Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models
Abstract
1. Introduction
2. Results
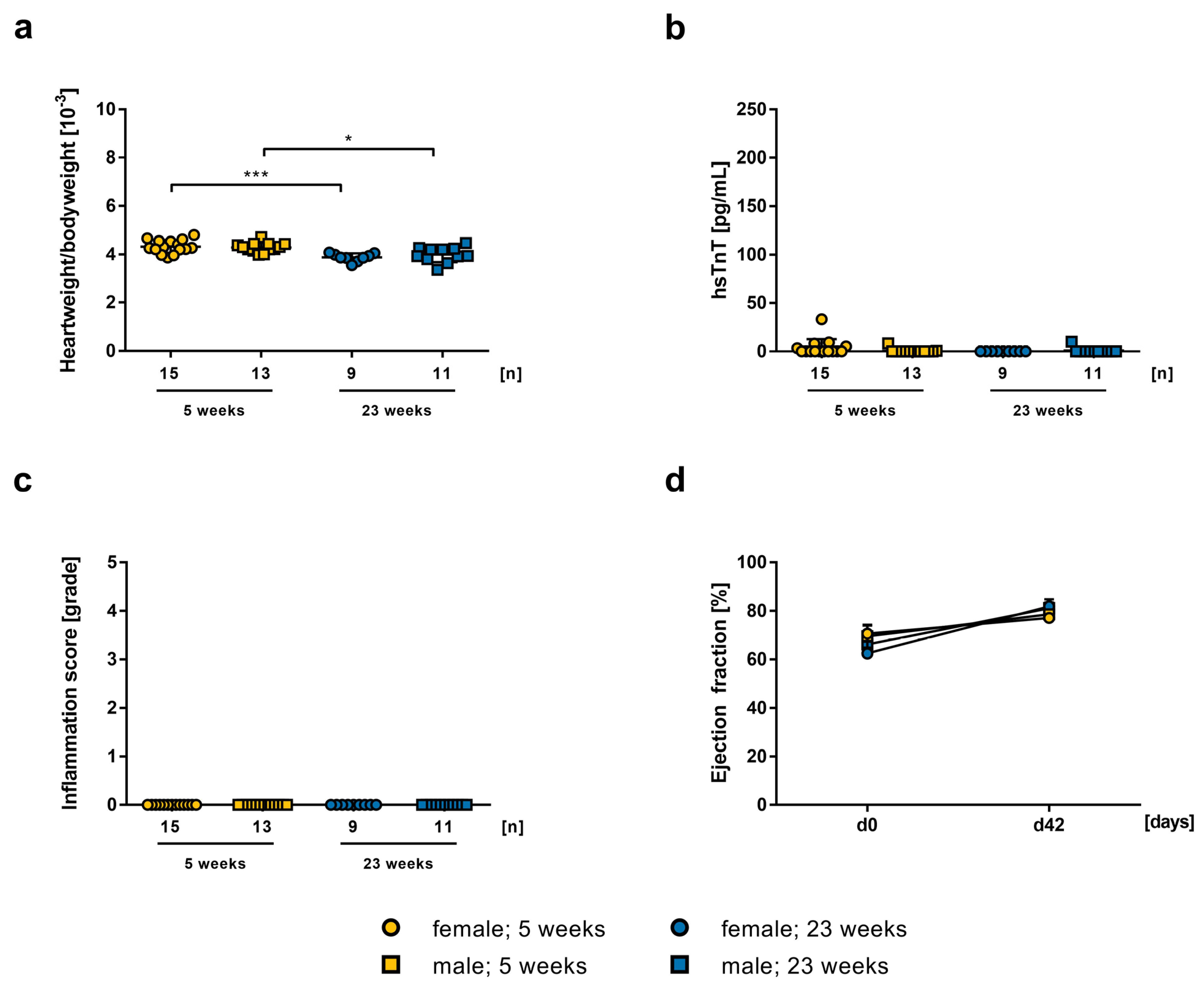
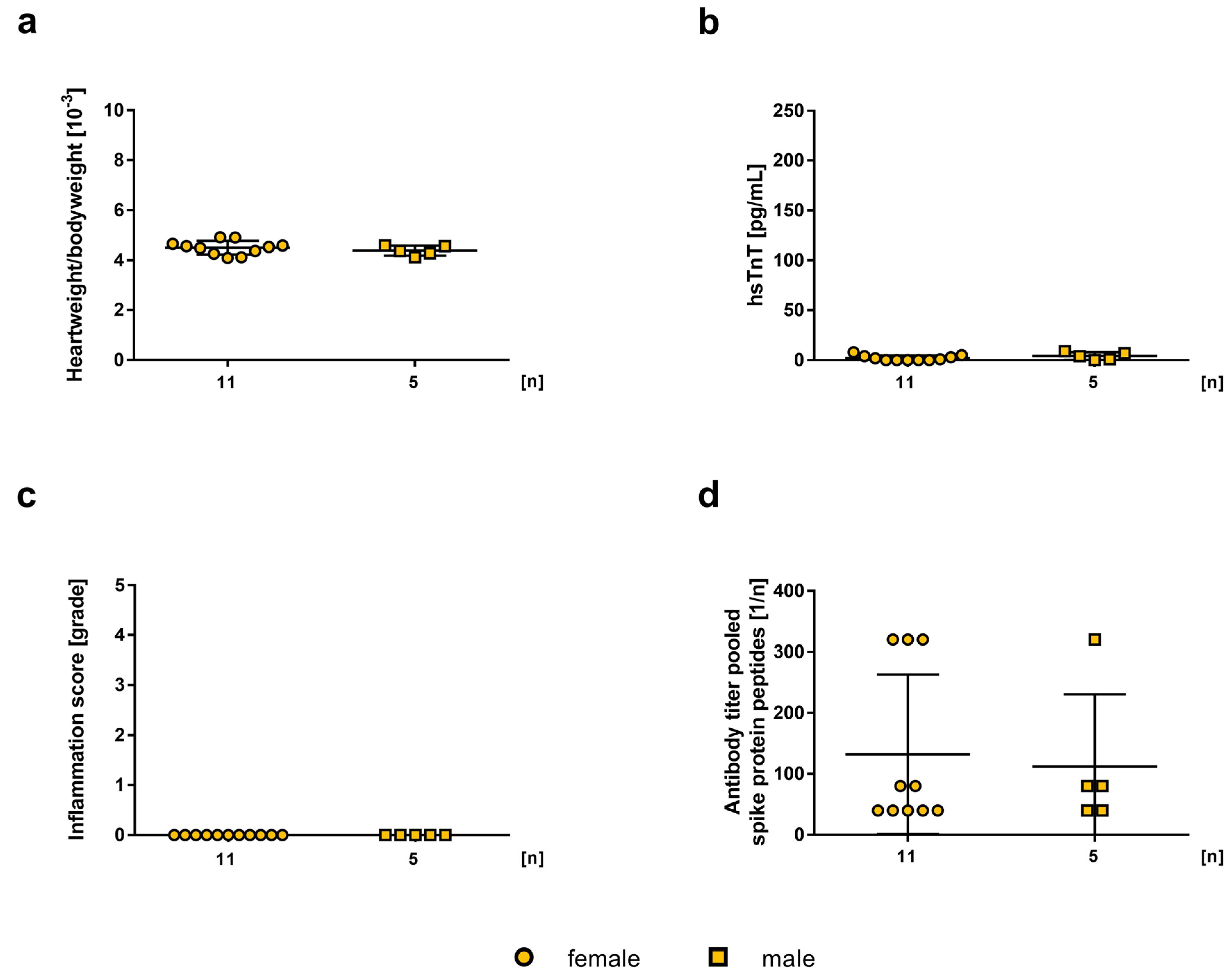
2.1. Influence of mRNA-Vaccine Depending on Age and Gender
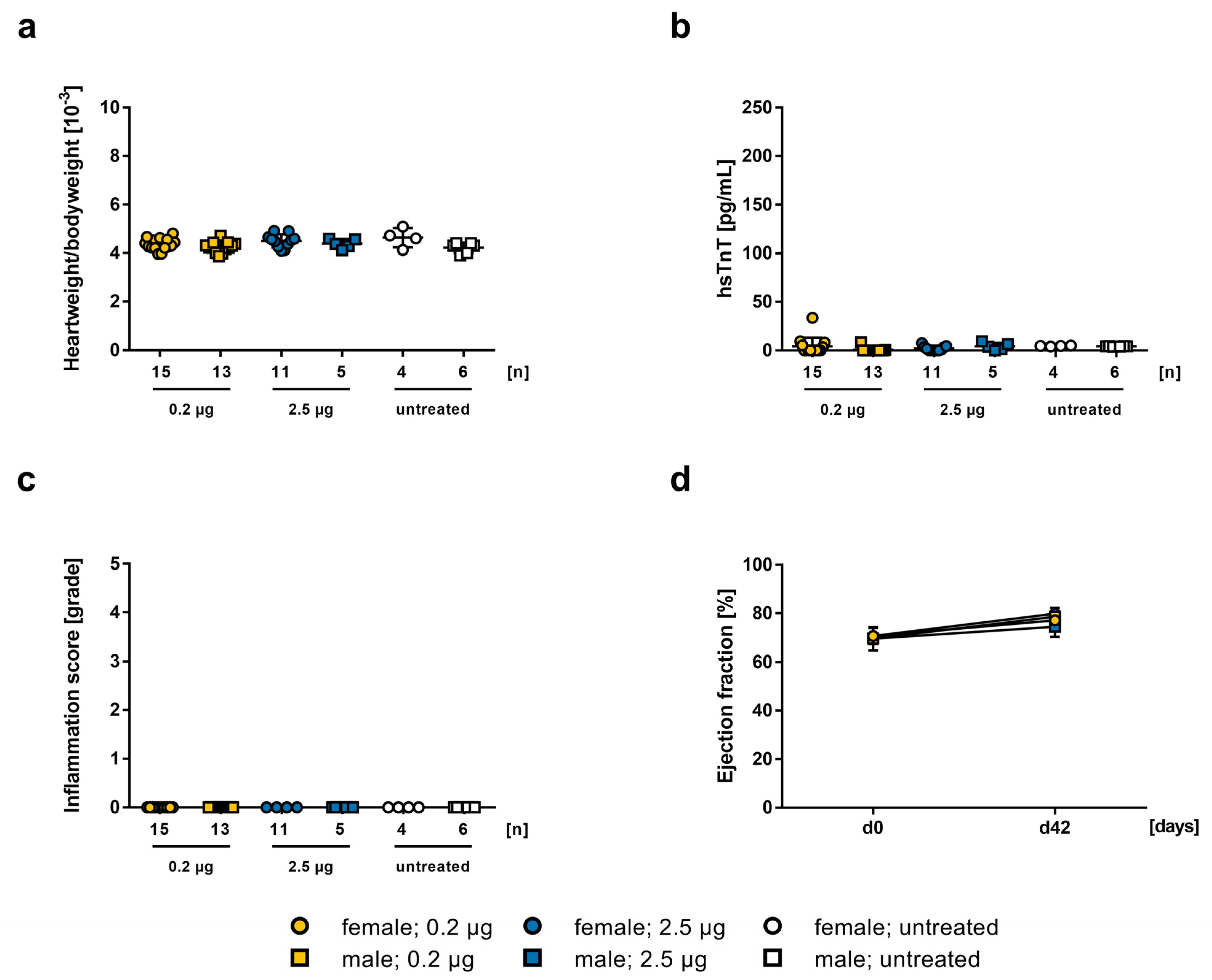
2.2. Influence of the mRNA-Vaccine Depending on the Mouse Strain
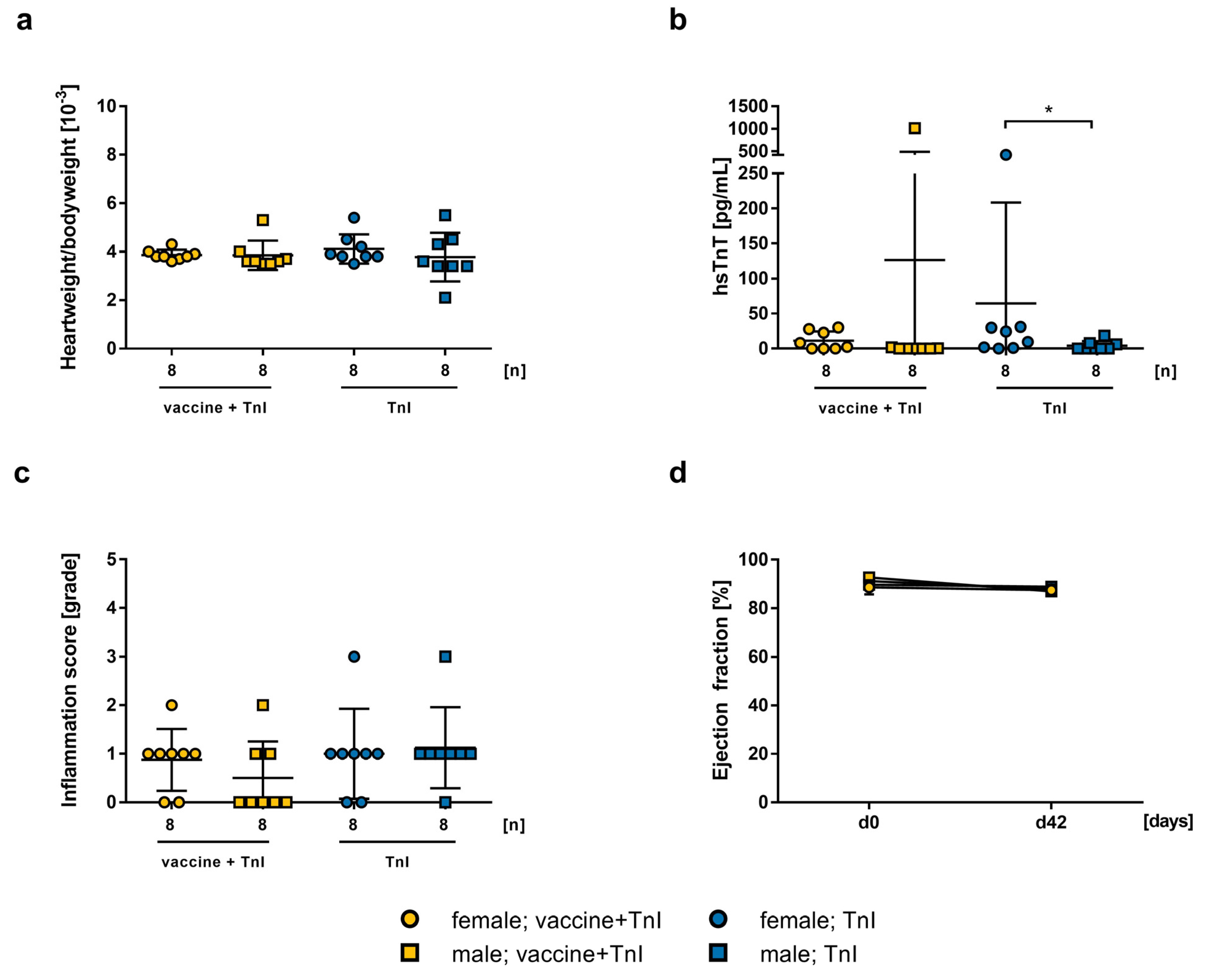
2.3. Influence of mRNA-Vaccination in Combination with Experimental Autoimmune Myocarditis
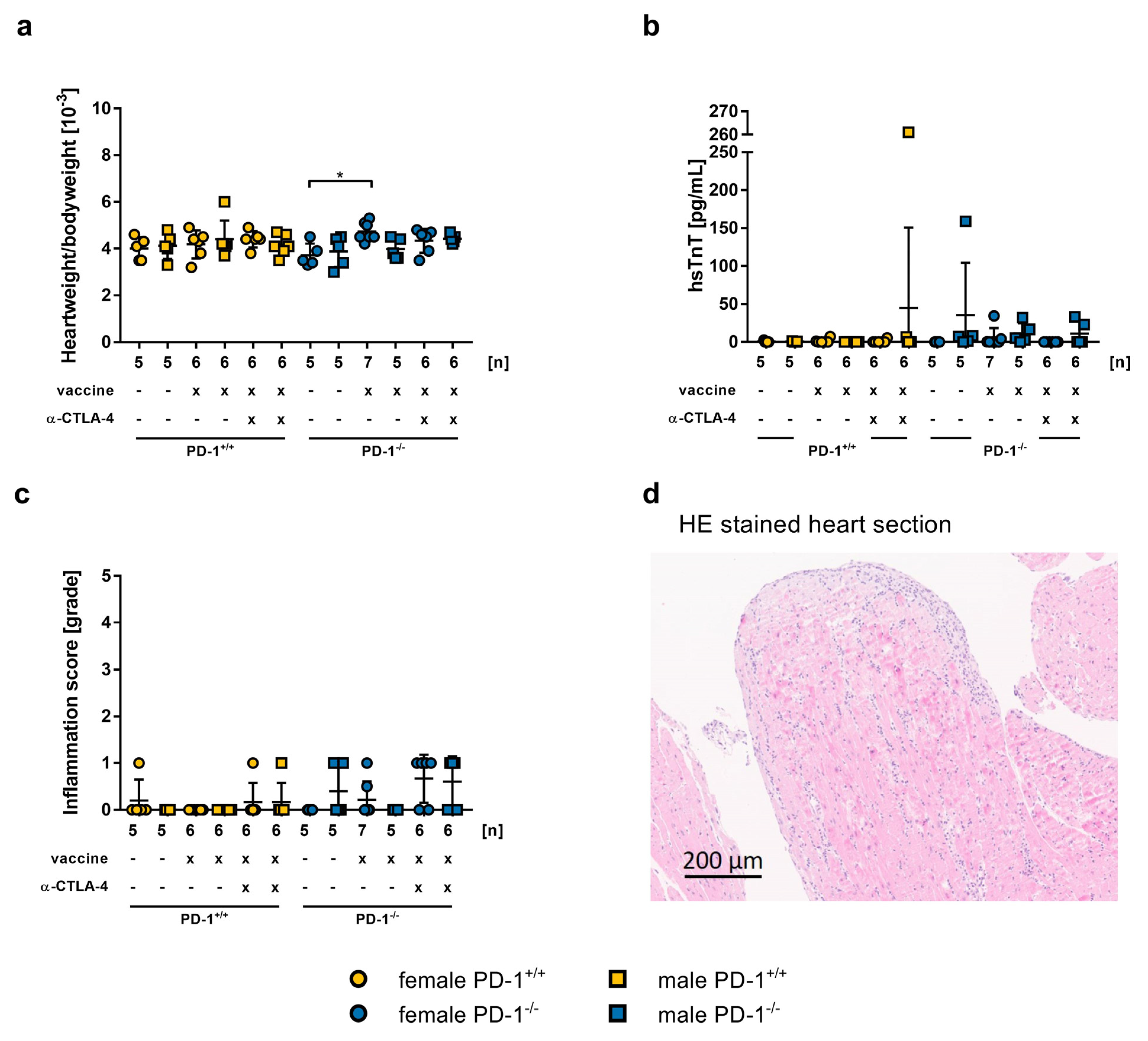
2.4. Safety of mRNA-Vaccination in PD-1−/− Mice and co-Treatment with CTLA-4 abs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Treatment and Induction of Experimental Autoimmune Myocarditis
4.3. Echocardiographic Examination of Cardiac Heart Function
4.4. Determination of High-Sensitive Troponin T Levels in Serum
4.5. Histopathological Examination of Inflammation and Fibrosis
4.6. Detection of Antibodies against Spike Protein Peptide Sequences via Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Comparative Values for All Measured Parameters Analyzing Myocarditis and Their Corresponding References Are Listed in Table S1
4.8. Statistical Analysis and Graphical Representation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Ladny, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183. [Google Scholar] [CrossRef]
- Ozma, M.A.; Maroufi, P.; Khodadadi, E.; Kose, S.; Esposito, I.; Ganbarov, K.; Dao, S.; Esposito, S.; Dal, T.; Zeinalzadeh, E.; et al. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez. Med. 2020, 28, 153–165. [Google Scholar]
