Effect of Chitosan-Diosgenin Combination on Wound Healing
Abstract
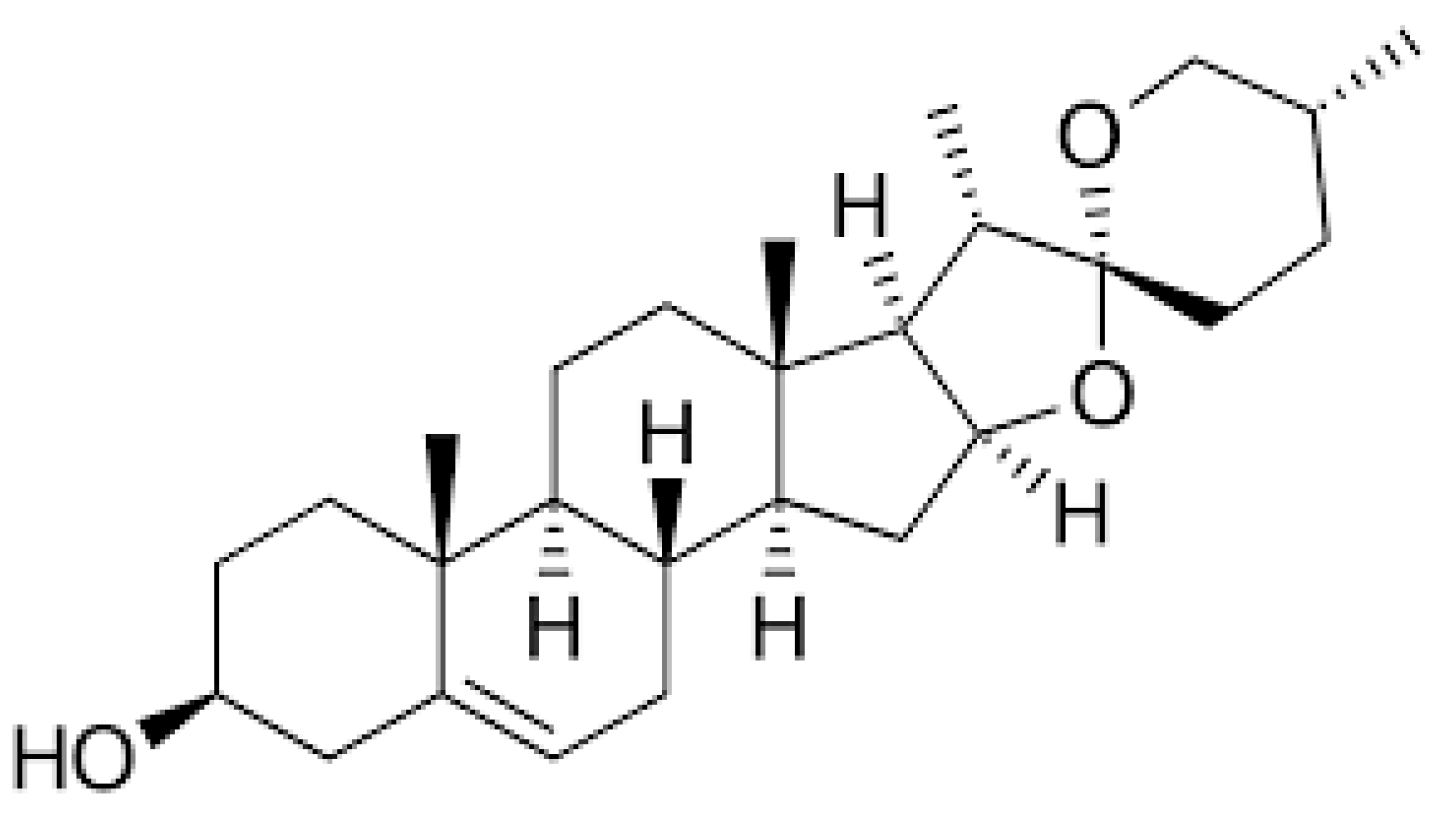
1. Introduction
2. Results
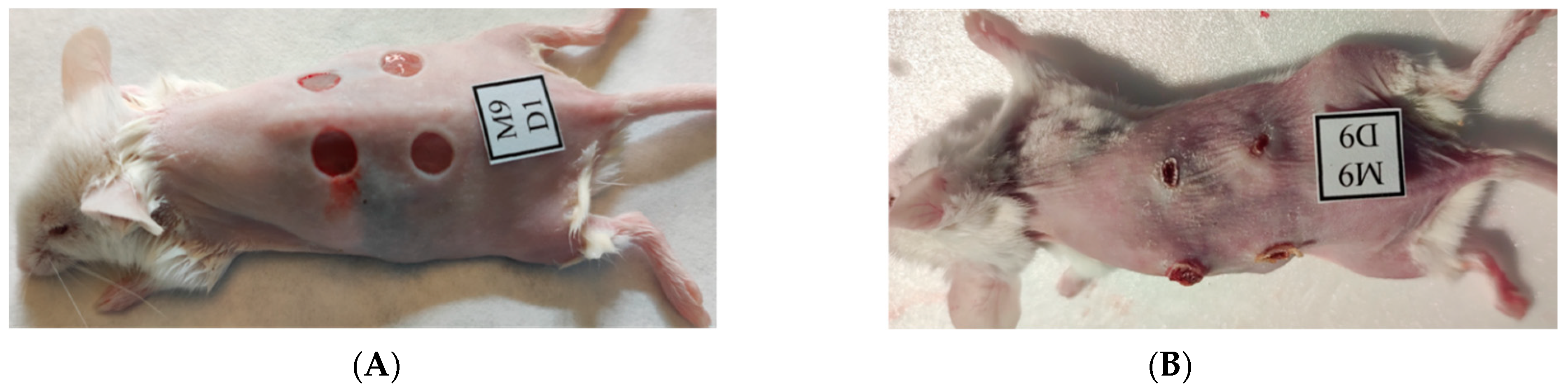
2.1. Macroscopic Observations
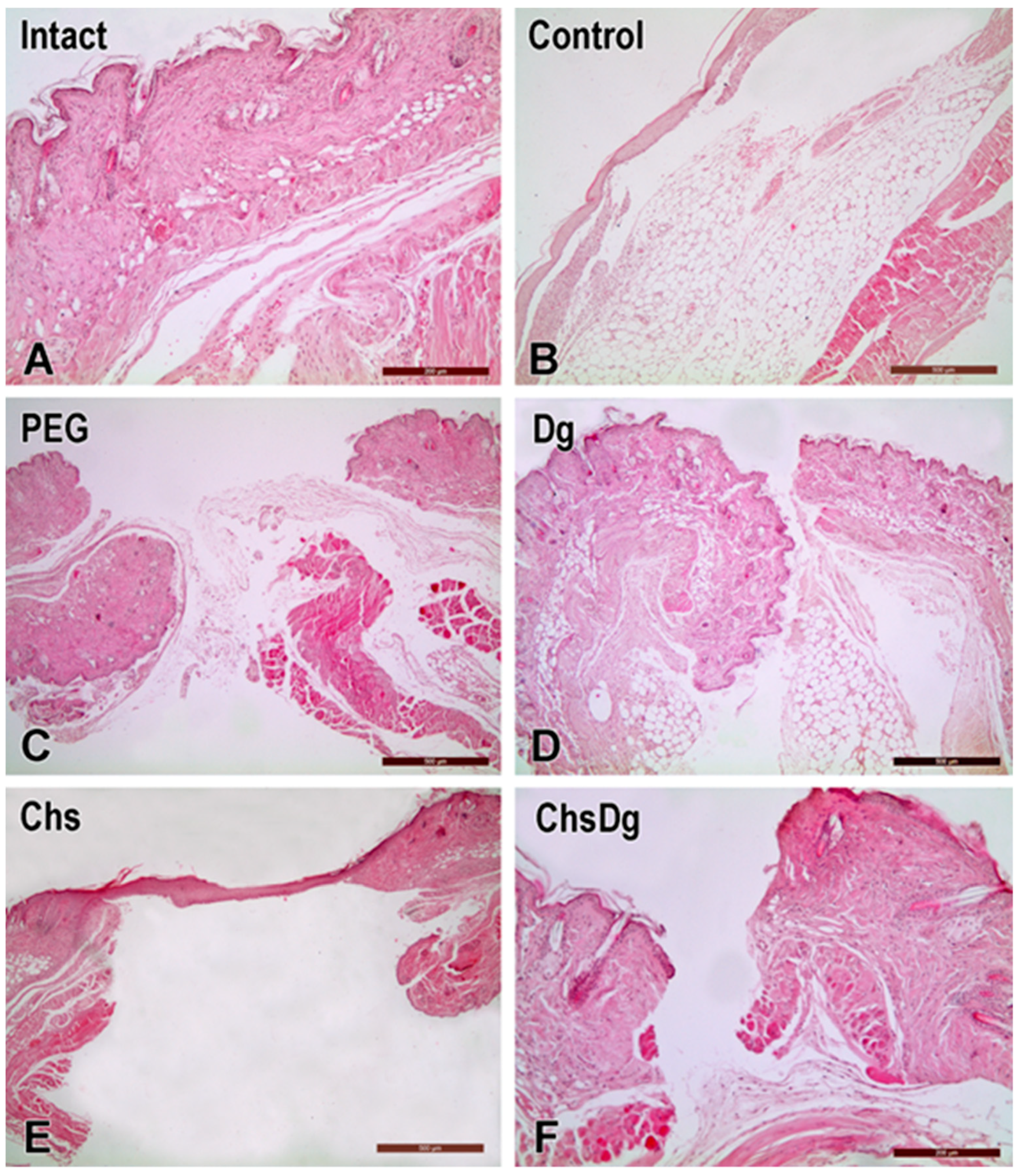
2.2. Microscopic Observations
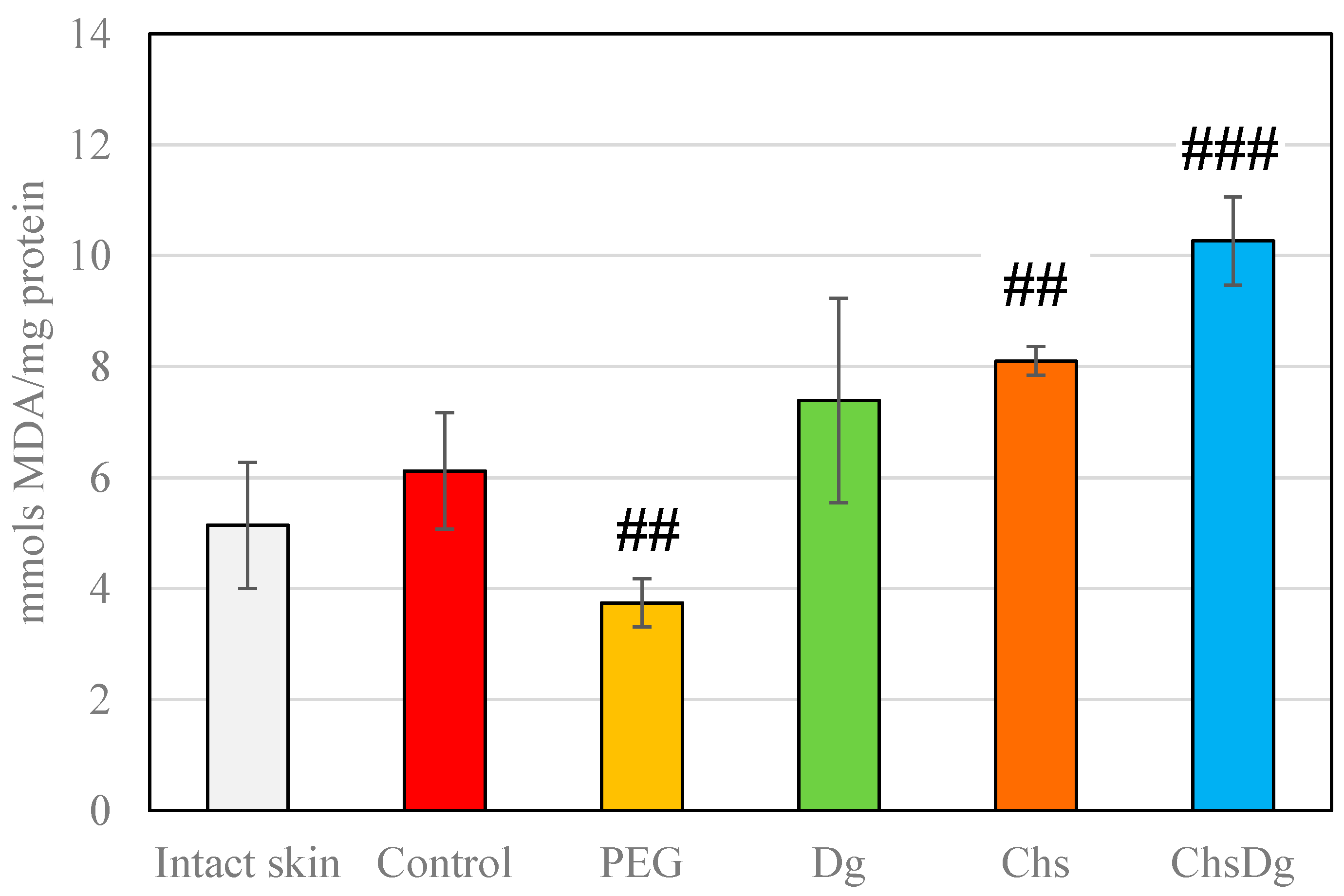
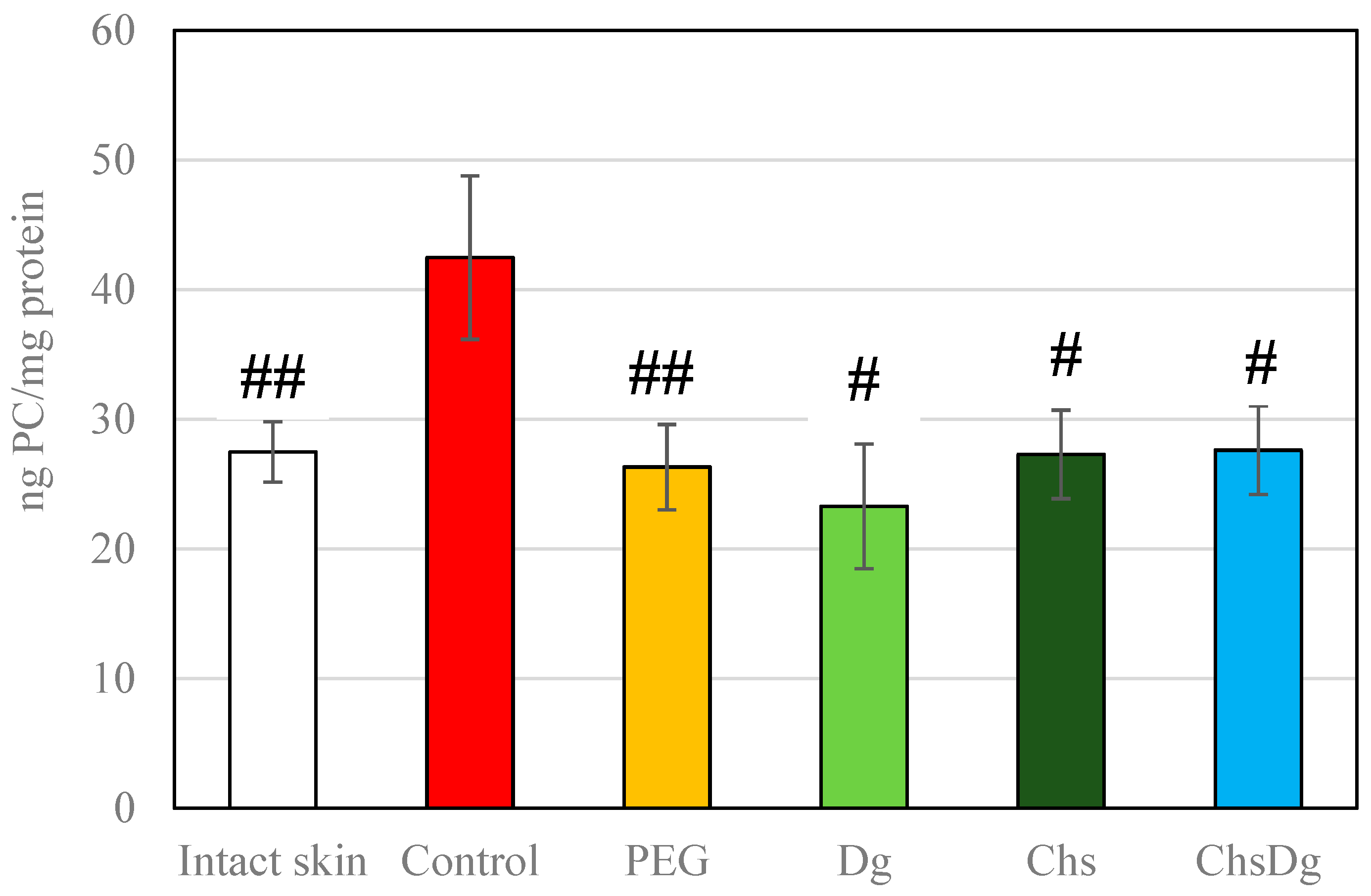
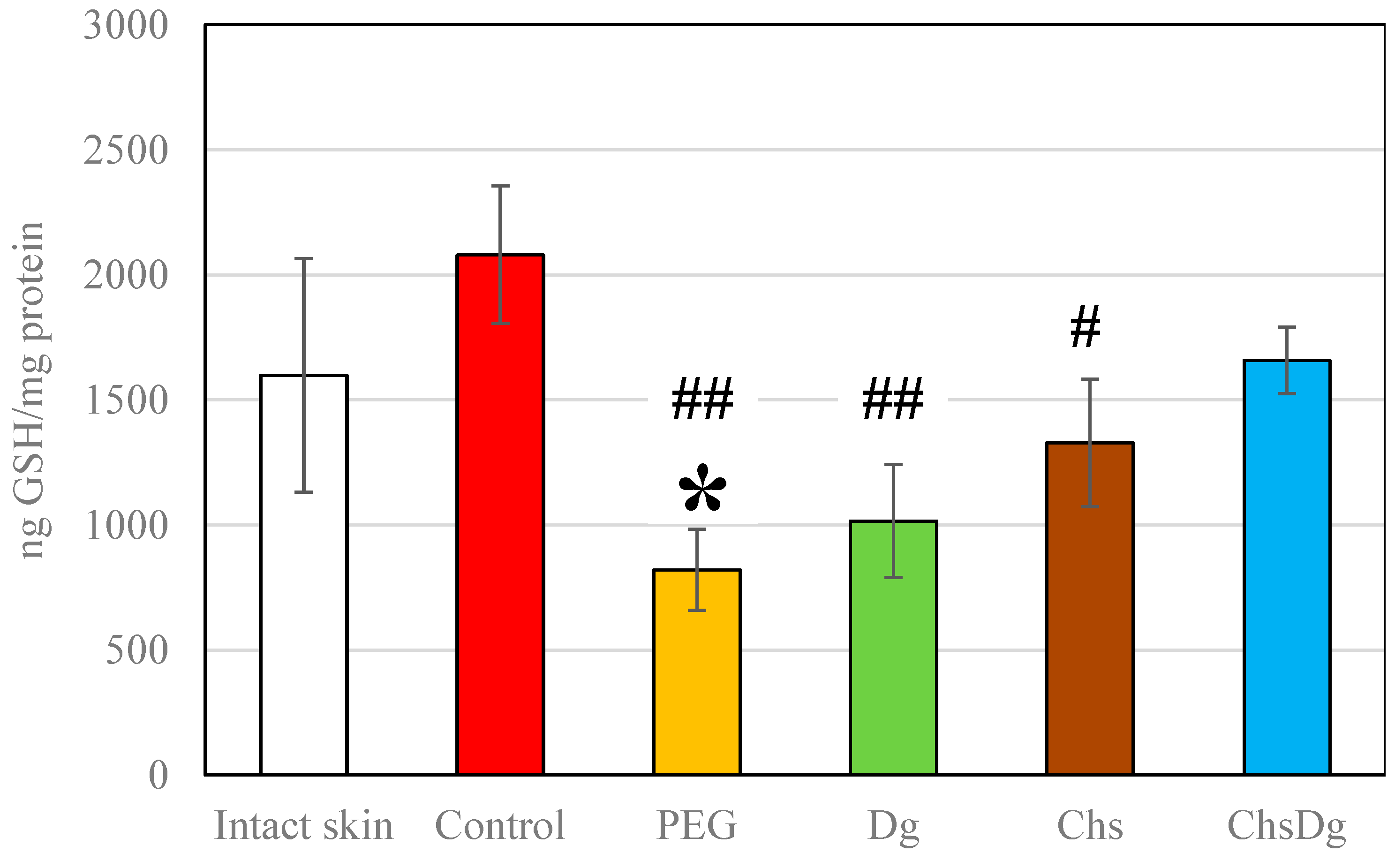
2.3. Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Materials
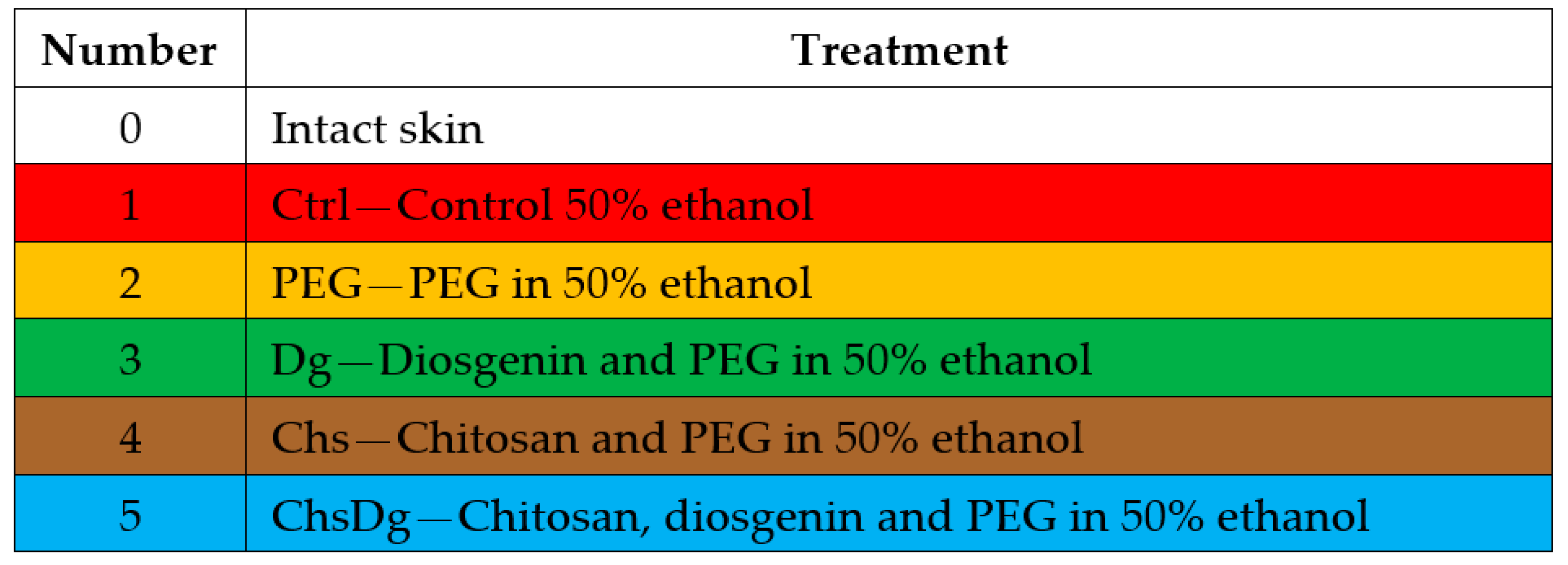
4.2. Preparation of the Combinations
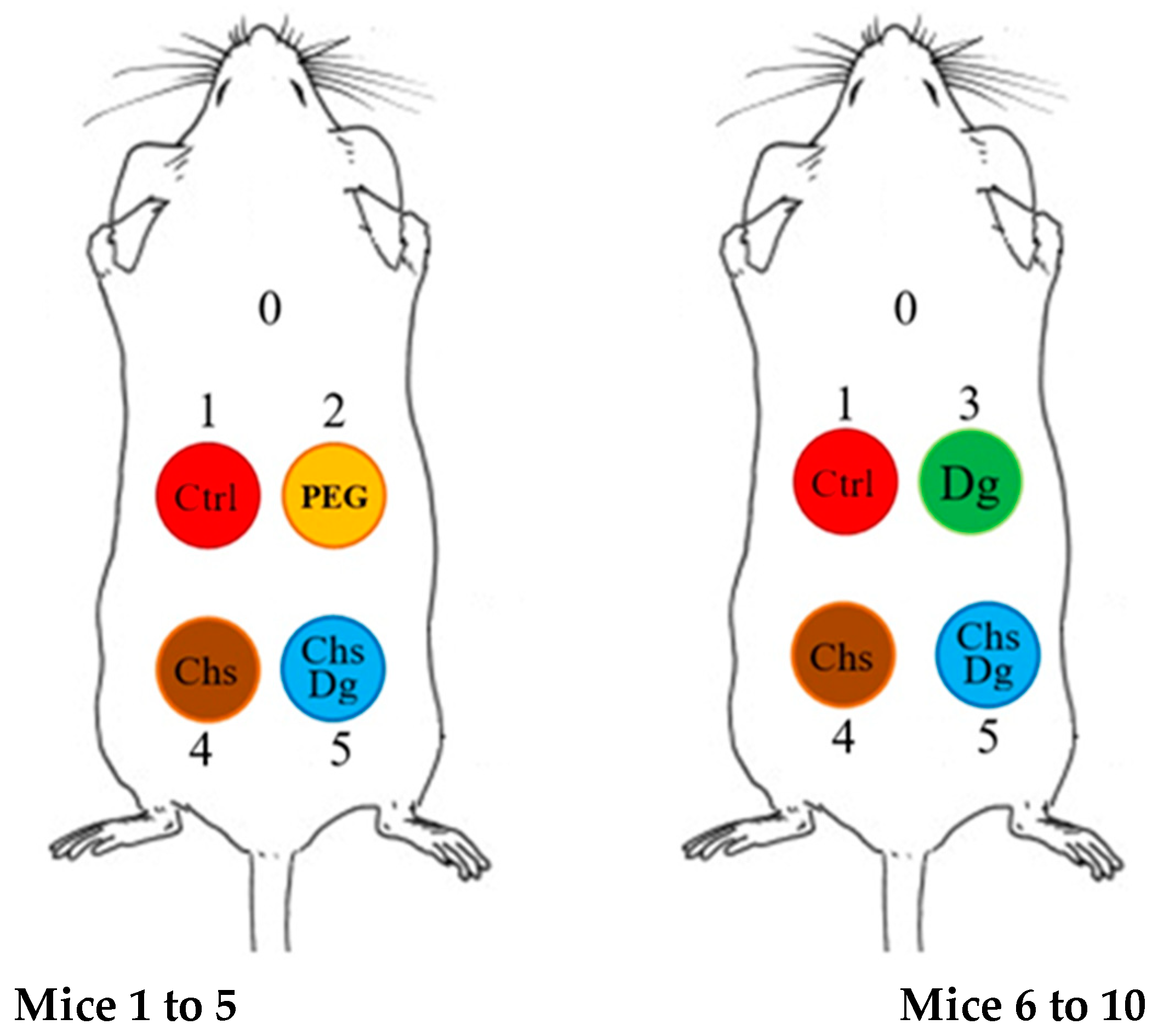
4.3. In Vivo Experiments
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr. Probl. Surg. 2001, 38, 72–140. [Google Scholar] [CrossRef]
- Rosenbaum, A.J.; Banerjee, S.; Rezak, K.M.; Uhl, R.L. Advances in wound management. J. Am. Acad. Orthop. Surg. 2018, 26, 833–843. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Kang, M.C.; Yumnam, S.; Park, W.S.; So, H.M.; Kim, K.H.; Shin, M.C.; Ahn, M.J.; Kim, S.Y. Ulmus Parvifolia accelerates skin wound healing by regulating the expression of MMPs and TGF-β. J. Clin. Med. 2020, 9, 59. [Google Scholar] [CrossRef]
- Glady, A.; Vandebroek, A.; Yasui, M. Human keratinocyte-derived extracellular vesicles activate the MAPKinase pathway and promote cell migration and proliferation in vitro. Inflamm. Regen 2021, 41, 4. [Google Scholar] [CrossRef]
