Pillararenes as Promising Carriers for Drug Delivery
Abstract
1. Introduction
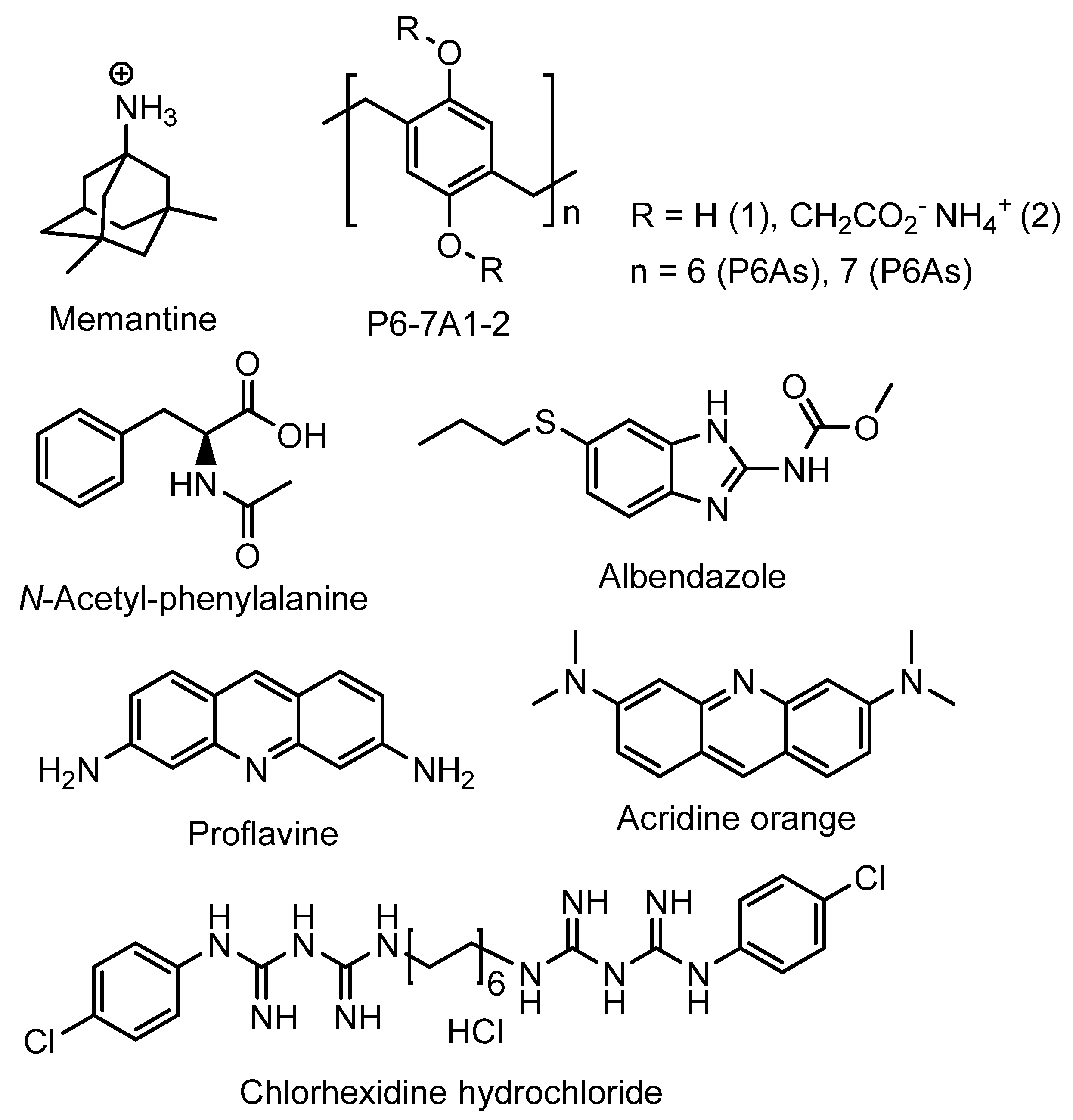
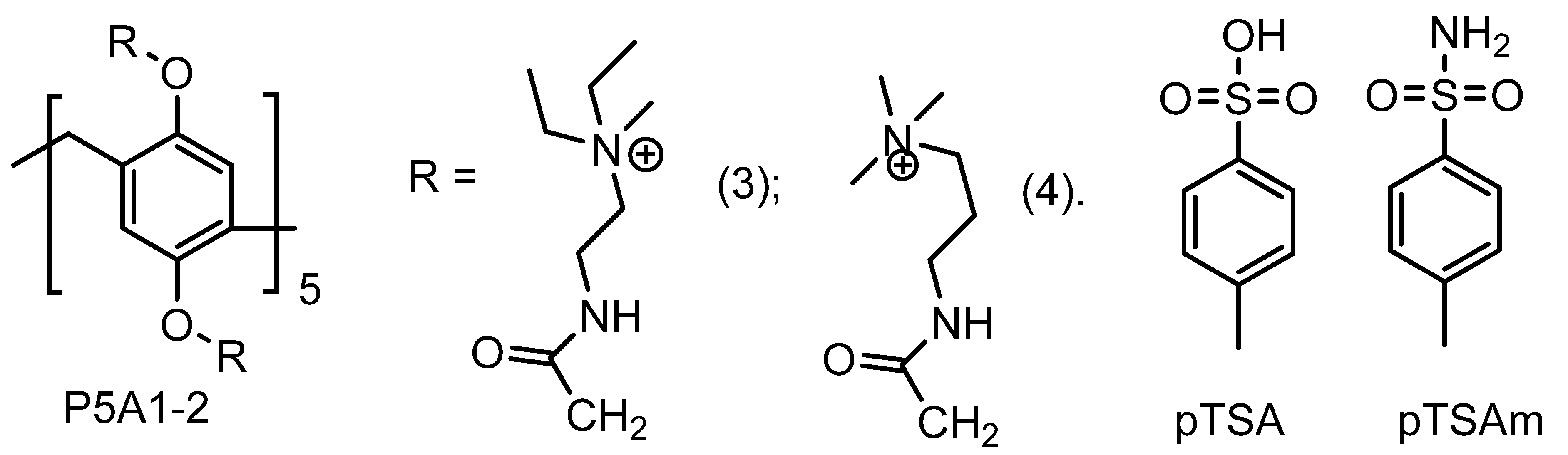
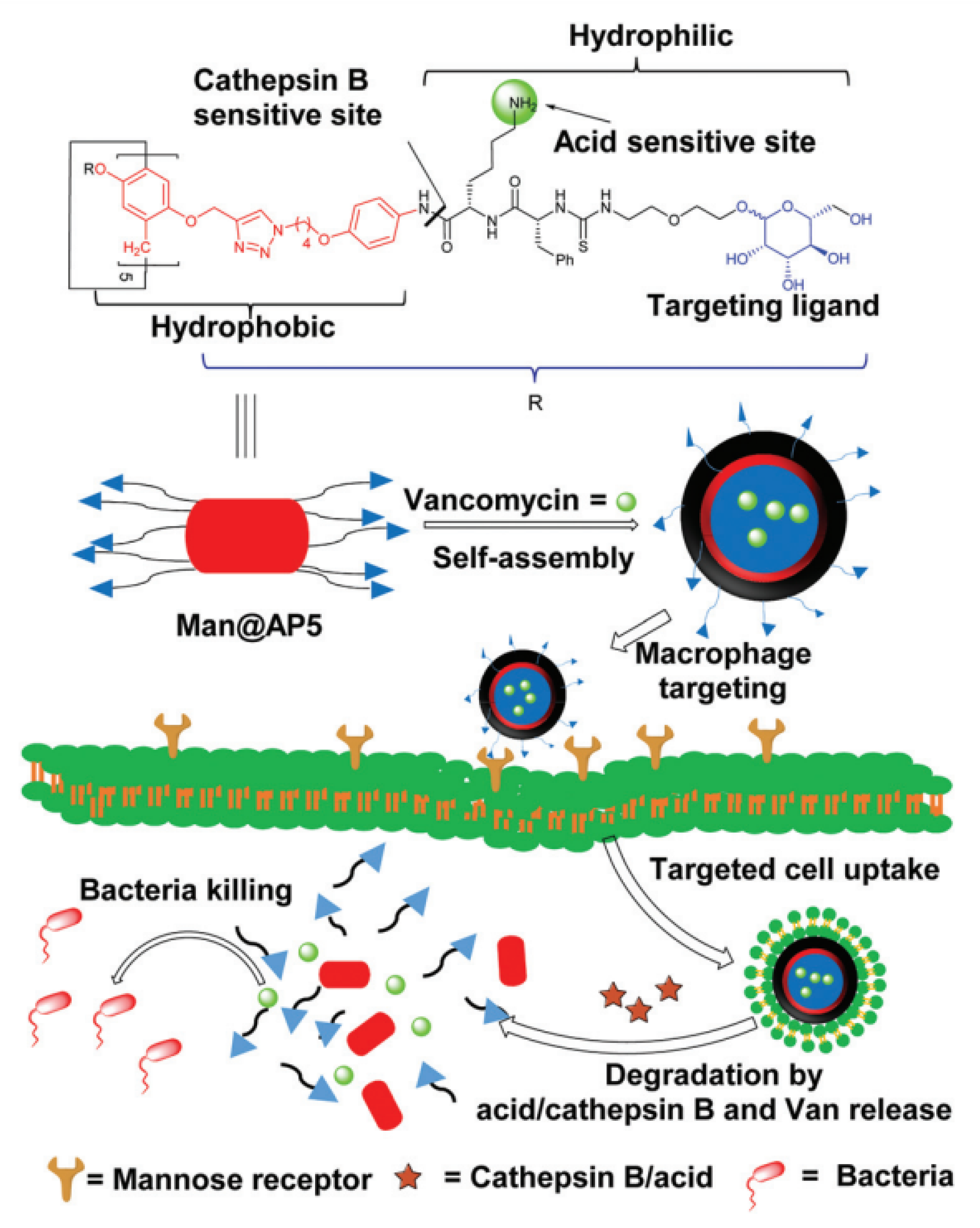
2. Pillararene-Based Antibacterial Systems
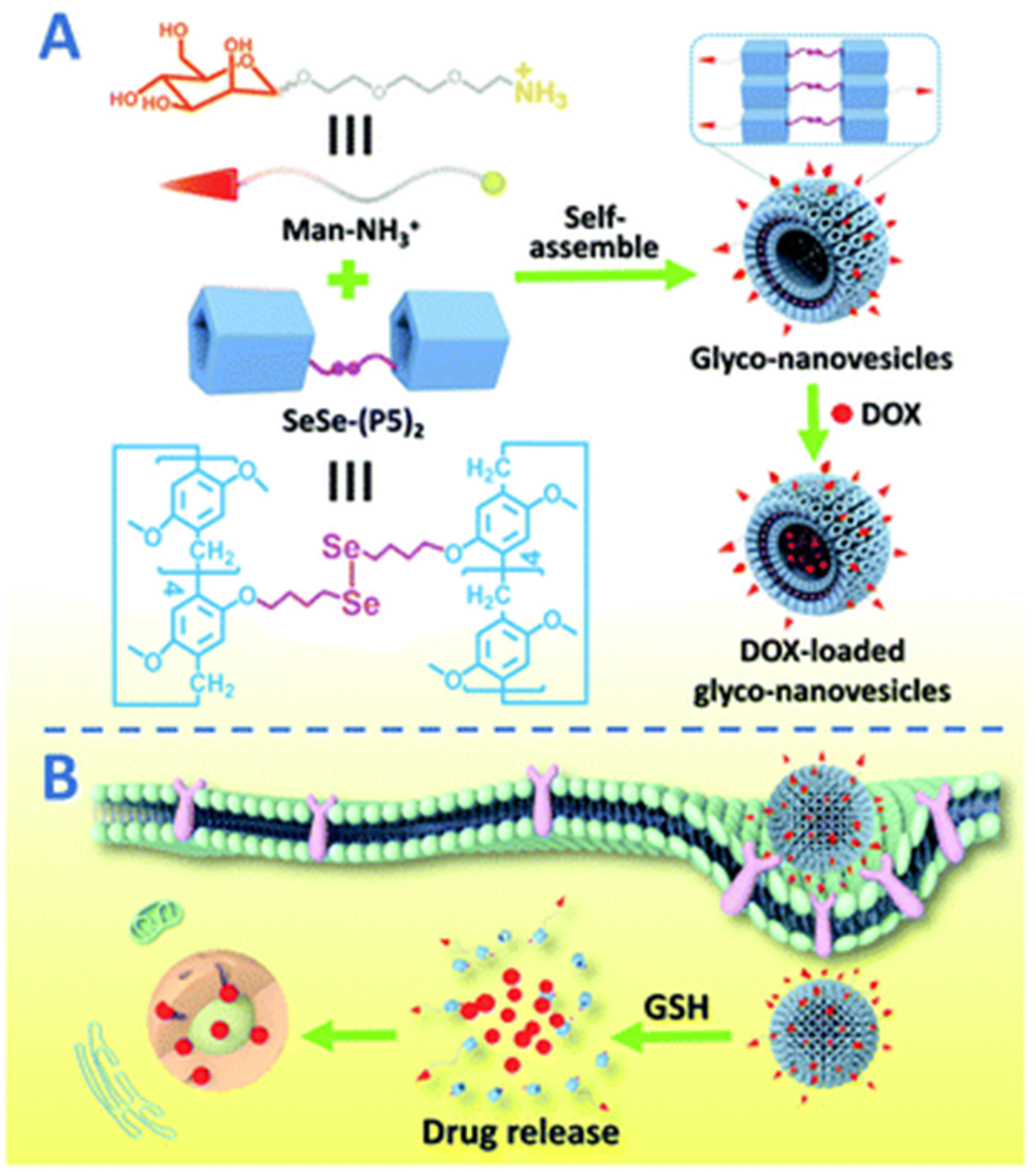
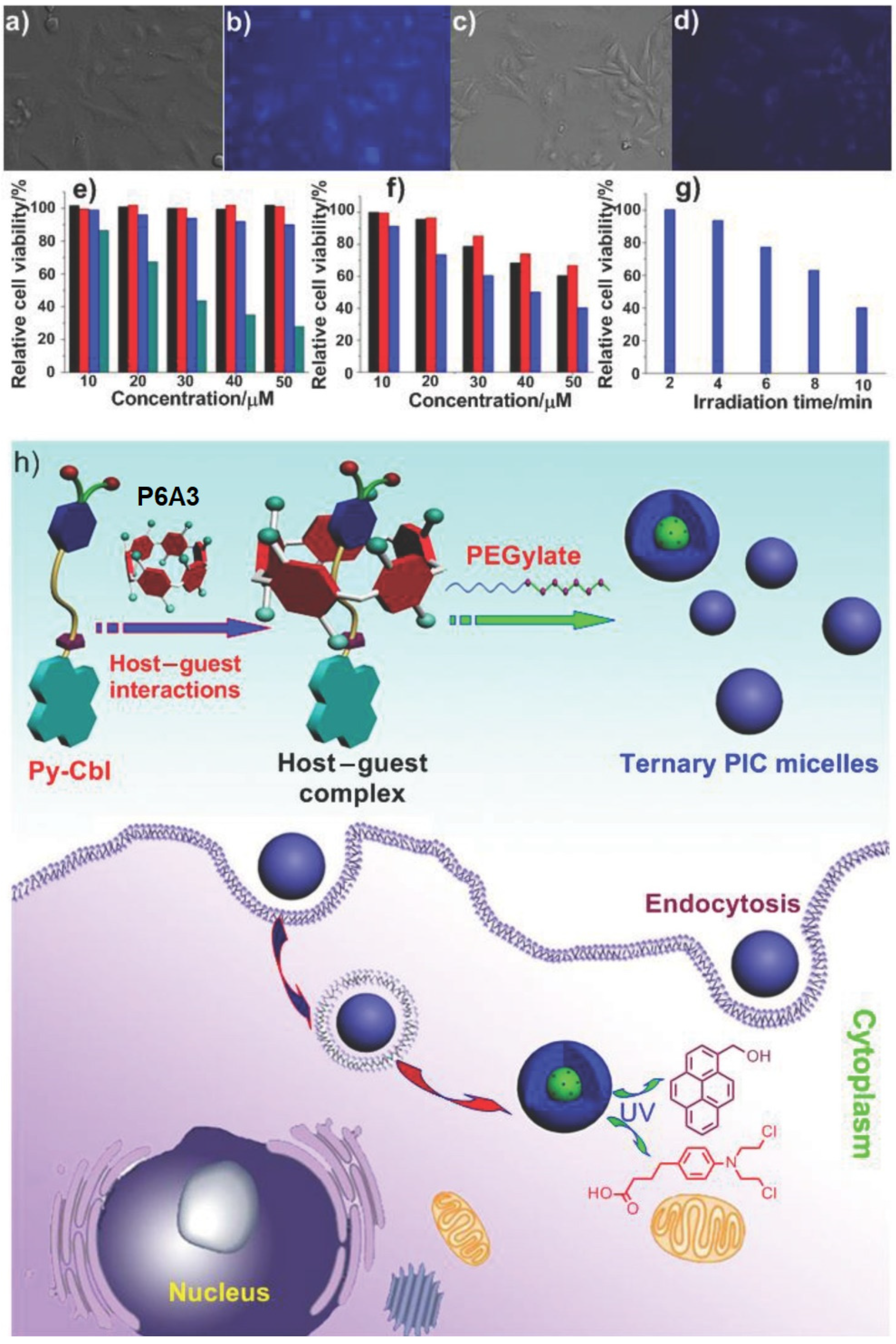
3. Pillararene-Based Systems for the Targeted Chemotherapy of Cancer
4. (Bio)thiol-Triggered Pillararene Drug Delivery Systems
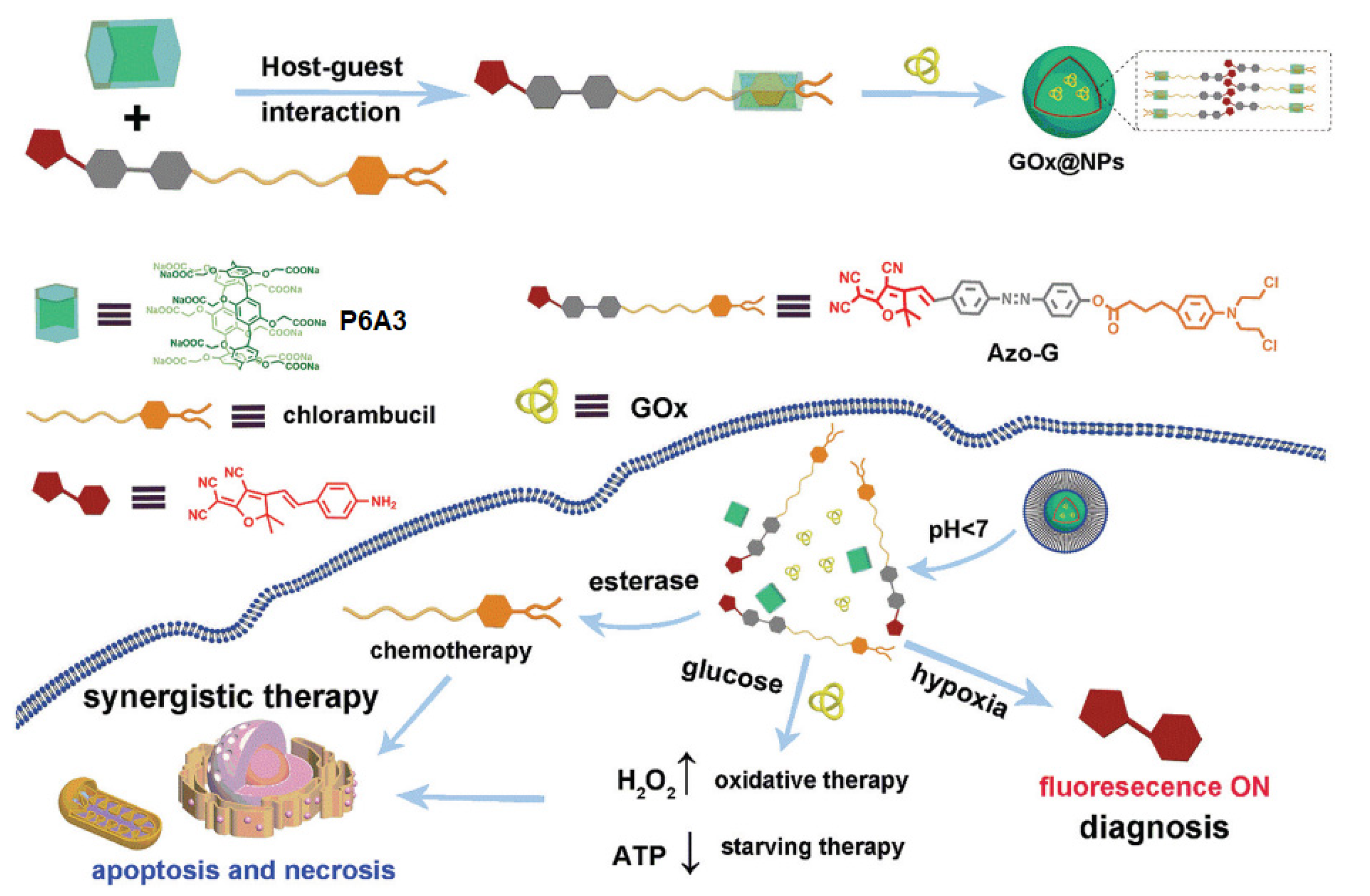
5. Hypoxia-Driven Drug Release Pillararene-Based Systems
6. ATP-Triggered Drug Release Pillararene-Based Systems
7. pH-Controlled Pillararene-Based Systems
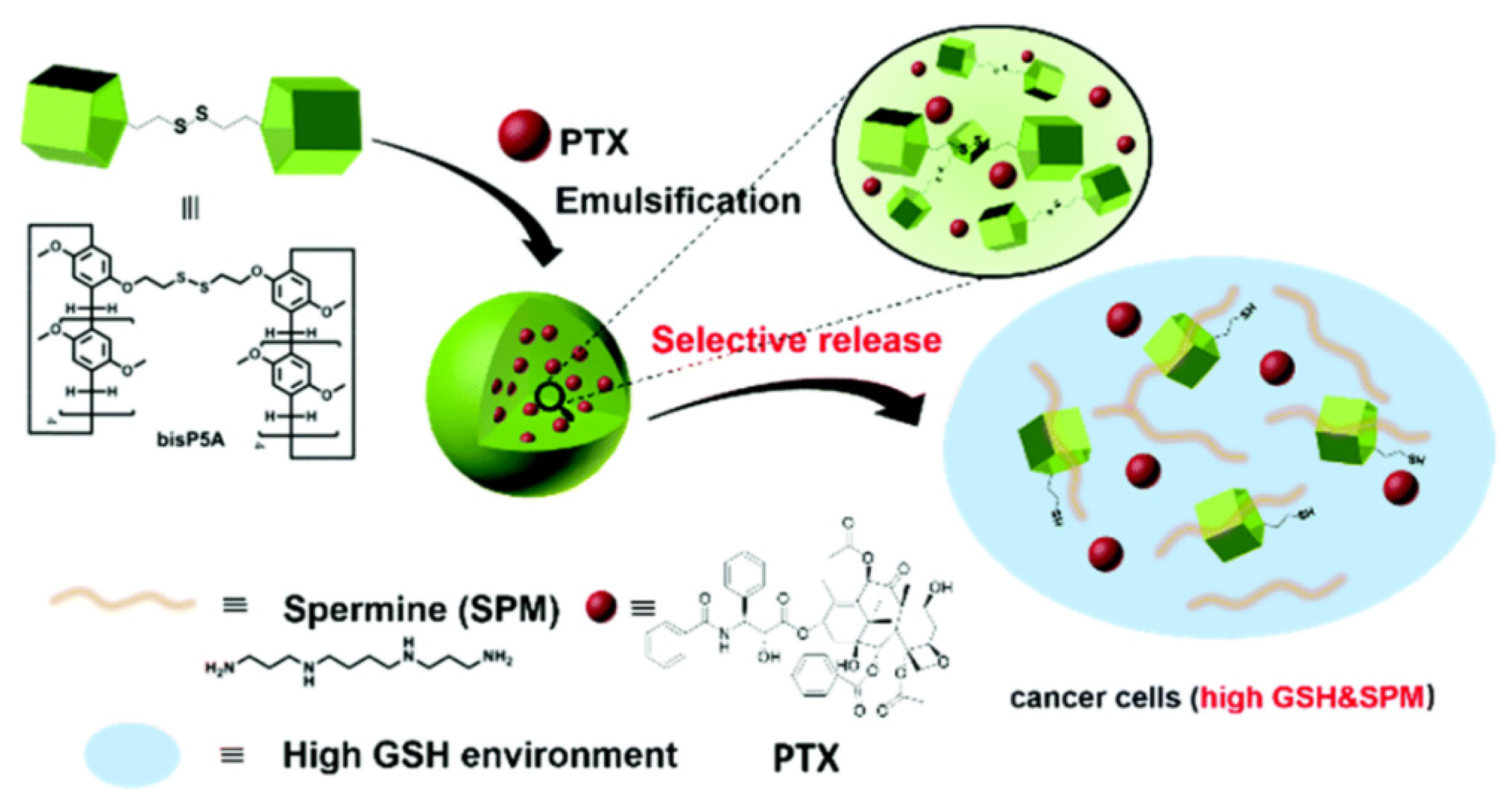
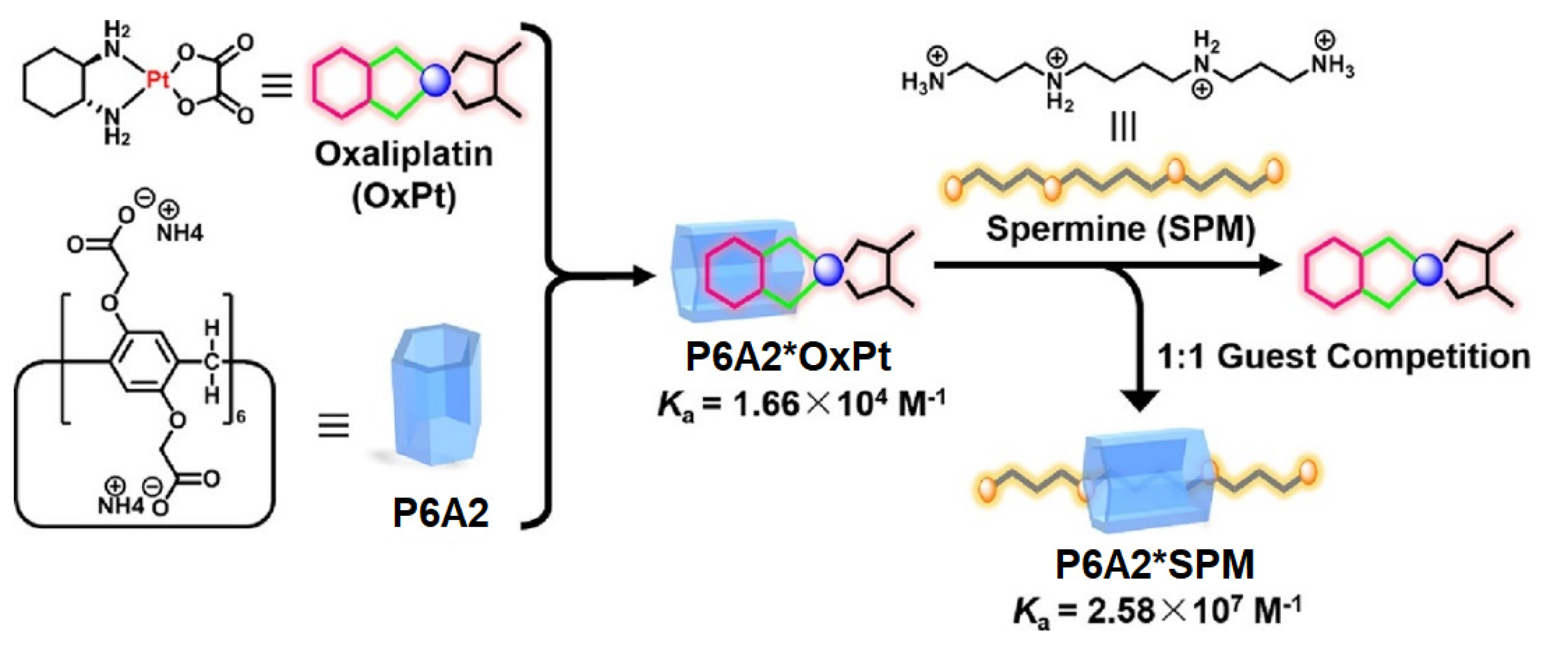
8. Spermine-Driven Drug Release Pillararene-Based Systems
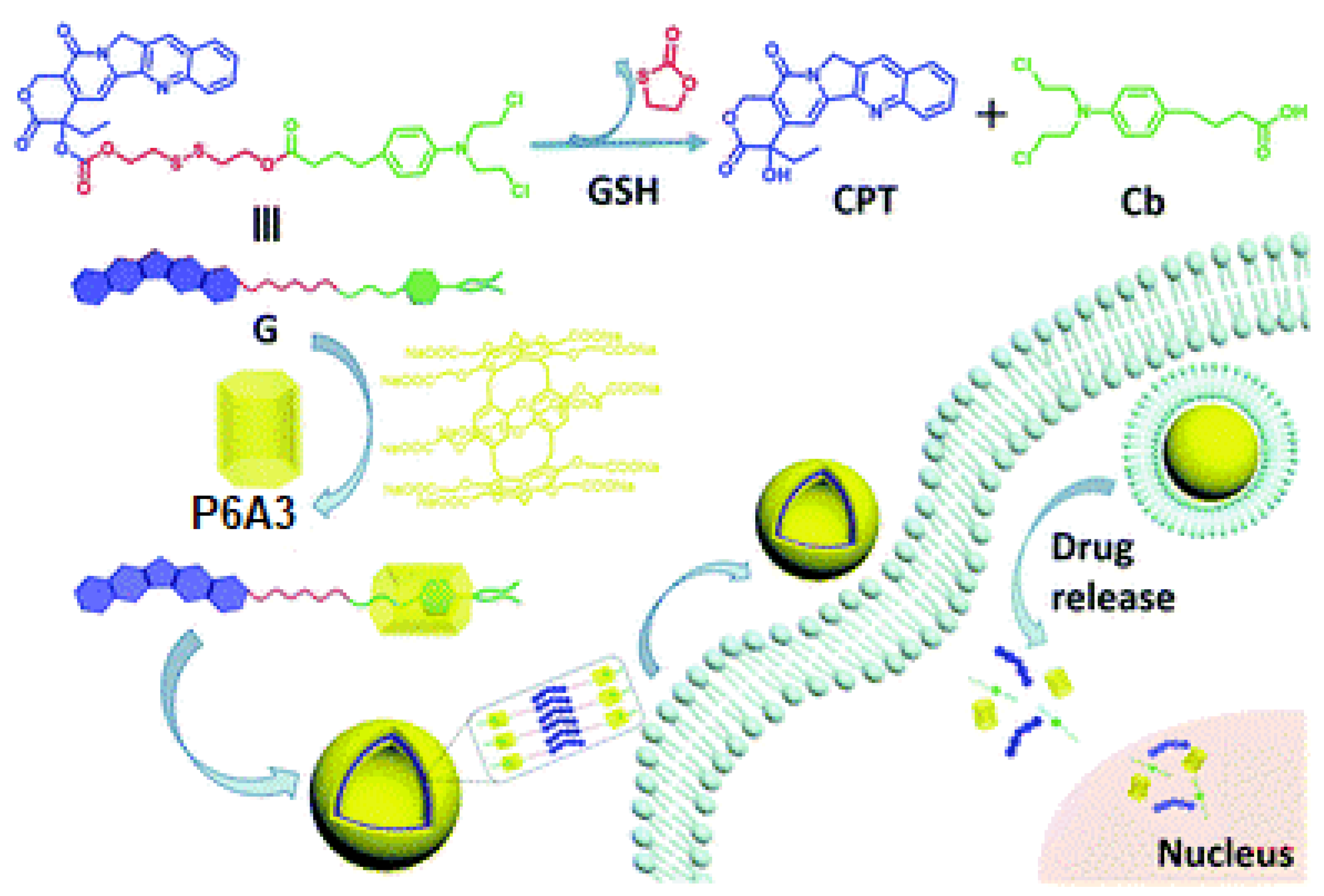
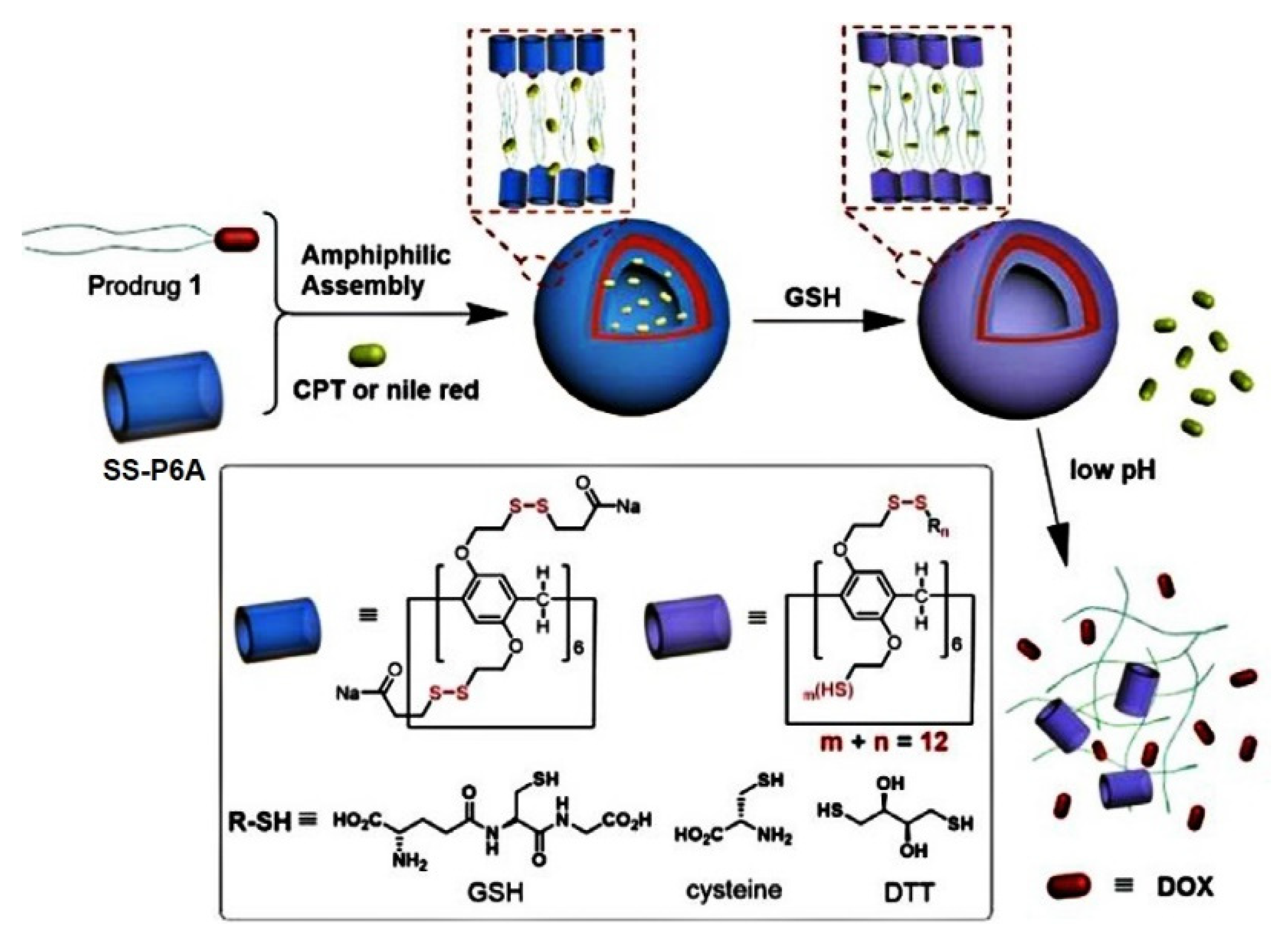
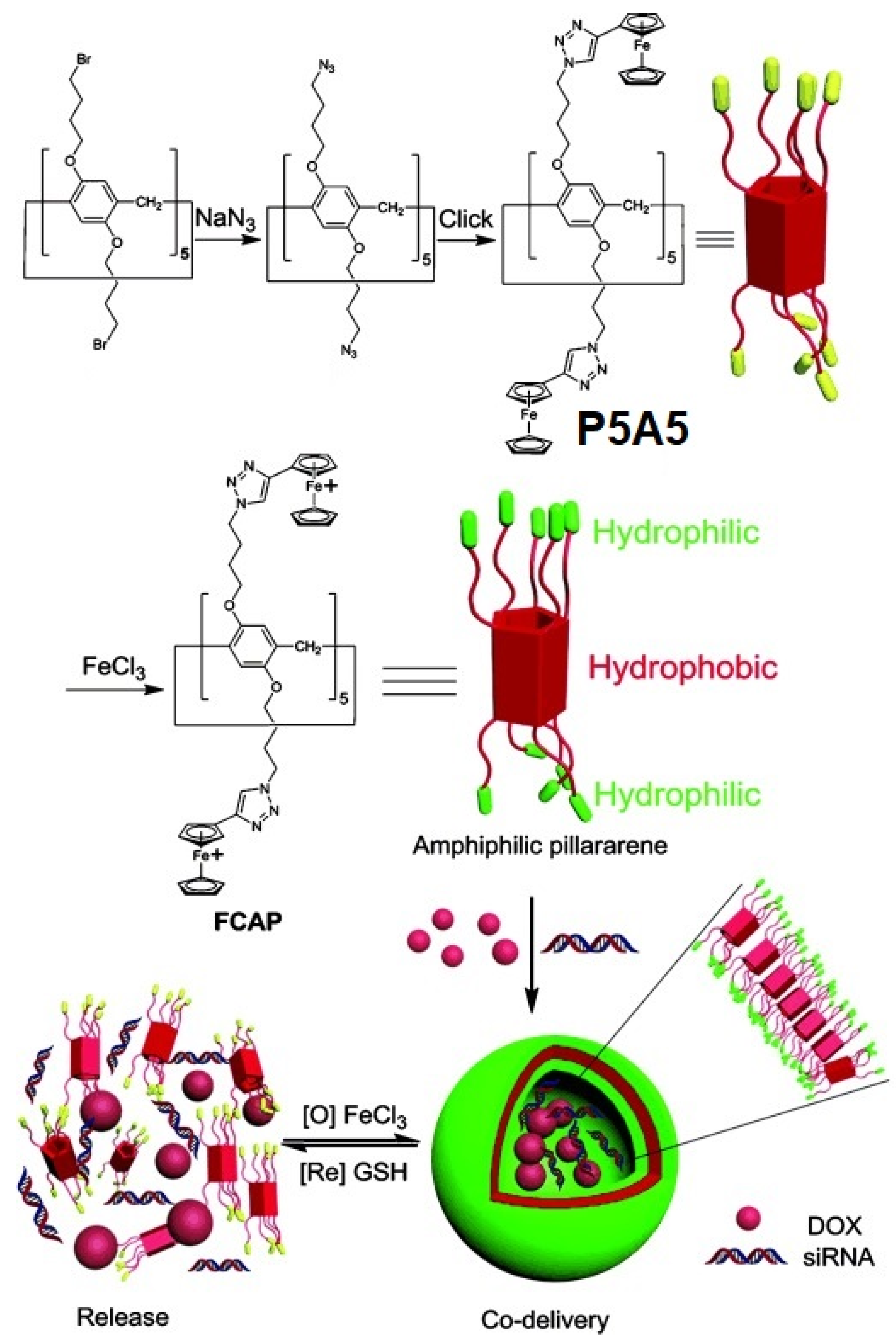
9. Redox-Responsive Drug Release Pillararene Systems
10. Light-Triggered Drug Release Pillararene Systems
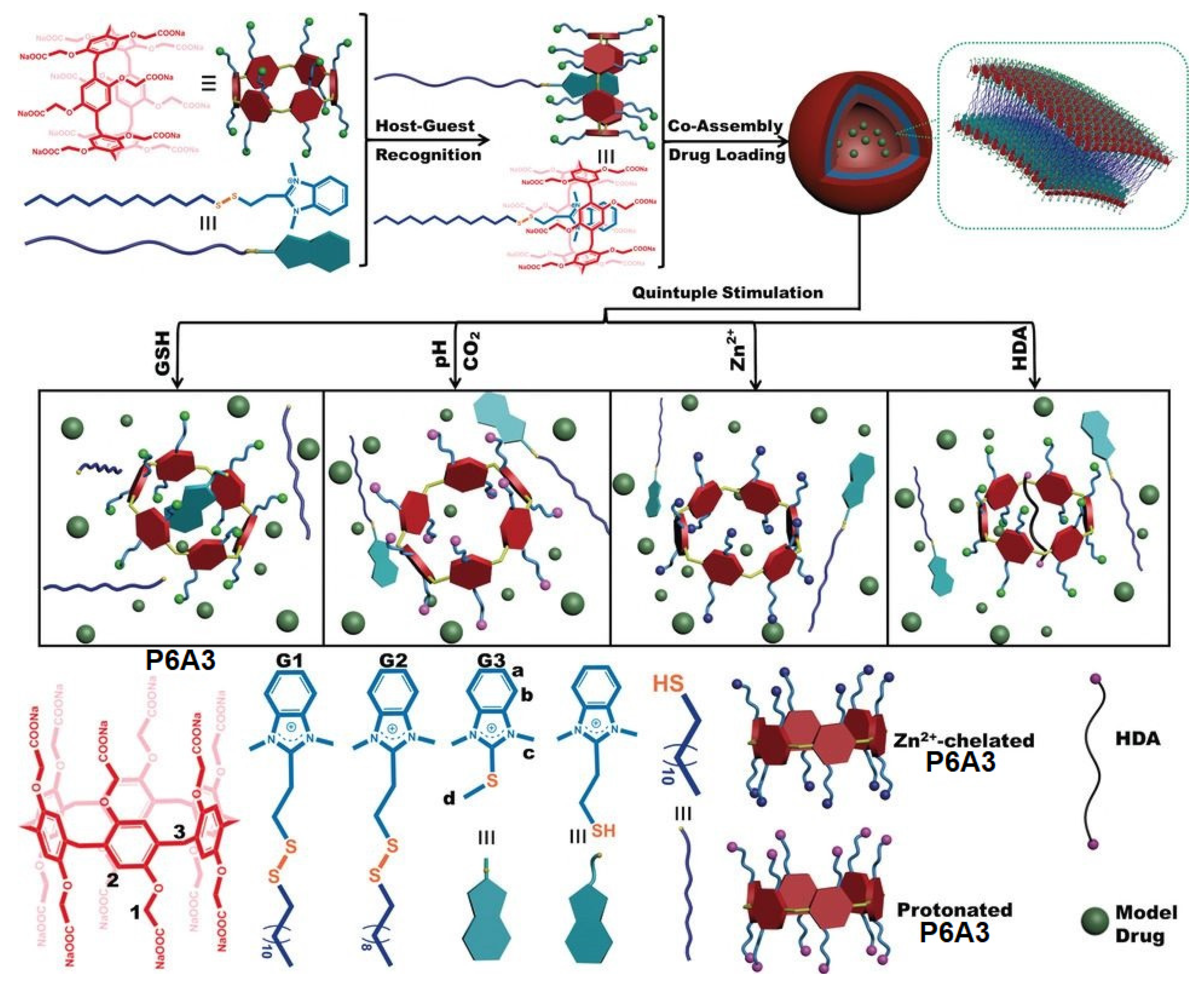
11. Multi-Response Drug Release Pillararene Systems
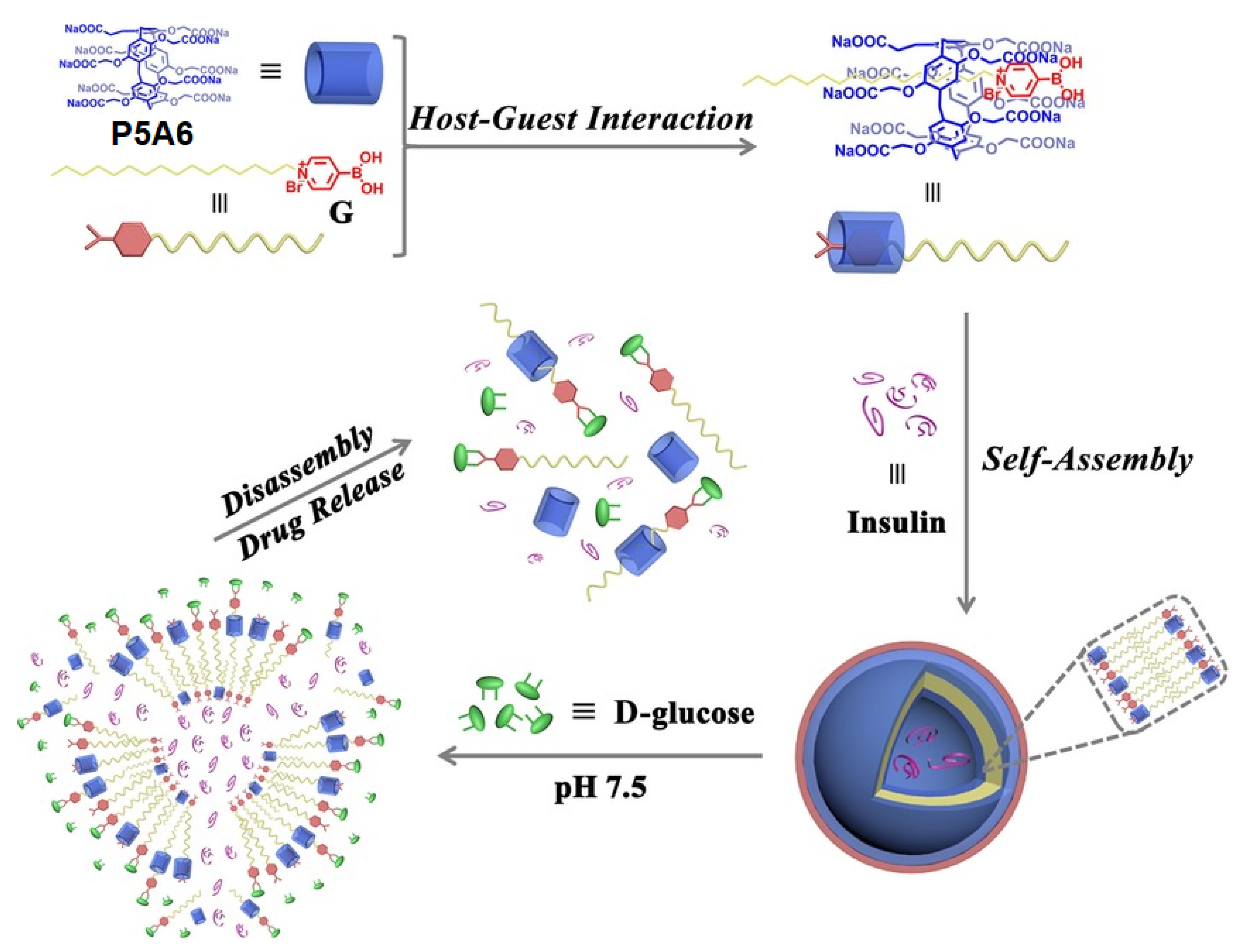
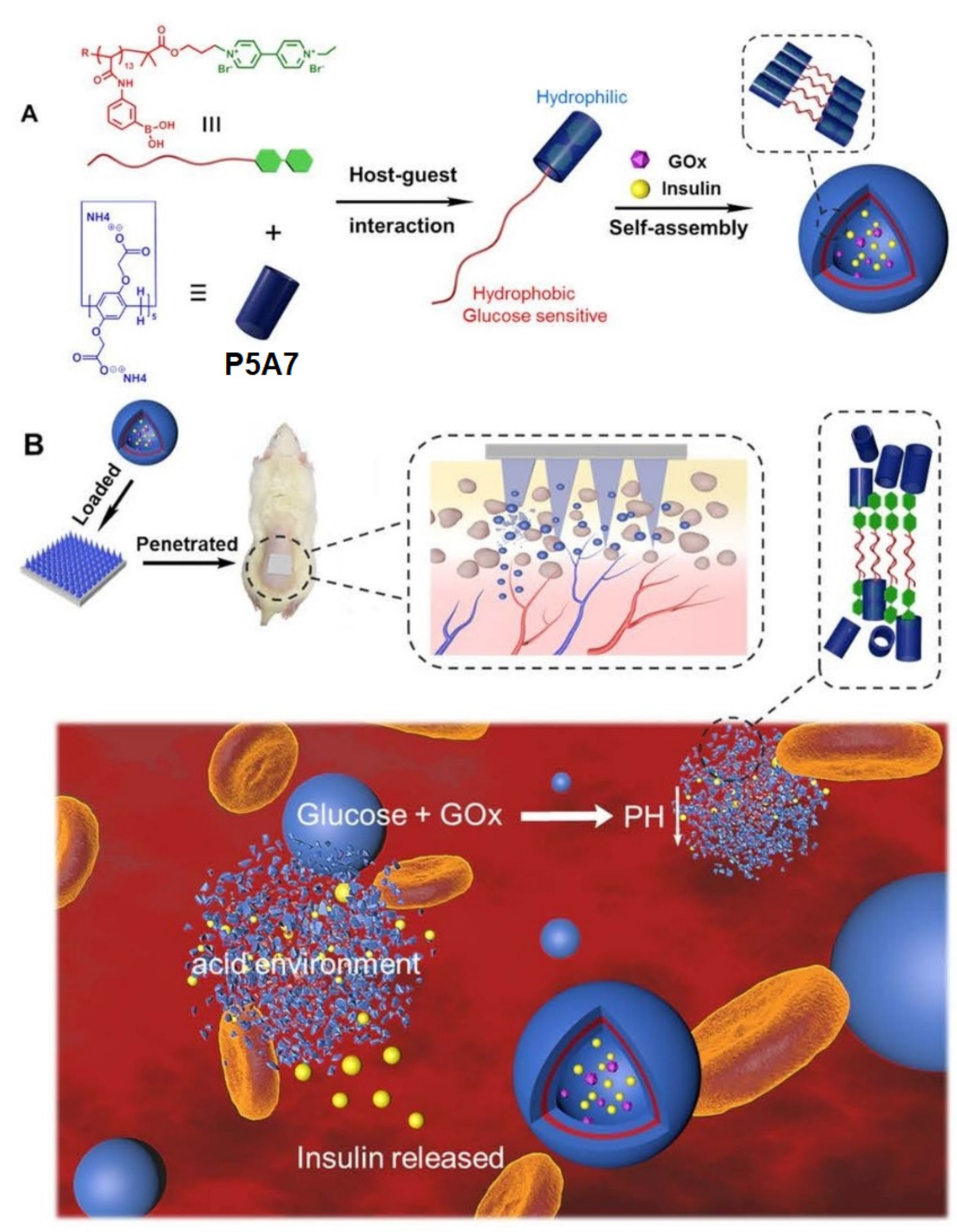
12. Insulin Delivery Systems Based on Pillararenes
13. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-A.; Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721–9723. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Kopchuk, D.S.; Kovalev, I.S.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N. Solvent-free synthesis of pillar[6]arenes. Green Chem. 2016, 18, 423–426. [Google Scholar] [CrossRef]
- Santra, S.; Kovalev, I.S.; Kopchuk, D.S.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N. Role of polar solvents for the synthesis of pillar[6]arenes. RSC Adv. 2015, 5, 104278–104282. [Google Scholar] [CrossRef]
- Yang, K.; Pei, Y.; Wen, J.; Pei, Z. Recent advances in pillar[n]arenes: Synthesis and applications based on host–guest interactions. Chem. Commun. 2016, 52, 9316–9326. [Google Scholar] [CrossRef]
- Ogoshi, T.; Ueshima, N.; Sakakibara, F.; Yamagishi, T.-a.; Haino, T. Conversion from pillar[5]arene to pillar[6–15]arenes by ring expansion and encapsulation of C60 by pillar[n]arenes with nanosize cavities. Org. Lett. 2014, 16, 2896–2899. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T. Pillararenes: Versatile synthetic receptors for supramolecular chemistry. Eur. J. Org. Chem. 2013, 2013, 2961–2975. [Google Scholar] [CrossRef]
- Xia, W.; Hu, X.-Y.; Chen, Y.; Lin, C.; Wang, L. A novel redox-responsive pillar[6]arene-based inclusion complex with a ferrocenium guest. Chem. Commun. 2013, 49, 5085–5087. [Google Scholar]
- Wang, Y.; Ping, G.; Li, C. Efficient complexation between pillar[5]arenes and neutral guests: From host–guest chemistry to functional materials. Chem. Commun. 2016, 52, 9858–9872. [Google Scholar] [CrossRef]
- Joseph, R. Pillar[n]arene derivatives as sensors for amino acids. ChemistrySelect 2021, 6, 3519–3533. [Google Scholar] [CrossRef]
- Li, C.-P.; Lu, Y.-X.; Zi, C.-T.; Zhao, Y.-T.; Zhao, H.; Zhang, Y.-P. Cationic pillar[6]arene induces cell apoptosis by inhibiting protein tyrosine phosphorylation via host–guest recognition. Int. J. Mol. Sci. 2020, 21, 4979. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wu, G.; Cai, Z.; Li, D.; Li, M.-H.; Wang, Y.; Yang, Y.-W. A water-soluble Leggero pillar[5]arene. Molecules 2022, 27, 6259. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Yao, Y. The first water-soluble pillar[5]arene dimer: Synthesis and construction of a reversible fluorescent supramolecular polymer network in water. Chem. Commun. 2017, 53, 165–167. [Google Scholar] [CrossRef]
