Neurodegenerative Changes in the Brains of the 5xFAD Alzheimer’s Disease Model Mice Investigated by High-Field and High-Resolution Magnetic Resonance Imaging and Multi-Nuclei Magnetic Resonance Spectroscopy
Abstract
1. Introduction
2. Results
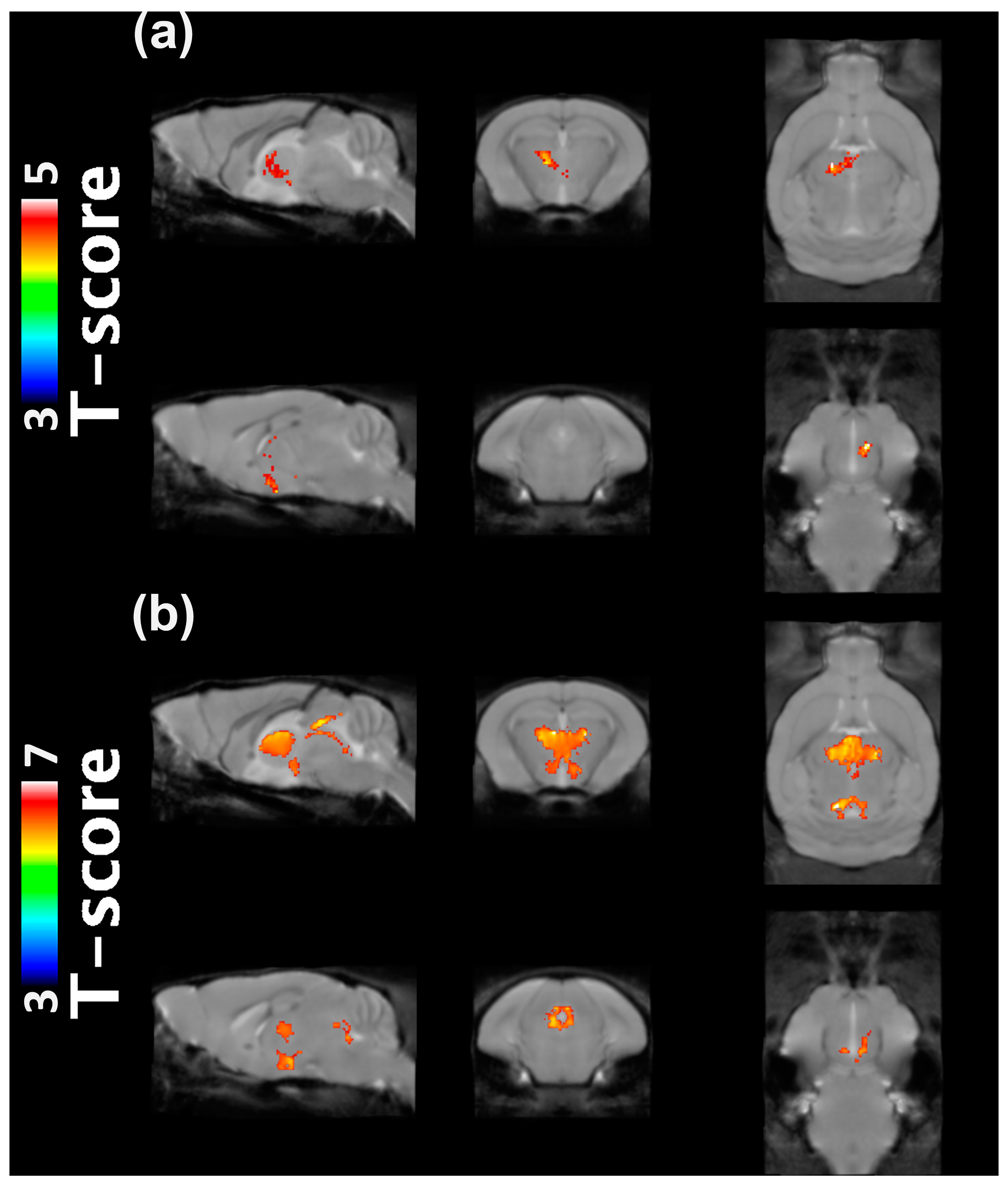
2.1. VBM Analysis
2.2. 1H MRS
2.3. 31P MRS
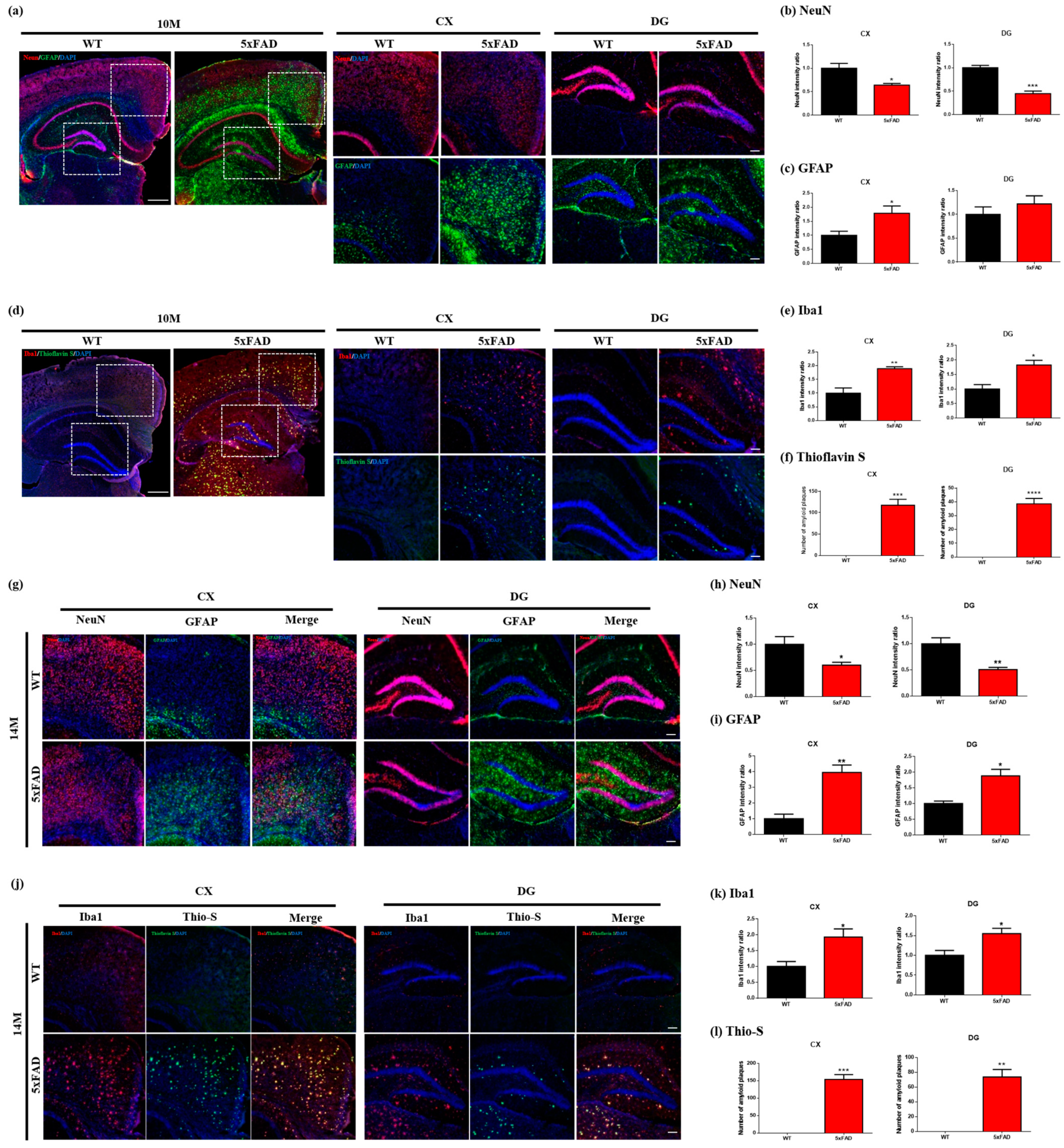
2.4. Immunofluorescence
3. Discussion
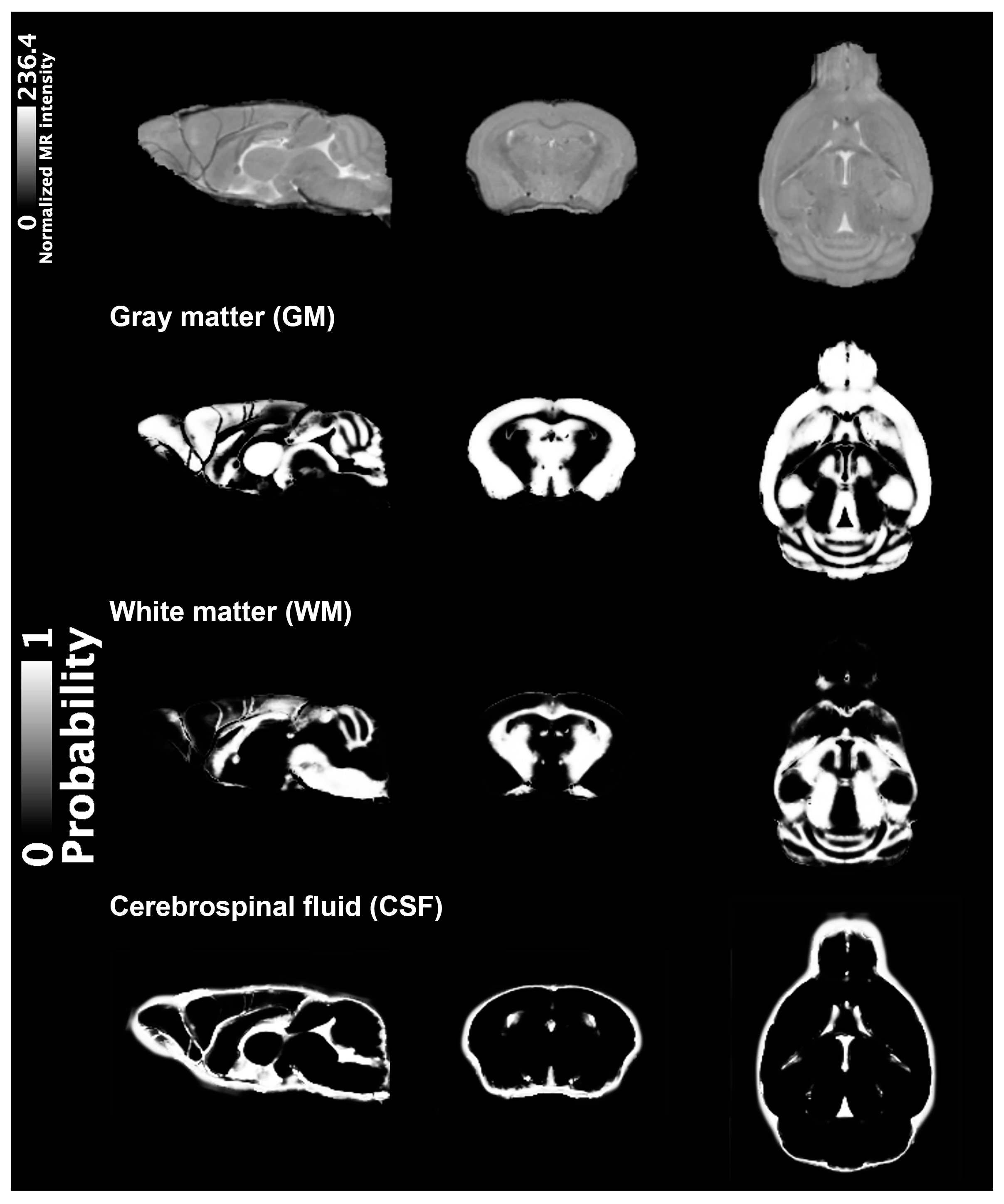
4. Materials and Methods
4.1. Animal Preparation
4.2. Acquisition of MRI/MRS
4.3. VBM Analysis
4.4. Preprocessing and Quantification for MRS
4.5. Immunofluorescence and Thioflavins S Staining
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morley, J.E.; Farr, S.A.; Nguyen, A.D. Alzheimer Disease. Clin. Geriatr. Med. 2018, 34, 591–601. [Google Scholar] [CrossRef]
- Song, C.; Guo, S.; Jin, S.; Chen, L.; Jung, Y.M. Biomarkers Determination Based on Surface-Enhanced Raman Scattering. Chemosensors 2020, 8, 118. [Google Scholar] [CrossRef]
- Adlard, P.A.; Tran, B.A.; Finkelstein, D.I.; Desmond, P.M.; Johnston, L.A.; Bush, A.I.; Egan, G.F. A Review of β-Amyloid Neuroimaging in Alzheimer’s Disease. Front. Neurosci. 2014, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Waerzeggers, Y.; Monfared, P.; Viel, T.; Winkeler, A.; Jacobs, A.H. Mouse Models in Neurological Disorders: Applications of Non-Invasive Imaging. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 819–839. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Bodea, L.G.; Goedert, M. Rodent Models for Alzheimer Disease. Nat. Rev. Neurosci. 2018, 19, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Barker, P.B. Various MRS Application Tools for Alzheimer Disease and Mild Cognitive Impairment. Am. J. Neuroradiol. 2014, 35, S4–S11. [Google Scholar] [CrossRef]
- Wang, H.; Tan, L.; Wang, H.F.; Liu, Y.; Yin, R.H.; Wang, W.Y.; Chang, X.L.; Jiang, T.; Yu, J.T. Magnetic Resonance Spectroscopy in Alzheimer’s Disease: Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2015, 46, 1049–1070. [Google Scholar] [CrossRef]
- Mandal, P.K. Magnetic Resonance Spectroscopy (MRS) and Its Application in Alzheimer’s Disease. Concepts Magn. Reson. Part A Bridg. Educ. Res. 2007, 30A, 40–64. [Google Scholar] [CrossRef]
