Coelenterazine-Type Bioluminescence-Induced Optical Probes for Sensing and Controlling Biological Processes
Abstract
1. Introduction
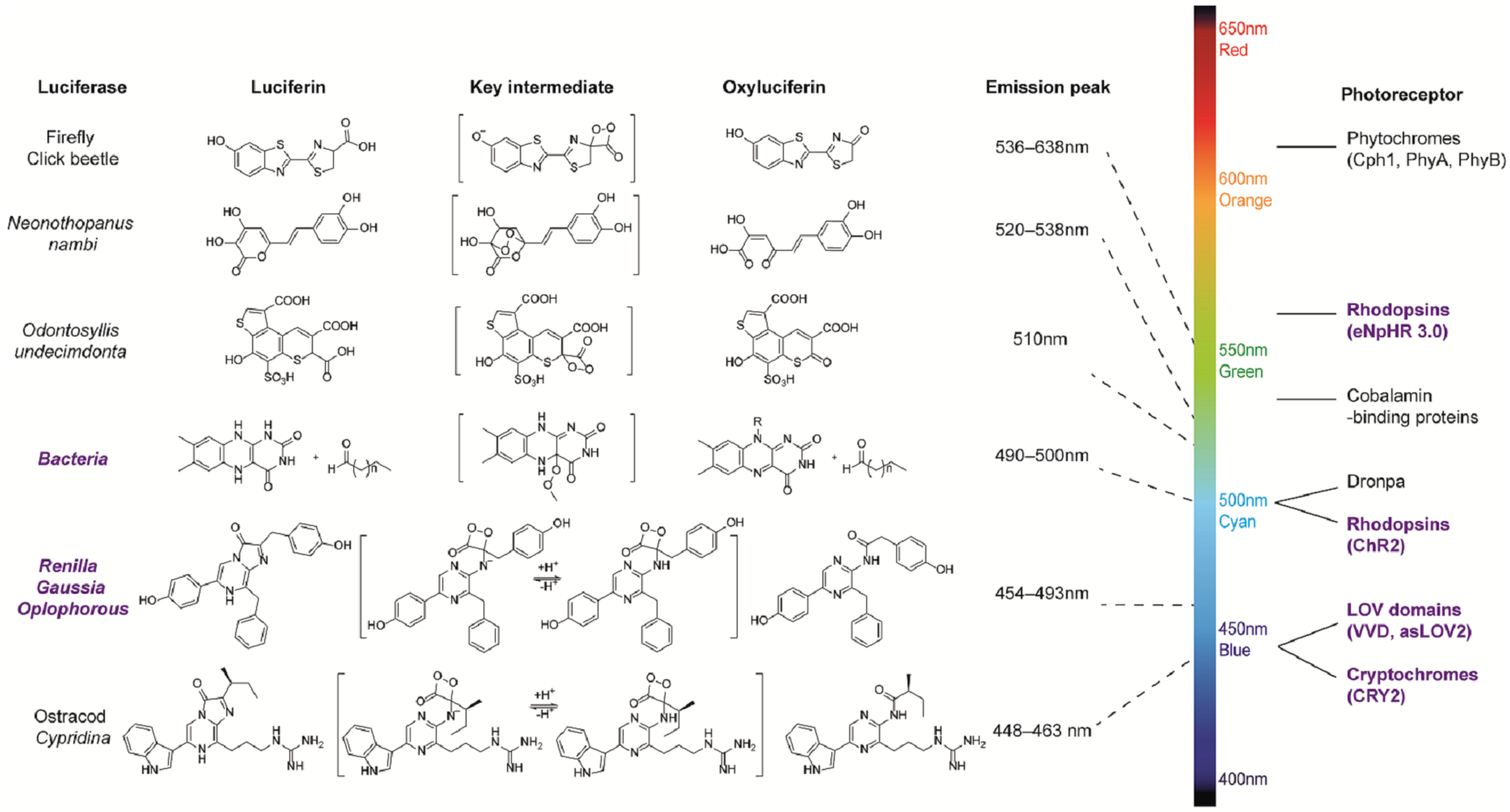
1.1. Bioluminescence and Bioluminescence Technology
1.2. Optogenetics and Photoswitchable Modules
2. Bioluminescence-Induced Photoswitchable Protein-Based Optical Probes: A Novel Strategy for Sensing and Controlling Biological Processes
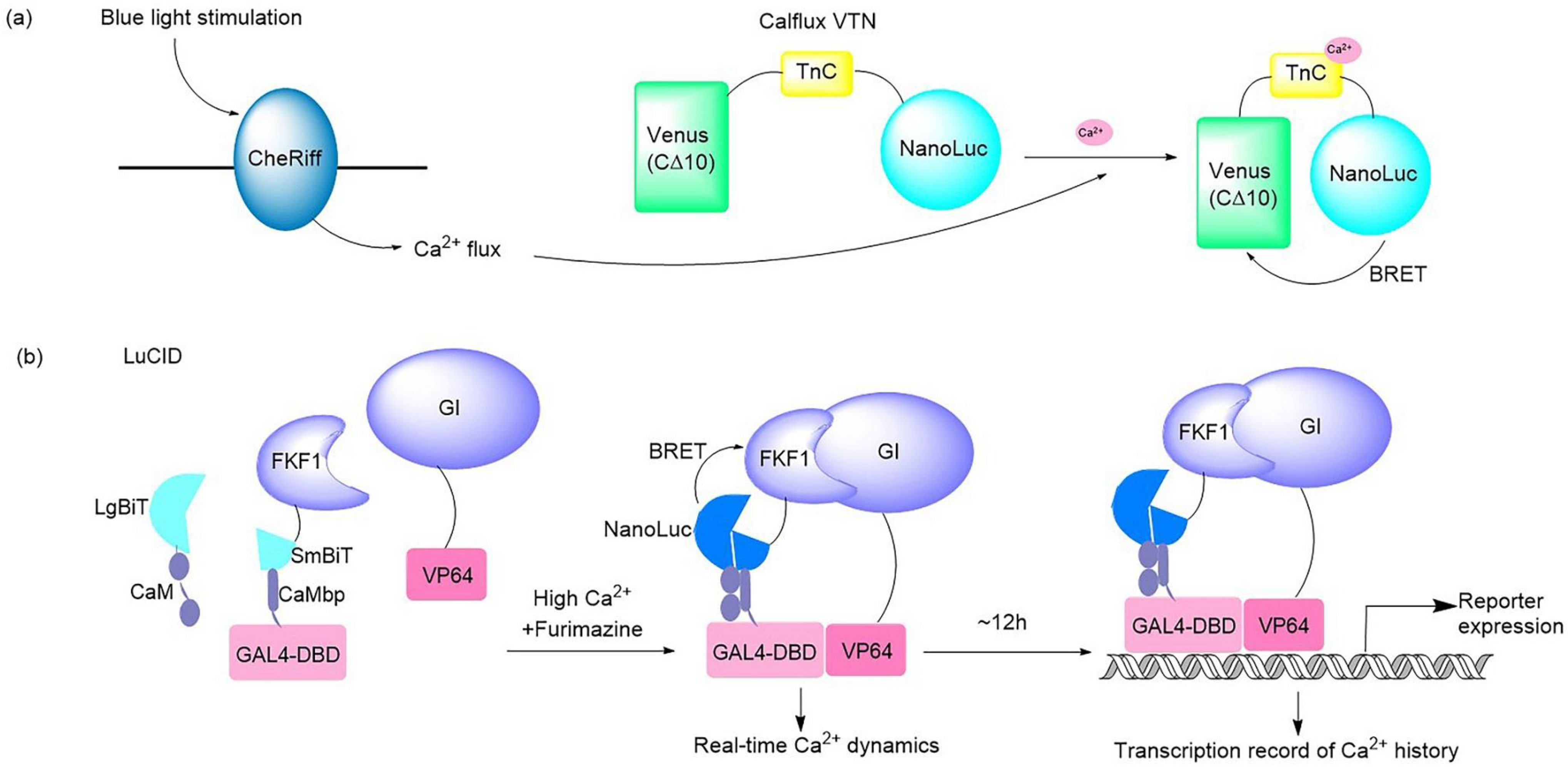
2.1. Bioluminescence-Induced Optical Biosensors for Ion Sensing
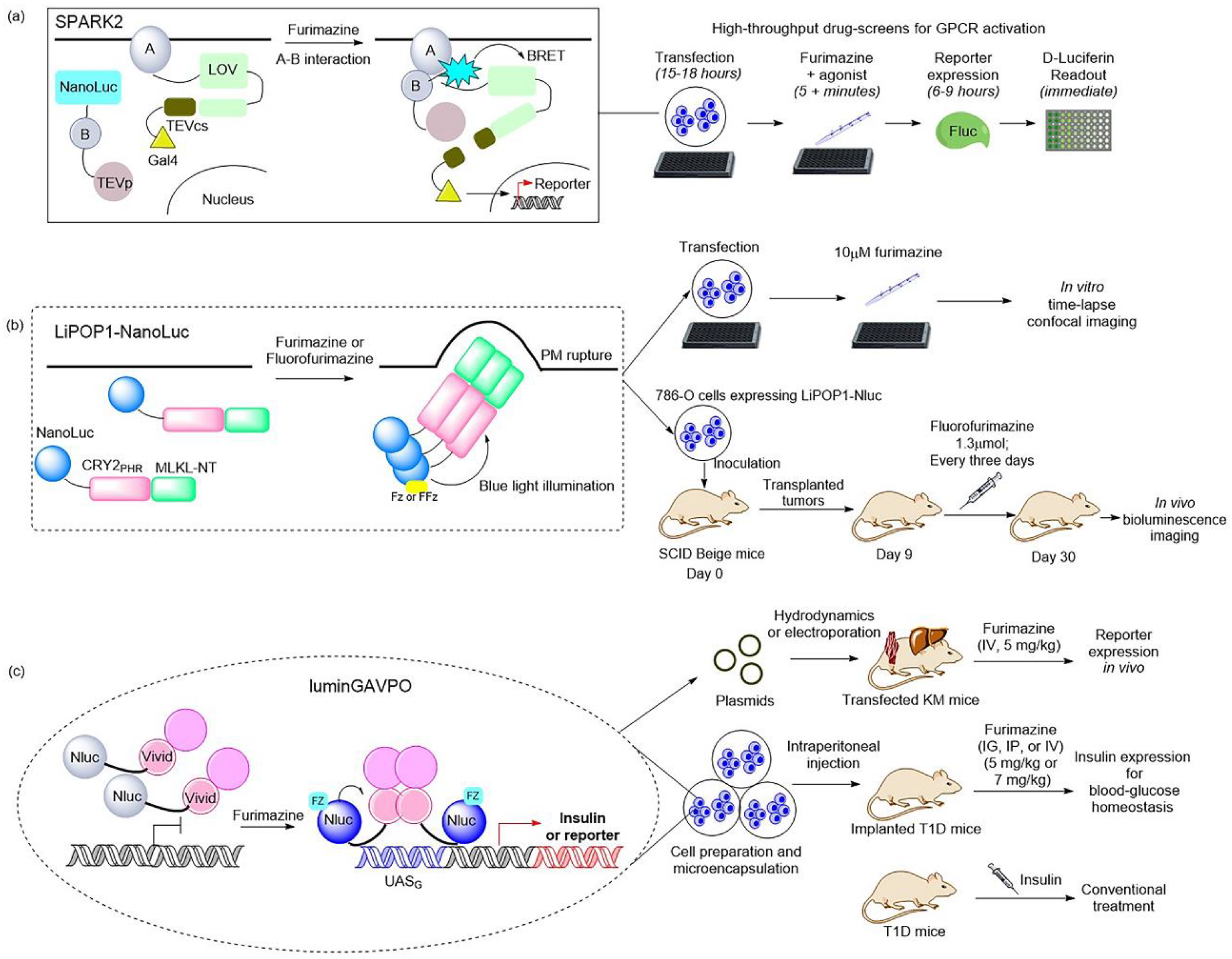
2.2. Bioluminescence-Aided Optical Tools for Reprogramming Cellular Activities
2.3. Luminopsins: Bioluminescent Optogenetics Probes in Neuroscience
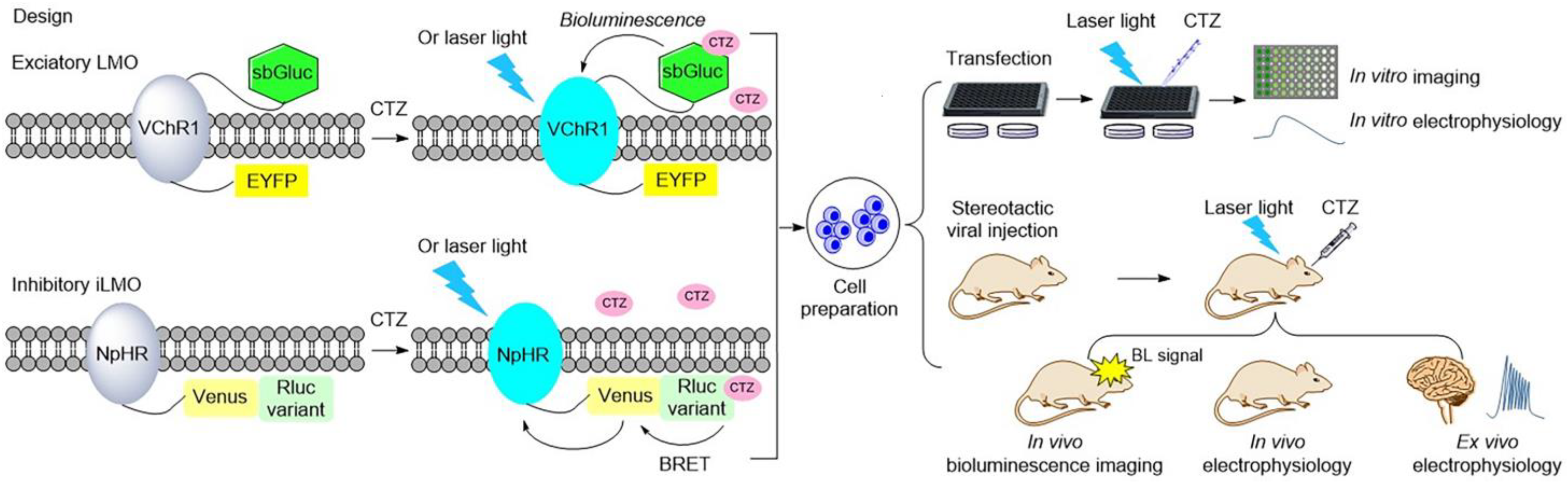
3. How to Optimize the Bioluminescence-Aided Photosensitive Probes
| Blue-Light-Emitting Luciferases | Peak Wavelength | Relative Brightness * | Decay Time | Potential Photoreceptor | In Application with Photosensory Domains | Reference |
|---|---|---|---|---|---|---|
| Rluc–CTZ | 482 nm | 1 | 0.6 h | Dronpa, rhodopsins, LOV domains, cryptochromes | iLMO1, hyBRET | [106,107] |
| Rluc8–CTZ | 487 nm | 4 | >86 h | hyBRET | [107,108] | |
| Gluc–CTZ | 480 nm | 24 | 1.3 ± 0.3 min | LMO1, LMO2 | [20,109] | |
| sbGluc–CTZ | 480 nm | 216 | 14.1 ± 3.2 min | LMO3, LMO3.2, eLMO3, SFLMO series | [20,109] | |
| GlucM23–CTZ | 480 nm | 240 | Not clear | LMO4, iLMO4 | [20,110] | |
| slGluc–CTZ | 481 nm | 72 | Not clear | iLMO | [20,111] | |
| LuxCDABE– Decanal and FMN | 490 nm | / | / | Bacterial biosensors for mercury | [112] | |
| NanoLuc–Fz | 456 nm | 96 | 2–3 h | LOV domains, cryptochromes, rhodopsins | bMCOⅡ, SPAPK2, LOTUS-V, BEACON, LiPOP, luminGAVPO, bPAC-nLuc | [113] |
| TeLuc–DTZ | 502 nm | 240 | ~40 min | Dronpa, rhodopsins | Not clear | [32] |
| RLuc8.6–CTZ | 535 nm | 6 | Not clear | Rhodopsins, cobalamin-binding protein | Not clear | [108] |
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AVPR2 | arginine vasopressin receptor 2 |
| AD | Alzheimer’s disease |
| AuNP | gold nanoparticle |
| asLOV2 | LOV domain from Avena sativa |
| bMCOII | bioluminescence multicharacteristic opsin II |
| BRET | bioluminescence resonance energy transfer |
| BEACON | BRET-activated optogenetics |
| bPAC | photoactivated adenylyl cyclase from Beggiatoa |
| CTZ | coelenterazine |
| CRY2 | cryptochrome 2 |
| ChR2 | channelrhodopsin-2 |
| CPH | caffeylpyruvate hydrolase |
| Cph1 | cyanobacterial phytochrome 1 |
| CHBT | 2-cyano-6-hydroxybenzothiazole |
| CalfluxVTN | calcium flux composed of Venus, troponin, and NanoLuc |
| CaM | calmodulin |
| CaMbp | calmodulin binding peptide |
| cAMP | cyclic AMP |
| DRD1 | dopamine receptor type I |
| DBD | DNA binding domain |
| ERα/β | estrogen receptor alpha/beta |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| EYFP | enhanced yellow fluorescent protein |
| EMCCD | electron-multiplying CCD |
| eNpHR | enhanced Natronomonas halorhodopsin |
| eLMO | excitatory luminopsin |
| FZ | furimazine |
| FFz | fluorofurimazine |
| FLuc | firefly luciferase |
| GFP | green fluorescent protein |
| GPCR | G protein-coupled receptor |
| GLuc | Gaussia luciferase |
| GeNL | green nanolantern |
| HispS | hispidin synthase |
| H3H | hispidin-3-hydroxylase |
| HBD | helix bundle domain |
| hAR | human androgen receptor |
| iLID | improved light-induced dimer |
| iLMO | inhibitory LMO |
| LOV | light-oxygen-voltage |
| LMO | luminopsin |
| LuCID | luciferin- and calcium-induced dimerization |
| LiPOP1 | light-induced nonapoptotic tool 1 |
| luminGAVPO | luminescent transcription factor GAVPO |
| MLKL | mixed-lineage kinase domain-like pseudokinase |
| Mac | Leptosphaeria maculans |
| NanoBiT | NanoLuc binary technology |
| NpHR | Natronomonas halorhodopsin |
| nLuc | nanoluciferase |
| Phy | phytochrome |
| PHR | photolyase-homologous region |
| Rluc | Renilla luciferase |
| RFP | red fluorescent protein |
| SR/ER | sarco/endoplasmic reticulum |
| SPARK2 | Specific protein association tool giving transcriptional readout with rapid kinetics |
| SFLMO | step-function luminopsin |
| sbGLuc | slow burn Gluc |
| slGLuc | super luminescent Gluc |
| TEVcs | tobacco etch virus cleavage site |