- Tan, L.-L.; Li, H.; Qiu, Y.-C.; Chen, D.-X.; Wang, X.; Pan, R.-Y.; Wang, Y.; Zhang, S.X.-A.; Wang, B.; Yang, Y.-W. Stimuli-responsive metal–organic frameworks gated by pillar[5]arene supramolecular switches. Chem. Sci. 2015, 6, 1640–1644. [Google Scholar] [CrossRef]
- Ding, C.D.; Li, Y.; Wang, T.; Fu, J. Triple-stimuli-responsive nanocontainers assembled by water-soluble pillar[5]arene-based pseudorotaxanes for controlled release. J. Mater. Chem. B 2016, 4, 2819–2827. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, J.; Sun, Y.; Boussouar, I.; Tian, D.; Li, H.; Jiang, L. Fabrication of a mercaptoacetic acid pillar[5]arene assembled nanochannel: A biomimetic gate for mercury poisoning. Chem. Sci. 2016, 7, 3227–3233. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, K.; Wei, P.; Huang, S.; Pei, Y.; Zhao, W.; Pei, Z. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: A redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. 2014, 53, 13126–13130. [Google Scholar] [CrossRef]
- Guo, S.; Liu, X.; Yao, C.; Lu, C.; Chen, Q.; Hu, X.-Y.; Wang, L. Photolysis of a bola-type supra-amphiphile promoted by water-soluble pillar[5]arene-induced assembly. Chem. Commun. 2016, 52, 10751–10754. [Google Scholar] [CrossRef]
- Yao, Y.; Xue, M.; Chi, X.; Ma, Y.; He, J.; Abliz, Z.; Huang, F. A new water-soluble pillar[5]arene: Synthesis and application in the preparation of gold nanoparticles. Chem. Commun. 2012, 48, 6505–6507. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, F.; Zuo, T.; Hua, J.; Diao, G. Stimulus-responsive light-harvesting complexes based on the pillararene-induced co-assembly of β-carotene and chlorophyll. Nat. Commun. 2016, 7, 12042. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, J.; Li, C. Pillararene-based conjugated porous polymers. Polym. Chem. 2021, 12, 2808–2824. [Google Scholar] [CrossRef]
- Li, M.-H.; Lou, X.-Y.; Yang, Y.-W. Pillararene-based molecular-scale porous materials. Chem. Commun. 2021, 57, 13429–13447. [Google Scholar] [CrossRef] [PubMed]
- Jie, K.; Zhou, Y.; Li, E.; Huang, F. Nonporous adaptive crystals of pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. [Google Scholar] [CrossRef]
- Wu, J.-R.; Yang, Y.-W. Synthetic macrocycle-based nonporous adaptive crystals for molecular separation. Angew. Chem. Int. Ed. 2021, 60, 1690–1701. [Google Scholar] [CrossRef]
- Yao, C.-Y.; de Silva, A.P. Recent developments in CO2 capture/storage/utilization with aromatic macrocycles. Carbon Capture Sci. Technol. 2022, 4, 100058. [Google Scholar] [CrossRef]
- Wang, G.; Hu, W.B.; Zhao, X.L.; Liu, Y.A.; Li, J.S.; Jiang, B.; Wen, K. Engineering a pillar[5]arene-based supramolecular organic framework by a co-crystallization method. Daltons Trans. 2018, 47, 5144–5148. [Google Scholar] [CrossRef]
- Jie, K.; Zhou, Y.; Yao, Y.; Shi, B.; Huang, F. CO2-responsive pillar[5]arene-based molecular recognition in water: Establishment and application in gas-controlled self-assembly and release. J. Am. Chem. Soc. 2015, 137, 10472–10475. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Zhang, Z.; Wang, J.; Yuan, X.; Zhao, Q.; Ding, Y.; Yao, Y. CO2 and photo-controlled reversible conversion of supramolecular assemblies based on water soluble pillar[5]arene and coumarin-containing guest. Chin. Chem. Lett. 2021, 32, 349–352. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Gong, W.; Sun, T.; Wang, Z.; Ning, G. Pillar[5]arene-derived microporous polyaminal networks with enhanced uptake performance for CO2 and iodine. Ind. Eng. Chem. Res. 2020, 59, 3269–3278. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Yang, Y.-W. Pillar[n]arene-based supramolecular switches in solution and on surfaces. Adv. Mater. 2020, 32, 2003263. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.-W. Functional materials with pillarene Struts. Acc. Mater. Res. 2021, 2, 292–305. [Google Scholar] [CrossRef]