- Watanabe, T.; Shiino, A.; Akiguchi, I. Absolute Quantification in Proton Magnetic Resonance Spectroscopy is Useful to Differentiate Amnesic Mild Cognitive Impairment from Alzheimer’s Disease and Healthy Aging. Dement. Geriatr. Cogn. Disord. 2010, 30, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Dedeoglu, A.; Choi, J.K.; Cormier, K.; Kowall, N.W.; Jenkins, B.G. Magnetic Resonance Spectroscopic Analysis of Alzheimer’s Disease Mouse Brain that Express Mutant Human APP Shows Altered Neurochemical Profile. Brain Res. 2004, 1012, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Rijpma, A.; van der Graaf, M.; Meulenbroek, O.; Olde Rikkert, M.G.M.; Heerschap, A. Altered Brain High-Energy Phosphate Metabolism in Mild Alzheimer’s Disease: A 3-Dimensional 31P MR Spectroscopic Imaging Study. NeuroImage Clin. 2018, 18, 254–261. [Google Scholar] [CrossRef]
- Pettegrew, J.W.; Panchalingam, K.; Klunk, W.E.; McClure, R.J.; Muenz, L.R. Alterations of Cerebral Metabolism in Probable Alzheimer’s Disease: A Preliminary Study. Neurobiol. Aging 1994, 15, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Cuénod, C.A.; Kaplan, D.B.; Michot, J.L.; Forette, F.; Jehenson, P.; Leroy Willig, A.; Syrota, A.; Boller, F. Phospholipid Abnormalities in Early Alzheimer’s Disease: In Vivo Phosphorus 31 Magnetic Resonance Spectroscopy. Arch. Neurol. 1995, 52, 89–94. [Google Scholar] [CrossRef]
- Chapleau, M.; Aldebert, J.; Montembeault, M.; Brambati, S.M. Atrophy in Alzheimer’s Disease and Semantic Dementia: An ALE Meta-Analysis of Voxel-Based Morphometry Studies. J. Alzheimers Dis. 2016, 54, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.G.; Filho, G.B. Voxel-Based Morphometry in Alzheimers Disease and Mild Cognitive Impairment: Systematic Review of Studies Addressing the Frontal Lobe. Dement. Neuropsychol. 2016, 10, 104–112. [Google Scholar] [CrossRef]
- Matsuda, H. Voxel-Based Morphometry of Brain MRI in Normal Aging and Alzheimer’s Disease. Aging Dis. 2013, 4, 29–37. [Google Scholar]
