankovich, J.M.; Jimbow, K.; Ito, S. 4-S-cysteaminylphenol and its analogues as substrates for tyrosinase and monoamine oxidase. Pigment Cell Res. 1990, 3, 146–149. [Google Scholar] [CrossRef]
- Ito, S.; Nishigaki, A.; Ishii-Osai, Y.; Ojika, M.; Wakamatsu, K.; Yamashita, T.; Tamura, Y.; Ito, A.; Honda, H.; Nakayama, E.; et al. Mechanism of putative neo-antigen formation from N-propionyl-4-S-cysteaminylphenol, a tyrosinase substrate, in melanoma models. Biochem. Pharmacol. 2012, 84, 646–653. [Google Scholar] [CrossRef]
- Takada, T.; Yamashita, T.; Sato, M.; Sato, A.; Ono, I.; Tamura, Y.; Sato, N.; Miyamoto, A.; Ito, A.; Honda, H.; et al. Growth inhibition of re-challenge B16 melanoma transplant by conjugates of melanogenesis substrate and magnetite nanoparticles as the basis for developing melanoma-targeted chemo-thermo-immunotherapy. J. Biomed. Biotechnol. 2009, 2009, 457936. [Google Scholar] [CrossRef]
- Sato, M.; Yamashita, T.; Ohkura, M.; Osai, Y.; Sato, A.; Takada, T.; Matsusaka, H.; Ono, I.; Tamura, Y.; Sato, N.; et al. N-propionyl-cysteaminylphenol-magnetite conjugate (NPrCAP/M) is a nanoparticle for the targeted growth suppression of melanoma cells. J. Investig. Dermatol. 2009, 129, 2233–2241. [Google Scholar] [CrossRef]
- Ito, A.; Honda, H.; Kobayashi, T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol. Immunother. 2006, 55, 320–328. [Google Scholar] [CrossRef]
- Sato, A.; Tamura, Y.; Sato, N.; Yamashita, T.; Takada, T.; Sato, M.; Osai, Y.; Okura, M.; Ono, I.; Ito, A.; et al. Melanoma-targeted chemo-thermo-immuno (CTI)-therapy using N-propionyl-4-S-cysteaminylphenol-magnetite nanoparticles elicits CTL response via heat shock protein-peptide complex release. Cancer Sci. 2010, 101, 1939–1946. [Google Scholar] [CrossRef]
- Tamura, Y.; Ito, A.; Wakamatsu, K.; Kamiya, T.; Torigoe, T.; Honda, H.; Yamashita, T.; Uhara, H.; Ito, S.; Jimbow, K. Immunomodulation of melanoma by chemo-thermo-immunotherapy using conjugates of melanogenesis substrate NPrCAP and magnetite nanoparticles: A Review. Int. J. Mol. Sci. 2022, 23, 6457. [Google Scholar] [CrossRef] [PubMed]
- Jimbow, K.; Ishii-Osai, Y.; Ito, S.; Tamura, Y.; Ito, A.; Yoneta, A.; Kamiya, T.; Yamashita, T.; Honda, H.; Wakamatsu, K.; et al. Melanoma-targeted chemothermotherapy and in situ peptide immunotherapy through HSP production by using melanogenesis substrate, NPrCAP, and magnetite nanoparticles. J. Skin. Cancer 2013, 2013, 742925. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Peng, P.; Liu, K.; Daou, M.; Srivastava, P.K. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science 1997, 278, 117–120. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Menoret, A.; Basu, S.; Binder, R.J.; McQuade, K.L. Heat shock proteins come of age: Primitive functions acquire new roles in an adaptive world. Immunity 1998, 8, 657–665. [Google Scholar] [CrossRef]
- Li, Z.; Menoret, A.; Srivastava, P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr. Opin. Immunol. 2002, 14, 45–51. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Yoshikawa, K.; Saga, S.; Wakabayashi, T.; Yoshida, J.; Kobayashi, T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol. Immunother 2003, 52, 80–88. [Google Scholar] [CrossRef]
- Ito, A.; Tanaka, K.; Kondo, K.; Shinkai, M.; Honda, H.; Matsumoto, K.; Saida, T.; Kobayashi, T. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci. 2003, 94, 308–313. [Google Scholar] [CrossRef]
- Mangana, J.; Cheng, P.F.; Kaufmann, C.; Amann, V.C.; Frauchiger, A.L.; Stögner, V.; Held, U.; von Moos, R.; Michielin, O.; Braun, R.P.; et al. Multicenter, real-life experience with checkpoint inhibitors and targeted therapy agents in advanced melanoma patients in Switzerland. Melanoma Res. 2017, 27, 358–368. [Google Scholar] [CrossRef]
- Alena, F.; Iwashina, T.; Gili, A.; Jimbow, K. Selective in vivo accumulation of N-acetyl-4-S-cysteaminylphenol in B16F10 murine melanoma and enhancement of its in vitro and in vivo antimelanoma effect by combination of buthionine sulfoximine. Cancer Res. 1994, 54, 2661–2666. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, Y.; Ito, A.; Wakamatsu, K.; Torigoe, T.; Honda, H.; Ito, S.; Jimbow, K. A Sulfur Containing Melanogenesis Substrate, N-Pr-4-S-CAP as a Potential Source for Selective Chemoimmunotherapy of Malignant Melanoma. Int. J. Mol. Sci. 2023, 24, 5235. https://doi.org/10.3390/ijms24065235
Tamura Y, Ito A, Wakamatsu K, Torigoe T, Honda H, Ito S, Jimbow K. A Sulfur Containing Melanogenesis Substrate, N-Pr-4-S-CAP as a Potential Source for Selective Chemoimmunotherapy of Malignant Melanoma. International Journal of Molecular Sciences. 2023; 24(6):5235. https://doi.org/10.3390/ijms24065235
Chicago/Turabian StyleTamura, Yasuaki, Akira Ito, Kazumasa Wakamatsu, Toshihiko Torigoe, Hiroyuki Honda, Shosuke Ito, and Kowichi Jimbow. 2023. "A Sulfur Containing Melanogenesis Substrate, N-Pr-4-S-CAP as a Potential Source for Selective Chemoimmunotherapy of Malignant Melanoma" International Journal of Molecular Sciences 24, no. 6: 5235. https://doi.org/10.3390/ijms24065235
APA StyleTamura, Y., Ito, A., Wakamatsu, K., Torigoe, T., Honda, H., Ito, S., & Jimbow, K. (2023). A Sulfur Containing Melanogenesis Substrate, N-Pr-4-S-CAP as a Potential Source for Selective Chemoimmunotherapy of Malignant Melanoma. International Journal of Molecular Sciences, 24(6), 5235. https://doi.org/10.3390/ijms24065235