- Poletti, P.; Tirani, M.; Cereda, D.; Trentini, F.; Guzzetta, G.; Marziano, V.; Buoro, S.; Riboli, S.; Crottogini, L.; Piccarreta, R.; et al. Age-specific SARS-CoV-2 infection fatality ratio and associated risk factors, Italy, February to April 2020. Eurosurveillance 2020, 25, 2001383. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper, L.T., Jr.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation 2021, 144, 506–508. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2021, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Karlstad, O.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600–612. [Google Scholar] [CrossRef]
- Hajjo, R.; Sabbah, D.A.; Bardaweel, S.K.; Tropsha, A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines 2021, 9, 1186. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Rojas, M.; Beltrán, S.; Polo, F.; Camacho-Domínguez, L.; Morales, S.D.; Gershwin, M.E.; Anaya, J.-M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022, 132, 102898. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Kadkhoda, K. Post RNA-based COVID vaccines myocarditis: Proposed mechanisms. Vaccine 2022, 40, 406–407. [Google Scholar] [CrossRef]
- Abu Mouch, S.; Roguin, A.; Hellou, E.; Ishai, A.; Shoshan, U.; Mahamid, L.; Zoabi, M.; Aisman, M.; Goldschmid, N.; Yanay, N.B. Myocarditis following COVID-19 mRNA vaccination. Vaccine 2021, 39, 3790–3793. [Google Scholar] [CrossRef]
- Ishay, Y.; Kenig, A.; Tsemach-Toren, T.; Amer, R.; Rubin, L.; Hershkovitz, Y.; Kharouf, F. Autoimmune phenomena following SARS-CoV-2 vaccination. Int. Immunopharmacol. 2021, 99, 107970. [Google Scholar] [CrossRef] [PubMed]
- Caron, P. Autoimmune and inflammatory thyroid diseases following vaccination with SARS-CoV-2 vaccines: From etiopathogenesis to clinical management. Endocrine 2022, 78, 406–417. [Google Scholar] [CrossRef]
