Abstract
Schistosomiasis, or also generally known as bilharzia or snail fever, is a parasitic disease that is caused by trematode flatworms of the genus Schistosoma. It is considered by the World Health Organisation as the second most prevalent parasitic disease after malaria and affects more than 230 million people in over 70 countries. People are infected via a variety of activities ranging from agricultural, domestic, occupational to recreational activities, where the freshwater snails Biomphalaria release Schistosoma cercariae larvae that penetrate the skin of humans when exposed in water. Understanding the biology of the intermediate host snail Biomphalaria is thus important to reveal the potential spread of schistosomiasis. In this article, we present an overview of the latest molecular studies focused on the snail Biomphalaria, including its ecology, evolution, and immune response; and propose using genomics as a foundation to further understand and control this disease vector and thus the transmission of schistosomiasis.
1. Introduction
Schistosomiasis is a tropical parasite-borne disease resulting from the infection of trematode blood flukes of the genus Schistosoma [1]. It is the second most prevalent parasitic disease after malaria. According to the World Health Organization, more than 230 million people were infected globally by schistosomiasis in 2019, and approximately 700 million people currently reside in 78 countries with a high risk of infection [2]. In Africa alone there is an annual death of 280,000 people from schistosomiasis [3,4].
1.1. Lifecycle of Schistosoma mansoni
To understand how this parasitic disease can become so successful in spreading amongst humans, it is necessary to understand its life cycle associated with the intermediate snail host and the definitive mammalian host (mainly humans, Figure 1). In brief, fertilized eggs are shed and released via the feces or urine of infected human hosts which further develop into ciliated miracidia in freshwater [3,5,6,7]. In the presence of Biomphalaria, miracidia will penetrate and infect this intermediate host [3]. Inside the snail, miracidia undergo asexual reproduction to produce sporocysts [3,5,6,7], which develop to form the motile cercariae with bifurcated tails and penetration glands [6,7]. Cercariae are released from the intermediate host approximately one month after the infection [3], and a single snail can shed up to 600 cercariae per day [8]. Humans are infected by cercariae via contact with contaminated water where the cercariae penetrate the human skin, causing “swimming itch” [5,9]. Inside the human body, cercariae lose their bifurcated tail and transform into schistosomula, which enter the blood circulatory system and migrate to the liver [3,7,9]. Schistosomula grow and mature into adult worms in the portal venous system four to six weeks after infection [3,5]. The adult worms pair with another individual with a opposite sex, then migrate into the bladder or intestines for mating and egg production [7,10]. Fertilized eggs are excreted after staying in the human body for around a week to start a new cycle [3] (Figure 1).
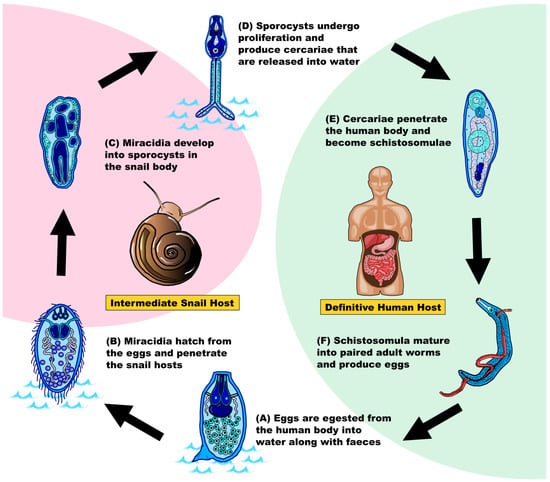
Figure 1.
Schematic diagram showing the lifecycle of Schistosoma mansoni (after [3,5,6,7]). Pink shading indicates the lifecycle in the intermediate snail host Biomphalaria, white shading indicates the lifecycle in the aquatic environment, and green shading indicates the lifecycle in the definitive human host. (A) Egg. (B) Ciliated miracidium. (C) Sporocyst (mother sporocyst and daughter sporocyst). (D) Free-living cercariae. (E) Schistosomulae. (F) Paired adult worms (male: blue; female: red).
1.2. Symptoms of Schistosomiasis
For infected persons, acute schistosomiasis or Katayama fever may occur due to hypersensitivity to schistosomula in the blood vessels [11,12]. Excess antigen-antibody complexes lead to various non-specific allergic symptoms such as fever, headache, myalgia, fatigue, and urticarial rash [3,5,7,12]. Most infected people recover and show no symptoms within three months [12]. Some patients, however, develop more severe clinical manifestations, such as shortness of breath, diarrhea, and enlarged liver and spleen [3,12]. Parasitism by Schistosoma can also cause chronic infection. The proteolytic enzyme that is secreted by the entrapped parasitic eggs stimulates granulomatous reactions and chronic eosinophilic inflammation [13,14]. The formation of granuloma in the liver results in severe abdominal pain and hematochezia [15]. Continuous liver inflammation leads to tissue fibrosis, causing portal vein thrombosis and pressure elevation [3,16] which disrupt the tissue and function of the infected liver [16].