- Di Paola, M.; Macaluso, E.; Carlesimo, G.A.; Tomaiuolo, F.; Worsley, K.J.; Fadda, L.; Caltagirone, C. Episodic Memory Impairment in Patients with Alzheimer’s Disease is Correlated with Entorhinal Cortex Atrophy: A Voxel-Based Morphometry Study. J. Neurol. 2007, 254, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Matsuda, H.; Nemoto, K.; Ohnishi, T.; Hirao, K.; Yamashita, F.; Asada, T.; Iwabuchi, S.; Samejima, H. Voxel-Based Morphometry to Discriminate Early Alzheimer’s Disease from Controls. Neurosci. Lett. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Schmidt-Wilcke, T.; Poljansky, S.; Hierlmeier, S.; Hausner, J.; Ibach, B. Memory Performance Correlates with Gray Matter Density in the Ento-/Perirhinal Cortex and Posterior Hippocampus in Patients with Mild Cognitive Impairment and Healthy Controls—A Voxel Based Morphometry Study. Neuroimage 2009, 47, 1914–1920. [Google Scholar] [CrossRef]
- Sawiak, S.J.; Wood, N.I.; Williams, G.B.; Morton, A.J.; Carpenter, T.A. Voxel-Based Morphometry with Templates and Validation in a Mouse Model of Huntington’s Disease. Magn. Reson. Imaging 2013, 31, 1522–1531. [Google Scholar] [CrossRef]
- Hikishima, K.; Komaki, Y.; Seki, F.; Ohnishi, Y.; Okano, H.J.; Okano, H. In Vivo Microscopic Voxel-Based Morphometry with a Brain Template to Characterize Strainspecific Structures in the Mouse Brain. Sci. Rep. 2017, 7, 85. [Google Scholar] [CrossRef]
- Hikishima, K.; Ando, K.; Komaki, Y.; Kawai, K.; Yano, R.; Inoue, T.; Itoh, T.; Yamada, M.; Momoshima, S.; Okano, H.J.; et al. Voxel-Based Morphometry of the Marmoset Brain: In Vivo Detection of Volume Loss in the Substantia Nigra of the MPTP-treated Parkinson’s Disease Model. Neuroscience 2015, 300, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Jullienne, A.; Trinh, M.V.; Obenaus, A. Neuroimaging of Mouse Models of Alzheimer’s Disease. Biomedicines 2022, 10, 305. [Google Scholar] [CrossRef]
- Girard, S.D.; Baranger, K.; Gauthier, C.; Jacquet, M.; Bernard, A.; Escoffier, G.; Marchetti, E.; Khrestchatisky, M.; Rivera, S.; Roman, F.S. Evidence for Early Cognitive Impairment Related to Frontal Cortex in the 5XFAD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, 781–796. [Google Scholar] [CrossRef]