- Kang, D.-H.; Na, J.-Y.; Yang, J.-H.; Moon, S.-H.; Kim, S.-H.; Jung, J.-J.; Cha, H.-J.; Ahn, J.-H.; Park, Y.-W.; Cho, S.-Y.; et al. Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina 2022, 58, 449. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for Diagnosis and Treatment of Myocarditis (JCS 2009): Digest version. Circ. J. 2011, 75, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Richter, A.; Sandmöller, A.; Portig, I.; Pankuweit, S. Inflammatory Dilated Cardiomyopathy (DCMI). Herz 2005, 30, 535–544. [Google Scholar] [CrossRef]
- Leuschner, F.; Katus, H.A.; Kaya, Z. Autoimmune myocarditis: Past, present and future. J. Autoimmun. 2009, 33, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Göser, S.; Andrassy, M.; Buss, S.J.; Leuschner, F.; Volz, C.H.; Ottl, R.; Zittrich, S.; Blaudeck, N.; Hardt, S.E.; Pfitzer, G.; et al. Cardiac Troponin I but Not Cardiac Troponin T Induces Severe Autoimmune Inflammation in the Myocardium. Circulation 2006, 114, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Göser, S.; Buss, S.J.; Leuschner, F.; Öttl, R.; Li, J.; Völkers, M.; Zittrich, S.; Pfitzer, G.; Rose, N.R.; et al. Identification of Cardiac Troponin I Sequence Motifs Leading to Heart Failure by Induction of Myocardial Inflammation and Fibrosis. Circulation 2008, 118, 2063–2072. [Google Scholar] [CrossRef]
- Leib, C.; Göser, S.; Lüthje, D.; Öttl, R.; Tretter, T.; Lasitschka, F.; Zittrich, S.; Pfitzer, G.; Katus, H.A.; Kaya, Z. Role of the Cholinergic Antiinflammatory Pathway in Murine Autoimmune Myocarditis. Circ. Res. 2011, 109, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Bockstahler, M.; Müller, A.-M.; Stroikova, V.; Leib, C.; Pfitzer, G.; Katus, H.A.; Kaya, Z. FN14 Signaling Plays a Pathogenic Role in a Mouse Model of Experimental Autoimmune Myocarditis. J. Card. Fail. 2019, 25, 674–685. [Google Scholar] [CrossRef]