1.3. Prevention and Control of Schistosomiasis
The World Health Organization (WHO) suggests the use of praziquantel for preventive chemotherapy [17], however this approach has been considered to have a limited effect on disease transmission management including the reports of praziquantel resistance in Schistosoma [18,19]. Given the fact that the exposure risk of schistosomiasis is highly determined by the population size of its intermediate host Biomphalaria, the development of a strategy to block the transmission of schistosomes by controlling the population of the snail is a strong control candidate [20]. Investigations of the efficiency of molluscicidal compounds in eliminating the snail host have been conducted for more than half a century [21]. Among the chemical compounds that have been tested, niclosamide was previously identified by WHO as the most commonly used [22], which restricts the production of ATP by the uncoupling of oxidative phosphorylation [23]. Despite a reduction in the prevalence of schistosomiasis in Brazil [24], the application of niclosamide has declined since 1986 due to the discovery of genotoxic and carcinogenic effects to non-targeted species and humans [25]. Aside from chemical molluscicides, recent research has focused on molluscicides extracted from medicinal plants. Earlier studies mentioned that plants from the families Euphorbiaceae (e.g., Euphorbia royleana) and Phytolaccaceae (e.g., Phytolacca dodecandra) have the most potent ability to kill snails [26,27]. The latex that is produced by Euphorbian is considered the most effective molluscicidal agent, which causes anaphylaxis and inflammatory responses in snails due to the effects of low pH, resulting in cell death and organ dysfunction [28,29]. Nevertheless, Euphorbian latex is a non-selective molluscicide that eliminates not only the intermediate snail host of Schistosoma but also other non-target aquatic species [30,31]. Recently, linalool (the extracts of Cinnamomum camphora) was found to cause gill damage and hepatopancreas shrinkage in snails with no observed adverse ecological impacts, thus linalool is considered as a potential powerful molluscicidal agent for Biomphalaria management in the future [32].
2. Biogeography and Evolutionary Trends of Snails Biomphalaria
Freshwater snails in the genus Biomphalaria are the intermediate host of the parasitic blood fluke Schistosoma mansoni and have a broad geographic distribution globally. To date, a total of 34 Biomphalaria species have been identified [33,34], 18 of which are potential intermediate hosts of Schistosoma mansoni [35]. Among the 18 Biomphalaria species which may act as potential intermediate host of S. mansoni, all 22 African species are susceptible to this parasite, but only 6 out of 12 neotropical species were tested to be infested naturally or experimentally [36,37,38,39]. To understand the spread of schistosomiasis, we, therefore, need to know how the different species of Biomphalaria snails have evolved. To date, different hypotheses of the evolution of species in the genera have been postulated.
2.1. Origin and Diversification of Biomphalaria
In the Gondwanaland origin hypothesis, based on fossil records, the last common ancestor of Biomphalaria was suggested to have originated and undergone speciation in Gondwanaland before the breakup of landmasses into today’s South America, Africa, Antarctica, and Australia at 100 million years ago (mya), while two major Biomphalaria lineages further split and separated on both sides of the Atlantic Ocean after the breakup of the supercontinent [40,41,42,43]. This hypothesis is not, however, supported by the degree of genetic differences between Biomphalaria species. If this hypothesis was correct, the genetic distances between Biomphalaria species within the same biogeographic realm should be in a range of 0.20 to 0.60 [44,45]. Woodruff and Mulvey [46], however, found that the American B. glabrata was distinctly separated from other Neotropical Biomphalaria species (mean D = 0.68) and clustered with the African species (mean D = 0.43), which implies that the formation of two lineages in South America and Africa is a more recent event than previously thought. In addition, the oldest fossil of Biomphalaria found in Africa was in the late Pleistocene strata (1–2 mya) [47], while those that have been found in South America occurred in the Paleocene (55–65 mya) [48,49], indicating the Biomphalaria that is found in Africa evolved at a later time. Similar results were also obtained from molecular analysis using mitochondrial COI gene, 16S rDNA, ITS1, and sequences, where the American Biomphalaria appear to be the basal taxa of their African congeners [50,51], and it is estimated that a B. glabrata-like species invaded Africa from 2.3 to 4.5 mya [46,49].
In the America origin hypothesis (Figure 2), a B. glabrata-like species experienced a trans-Atlantic radiation from South America to Africa either by attaching to the feathers of waterfowl or rafting on vegetation as egg masses, planktonic larvae, or even adults [46,51]. During the Quaternary, fluctuation in the amount of solar radiation received, repeated drought, and flooding all occurred, which resulted in periodic glaciations, and the subsequent fragmentation and coalescence of habitats [52,53]. The changes in landform and terrain facilitated the genetic divergence and speciation among populations of Biomphalaria [50,51], with B. glabrata-like species invading West Africa and evolving into other African species during the Pliocene in approximately 1.8–4.5 mya [46,50,51]. The B. glabrata-like species diverged into B. camerunensis and B. pfeifferi in West Africa after its colonization [51,54], with B. pfeifferi expanding the population range during the warm interglacial period from 30–70 kya after the last ice age [54,55,56,57]. Based on molecular phylogenetic trees, B. pfeifferi-like species further diverged into B. angulosa in East Africa, which acts as the basal member of the Nilotic species complex (B. alexandrina, B. choanomphala, B. smithi, B. stanleyi, and B. sudanica) [51,54].