- Kim, D.; Ku, B.; Choi, E.M. Se-methylselenocysteine stimulates migration and antioxidant response in HaCaT keratinocytes: Implications for wound healing. J. Trace Elem. Med. Biol. 2020, 58, 126426. [Google Scholar] [CrossRef]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Broszczak, D.A.; Mani, J.S.; Anesi, J.; Naiker, M. A cut above the rest: Oxidative stress in chronic wounds and the potential role of polyphenols as therapeutics. J. Pharm. Pharmacol. 2022, 74, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Alsbjörn, B. In search of an ideal skin substitute. Scand. J. Plast. Reconstr. Surg. 1984, 18, 127–133. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced therapeutic dressings for effective wound healing–A review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Chitosan, a drug carrier for the 21st century: A review. STP Pharma Sci. 2000, 10, 5–22. [Google Scholar]
- Paul, W.; Sharma, C.P. Chitosan and alginate wound dressings: A short review. Trends Biomater. Artif. Organs 2004, 18, 18–23. [Google Scholar]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Chen, G.; Li, Z.; Liu, Y.; Wang, G.; Wu, X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 213–222. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.H.; Gong, Y.D.; Zhao, N.M.; Zhang, X.F. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 2002, 23, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, T.; Qi, Y.; Han, X.; Xu, Y.; Peng, J.; Tang, X. Development and validation of a sensitive and rapid non-aqueous LC-ESI-MS/MS method for measurement of diosgenin in the plasma of normal and hyperlipidemic rats: A comparative study. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2009, 877, 1530–1536. [Google Scholar] [CrossRef]
- Lepage, C.; Léger, D.Y.; Bertrand, J.; Martin, F.; Beneytout, J.L.; Liagre, B. Diosgenin induces death receptor-5 through activation of P38 pathway and promotes TRAIL-Induced apoptosis in colon cancer cells. Cancer Lett. 2011, 301, 193–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L.; An, X.; Fu, E.; Ma, C. Modification of cellulase and its application to extraction of diosgenin from Dioscorea zingiberensis CH Wright. Biochem. Eng. J. 2009, 47, 80–86. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An updated pharmacological review and therapeutic perspectives. Oxid. Med. Cell. Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef]
- Shao, B.; Guo, H.; Cui, Y.; Ye, M.; Han, J.; Guo, D. Steroidal saponins from Smilax China and their anti-inflammatory activities. Phytochemistry 2007, 68, 623–630. [Google Scholar] [CrossRef]
- Gupta, D.D.; Mishra, S.; Verma, S.S.; Shekher, A.; Rai, V.; Awasthee, N.; Das, T.J.; Paul, D.; Das, S.K.; Tag, H.; et al. Evaluation of antioxidant, anti-inflammatory and anticancer activities of diosgenin enriched Paris polyphylla rhizome extract of Indian Himalayan Landraces. J. Ethnopharmacol. 2021, 270, 113842. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Zhang, X.; Bing, B.; Zhang, L.; Wang, H.; Zhao, W. Anti-tumour and immunomodulating activities of diosgenin, a naturally occurring steroidal saponin. Nat. Prod. Res. 2012, 26, 2243–2246. [Google Scholar] [CrossRef]
- Selim, S.; al Jaouni, S. Anti-inflammatory, Antioxidant and antiangiogenic activities of diosgenin isolated from traditional medicinal plant, Costus Speciosus (Koen Ex.Retz.) Sm. Nat. Prod. Res. 2016, 30, 1830–1833. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Y.M.; Yu, S.L.; Han, Y.W.; Kou, J.P.; Liu, B.L.; Yu, B.Y. Advances in the pharmacological activities and mechanisms of diosgenin. Chin. J. Nat. Med. 2015, 13, 578–587. [Google Scholar] [CrossRef]
- Fei-Xiong, C.; Ming-Rui, Z.; Chuo-Chuo, L.; Fei-Fei, P.; Bao-zeng, R. Determination and correlation of the solubility for diosgenin in alcohol solvents. J. Chem. Thermodyn. 2012, 50, 1–6. [Google Scholar]
- Kumar, R.; Mehndiratta, P.; Mishra, N.; Thukral, A.; Pathak, S.; Singh, R. Bioavailability enhancement of poorly water-soluble nano diosgenin by encapsulation using chitosan/bovine serum albumin bilayers. Asian J. Pharm. 2018, 12, 115–119. [Google Scholar]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 18, 650598. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Kratz, G. Modeling of wound healing processes in human skin using tissue culture. Microsc. Res. Tech. 1998, 42, 345–350. [Google Scholar] [CrossRef]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A. Wound healing models: A systematic review of animal and non-animal models. Wound Med. 2019, 24, 8–17. [Google Scholar] [CrossRef]