- Wang, K.; Tian, X.; Jordan, J.H.; Velmurugan, K.; Wang, L.; Hu, X.-Y. The emerging applications of pillararene architectures in supramolecular catalysis. Chin. Chem. Lett. 2022, 33, 89–96. [Google Scholar] [CrossRef]
- Li, M.-H.; Yang, Z.; Li, Z.; Wu, J.-R.; Yang, B.; Yang, Y.-W. Construction of hydrazone-linked macrocycle-enriched covalent organic frameworks for highly efficient photocatalysis. Chem. Mater. 2022, 34, 5726–5739. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Yang, Y.-W. Conjugated macrocycle polymer nanoparticles with alternating pillarenes and porphyrins as struts and cyclic nodes. Small 2019, 15, 1805509. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Wang, Y.; Yang, Y.-W. Pillararene-enriched linear conjugated polymer materials with thiazolo[5,4-d]thiazole linkages for photocatalysis. Chem. Commun. 2021, 57, 6546–6549. [Google Scholar] [CrossRef]
- Kato, K.; Fa, S.; Ohtani, S.; Shi, T.-h.; Brouwer, A.M.; Ogoshi, T. Noncovalently bound and mechanically interlocked systems using pillar[n]arenes. Chem. Soc. Rev. 2022, 51, 3648–3687. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Yan, Y.; Shan, X.; Zhao, M.; Zhao, Q.; Liao, X.; Xie, M. Pillararene-containing polymers with tunable conductivity based on host–guest complexations. ACS Macro Lett. 2019, 8, 1588–1593. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Zhu, W.; Huang, F. Pillararenes as versatile building blocks for fluorescent materials. Acc. Mater. Res. 2022, 3, 658–668. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Jiang, X.-M.; Gong, G.-F.; Yao, H.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Pillararene-based AIEgens: Research progress and appealing applications. Chem. Commun. 2021, 57, 284–301. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Xu, W.; Huang, J.; Sun, Z.; Liao, T.; Kovaleva, E.G.; Xu, C.; Cheng, J.; Li, H. Specific extraction of nucleic acids employing pillar[6]arene-functionalized nanochannel platforms and even nanomaterials for theranostics. Chem. Commun. 2022, 58, 9278. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Lou, X.-Y.; Ma, L.; Gao, H.; Yang, Y.-W. Supramolecular nanotheranostics based on pillarenes. Theranostics 2019, 9, 3075–3093. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, C.; Ji, G.; Liu, J.; Zhu, P.; Qian, J.; Zhu, S.-X.; Zhang, Y.; Ling, Y. Tumor microenvironment-activatable Boolean logic supramolecular nanotheranostics based on a pillar[6]arene for tumor hypoxia imaging and multimodal synergistic therapy. Mater. Chem. Front. 2021, 5, 5846. [Google Scholar] [CrossRef]
- Song, N.; Zhang, Z.; Liu, P.; Dai, D.; Chen, C.; Li, Y.; Wang, L.; Han, T.; Yang, Y.-W.; Wang, D.; et al. Pillar[5]arene-modified gold nanorods as nanocarriers for multi-modal imaging-guided synergistic photodynamic-photothermal therapy. Adv. Funct. Mater. 2021, 31, 2009924. [Google Scholar] [CrossRef]