| T1D | type 1 diabetes |
| TnC | troponin C domain |
| UCNPs | upconversion nanoparticles |
| VVD | photoreceptor vivid |
| VChR1 | Volvox channelrhodopsin-1 |
References
- Yeh, H.W.; Ai, H.W. Development and Applications of Bioluminescent and Chemiluminescent Reporters and Biosensors. Annu. Rev. Anal. Chem. (Palo Alto Calif.) 2019, 12, 129–150. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Syed, A.J.; Anderson, J.C. Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev. 2021, 50, 5668–5705. [Google Scholar] [CrossRef]
- Shimomura, O. Bioluminescence: Chemical Principles and Methods; World Scientific Publishing Co., Pte. Ltd.: Singapore, 2006. [Google Scholar] [CrossRef]
- de Wet, J.R.; Wood, K.V.; DeLuca, M.; Helinski, D.R.; Subramani, S. Firefly luciferase gene: Structure and expression in mammalian cells. Mol. Cell. Biol. 1987, 7, 725–737. [Google Scholar] [CrossRef] [PubMed]
- White, E.H.; McCapra, F.; Field, G.F.; McElroy, W.D. The structure and synthesis of firefly luciferin. J. Am. Chem. Soc. 1961, 83, 2402–2403. [Google Scholar] [CrossRef]
- Malmqvist, M. Biospecific interaction analysis using biosensor technology. Nature 1993, 361, 186–187. [Google Scholar] [CrossRef]
- Masud, M.K.; Na, J.; Younus, M.; Hossain, M.S.A.; Bando, Y.; Shiddiky, M.J.A.; Yamauchi, Y. Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem. Soc. Rev. 2019, 48, 5717–5751. [Google Scholar] [CrossRef]
- Love, A.C.; Prescher, J.A. Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology. Cell Chem. Biol. 2020, 27, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, X.; Ji, H.; Du, L.; Li, M. Constructing firefly luciferin bioluminescence probes for in vivo imaging. Org. Biomol. Chem. 2022, 20, 1360–1372. [Google Scholar] [CrossRef]
- Baljinnyam, B.; Ronzetti, M.; Simeonov, A. Advances in luminescence-based technologies for drug discovery. Expert Opin. Drug Discov. 2023, 18, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Cheon, S.Y.; Park, S.; Lee, D.; Lee, Y.; Han, S.; Kim, M.; Koo, H. Recent advances in optical imaging through deep tissue: Imaging probes and techniques. Biomater. Res. 2022, 26, 57. [Google Scholar] [CrossRef]
- Anand, U.; Chandel, A.K.S.; Oleksak, P.; Mishra, A.; Krejcar, O.; Raval, I.H.; Dey, A.; Kuca, K. Recent advances in the potential applications of luminescence-based, SPR-based, and carbon-based biosensors. Appl. Microbiol. Biotechnol. 2022, 106, 2827–2853. [Google Scholar] [CrossRef]
- Kolar, K.; Knobloch, C.; Stork, H.; Žnidarič, M.; Weber, W. OptoBase: A Web Platform for Molecular Optogenetics. ACS Synth. Biol. 2018, 7, 1825–1828. [Google Scholar] [CrossRef]
- Hongdusit, A.; Liechty, E.T.; Fox, J.M. Optogenetic interrogation and control of cell signaling. Curr. Opin. Biotechnol. 2020, 66, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; He, L.; Huang, Y.; Zhou, Y. Optophysiology: Illuminating cell physiology with optogenetics. Physiol. Rev. 2022, 102, 1263–1325. [Google Scholar] [CrossRef] [PubMed]
- Berglund, K.; Birkner, E.; Augustine, G.J.; Hochgeschwender, U. Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons. PLoS ONE 2013, 8, e59759. [Google Scholar] [CrossRef] [PubMed]
- Erdenee, E.; Ting, A.Y. A Dual-Purpose Real-Time Indicator and Transcriptional Integrator for Calcium Detection in Living Cells. ACS Synth. Biol. 2022, 11, 1086–1095. [Google Scholar] [CrossRef]
- Kim, C.K.; Cho, K.F.; Kim, M.W.; Ting, A.Y. Luciferase-LOV BRET enables versatile and specific transcriptional readout of cellular protein-protein interactions. Elife 2019, 8, e43826. [Google Scholar] [CrossRef]
- Park, S.Y.; Song, S.-H.; Palmateer, B.; Pal, A.; Petersen, E.D.; Shall, G.P.; Welchko, R.M.; Ibata, K.; Miyawaki, A.; Augustine, G.J.; et al. Novel luciferase–opsin combinations for improved luminopsins. J. Neurosci. Res. 2020, 98, 410–421. [Google Scholar] [CrossRef]
- Narcisse, D.; Mustafi, S.M.; Carlson, M.; Batabyal, S.; Kim, S.; Wright, W.; Kumar Mohanty, S. Bioluminescent Multi-Characteristic Opsin for Simultaneous Optical Stimulation and Continuous Monitoring of Cortical Activities. Front. Cell Neurosci. 2021, 15, 750663. [Google Scholar] [CrossRef]