- Burkatovskaya, M.; Tegos, G.P.; Swietlik, E.; Demidova, T.N.; Castano, A.P.; Hamblin, M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006, 27, 4157–4164. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Harti, A.S.; Sulisetyawati, S.D.; Murharyati, A.; Oktariani, M.; Wijayanti, I.B. The effectiveness of snail slime and chitosan in wound healing. Int. J. Pharma. Biol. Sci. 2016, 5, 76–80. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Uddin, M.F.; Zabin, N.; Shakil, M.S.; Alam, M.; Achal, F.J.; Ara Begum, M.H.; Hossen, M.S.; Hasan, M.A.; Morshed, M.M. Fabrication and characterization of chitosan-polyethylene glycol (Ch-PEG) based hydrogels and evaluation of their potency in rat skin wound model. Int. J. Biomater. 2021, 2021, 4877344. [Google Scholar] [CrossRef]
- Park, C.J.; Gabrielson, N.P.; Pack, D.W.; Jamison, R.D.; Wagoner Johnson, A.J. The effect of chitosan on the migration of neutrophil-like HL60 cells, mediated by IL-8. Biomaterials 2009, 30, 436–444. [Google Scholar] [CrossRef]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Future Sci. OA 2018, 4, FSO225. [Google Scholar] [CrossRef]