- Bockstahler, M.; Fischer, A.; Goetzke, C.C.; Neumaier, H.L.; Sauter, M.; Kespohl, M.; Müller, A.-M.; Meckes, C.; Salbach, C.; Schenk, M.; et al. Heart-Specific Immune Responses in an Animal Model of Autoimmune-Related Myocarditis Mitigated by an Immunoproteasome Inhibitor and Genetic Ablation. Circulation 2020, 141, 1885–1902. [Google Scholar] [CrossRef]
- Rose, N.R.; Hill, S.L. Autoimmune myocarditis. Int. J. Cardiol. 1996, 54, 171–175. [Google Scholar] [CrossRef]
- Neu, N.; Rose, N.R.; Beisel, K.W.; Herskowitz, A.; Gurri-Glass, G.; Craig, S.W. Cardiac myosin induces myocarditis in genetically predisposed mice. J. Immunol. 1987, 139, 3630–3636. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Meijers, W.C.; Screever, E.M.; Qin, J.; Carroll, M.G.; Sun, X.; Tannous, E.; Zhang, Y.; Sugiura, A.; Taylor, B.C.; et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 2022, 611, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Göser, S.; Ottl, R.; Brodner, A.; Dengler, T.J.; Torzewski, J.; Egashira, K.; Rose, N.R.; Katus, H.A.; Kaya, Z. Critical Role for Monocyte Chemoattractant Protein-1 and Macrophage Inflammatory Protein-1α in Induction of Experimental Autoimmune Myocarditis and Effective Anti–Monocyte Chemoattractant Protein-1 Gene Therapy. Circulation 2005, 112, 3400–3407. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef]