- He, L.; Huang, Z.; Huang, K.; Chen, R.; Nguyen, N.T.; Wang, R.; Cai, X.; Huang, Z.; Siwko, S.; Walker, J.R.; et al. Optogenetic Control of Non-Apoptotic Cell Death. Adv. Sci. (Weinh) 2021, 8, 2100424. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Qian, Y.; Shao, J.; Li, X.; Liu, S.; Zhu, L.; Zhao, Y.; Ye, H.; Yang, Y. A synthetic BRET-based optogenetic device for pulsatile transgene expression enabling glucose homeostasis in mice. Nat. Commun. 2021, 12, 615. [Google Scholar] [CrossRef]
- Sureda-Vives, M.; Sarkisyan, K.S. Bioluminescence-Driven Optogenetics. Life 2020, 10, 318. [Google Scholar] [CrossRef]
- Kamiya, G.; Kitada, N.; Furuta, T.; Hirano, T.; Maki, S.A.; Kim, S.B. S-Series Coelenterazine-Driven Combinatorial Bioluminescence Imaging Systems for Mammalian Cells. Int. J. Mol. Sci. 2023, 24, 1420. [Google Scholar] [CrossRef]
- Kamiya, G.; Kitada, N.; Furuta, T.; Hirano, T.; Maki, S.; Kim, S.B. C-Series Coelenterazine-Driven Bioluminescence Signature Imaging. Int. J. Mol. Sci. 2022, 23, 13047. [Google Scholar] [CrossRef] [PubMed]
- Coutant, E.P.; Gagnot, G.; Hervin, V.; Baatallah, R.; Goyard, S.; Jacob, Y.; Rose, T.; Janin, Y.L. Bioluminescence Profiling of NanoKAZ/NanoLuc Luciferase Using a Chemical Library of Coelenterazine Analogues. Chem. (Weinh. Der Bergstr. Ger.) 2020, 26, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, R.; Hoshino, E.; Kakudate, Y.; Kishigami, S.; Iwasawa, N.; Sasaki, S.I.; Nakajima, T.; Sato, M.; Nishiyama, S.; Citterio, D.; et al. Azide- and Dye-Conjugated Coelenterazine Analogues for a Multiplex Molecular Imaging Platform. Bioconjug Chem. 2018, 29, 1922–1931. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Du, L.; Li, M. Lighting up bioluminescence with coelenterazine: Strategies and applications. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2016, 15, 466–480. [Google Scholar] [CrossRef]
- Krasitskaya, V.V.; Bashmakova, E.E.; Frank, L.A. Coelenterazine-Dependent Luciferases as a Powerful Analytical Tool for Research and Biomedical Applications. Int. J. Mol. Sci. 2020, 21, 7465. [Google Scholar] [CrossRef]
- Su, Y.; Walker, J.R.; Park, Y.; Smith, T.P.; Liu, L.X.; Hall, M.P.; Labanieh, L.; Hurst, R.; Wang, D.C.; Encell, L.P.; et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020, 17, 852–860. [Google Scholar] [CrossRef]
- Yeh, H.W.; Karmach, O.; Ji, A.; Carter, D.; Martins-Green, M.M.; Ai, H.W. Red-shifted luciferase-luciferin pairs for enhanced bioluminescence imaging. Nat. Methods 2017, 14, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cumberbatch, D.; Centanni, S.; Shi, S.-Q.; Winder, D.; Webb, D.; Johnson, C.H. Coupling optogenetic stimulation with NanoLuc-based luminescence (BRET) Ca++ sensing. Nat. Commun. 2016, 7, 13268. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Tsutsui, H.; Suzuki, K.; Agetsuma, M.; Arai, Y.; Jinno, Y.; Bai, G.; Daniels, M.J.; Okamura, Y.; Matsuda, T.; et al. Genetically encoded bioluminescent voltage indicator for multi-purpose use in wide range of bioimaging. Sci. Rep. 2017, 7, 42398. [Google Scholar] [CrossRef]
- Inagaki, S.; Agetsuma, M.; Ohara, S.; Iijima, T.; Yokota, H.; Wazawa, T.; Arai, Y.; Nagai, T. Imaging local brain activity of multiple freely moving mice sharing the same environment. Sci. Rep. 2019, 9, 7460. [Google Scholar] [CrossRef]
- Parag-Sharma, K.; O’Banion, C.P.; Henry, E.C.; Musicant, A.M.; Cleveland, J.L.; Lawrence, D.S.; Amelio, A.L. Engineered BRET-Based Biologic Light Sources Enable Spatiotemporal Control over Diverse Optogenetic Systems. ACS Synth. Biol. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Naim, N.; White, A.D.; Reece, J.M.; Wankhede, M.; Zhang, X.; Vilardaga, J.-P.; Altschuler, D.L. Luminescence-activated nucleotide cyclase regulates spatial and temporal cAMP synthesis. J. Biol. Chem. 2019, 294, 1095–1103. [Google Scholar] [CrossRef]
- Saito-Moriya, R.; Nakayama, J.; Kamiya, G.; Kitada, N.; Obata, R.; Maki, S.A.; Aoyama, H. How to Select Firefly Luciferin Analogues for In Vivo Imaging. Int. J. Mol. Sci. 2021, 22, 1848. [Google Scholar] [CrossRef]
- Kotlobay, A.A.; Sarkisyan, K.S.; Mokrushina, Y.A.; Marcet-Houben, M.; Serebrovskaya, E.O.; Markina, N.M.; Gonzalez Somermeyer, L.; Gorokhovatsky, A.Y.; Vvedensky, A.; Purtov, K.V.; et al. Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. USA 2018, 115, 12728–12732. [Google Scholar] [CrossRef] [PubMed]