- Li, Z.; Song, N.; Yang, Y.-W. Stimuli-responsive drug delivery systems based on supramolecular nanovalves. Matter 2019, 1, 345–368. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, W.; Cheng, M.; Zhang, S.; Kovaleva, E.G.; Liang, F.; Tian, D.; Liu, J.-a.; Abdelhameed, R.M.; Cheng, J.; et al. Controlled release of drug molecules by pillararene-modified nanosystems. Chem. Commun. 2022, 58, 3255–3269. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Dong, J.; Chen, Y.; Luan, X.; Li, X.; Du, X. Pillararene-based supramolecular vesicles for stimuli-responsive drug delivery. Chem. Eur. J. 2022, 28, e202202050. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Khalil-cruz, L.E.; Khashab, N.M.; Yu, G.; Huang, F. Pillararene-based supramolecular systems for theranostics and bioapplications. Sci. China Chem. 2021, 64, 688–700. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structure on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef]
- Wilkins, M.; Hall-Stoodley, L.; Allan, R.N.; Faust, S.N. New approaches to the treatment of biofilm-related infections. J. Infect. 2014, 69, S47–S52. [Google Scholar] [CrossRef]
- Prosser, B.L.; Taylor, D.; Dix, B.A.; Cleeland, R. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob. Agents Chemother. 1987, 31, 1502–1506. [Google Scholar] [CrossRef]
- Nickel, J.C.; Ruseska, I.; Wright, J.B.; Costerton, J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985, 27, 619–624. [Google Scholar] [CrossRef]
- Gristina, A.G.; Hobgooda, C.D.; Webb, L.X.; Myrvik, Q.N. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 1987, 8, 423–426. [Google Scholar] [CrossRef]
- Evans, R.C.; Holmes, C.J. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob. Agents Chemother. 1987, 31, 889–894. [Google Scholar] [CrossRef]
- Mah, T.-F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Joseph, R.; Naugolny, A.; Feldman, M.; Herzog, I.M.; Fridman, M.; Cohen, Y. Cationic pillararenes potently inhibit biofilm formation without affecting bacterial growth and viability. J. Am. Chem. Soc. 2016, 138, 754–775. [Google Scholar] [CrossRef]
- Joseph, R.; Kaizerman, D.; Herzog, I.M.; Hadar, M.; Feldman, M.; Fridman, M.; Cohen, Y. Phosphonium pillar[5]arenes as a new class of efficient biofilm inhibitors: Importance of charge cooperativity and the pillar platform. Chem. Commun. 2016, 52, 10656–10659. [Google Scholar] [CrossRef]
- Kaizerman-Kane, D.; Hadar, M.; Joseph, R.; Logviniuk, D.; Zafrani, Y.; Fridman, M.; Cohen, Y. Design guidelines for cationic pillar[n]arenes that prevent biofilm formation by gram-positive pathogens. ACS Infect. Dis. 2021, 7, 579–585. [Google Scholar] [CrossRef]
- Galanos, N.; Gillon, E.; Imberty, A.; Matthews, S.E.; Vidal, S. Pentavalent pillar[5]arene-based glycoclusters and theirmultivalent binding to pathogenic bacterial lectins. Org. Biomol. Chem. 2016, 14, 3476–3481. [Google Scholar] [CrossRef] [PubMed]
- Buet, K.; Nierengarten, I.; Galanos, N.; Gillon, E.; Holler, M.; Imberty, A.; Matthews, S.E.; Vidal, S.; Vincent, S.P.; Nierengarten, J.-F. Pillar[5]arene-based glycoclusters: Synthesis and multivalent binding to pathogenic bacterial lectins. Chem. Eur. J. 2016, 22, 2955–2963. [Google Scholar]
- Vincent, S.P.; Buet, K.; Nierengarten, I.; Imberty, A.; Nierengarten, J.-F. Biologically active heteroglycoclusters constructed on a pillar[5]arene-containing [2]rotaxane scaffold. Chem. Eur. J. 2016, 22, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, M.-j.; Meng, Y.; Chen, D. Epoxidation of pillar[5]arene with three dimensional macrocyclic structure and host-guest capability for epoxy coatings. Prog. Org. Coat. 2022, 166, 106821. [Google Scholar] [CrossRef]