- Lute, K.D.; May, K.F., Jr.; Lu, P.; Zhang, H.; Kocak, E.; Mosinger, B.; Wolford, C.; Phillips, G.; Caligiuri, M.A.; Zheng, P.; et al. Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti–CTLA-4 antibodies. Blood 2005, 106, 3127–3133. [Google Scholar] [CrossRef]
- Wei, S.C.; Meijers, W.C.; Axelrod, M.L.; Anang, N.-A.A.; Screever, E.M.; Wescott, E.C.; Johnson, D.B.; Whitley, E.; Lehmann, L.; Courand, P.-Y.; et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor–Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021, 11, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. bioRxiv 2020. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Midoux, P.; Pichon, C. Lipid-based mRNA vaccine delivery systems. Expert Rev. Vaccines 2015, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- McLean, K.; Johnson, T.J. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: A case report. Acad. Emerg. Med. 2021, 28, 918–921. [Google Scholar] [CrossRef]
- Marshall, M.; Ferguson, I.D.; Lewis, P.; Jaggi, P.; Gagliardo, C.; Collins, J.S.; Shaughnessy, R.; Caron, R.; Fuss, C.; Corbin, K.J.E.; et al. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics 2021, 148, e2021052478. [Google Scholar] [CrossRef]
- Hana, D.; Patel, K.; Roman, S.; Gattas, B.; Sofka, S. Clinical Cardiovascular Adverse Events Reported Post-COVID-19 Vaccination: Are They a Real Risk? Curr. Probl. Cardiol. 2021, 47, 101077. [Google Scholar] [CrossRef] [PubMed]
- Shiravi, A.A.; Ardekani, A.; Sheikhbahaei, E.; Heshmat-Ghahdarijani, K. Cardiovascular Complications of SARS-CoV-2 Vaccines: An Overview. Cardiol. Ther. 2021, 11, 13–21. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Nguyen, M.; Martin, D.; DeStefano, F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015, 33, 4398–4405. [Google Scholar] [CrossRef]