- Yevtodiyenko, A.; Bazhin, A.; Khodakivskyi, P.; Godinat, A.; Budin, G.; Maric, T.; Pietramaggiori, G.; Scherer, S.S.; Kunchulia, M.; Eppeldauer, G.; et al. Portable bioluminescent platform for in vivo monitoring of biological processes in non-transgenic animals. Nat. Commun. 2021, 12, 2680. [Google Scholar] [CrossRef]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef]
- Yao, Z.; Caldwell, D.R.; Love, A.C.; Kolbaba-Kartchner, B.; Mills, J.H.; Schnermann, M.J.; Prescher, J.A. Coumarin luciferins and mutant luciferases for robust multi-component bioluminescence imaging. Chem. Sci. 2021, 12, 11684–11691. [Google Scholar] [CrossRef]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like Protein from the Purple Membrane of Halobacterium halobium. Nat. New Biol. 1971, 233, 149–152. [Google Scholar] [CrossRef]
- Zhang, F.; Vierock, J.; Yizhar, O.; Fenno, L.E.; Tsunoda, S.; Kianianmomeni, A.; Prigge, M.; Berndt, A.; Cushman, J.; Polle, J.; et al. The microbial opsin family of optogenetic tools. Cell 2011, 147, 1446–1457. [Google Scholar] [CrossRef]
- Glantz, S.T.; Carpenter, E.J.; Melkonian, M.; Gardner, K.H.; Boyden, E.S.; Wong, G.K.-S.; Chow, B.Y. Functional and topological diversity of LOV domain photoreceptors. Proc. Natl. Acad. Sci. USA 2016, 113, E1442–E1451. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S. A history of optogenetics: The development of tools for controlling brain circuits with light. F1000 Biol. Rep. 2011, 3, 11. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Han, X.; Qian, X.; Bernstein, J.G.; Zhou, H.-H.; Franzesi, G.T.; Stern, P.; Bronson, R.T.; Graybiel, A.M.; Desimone, R.; Boyden, E.S. Millisecond-Timescale Optical Control of Neural Dynamics in the Nonhuman Primate Brain. Neuron 2009, 62, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cui, B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015, 33, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.-H.; He, L.; Huang, Y.; Zhou, Y. Optogenetics for transcriptional programming and genetic engineering. Trends Genet. 2022, 38, 1253–1270. [Google Scholar] [CrossRef]
- Chen, F.; Warnock, R.L.; Van der Meer, J.R.; Wegner, S.V. Bioluminescence-Triggered Photoswitchable Bacterial Adhesions Enable Higher Sensitivity and Dual-Readout Bacterial Biosensors for Mercury. ACS Sens. 2020, 5, 2205–2210. [Google Scholar] [CrossRef]
- Komatsu, N.; Terai, K.; Imanishi, A.; Kamioka, Y.; Sumiyama, K.; Jin, T.; Okada, Y.; Nagai, T.; Matsuda, M. A platform of BRET-FRET hybrid biosensors for optogenetics, chemical screening, and in vivo imaging. Sci. Rep. 2018, 8, 8984. [Google Scholar] [CrossRef]
- Adir, O.; Albalak, M.R.; Abel, R.; Weiss, L.E.; Chen, G.; Gruber, A.; Staufer, O.; Kurman, Y.; Kaminer, I.; Shklover, J.; et al. Synthetic cells with self-activating optogenetic proteins communicate with natural cells. Nat. Commun. 2022, 13, 2328. [Google Scholar] [CrossRef] [PubMed]
- Birkner, E.; Berglund, K.; Klein, M.E.; Augustine, G.J.; Hochgeschwender, U. Non-invasive activation of optogenetic actuators. Proc. SPIE Int. Soc. Opt. Eng. 2014, 8928, 89282F. [Google Scholar] [CrossRef] [PubMed]
- Berglund, K.; Clissold, K.; Li, H.E.; Wen, L.; Park, S.Y.; Gleixner, J.; Klein, M.E.; Lu, D.; Barter, J.W.; Rossi, M.A.; et al. Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation. Proc. Natl. Acad. Sci. USA 2016, 113, E358–E367. [Google Scholar] [CrossRef]
- Yu, S.P.; Tung, J.K.; Wei, Z.Z.; Chen, D.; Berglund, K.; Zhong, W.; Zhang, J.Y.; Gu, X.; Song, M.; Gross, R.E.; et al. Optochemogenetic Stimulation of Transplanted iPS-NPCs Enhances Neuronal Repair and Functional Recovery after Ischemic Stroke. J. Neurosci. 2019, 39, 6571–6594. [Google Scholar] [CrossRef]
- Zenchak, J.R.; Palmateer, B.; Dorka, N.; Brown, T.M.; Wagner, L.-M.; Medendorp, W.E.; Petersen, E.D.; Prakash, M.; Hochgeschwender, U. Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model. J. Neurosci. Res. 2020, 98, 458–468. [Google Scholar] [CrossRef]