- Torres, I.M.; Patankar, Y.R.; Shabaneh, T.B.; Dolben, E.; Hogan, D.H.; Leib, D.A.; Berwin, B.L. Acidosis potentiates the host proinflammatory interleukin-1β response to Pseudomonas aeruginosa infection. Infect. Immun. 2014, 82, 4689–4697. [Google Scholar] [CrossRef]
- Barbera, L.; Franco, D.; De Plano, L.M.; Gattuso, G.; Guglielmino, S.P.P.; Lentini, G.; Manganaro, N.; Marino, N.; Pappalardo, S.; Parisi, M.F.; et al. A water-soluble pillar[5]arene as a new carrier for an old drug. Org. Biomol. Chem. 2017, 15, 3192–3195. [Google Scholar] [CrossRef]
- Barbera, L.; De Plano, L.M.; Franco, D.; Gattuso, G.; Guglielmino, S.P.P.; Lando, G.; Notti, A.; Parisi, M.F.; Pisagatti, I. Antiadhesive and antibacterial properties of pillar[5]arene-based multilayers. Chem. Commun. 2018, 54, 10203–10206. [Google Scholar] [CrossRef]
- Wheate, N.J.; Dickson, K.-A.; Kim, R.R.; Nematollahi, A.; Macquart, R.B.; Kayser, V.; Yu, G.; Church, W.B.; Marsh, D.J. Host-guest complexes of carboxylated pillar[n]arenes with drugs. J. Pharm. Sci. 2016, 105, 3615–3625. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Yakimova, L.S.; Rizvanov, I.K.; Plemenkov, V.V.; Stoikov, I.I. Water-soluble pillar[5]arenes: Synthesis and characterization of the inclusion complexes with p-toluenesulfonic acid. Macroheterocycles 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Gelmo, P. Über sulfamide der p-amidobenzolsulfonsäure. J. Prakt. Chem. 1908, 77, 369–382. [Google Scholar] [CrossRef]
- Peng, H.; Xie, B.; Yang, X.; Dai, J.; Wei, G.; He, Y. Pillar[5]arene-based, dual pH and enzyme responsive supramolecular vesicles for targeted antibiotic delivery against intracellular MRSA. Chem. Commun. 2020, 56, 8115–8118. [Google Scholar] [CrossRef]
- Choi, E.S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-modified serum exosomes for the elevated uptake to murine dendritic cells and Lymphatic accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Ajaj, K.A.; Biniossek, M.L.; Kratz, F. Development of protein-binding bifunctional linkers for a new generation of dual-acting prodrugs. Bioconjugate Chem. 2009, 20, 390–396. [Google Scholar] [CrossRef]
- Humphrey, C.; Veve, M.P.; Walker, B.; Shorman, M.A. Long-term vancomycin use had low risk of ototoxicity. PLoS ONE 2019, 14, e0224561. [Google Scholar] [CrossRef]
- Peng, H.; Xie, B.; Cen, X.; Dai, J.; Dai, Y.; Yang, X.; He, Y. Glutathione-responsive multifunctional nanoparticles based on mannose-modified pillar[5]arene for targeted antibiotic delivery against intracellular methicillin-resistant S. aureus. Mater. Chem. Front. 2022, 6, 360–367. [Google Scholar] [CrossRef]
- Lee, M.H.; Sharma, A.; Chang, M.J.; Lee, J.; Son, S.; Sessler, J.L.; Kang, C.; Kim, J.S. Fluorogenic reaction-based prodrug conjugates as targeted cancer theranostics. Chem. Soc. Rev. 2018, 47, 28–52. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Guan, X.; Guo, Z.; Wang, T.; Lin, L.; Chen, J.; Tian, H.; Chen, X. A pH-responsive detachable PEG shielding strategy for gene delivery system in cancer therapy. Biomacromolecules 2017, 18, 1342–1349. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A., Jr.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A feasible target for cancer therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, M.; Chen, Z.; Hu, X.; Pu, L.; Pei, Z.; Pei, Y. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar[5]arene dimer for targeted chemotherapy. Chem. Commun. 2020, 56, 10642–10645. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Shen, Z.; Pei, Y.; Pei, Z. Covalently bridged pillararene-based oligomers: From construction to applications. Chem. Commun. 2021, 57, 10983–10997. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Teng, K.-X.; Ding, Y.-F.; Yue, L.; Yang, Q.-Z.; Wang, R. Dual stimuli-responsive bispillar[5]arene-based nanoparticles for precisely selective drug delivery in cancer cells. Chem. Commun. 2019, 55, 2340–2343. [Google Scholar] [CrossRef] [PubMed]