- Kytö, V.; Sipilä, J.; Rautava, P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart 2013, 99, 1681–1684. [Google Scholar] [CrossRef]
- Vasudeva, R.; Bhatt, P.; Lilje, C.; Desai, P.; Amponsah, J.; Umscheid, J.; Parmar, N.; Bhatt, N.; Adupa, R.; Pagad, S.; et al. Trends in Acute Myocarditis Related Pediatric Hospitalizations in the United States, 2007–2016. Am. J. Cardiol. 2021, 149, 95–102. [Google Scholar] [CrossRef]
- Mohiddin, S.A.; Guttmann, O.; Marelli-Berg, F. Vaccine-Triggered Acute Autoimmune Myocarditis: Defining, Detecting, and Managing an Apparently Novel Condition. J. Am. Heart Assoc. 2022, 11, e026873. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, N.; Fairchok, M.P.; Hasbani, K. Myocarditis After COVID-19 Vaccination in Pediatrics: A Proposed Pathway for Triage and Treatment. J. Am. Heart Assoc. 2022, 11, e026097. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Stein-Merlob, A.F.; Rothberg, M.V.; Ribas, A.; Yang, E.H. Cardiotoxicities of novel cancer immunotherapies. Heart 2021, 107, 1694–1703. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Qu, Q.; Lou, Y.-X.; Hua, Y.; Sun, G.-Z.; Sun, W.; Kong, X.-Q. Cardiotoxicity in cancer immune-checkpoint therapy: Mechanisms, clinical evidence, and management strategies. Int. J. Cardiol. 2021, 344, 170–178. [Google Scholar] [CrossRef]
- Arangalage, D.; Degrauwe, N.; Michielin, O.; Monney, P.; Özdemir, B.C. Pathophysiology, diagnosis and management of cardiac toxicity induced by immune checkpoint inhibitors and BRAF and MEK inhibitors. Cancer Treat. Rev. 2021, 100, 102282. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, J.; Hou, X.; Yang, Q.; Zhou, Y.; Ye, J.; Wu, X.; Feng, Y.; Hu, T.; Xu, Z.; et al. Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol. 2021, 17, 3477–3484. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef]