- Song, D.; Yang, Q.; Lang, Y.; Wen, Z.; Xie, Z.; Zheng, D.; Yan, T.; Deng, Y.; Nakanishi, H.; Quan, Z.; et al. Manipulation of hippocampal CA3 firing via luminopsins modulates spatial and episodic short-term memory, especially working memory, but not long-term memory. Neurobiol. Learn. Mem. 2018, 155, 435–445. [Google Scholar] [CrossRef]
- Petersen, E.D.; Sharkey, E.D.; Pal, A.; Shafau, L.O.; Zenchak-Petersen, J.; Peña, A.J.; Aggarwal, A.; Prakash, M.; Hochgeschwender, U. Restoring Function After Severe Spinal Cord Injury through BioLuminescent-OptoGenetics. Front. Neurol. 2022, 12, 792643. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Tung, J.K.; Wang, Z.; Yu, S.P.; Gross, R.E.; Wei, L.; Berglund, K. Improved trafficking and expression of luminopsins for more efficient optical and pharmacological control of neuronal activity. J. Neurosci. Res. 2020, 98, 481–490. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Berglund, K.; Carrasco, D.; Goebel, K.; Gross, R.E.; Isaacson, R.; Mistretta, O.C.; Wynans, C. Bioluminescent Optogenetics: A Novel Experimental Therapy to Promote Axon Regeneration after Peripheral Nerve Injury. Int. J. Mol. Sci. 2021, 22, 7217. [Google Scholar] [CrossRef]
- Ecanow, A.; Berglund, K.; Carrasco, D.; Isaacson, R.; English, A.W. Enhancing Motor and Sensory Axon Regeneration after Peripheral Nerve Injury Using Bioluminescent Optogenetics. Int. J. Mol. Sci. 2022, 23, 16084. [Google Scholar] [CrossRef]
- Ikefuama, E.C.; Kendziorski, G.E.; Anderson, K.; Shafau, L.; Prakash, M.; Hochgeschwender, U.; Petersen, E.D. Improved Locomotor Recovery in a Rat Model of Spinal Cord Injury by BioLuminescent-OptoGenetic (BL-OG) Stimulation with an Enhanced Luminopsin. Int. J. Mol. Sci. 2022, 23, 12994. [Google Scholar] [CrossRef]
- Berglund, K.; Fernandez, A.M.; Gutekunst, C.-A.N.; Hochgeschwender, U.; Gross, R.E. Step-function luminopsins for bimodal prolonged neuromodulation. J. Neurosci. Res. 2020, 98, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.K.; Gutekunst, C.A.; Gross, R.E. Inhibitory luminopsins: Genetically-encoded bioluminescent opsins for versatile, scalable, and hardware-independent optogenetic inhibition. Sci. Rep. 2015, 5, 14366. [Google Scholar] [CrossRef]
- Tung, J.K.; Shiu, F.H.; Ding, K.; Gross, R.E. Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol. Dis. 2018, 109, 1–10. [Google Scholar] [CrossRef]
- Jaiswal, P.B.; Tung, J.K.; Gross, R.E.; English, A.W. Motoneuron activity is required for enhancements in functional recovery after peripheral nerve injury in exercised female mice. J. Neurosci. Res. 2020, 98, 448–457. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ma, G.; Zhou, Y.; Jing, J. Optogenetic approaches to control Ca(2+)-modulated physiological processes. Curr. Opin. Physiol. 2020, 17, 187–196. [Google Scholar] [CrossRef]
- Broyles, C.N.; Robinson, P.; Daniels, M.J. Fluorescent, Bioluminescent, and Optogenetic Approaches to Study Excitable Physiology in the Single Cardiomyocyte. Cells 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Takai, A.; Nakano, M.; Saito, K.; Haruno, R.; Watanabe, T.M.; Ohyanagi, T.; Jin, T.; Okada, Y.; Nagai, T. Expanded palette of Nano-lanterns for real-time multicolor luminescence imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 4352–4356. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, M.; Sadaghiani, A.M.; Hsueh, B.; Dolmetsch, R.E. Induction of protein-protein interactions in live cells using light. Nat. Biotechnol. 2009, 27, 941–945. [Google Scholar] [CrossRef]
- Saito, K.; Chang, Y.F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3, 1262. [Google Scholar] [CrossRef]
- Suzuki, K.; Kimura, T.; Shinoda, H.; Bai, G.; Daniels, M.J.; Arai, Y.; Nakano, M.; Nagai, T. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 2016, 7, 13718. [Google Scholar] [CrossRef]
- Hossain, M.N.; Suzuki, K.; Iwano, M.; Matsuda, T.; Nagai, T. Bioluminescent Low-Affinity Ca2+ Indicator for ER with Multicolor Calcium Imaging in Single Living Cells. ACS Chem. Biol. 2018, 13, 1862–1871. [Google Scholar] [CrossRef]
- Farhana, I.; Hossain, M.N.; Suzuki, K.; Matsuda, T.; Nagai, T. Genetically Encoded Fluorescence/Bioluminescence Bimodal Indicators for Ca2+ Imaging. ACS Sens. 2019, 4, 1825–1834. [Google Scholar] [CrossRef]