- Girard, S.D.; Jacquet, M.; Baranger, K.; Migliorati, M.; Escoffier, G.; Bernard, A.; Khrestchatisky, M.; Féron, F.; Rivera, S.; Roman, F.S.; et al. Onset of Hippocampus-Dependent Memory Impairments in 5XFAD Transgenic Mouse Model of Alzheimer’s Disease. Hippocampus 2014, 24, 762–772. [Google Scholar] [CrossRef]
- Macdonald, I.R.; DeBay, D.R.; Reid, G.A.; O’Leary, T.P.; Jollymore, C.T.; Mawko, G.; Burrell, S.; Martin, E.; Bowen, C.V.; Brown, R.E.; et al. Early Detection of Cerebral Glucose Uptake Changes in the 5XFAD Mouse. Curr. Alzheimer Res. 2014, 11, 450–460. [Google Scholar] [CrossRef]
- Near, J.; Edden, R.; Evans, C.J.; Paquin, R.; Harris, A.; Jezzard, P. Frequency and Phase Drift Correction of Magnetic Resonance Spectroscopy data by Spectral Registration in the Time Domain. Magn. Reson. Med. 2015, 73, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Lalande, J.; Halley, H.; Balayssac, S.; Gilard, V.; Déjean, S.; Martino, R.; Francés, B.; Lassalle, J.M.; Malet-Martino, M. 1H NMR Metabolomic Signatures in Five Brain Regions of the aβPPswe Tg2576 Mouse Model of Alzheimer’s Disease at Four Ages. J. Alzheimers Dis. 2014, 39, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Mlynárik, V.; Cacquevel, M.; Sun-Reimer, L.; Janssens, S.; Cudalbu, C.; Lei, H.; Schneider, B.L.; Aebischer, P.; Gruetter, R. Proton and Phosphorus Magnetic Resonance Spectroscopy of a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2012, 31, S87–S99. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, H.J.; Jeong, Y.J.; Oh, S.J.; Kang, K.J.; Han, S.J.; Nam, K.R.; Lee, Y.J.; Lee, K.C.; Ryu, Y.H.; et al. Age Dependency of mGluR5 Availability in 5xFAD Mice Measured by PET. Neurobiol. Aging 2019, 84, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Edden, R.A.E.; Gao, F.; Wang, G.; Wu, L.; Zhao, B.; Wang, M.; Chan, Q.; Chen, W.; Barker, P.B. Decreased γ-Aminobutyric Acid Levels in the Parietal Region of Patients with Alzheimer’s Disease. J. Magn. Reson. Imaging 2015, 41, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. Changes in Intracellular Calcium and Glutathione in Astrocytes as the Primary Mechanism of Amyloid Neurotoxicity. J. Neurosci. 2003, 23, 5088–5095. [Google Scholar] [CrossRef]