- Saenger, A.; Beyrau, R.; Braun, S.; Cooray, R.; Dolci, A.; Freidank, H.; Giannitsis, E.; Gustafson, S.; Handy, B.; Katus, H.; et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin. Chim. Acta 2011, 412, 748–754. [Google Scholar] [CrossRef]
- Wang, Y.; Afanasyeva, M.; Hill, S.L.; Kaya, Z.; Rose, N.R. Nasal administration of cardiac myosin suppresses autoimmune myocarditis in mice. J. Am. Coll. Cardiol. 2000, 36, 1992–1999. [Google Scholar] [CrossRef]
- Kaya, Z.; Afanasyeva, M.; Wang, Y.; Dohmen, K.M.; Schlichting, J.; Tretter, T.; Fairweather, D.; Holers, V.M.; Rose, N.R. Contribution of the innate immune system to autoimmune myocarditis: A role for complement. Nat. Immunol. 2001, 2, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Bangert, A.; Andrassy, M.; Müller, A.-M.; Bockstahler, M.; Fischer, A.; Volz, C.H.; Leib, C.; Göser, S.; Korkmaz-Icöz, S.; Zittrich, S.; et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc. Natl. Acad. Sci. USA 2016, 113, E155–E164. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Leib, C.; Werfel, S.; Göser, S.; Öttl, R.; Leuchs, B.; Pfitzer, G.; Katus, H.A.; Müller, O.J. Comparison of IL-10 and MCP-1-7ND gene transfer with AAV9 vectors for protection from murine autoimmune myocarditis. Cardiovasc. Res. 2011, 91, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Stypmann, J.; Engelen, M.A.; Troatz, C.; Rothenburger, M.; Eckardt, L.; Tiemann, K. Echocardiographic assessment of global left ventricular function in mice. Lab. Anim. 2009, 43, 127–137. [Google Scholar] [CrossRef]