- Qian, Y.; Rancic, V.; Wu, J.; Ballanyi, K.; Campbell, R.E. A Bioluminescent Ca2+ Indicator Based on a Topological Variant of GCaMP6s. ChemBioChem 2019, 20, 516–520. [Google Scholar] [CrossRef]
- Picaud, S.; Lambolez, B.; Tricoire, L. Bioluminescence Imaging of Neuronal Network Dynamics Using Aequorin-Based Calcium Sensors. In Live Cell Imaging: Methods and Protocols; Kim, S.-B., Ed.; Springer: New York, NY, USA, 2021; pp. 281–294. [Google Scholar]
- Roychaudhuri, R.; Gadalla, M.M.; West, T.; Snyder, S.H. A Novel Stereospecific Bioluminescent Assay for Detection of Endogenous d-Cysteine. ACS Chem. Neurosci. 2022, 13, 3257–3262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Seo, H.; Park, Y.; Lee, H.S.; Lee, S.H.; Ko, K.S. Development of a Human Estrogen Receptor Dimerization Assay for the Estrogenic Endocrine-Disrupting Chemicals Using Bioluminescence Resonance Energy Transfer. Int. J. Environ. Res. Public Health 2021, 18, 8875. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Seo, H.; Byrd, N.; Kim, H.; Lee, K.G.; Lee, S.H.; Park, Y. Human cell-based estrogen receptor beta dimerization assay. Chem. Biol. Interact 2022, 369, 110264. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Lopreside, A.; Montali, L.; Cevenini, L.; Roda, A.; Michelini, E. A Genetically Encoded Bioluminescence Intracellular Nanosensor for Androgen Receptor Activation Monitoring in 3D Cell Models. Sensors 2021, 21, 893. [Google Scholar] [CrossRef]
- Nagai, T.; Horikawa, K.; Saito, K.; Matsuda, T. Genetically encoded Ca(2+) indicators; expanded affinity range, color hue and compatibility with optogenetics. Front. Mol. Neurosci. 2014, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Levskaya, A.; Chevalier, A.A.; Tabor, J.J.; Simpson, Z.B.; Lavery, L.A.; Levy, M.; Davidson, E.A.; Scouras, A.; Ellington, A.D.; Marcotte, E.M.; et al. Engineering Escherichia coli to see light. Nature 2005, 438, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Kroning, K.E.; Wang, W. SPARK: A Transcriptional Assay for Recording Protein-Protein Interactions in a Defined Time Window. Curr. Protoc. 2021, 1, e190. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 2012, 9, 266–269. [Google Scholar] [CrossRef]
- Berglund, K.; Tung, J.K.; Higashikubo, B.; Gross, R.E.; Moore, C.I.; Hochgeschwender, U. Combined Optogenetic and Chemogenetic Control of Neurons. Methods Mol. Biol. 2016, 1408, 207–225. [Google Scholar] [CrossRef]
- Crespo, E.L.; Prakash, M.; Bjorefeldt, A.; Medendorp, W.E.; Shaner, N.C.; Lipscombe, D.; Moore, C.I.; Hochgeschwender, U. Bioluminescent optogenetic (BL-OG) activation of neurons during mouse postnatal brain development. STAR Protoc. 2021, 2, 100667. [Google Scholar] [CrossRef]
- Gomez-Ramirez, M.; More, A.I.; Friedman, N.G.; Hochgeschwender, U.; Moore, C.I. The BioLuminescent-OptoGenetic in vivo response to coelenterazine is proportional, sensitive, and specific in neocortex. J. Neurosci. Res. 2020, 98, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Tackenberg, M.C.; McMahon, D.G. Optogenetic Methods for the Study of Circadian Rhythms. In Circadian Clocks: Methods and Protocols; Brown, S.A., Ed.; Springer: New York, NY, USA, 2021; pp. 325–336. [Google Scholar]
- Chrobok, L.; Pradel, K.; Janik, M.E.; Sanetra, A.M.; Bubka, M.; Myung, J.; Ridla Rahim, A.; Klich, J.D.; Jeczmien-Lazur, J.S.; Palus-Chramiec, K.; et al. Intrinsic circadian timekeeping properties of the thalamic lateral geniculate nucleus. J. Neurosci. Res. 2021, 99, 3306–3324. [Google Scholar] [CrossRef]
- Tackenberg, M.C.; Hughey, J.J.; McMahon, D.G. Optogenetic stimulation of VIPergic SCN neurons induces photoperiodic-like changes in the mammalian circadian clock. Eur. J. Neurosci. 2021, 54, 7063–7071. [Google Scholar] [CrossRef]
- Prakash, M.; Murphy, J.; St Laurent, R.; Friedman, N.; Crespo, E.L.; Bjorefeldt, A.; Pal, A.; Bhagat, Y.; Kauer, J.A.; Shaner, N.C.; et al. Selective control of synaptically-connected circuit elements by all-optical synapses. Commun. Biol. 2022, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Zambito, G.; Hall, M.P.; Wood, M.G.; Gaspar, N.; Ridwan, Y.; Stellari, F.F.; Shi, C.; Kirkland, T.A.; Encell, L.P.; Löwik, C.; et al. Red-shifted click beetle luciferase mutant expands the multicolor bioluminescent palette for deep tissue imaging. iScience 2021, 24, 101986. [Google Scholar] [CrossRef]