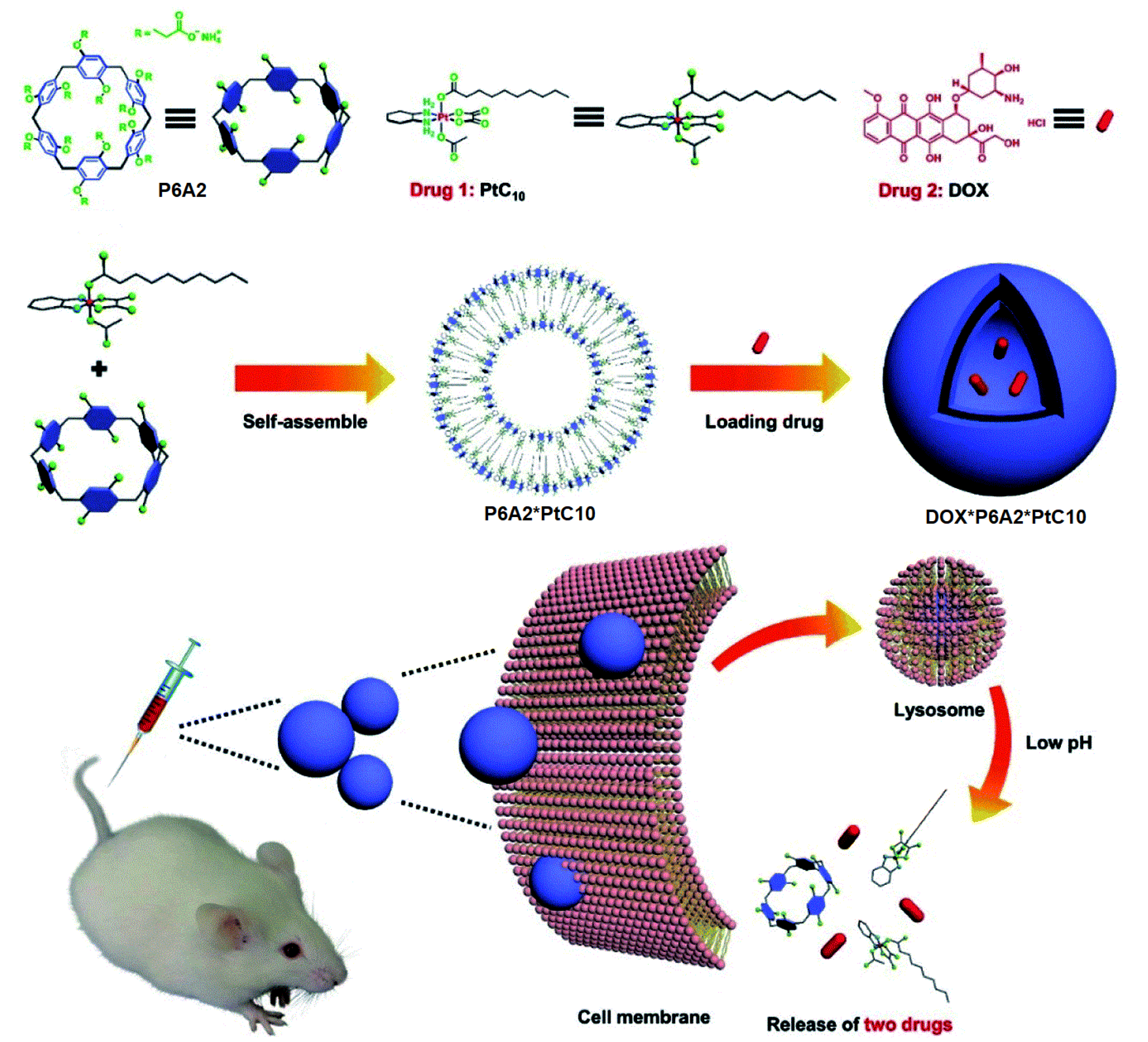
- Shao, W.; Liu, X.; Sun, G.; Hu, X.-Y.; Zhu, J.-J.; Wang, L. Construction of drug–drug conjugate supramolecular nanocarriers based on water-soluble pillar[6]arene for combination chemotherapy. Chem. Commun. 2018, 54, 9462–9465. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Mao, W.; Li, P.; Zhou, F.; Ma, D. Smart supramolecular vesicles based on glutathione-reactive pillar[6]arene and acid-labile prodrug: Dual drug loading and sequential release. Chin. Chem. Lett. 2022, 33, 209–212. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. Supramolecular drug delivery system from macrocycle-based self-assembled amphiphiles for effective tumor therapy. ACS Appl. Mater. Inter. 2021, 13, 53564–53573. [Google Scholar] [CrossRef]
- Gribble, F.M.; Loussouarn, G.; Tucker, S.J.; Zhao, C.; Nichols, C.G.; Ashcroft, F.M. A novel method for measurement of submembrane ATP concentration. J. Biol. Chem. 2000, 275, 30046–30049. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Geng, W.-C.; Chen, F.-Y.; Duan, X.-C.; Zheng, Z.; Ding, D.; Guo, D.-S. Biomarker displacement activation: A general host–guest strategy for targeted phototheranostics in vivo. J. Am. Chem. Soc. 2018, 140, 4945–4953. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Extracellular ATP metabolism on vascular endothelial cells: A pathway with pro-phrombotic and anti-thrombotic molecules. Vasc. Pharmacol. 2015, 75, 1–6. [Google Scholar] [CrossRef]
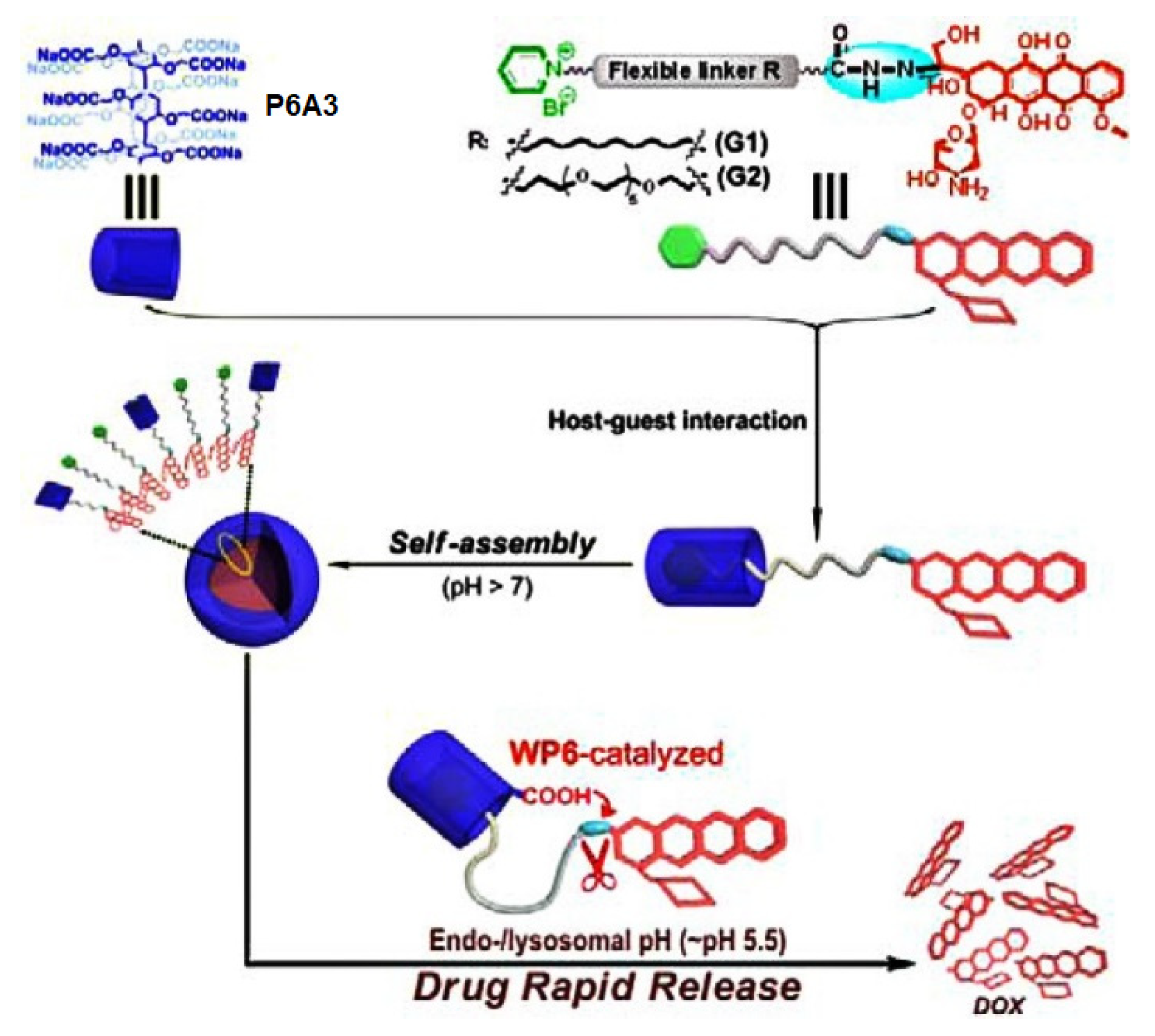
- Cao, Y.; Li, Y.; Hu, X.-Y.; Zou, X.; Xiong, S.; Lin, C.; Wang, L. Supramolecular nanoparticles constructed by DOX-based prodrug with water-soluble pillar[6]arene for self-catalyzed rapid drug release. Chem. Mater. 2015, 27, 1110–1119. [Google Scholar] [CrossRef]
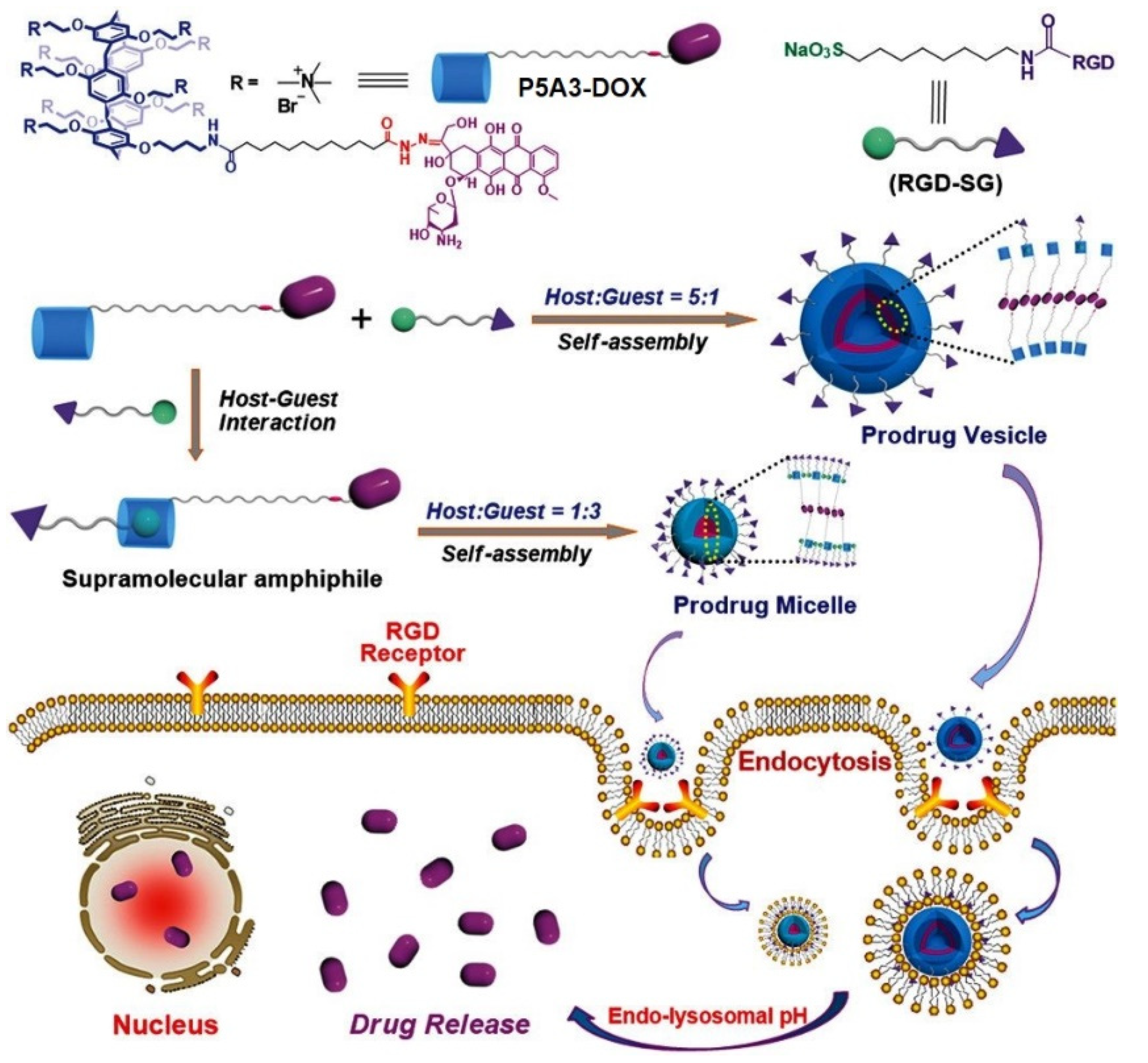
- Hu, X.-Y.; Gao, L.; Mosel, S.; Ehlers, M.; Zellermann, E.; Jiang, H.; Knauer, S.K.; Wang, L.; Schmuck, C. From supramolecular vesicles to micelles: Controllable construction of tumor-targeting nanocarriers based on host–guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small 2018, 14, 1803952. [Google Scholar] [CrossRef]
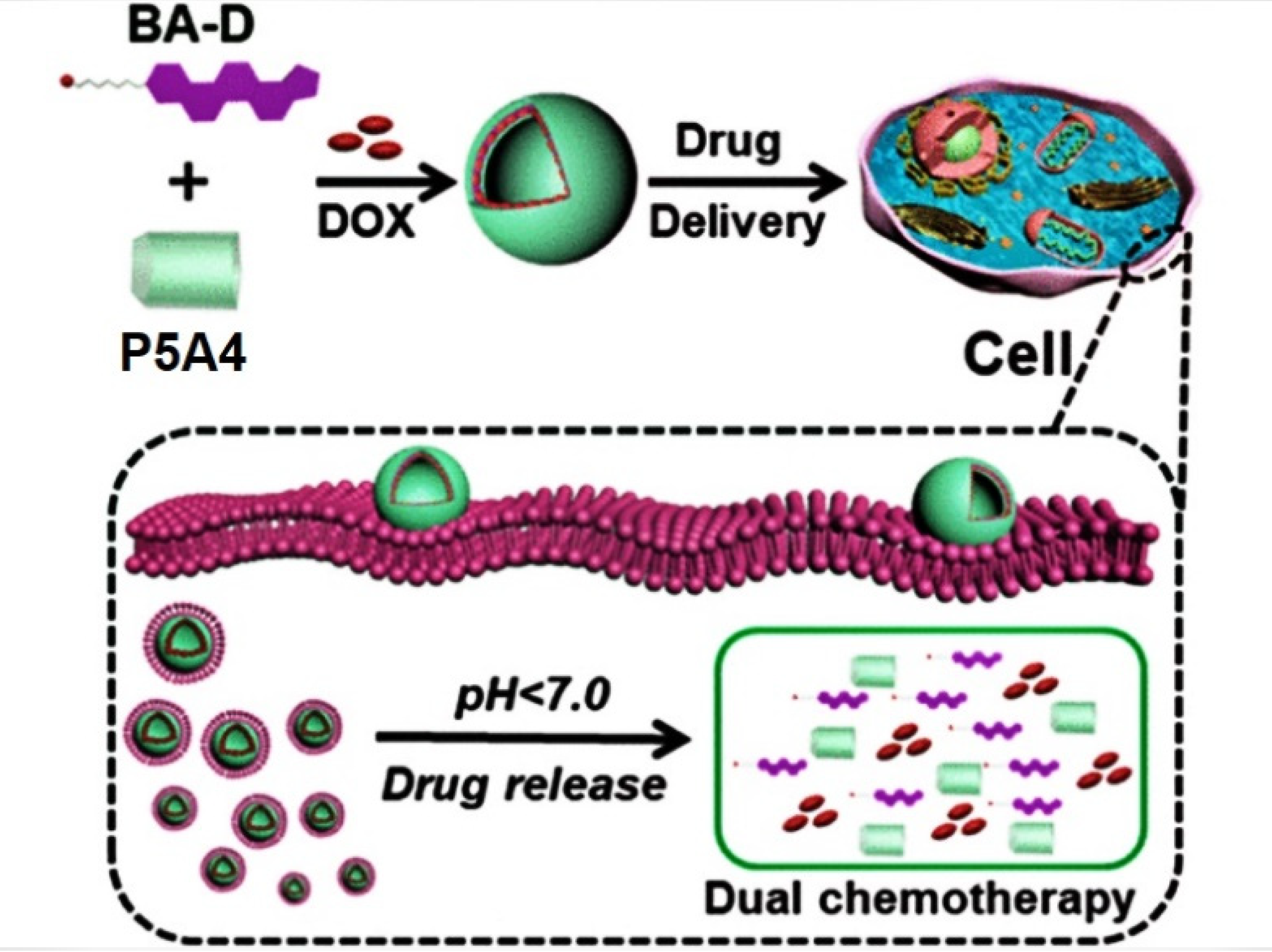
- Sun, G.; Zuo, M.; Xu, Z.; Wang, K.; Wang, L.; Hu, X.-Y. Orthogonal design of supramolecular prodrug vesicles via water-soluble pillar[5]arene and betulinic acid derivative for dual chemotherapy. ACS Appl. Bio Mater. 2022, 5, 3320–3328. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Meng, Z.; Guo, L.; Yuan, X.; Zhang, Y.; Chai, Y.; Sessler, J.L.; Meng, Q.; Li, C. Supramolecular combination chemotherapy: A pH-responsive co-encapsulation drug delivery system. Chem. Sci. 2020, 11, 6275–6282. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.-F.; Sun, Z.; Zhang, X. Supramolecular chemotherapy: Carboxylated pillar[6]arene for decreasing cytotoxicity of oxaliplatin to normal cells and improving its anticancer bioactivity against colorectal cancer. ACS Appl. Mater. Inter. 2018, 10, 5365–5372. [Google Scholar] [CrossRef]