- Leuschner, F.; Li, J.; Göser, S.; Reinhardt, L.; Öttl, R.; Bride, P.; Zehelein, J.; Pfitzer, G.; Remppis, A.; Giannitsis, E.; et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur. Heart J. 2008, 29, 1949–1955. [Google Scholar] [CrossRef]

| Inflammation Score [Grade] | Inflammation [%] |
|---|---|
| 0 | 0 |
| 1 | 1–20 |
| 2 | 21–40 |
| 3 | 41–60 |
| 4 | 61–80 |
| 5 | >80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zirkenbach, V.A.; Ignatz, R.M.; Öttl, R.; Cehreli, Z.; Stroikova, V.; Kaya, M.; Lehmann, L.H.; Preusch, M.R.; Frey, N.; Kaya, Z. Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models. Int. J. Mol. Sci. 2023, 24, 5011. https://doi.org/10.3390/ijms24055011
Zirkenbach VA, Ignatz RM, Öttl R, Cehreli Z, Stroikova V, Kaya M, Lehmann LH, Preusch MR, Frey N, Kaya Z. Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models. International Journal of Molecular Sciences. 2023; 24(5):5011. https://doi.org/10.3390/ijms24055011
Chicago/Turabian StyleZirkenbach, Vanessa A., Rebecca M. Ignatz, Renate Öttl, Zeynep Cehreli, Vera Stroikova, Mansur Kaya, Lorenz H. Lehmann, Michael R. Preusch, Norbert Frey, and Ziya Kaya. 2023. "Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models" International Journal of Molecular Sciences 24, no. 5: 5011. https://doi.org/10.3390/ijms24055011
APA StyleZirkenbach, V. A., Ignatz, R. M., Öttl, R., Cehreli, Z., Stroikova, V., Kaya, M., Lehmann, L. H., Preusch, M. R., Frey, N., & Kaya, Z. (2023). Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models. International Journal of Molecular Sciences, 24(5), 5011. https://doi.org/10.3390/ijms24055011