- Bains, J.S.; Shaw, C.A. Neurodegenerative Disorders in Humans: The Role of Glutathione in Oxidative Stress-Mediated Neuronal Death. Brain Res. Rev. 1997, 25, 335–358. [Google Scholar] [CrossRef]
- Cecchi, C.; Latorraca, S.; Sorbi, S.; Iantomasi, T.; Favilli, F.; Vincenzini, M.T.; Liguri, G. Gluthatione level is altered in lymphoblasts from patients with familial Alzheimer’s disease. Neurosci. Lett. 1999, 275, 152–154. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Oliveira, C.R. Glutathione Cycle Impairment Mediates Aβ-Induced Cell Toxicity. Free Radic. Res. 2003, 37, 241–250. [Google Scholar] [CrossRef]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR Studies on the Energy Metabolism of Glial and Neuronal Cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef]
- Burg, M.B.; Kwon, E.D.; Kültz, D. Regulation of Gene Expression by Hypertonicity. Annu. Rev. Physiol. 1997, 59, 437–455. [Google Scholar] [CrossRef]
- Law, R.O. Regulation of Mammalian Brain Cell Volume. J. Exp. Zool. 1994, 268, 90–96. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Wacker, P.; Nunes, P.V.; Yacubian, J.; Castro, C.C.; Otaduy, M.C.G.; Gattaz, W.F. Reduced Phospholipid Breakdown in Alzheimer’s Brains: A 31P Spectroscopy Study. Psychopharmacology 2005, 180, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Akolkar, H.; Tripathi, M. Mapping of Hippocampal pH and Neurochemicals from In Vivo Multi-Voxel 31P Study in Healthy Normal Young Male/Female, Mild Cognitive Impairment, and Alzheimer’s Disease. J. Alzheimers Dis. 2012, 31, S75–S86. [Google Scholar] [CrossRef]
- Pollak, N.; Dölle, C.; Ziegler, M. The Power to Reduce: Pyridine Nucleotides—Small Molecules with a Multitude of Functions. Biochem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Hérard, A.S.; Flandin, G.; Duchesnay, E.; Besret, L.; Frouin, V.; Hantraye, P.; Bonvento, G.; Delzescaux, T. Quantitative Validation of Voxel-Wise Statistical Analyses of Autoradiographic Rat Brain Volumes: Application to Unilateral Visual Stimulation. Neuroimage 2008, 40, 482–494. [Google Scholar] [CrossRef]