- Casini, A.; Ceni, E.; Salzano, R.; Biondi, P.; Parola, M.; Galli, A.; Foschi, M.; Caligiuri, A.; Pinzani, M.; Surrenti, C. Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: Role of nitric oxide. Hepatology 1997, 25, 361–367. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef]
- Sinha, M.; Banik, R.M.; Haldar, C.; Maiti, P. Development of ciprofloxacin hydrochloride loaded poly(ethylene glycol)/chitosan scaffold as wound dressing. J. Porous Mater. 2013, 20, 799–807. [Google Scholar] [CrossRef]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- Kim, S. Competitive biological activities of chitosan and its derivatives: Antimicrobial, antioxidant, anticancer, and anti-inflammatory activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Höhn, T.J.A.; Grune, T. The proteasome and the degradation of oxidized proteins: Part II—Protein oxidation and proteasomal degradation. Redox Biol. 2014, 2, 99–104. [Google Scholar]
- Badawy, M.E.I. A new rapid and sensitive spectrophotometric method for determination of a biopolymer chitosan. Int. J. Carbohydr. Chem. 2012, 2012, 139328. [Google Scholar] [CrossRef]
- Mudge, B.P.; Harris, C.; Gilmont, R.R.; Adamson, B.S.; Rees, R.S. Role of glutathione redox dysfunction in diabetic wounds. Wound Repair Regen. 2002, 10, 52–58. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Rasik, A.M.; Shukla, A. Antioxidant status in delayed healing type of wounds. Int. J. Exp. Pathol. 2000, 81, 257. [Google Scholar] [CrossRef]