- Bauhuber, S.; Hozsa, C.; Breunig, M.; Göpferich, A. Delivery of nucleic acids via disulfide-based carrier systems. Adv. Mater. 2009, 21, 3286–3306. [Google Scholar] [CrossRef]
- Gu, Y.; Zhong, Y.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Acetal-linked paclitaxel prodrug micellar nanoparticles as a versatile and potent platform for cancer therapy. Biomacromolecules 2013, 14, 2772–2780. [Google Scholar] [CrossRef]
- Lee, G.Y.; Pan, W.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satapathy, M.; Nie, S.; Mao, H.; Yang, L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef]
- Bhuniya, S.; Maiti, S.; Kim, E.-J.; Lee, H.; Sessler, J.L.; Hong, K.S.; Kim, J.S. An activatable theranostic for targeted cancer therapy and imaging. Angew. Chem. Int. Ed. 2014, 53, 4469–4474. [Google Scholar] [CrossRef]
- Yu, G.; Yu, W.; Mao, Z.; Gao, C.; Huang, F. A pillararene-based ternary drug-delivery system with photocontrolled anticancer drug release. Small 2015, 11, 919–925. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Jia, K.; Cao, Y.; Li, Y.; Qin, S.; Zhou, F.; Lin, C.; Zhang, D.; Wang, L. Dual photo- and pH-responsive supramolecular nanocarriers based on water-soluble pillar[6]arene and different azobenzene derivatives for intracellular anticancer drug delivery. Chem. Eur. J. 2015, 21, 1208–1220. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, X.; Chen, D.; Yan, H.; Li, X.; Du, X. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar[6]arenes that are responsive to five stimuli. Angew. Chem. Int. Ed. 2017, 56, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Kalra, B.; Agrawal, N. Oral Insulin. Diabetol. Metab. Syndr. 2010, 2, 66. [Google Scholar] [CrossRef]
- Gao, L.; Wang, T.; Jia, K.; Wu, X.; Yao, C.; Shao, W.; Zhang, D.; Hu, X.-Y.; Wang, L. Glucose-responsive supramolecular vesicles based on water-soluble pillar[5]arene and pyridylboronic acid derivatives for controlled insulin delivery. Chem. Eur. J. 2017, 23, 6605–6614. [Google Scholar] [CrossRef] [PubMed]
- James, T.D.; Shinkai, S. Artificial receptors as chemosensors for carbohydrates. Top. Curr. Chem. 2002, 218, 159–200. [Google Scholar]
- Wang, W.; Gao, X.; Wang, B. Boronic acid-based sensors. Curr. Org. Chem. 2002, 6, 1285–1317. [Google Scholar] [CrossRef]
- Wiskur, S.L.; Ait-Haddou, H.; Lavigne, J.J.; Anslyn, E.V. Teaching old indicators new tricks. Acc. Chem. Res. 2001, 34, 963–972. [Google Scholar] [CrossRef]
- Zuo, M.; Qian, W.; Xu, Z.; Shao, W.; Hu, X.-Y.; Zhang, D.; Jiang, J.; Sun, X.; Wang, L. Multiresponsive supramolecular theranostic nanoplatform based on pillar[5]arene and diphenylboronic acid derivatives for integrated glucose sensing and insulin delivery. Small 2018, 14, 1801942. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, W.; Bai, X.; Ju, Y.; Cao, C.; Zou, S.; Tong, Z.; Cen, C.; Jiang, G.; Kong, X. Glucose- and pH-responsive supramolecular polymer vesicles based on host–guest interaction for transcutaneous delivery of insulin. ACS Appl. Bio Mater. 2020, 3, 6376–6383. [Google Scholar]
- Song, N.; Kakuta, T.; Yamagishi, T.; Yang, Y.-W.; Ogoshi, T. Molecular-Scale Porous Materials Based on Pillar[n]arenes. Chem 2018, 4, 2029–2053. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T. Pillar[5]- and pillar[6]arene-based supramolecular assemblies built by using their cavity-size-dependent host–guest interactions. Chem. Commun. 2014, 50, 4776–4787. [Google Scholar] [CrossRef]