- Dubois, A.; Hérard, A.S.; Delatour, B.; Hantraye, P.; Bonvento, G.; Dhenain, M.; Delzescaux, T. Detection by Voxel-Wise Statistical Analysis of Significant Changes in Regional Cerebral Glucose Uptake in an APP/PS1 Transgenic Mouse Model of Alzheimer’s Disease. Neuroimage 2010, 51, 586–598. [Google Scholar] [CrossRef]
- Yang, J.; Zaim Wadghiri, Y.; Minh Hoang, D.; Tsui, W.; Sun, Y.; Chung, E.; Li, Y.; Wang, A.; de Leon, M.; Wisniewski, T. Detection of Amyloid Plaques Targeted by USPIO-Aβ1-42 in Alzheimer’s Disease Transgenic Mice Using Magnetic Resonance Microimaging. Neuroimage 2011, 55, 1600–1609. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2001; ISBN 0125476361. [Google Scholar]
- Govindaraju, V.; Young, K.; Maudsley, A.A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000, 13, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Automatic Quantitation of Localized in Vivo1H Spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.; Cesare, F.D.; Andrasescu, A.; Popa, E.; Lazariev, A.; Vescovo, E.; Strbak, O.; Williams, S.; Starcuk, Z.; Cabanas, M.; et al. Quantitation of Magnetic Resonance Spectroscopy Signals: The jMRUI Software Package. Meas. Sci. Technol. 2009, 20, 104035. [Google Scholar] [CrossRef]


| 10-Month-Old | 14-Month-Old | |||||||
| WT | 5xFAD | WT | 5xFAD | |||||
| Global Measures | Average | SD | Average | SD | Average | SD | Average | SD |
| GMV | 0.24 | 0.01 | 0.25 | 0.02 | 0.24 | 0.01 | 0.22 | 0.08 |
| ICV | 0.56 | 0.08 | 0.53 | 0.03 | 0.57 | 0.05 | 0.49 | 0.17 |
| TBV | 0.38 | 0.01 | 0.39 | 0.02 | 0.39 | 0.01 | 0.35 | 0.13 |
| Brain regions | Modulated GM volume | |||||||
| Caudate-putamen | 67.62 | 22.9 | 73.11 | 24.81 | 67.83 | 24.02 | 68.15 | 24.19 |
| Globus pallidus | 16.35 | 14.4 | 18.5 | 16.21 | 15.69 | 14.38 | 15.18 | 13.88 |
| Hippocampus | 62.78 | 20.9 | 69.68 | 22.87 | 65.97 | 21.47 | 64.12 | 20.92 |
| Amygdala | 68.76 | 21.18 | 75.55 | 22.93 | 71.74 | 21.83 | 69.79 | 21.39 |
| Thalamus | 31.95 | 29.6 | 33.45 | 30.81 | 32.18 | 29.71 | 32.2 | 29.77 |
| Hypothalamus | 48.48 | 26.12 | 49.34 | 26.71 | 48.73 | 25.84 | 48.59 | 25.8 |