- Endo, M.; Ozawa, T. Advanced Bioluminescence System for In Vivo Imaging with Brighter and Red-Shifted Light Emission. Int. J. Mol. Sci. 2020, 21, 6538. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, E.; Ingles-Prieto, A.; Tichy, A.-M.; McKenzie, C.; Janovjak, H. A Phytochrome Sensory Domain Permits Receptor Activation by Red Light. Angew. Chem. Int. Ed. 2016, 55, 6339–6342. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.; Lin, M.Z. Optobiochemistry: Genetically Encoded Control of Protein Activity by Light. Annu. Rev. Biochem. 2021, 90, 475–501. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Schoenenberger, P.; Mattis, J.; Tye, K.M.; Deisseroth, K.; Hegemann, P.; Oertner, T.G. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. USA 2011, 108, 7595–7600. [Google Scholar] [CrossRef]
- Yi, Z.; Luo, Z.; Qin, X.; Chen, Q.; Liu, X. Lanthanide-Activated Nanoparticles: A Toolbox for Bioimaging, Therapeutics, and Neuromodulation. Acc. Chem. Res. 2020, 53, 2692–2704. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Ma, G.; Tan, P.; Li, Z.; Zang, S.; Wu, X.; Jing, J.; Fang, S.; Zhou, L.; et al. Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. eLife 2015, 4, e10024. [Google Scholar] [CrossRef]
- He, L.; Tan, P.; Zhu, L.; Huang, K.; Nguyen, N.T.; Wang, R.; Guo, L.; Li, L.; Yang, Y.; Huang, Z.; et al. Circularly permuted LOV2 as a modular photoswitch for optogenetic engineering. Nat. Chem. Biol. 2021, 17, 915–923. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Huang, K.; Zeng, H.; Jing, J.; Wang, R.; Fang, S.; Chen, J.; Liu, X.; Huang, Z.; You, M.J.; et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat. Nanotechnol. 2021, 16, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.; Xin, H.; Wan, T.; Ping, Y. Near-infrared optogenetic engineering of photothermal nanoCRISPR for programmable genome editing. Proc. Natl. Acad. Sci. USA 2020, 117, 2395–2405. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, X.; Chong, P.; Liu, J.; Andre, L.N.; Ong, K.S.; Brinson, K., Jr.; Mahdi, A.I.; Li, J.; Fenno, L.E.; et al. Sono-optogenetics facilitated by a circulation-delivered rechargeable light source for minimally invasive optogenetics. Proc. Natl. Acad. Sci. USA 2019, 116, 26332–26342. [Google Scholar] [CrossRef]
- Chen, Z.; Tsytsarev, V.; Finfrock, Y.Z.; Antipova, O.A.; Cai, Z.; Arakawa, H.; Lischka, F.W.; Hooks, B.M.; Wilton, R.; Wang, D.; et al. Wireless Optogenetic Modulation of Cortical Neurons Enabled by Radioluminescent Nanoparticles. ACS Nano 2021, 15, 5201–5208. [Google Scholar] [CrossRef]
- Liu, J.; O’Kane, D.J.; Escher, A. Secretion of functional Renilla reniformis luciferase by mammalian cells. Gene 1997, 203, 141–148. [Google Scholar] [CrossRef]
- Loening, A.M.; Fenn, T.D.; Wu, A.M.; Gambhir, S.S. Consensus guided mutagenesis of Renilla luciferase yields enhanced stability and light output. Protein Eng. Des. Sel. PEDS 2006, 19, 391–400. [Google Scholar] [CrossRef]
- Loening, A.M.; Wu, A.M.; Gambhir, S.S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat. Methods 2007, 4, 641–643. [Google Scholar] [CrossRef]
- Welsh, J.P.; Patel, K.G.; Manthiram, K.; Swartz, J.R. Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochem. Biophys. Res. Commun. 2009, 389, 563–568. [Google Scholar] [CrossRef]
- Lindberg, E.; Mizukami, S.; Ibata, K.; Miyawaki, A.; Kikuchi, K. Development of luminescent coelenterazine derivatives activatable by β-galactosidase for monitoring dual gene expression. Chem. (Weinh. Der Bergstr. Ger.) 2013, 19, 14970–14976. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Suzuki, H.; Sato, M.; Tao, H. Superluminescent variants of marine luciferases for bioassays. Anal. Chem. 2011, 83, 8732–8740. [Google Scholar] [CrossRef]
- Francis, K.P.; Yu, J.; Bellinger-Kawahara, C.; Joh, D.; Hawkinson, M.J.; Xiao, G.; Purchio, T.F.; Caparon, M.G.; Lipsitch, M.; Contag, P.R. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect. Immun. 2001, 69, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P.; Unch, J.; Binkowski, B.F.; Valley, M.P.; Butler, B.L.; Wood, M.G.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Machleidt, T.; et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012, 7, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