- Telorack, M.; Abplanalp, J.; Werner, S. Low levels of glutathione are sufficient for survival of keratinocytes after UV irradiation and for healing of mouse skin wounds. Arch. Dermatol. Res. 2016, 308, 443–448. [Google Scholar] [CrossRef]
- ben Mansour, R.; Gargouri, B.; Bouaziz, M.; Elloumi, N.; Belhadj Jilani, I.; Ghrabi, Z.; Lassoued, S. Antioxidant activity of ethanolic Extract of inflorescence of Ormenis Africana in vitro and in cell cultures. Lipids Health Dis. 2011, 10, 1–7. [Google Scholar] [CrossRef]
- Whitekus, M.J.; Li, N.; Zhang, M.; Wang, M.; Horwitz, M.A.; Nelson, S.K.; Horwitz, L.D.; Brechun, N.; Diaz-Sanchez, D.; Nel, A.E. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J. Immunol. 2002, 168, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Intact | Control | PEG | Dg | Chs | ChsDg |
|---|---|---|---|---|---|---|
| Erythema | ||||||
| Absence | 0 | |||||
| Mild | 1 | 1 | 1 | 1 | ||
| Moderately expressed with slight edema | 2 | |||||
| Strongly expressed with edema | ||||||
| Pronounced with swelling crossing the border of the treated field | ||||||
| Skin corrosive effect/after repeated contact/ | ||||||
| Absence | 0 | 0 | 0 | |||
| Mild | 1 | 1 | 1 | |||
| Moderate | ||||||
| Intense/Strong | ||||||
| Very strong | ||||||
| Ratio of stratum corneum:epidermis:papillary layer of the dermis | 1:10:20 | 1:5:10 | 1:8:15 | 1:8:15 | 1:8:15 | 1:5:10 |
| Parameters | Intact | Control | PEG | Dg | Chs | ChsDg |
|---|---|---|---|---|---|---|