- Tang, R.; Ye, Y.; Zhu, S.; Wang, Y.; Lu, B.; Yao, Y. Pillar[6]arenes: From preparation, host-guest property to self-assembly and applications. Chin. Chem. Lett. 2023, 34, 107734. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, K.T.; Oh, Y.T.; Oh, N.M.; Yound, Y.S.; Lee, E.S. An artificial photosensitizer drug network for mitochondria-selective photodynamic therapy. Chem. Commun. 2012, 48, 2522–2524. [Google Scholar] [CrossRef]
- Lesnik, C.; Cohen, Y.; Atir-Lande, A.; Schuldiner, M.; Arava, Y. OM14 is a mitochondrial receptor for cytosolic ribosomes that supports co-translational import into mitochondria. Nat. Commun. 2014, 5, 5711–6711. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, J.; Lee, J.-H.; Lee, J.H.; Choi, J.-M.; Kweon, H.-S.; Han, J.H.; Kim, J.-H.; Byun, K.M.; Jung, J.H.; et al. Enhanced NIR Radiation-Triggered Hyperthermia by Mitochondrial Targeting. J. Am. Chem. Soc. 2015, 137, 3017–3023. [Google Scholar] [CrossRef]
- Yu, G.; Wu, D.; Li, Y.; Zhang, Z.; Shao, L.; Zhou, J.; Hu, Q.; Tang, G.; Huang, F. A pillar[5]arene-based [2]rotaxane lights up mitochondria. Chem. Sci. 2016, 7, 3017–3024. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Zabirov, N.; Pozdeev, O.; Shcherbakova, V.; Shumilova, T.; Cherkasov, R.; Gil’manova, G.K. Antiviral activity of macrocyclic polyethers and their complexes with the alkaline metal salts of N-phosphorylated amides and thioamides. Pharm. Chem. J. 1991, 25, 321–324. [Google Scholar] [CrossRef]
- Zheng, D.-D.; Fu, D.-Y.; Wu, Y.; Sun, Y.-L.; Tan, L.-L.; Zhou, T.; Ma, S.-Q.; Zha, X.; Yang, Y.-W. Efficient inhibition of human papillomavirus 16 L1 pentamer formation by a carboxylatopillarene and a p-sulfonatocalixarene. Chem. Commun. 2014, 50, 3201–3203. [Google Scholar] [CrossRef]
- Jones, S.T.; Cagno, V.; Janeček, M.; Ortiz, D.; Gasilova, N.; Piret, J.; Gasbarri, M.; Constant, D.A.; Han, Y.; Vuković, L. Modified cyclodextrins as broad-spectrum antivirals. Sci. Adv. 2020, 6, eaax9318. [Google Scholar] [CrossRef]
- Graham, D.R.; Chertova, E.; Hilburn, J.M.; Arthur, L.O.; Hildreth, J.E. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with β-cyclodextrin inactivates and permeabilizes the virions: Evidence for virion-associated lipid rafts. J. Virol. 2003, 77, 8237–8248. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.M.; Super, E.H.; Batt, L.J.; Gasbarri, M.; Coppola, F.; Bhebhe, L.M.; Cheesman, B.T.; Howe, A.M.; Král, P.; Coulston, R.; et al. Broad-spectrum extracellular antiviral properties of cucurbit[n]urils. ACS Infect. Dis. 2022, 8, 2084–2095. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zyryanov, G.V.; Kopchuk, D.S.; Kovalev, I.S.; Santra, S.; Majee, A.; Ranu, B.C. Pillararenes as Promising Carriers for Drug Delivery. Int. J. Mol. Sci. 2023, 24, 5167. https://doi.org/10.3390/ijms24065167
Zyryanov GV, Kopchuk DS, Kovalev IS, Santra S, Majee A, Ranu BC. Pillararenes as Promising Carriers for Drug Delivery. International Journal of Molecular Sciences. 2023; 24(6):5167. https://doi.org/10.3390/ijms24065167
Chicago/Turabian StyleZyryanov, Grigory V., Dmitry S. Kopchuk, Igor S. Kovalev, Sougata Santra, Adinath Majee, and Brindaban C. Ranu. 2023. "Pillararenes as Promising Carriers for Drug Delivery" International Journal of Molecular Sciences 24, no. 6: 5167. https://doi.org/10.3390/ijms24065167
APA StyleZyryanov, G. V., Kopchuk, D. S., Kovalev, I. S., Santra, S., Majee, A., & Ranu, B. C. (2023). Pillararenes as Promising Carriers for Drug Delivery. International Journal of Molecular Sciences, 24(6), 5167. https://doi.org/10.3390/ijms24065167