| Name | Coupling Method | Photoreceptor | Luciferase | Luciferin | Intracellular Effect | Purpose | Reference |
|---|---|---|---|---|---|---|---|
| bMCOII | Fusion | Mutant opsin from algae | GFP–NanoLuc | FZ | Ca2+ sensing | Cortical activities | [21] |
| / | Co-transfection | ChR2, melanopsin | Venus–NanoLuc | FZ | Ca2+ sensing | Ca2+ signaling in neuron | [33] |
| LuCID | Fusion | LOV domain in FKF1 | Reconstituted NanoLuc (NanoBiT) | FZ | Ca2+ sensing | Study calcium signaling | [18] |
| LOTUS-V | Co-transfection | ChR2, NpHR3.0 | NanoLuc–Venus | FZ | Voltage indicator | Imaging | [34,35] |
| / | Nonfusion | Magnets (from VVD) | LuxCDABE | Decanal and FMN | Bacterial adhesions | Hg2+ sensing | [51] |
| hyBRET | Co-transfection | CRY2 | YFP–CFP–Rluc8 | CTZh, diacetyl CTZ h | ERK activation | ERK activity | [52] |
| SPARK2 | Co-transfection | asLOV2 (LOV from Avena sativa) | NanoLuc | FZ | Protein dissociation/transcription | Screening for GPCR agonists | [19] |
| BEACON | Co-transfection | CRY2, LOV, VVD | mCerulean3–NanoLuc, eGFP–NanoLuc | FZ | Transgene expression | Study signaling pathway | [36] |
| LiPOP | Fusion | CRY2 | NanoLuc | FZ, FFz | Cell death | Nonapoptotic cell death | [22] |
| luminGAVPO | Fusion | VVD domain in transcription factor | NanoLuc | FZ | Transgene expression | Type 1 diabetes | [23] |
| / | Fusion | LOV domain in EL222 | Gluc | CTZ | Cell communications | Control synthetic processes | [53] |
| bPAC–nLuc | Fusion | A light-activated adenylyl cyclase from Beggiatoa | NanoLuc | FZ, Fz-4377, CTZ h | cAMP synthesis | Control cAMP signaling | [37] |
| LMO1 | Fusion | ChR2 | Gluc | CTZ | Excite neurons | Control neuronal activity | [17,54] |
| LMO2 | Fusion | VChR1 | Gluc | CTZ | Excite neurons | ||
| LMO3 | Fusion | VChR1 | sbGluc | CTZ | Excite neurons | Treatment for Alzheimer’s disease, Parkinson’s disease, and spinal cord injury. Study for postnatal brain development | [55,56,57,58,59] |
| eLMO3 | Fusion | VChR1 | sbGluc | CTZ | Excite neurons | Control neuronal activity, enhance axon regeneration after peripheral nerve injury | [60,61,62] |
| LMO3.2 | Fusion | Opsin CheRiff | sbGluc | CTZ | Excite neurons | Control neuronal activity. Treatment for spinal cord injury | [63] |
| LMO4 | Fusion | VChR1 | GlucM23 | CTZ | Excite neurons | Control neuronal activity | [20] |
| SFLMO series | Fusion | ChR2CS, ChR2DA, ChR2CS/DA | sbGluc | CTZ | Excite neurons | Epileptic networks | [64] |
| iLMO | Fusion | Mac | slGluc | CTZ | Inhibit neurons | Treatment for Alzheimer’s disease | [55,58] |
| iLMO1 | Fusion | NpHR | TagRFP–Rluc | CTZ | Inhibit neurons | Control neuronal activity | [65] |
| iLMO2 | Fusion | NpHR | Nanolantern (Venus–Rluc8) | CTZ | Inhibit neurons | Control neuronal activity, functional recovery after peripheral nerve injury | [65,66,67] |
| iLMO4 | Fusion | iChloC | GLucM23 | CTZ | Inhibit neurons | Control neuronal activity | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Song, J.; Zhang, Y. Coelenterazine-Type Bioluminescence-Induced Optical Probes for Sensing and Controlling Biological Processes. Int. J. Mol. Sci. 2023, 24, 5074. https://doi.org/10.3390/ijms24065074
Jiang T, Song J, Zhang Y. Coelenterazine-Type Bioluminescence-Induced Optical Probes for Sensing and Controlling Biological Processes. International Journal of Molecular Sciences. 2023; 24(6):5074. https://doi.org/10.3390/ijms24065074
Chicago/Turabian StyleJiang, Tianyu, Jingwen Song, and Youming Zhang. 2023. "Coelenterazine-Type Bioluminescence-Induced Optical Probes for Sensing and Controlling Biological Processes" International Journal of Molecular Sciences 24, no. 6: 5074. https://doi.org/10.3390/ijms24065074
APA StyleJiang, T., Song, J., & Zhang, Y. (2023). Coelenterazine-Type Bioluminescence-Induced Optical Probes for Sensing and Controlling Biological Processes. International Journal of Molecular Sciences, 24(6), 5074. https://doi.org/10.3390/ijms24065074