| Neo cortex | 59.69 | 23.49 | 65.9 | 25.45 | 61.25 | 24.09 | 60.87 | 24.02 |
| Central gray | 55.98 | 17.54 | 56.86 | 18.54 | 56.28 | 16.89 | 54.23 | 16.73 |
| Cerebellum | 38.87 | 20.54 | 41.62 | 21.9 | 39.04 | 20.92 | 38.37 | 20.36 |
| Midbrain | 15.48 | 14.24 | 15.56 | 14.75 | 15.36 | 14.47 | 14.99 | 14.15 |
| Olfactory bulb | 60.45 | 26.4 | 59.17 | 26.61 | 60.09 | 25.48 | 60.36 | 25.4 |
| Internal capsule | 12.7 | 9.89 | 14.65 | 11.22 | 12.14 | 10.13 | 11.78 | 9.53 |
| External capsule | 47.49 | 21.7 | 52.88 | 23.75 | 49.47 | 22.25 | 48.9 | 22.06 |
| Anterior commissure | 67.22 | 12.48 | 70.58 | 12.51 | 66.7 | 12.37 | 68.24 | 12.07 |
| Superior colliculi | 19.17 | 13.87 | 20.46 | 15.1 | 20.1 | 14.43 | 19.1 | 13.88 |
| Inferior colliculi | 15.47 | 13.59 | 16.94 | 15.13 | 17.29 | 14.74 | 16.86 | 14.37 |
| Fimbria | 15.2 | 7.08 | 16.56 | 7.45 | 14.55 | 6.19 | 14.57 | 6.09 |
| Basal forebrain septa | 57.91 | 18.44 | 62.33 | 19.47 | 59.82 | 18.18 | 60.03 | 18.57 |
| In Vivo 1H Spectroscopy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cortex | Hippocampus | |||||||
| 10 Month | 14 Month | 10 Month | 14 Month | |||||
| Metabolites | 5xFAD | WT | 5xFAD | WT | 5xFAD | WT | 5xFAD | WT |
| GABA | 3.67 ± 0.67 | 3.49 ± 0.70 | 2.90 ± 0.71 | 3.18 ± 0.72 | 2.80 ± 0.88 | 3.74 ± 0.93 | 2.79 ± 1.24 | 3.86 ± 0.75 |
| Gln | 4.90 ± 1.15 | 5.39 ± 1.17 | 5.06 ± 1.06 | 5.13 ± 0.98 | 4.37 ± 0.99 | 4.06 ± 0.67 | 3.99 ± 1.01 | 4.29 ± 0.56 |
| Glu | 13.07 ± 1.27 | 13.61 ± 1.45 | 13.56 ± 1.15 | 12.68 ± 1.18 | 8.38 ± 0.81 | 8.74 ± 0.93 | 8.92 ± 1.12 | 9.08 ± 0.55 |
| GSH | 1.77 ± 0.31 | 2.52 ± 0.49 | 2.44 ± 0.69 | 1.81 ± 0.42 | 1.74 ± 0.55 | 1.93 ± 0.36 | 2.34 ± 0.48 | 1.99 ± 0.40 |
| mIns | 6.93 ± 0.45 | 7.01 ± 0.85 | 7.01 ± 1.43 | 6.20 ± 0.89 | 6.92 ± 0.86 | 6.16 ± 0.41 | 7.92 ± 0.83 | 6.18 ± 0.67 |
| Tau | 13.82 ± 1.41 | 15.80 ± 1.08 | 15.07 ± 2.50 | 15.00 ± 1.29 | 11.83 ± 1.71 | 11.75 ± 0.95 | 13.71 ± 1.02 | 12.32 ± 1.45 |
| tCho | 2.24 ± 0.56 | 2.46 ± 0.22 | 2.12 ± 0.57 | 2.26 ± 0.25 | 1.75 ± 0.34 | 1.71 ± 0.13 | 1.65 ± 0.37 | 1.88 ± 0.17 |
| tNAA | 10.37 ± 0.55 | 11.53 ± 1.05 | 10.39 ± 0.78 | 11.07 ± 1.07 | 8.27 ± 0.64 | 8.94 ± 0.68 | 8.57 ± 0.92 | 9.60 ± 0.71 |