| Desquamation | − | + | + | + | + | − |
| Rhagades | − | − | + | − | + | − |
| Loss of skin appendages | − | − | Focal | − | Focal | Focal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, L.; Stoilova, O.; Pramatarov, G.; Kanzova, H.; Tsvetanova, E.; Andreeva, M.; Georgieva, A.; Atanasova, D.; Philipov, S.; Alexandrova, A. Effect of Chitosan-Diosgenin Combination on Wound Healing. Int. J. Mol. Sci. 2023, 24, 5049. https://doi.org/10.3390/ijms24055049
Petrov L, Stoilova O, Pramatarov G, Kanzova H, Tsvetanova E, Andreeva M, Georgieva A, Atanasova D, Philipov S, Alexandrova A. Effect of Chitosan-Diosgenin Combination on Wound Healing. International Journal of Molecular Sciences. 2023; 24(5):5049. https://doi.org/10.3390/ijms24055049
Chicago/Turabian StylePetrov, Lubomir, Olya Stoilova, Georgi Pramatarov, Hristiyana Kanzova, Elina Tsvetanova, Madlena Andreeva, Almira Georgieva, Dimitrinka Atanasova, Stanislav Philipov, and Albena Alexandrova. 2023. "Effect of Chitosan-Diosgenin Combination on Wound Healing" International Journal of Molecular Sciences 24, no. 5: 5049. https://doi.org/10.3390/ijms24055049
APA StylePetrov, L., Stoilova, O., Pramatarov, G., Kanzova, H., Tsvetanova, E., Andreeva, M., Georgieva, A., Atanasova, D., Philipov, S., & Alexandrova, A. (2023). Effect of Chitosan-Diosgenin Combination on Wound Healing. International Journal of Molecular Sciences, 24(5), 5049. https://doi.org/10.3390/ijms24055049