| tCr | 9.49 ± 0.88 | 10.80 ± 1.05 | 10.44 ± 2.25 | 10.06 ± 1.07 | 10.03 ± 0.70 | 9.36 ± 0.71 | 10.46 ± 1.01 | 10.09 ± 0.96 |
| Glx | 17.96 ± 1.57 | 18.99 ± 2.02 | 18.62 ± 1.74 | 17.81 ± 1.79 | 12.75 ± 1.53 | 12.81 ± 1.25 | 12.90 ± 1.76 | 13.37 ± 0.93 |
| In vivo 31P spectroscopy in the whole brain | ||||||||
| 11 month | ||||||||
| Metabolites | 5xFAD | WT | ||||||
| tATP/PCr | 1.86 ± 0.22 | 1.78 ± 0.15 | ||||||
| Pi/PCr | 0.40 ± 0.13 | 0.45 ± 0.08 | ||||||
| PME/PCr | 0.66 ± 0.07 | 0.76 ± 0.03 | ||||||
| PDE/PCr | 0.36 ± 0.07 | 0.26 ± 0.06 | ||||||
| NADP/PCr | 0.54 ± 0.14 | 0.81 ± 0.11 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, C.-H.; Kim, J.; Baek, H.-M.; Chang, K.-A.; Choe, B.-Y. Neurodegenerative Changes in the Brains of the 5xFAD Alzheimer’s Disease Model Mice Investigated by High-Field and High-Resolution Magnetic Resonance Imaging and Multi-Nuclei Magnetic Resonance Spectroscopy. Int. J. Mol. Sci. 2023, 24, 5073. https://doi.org/10.3390/ijms24065073
Yoo C-H, Kim J, Baek H-M, Chang K-A, Choe B-Y. Neurodegenerative Changes in the Brains of the 5xFAD Alzheimer’s Disease Model Mice Investigated by High-Field and High-Resolution Magnetic Resonance Imaging and Multi-Nuclei Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences. 2023; 24(6):5073. https://doi.org/10.3390/ijms24065073
Chicago/Turabian StyleYoo, Chi-Hyeon, Jinho Kim, Hyeon-Man Baek, Keun-A Chang, and Bo-Young Choe. 2023. "Neurodegenerative Changes in the Brains of the 5xFAD Alzheimer’s Disease Model Mice Investigated by High-Field and High-Resolution Magnetic Resonance Imaging and Multi-Nuclei Magnetic Resonance Spectroscopy" International Journal of Molecular Sciences 24, no. 6: 5073. https://doi.org/10.3390/ijms24065073
APA StyleYoo, C.-H., Kim, J., Baek, H.-M., Chang, K.-A., & Choe, B.-Y. (2023). Neurodegenerative Changes in the Brains of the 5xFAD Alzheimer’s Disease Model Mice Investigated by High-Field and High-Resolution Magnetic Resonance Imaging and Multi-Nuclei Magnetic Resonance Spectroscopy. International Journal of Molecular Sciences, 24(6), 5073. https://doi.org/10.3390/ijms24065073