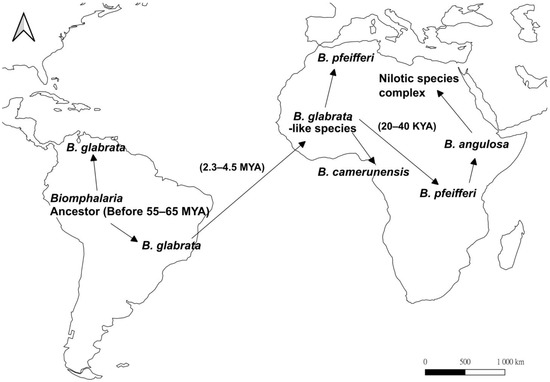
Figure 2.
Map showing the proposed evolutionary history of the snail genus, Biomphalaria based on the American origin hypothesis (after [46,51]). Arrows represent proposed dispersal direction. The ancestor of Biomphalaria existed in South America from 55 to 65 million years ago (mya), and later diverged into more than 20 species. B. glabrata-like species underwent trans-Atlantic dispersal in 2.3–4.5 mya, separating into B. camerunensis and B. pfeifferi in West Africa. B. pfeifferi colonized East Africa in approximately 20–40 kya, which further diverged into B. angulosa and subsequently the Nilotic species complex.
2.2. Origin and Diversification of Schistosoma
In the Asian origin hypothesis (Figure 3), the common ancestor of Schistosoma arose in Asia and expanded to Africa [58], and this is well supported by molecular phylogenetic analyses where the Asian species (except S. indicum) forms a basal clade to the African congeners [58,59,60]. Using the ITS2 sequences, the separation of African species from the Asian ancestor was estimated to occur at around 24–70 mya, while the speciation of S. mansoni happened between 10 and 30 mya [61], suggesting that Schistosoma colonized Africa much earlier than Biomphalaria. The long-term coexistence of Schistosoma and Biomphalaria in Africa resulted in host-parasite coevolution. It has been suggested that the schistosome susceptible B. glabrata-like species in Africa developed parasitic resistance genes under selective pressure, causing natural selection to favor S. mansoni with high infectivity, such that all Biomphalaria in Africa are susceptible to infection [51,62,63]. On the other hand, during the 16th to 19th centuries, S. mansoni was introduced from the Old World to the Americas through the Atlantic slave trade as suggested by the low electrophoretic variation in enzymes between these populations [64,65]. Due to the relatively recent introduction of the parasite, it has also been suggested that no coevolutionary relationship has been established for Schistosoma and Biomphalaria in the Americas [51], and only three Biomphalaria species are now the natural host of S. mansoni in the New World [35,66,67].
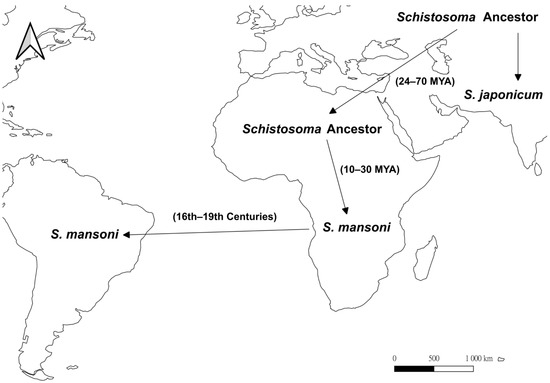
Figure 3.
Map showing the proposed evolutionary history of the blood fluke, Schistosoma based on the Asian origin hypothesis (after [58,59,60,61]). Arrows represent the suggested dispersal directions. In this hypothesis, the ancestor of Schistosoma originated in Asia, migrated to Africa at 24 to 70 mya, and separated into S. mansoni and other Schistosoma species at 10 to 30 mya. Later in the 16th to 19th centuries, S. mansoni was accidentally introduced into South America through human activities such as slave trade.
3. Distribution and Population of Biomphalaria in Asia
In the Neotropical region, Biomphalaria straminea is the most important intermediate host of Schistosoma mansoni [68]. Originally native to the freshwaters of northeast Brazil [69,70], the population has now spread to the Caribbean [71,72] and other South American countries, including Paraguay, Argentina [73], and Uruguay [74] due their high desiccation tolerance and reproductive potential [75,76].
Apart from this regional expansion, transoceanic dispersal of B. straminea has also been reported (i.e., from Latin America to Asia). In 1973, B. straminea was first discovered in the Lam Tsuen River of Hong Kong [77]. This introduction has been suggested to be linked with aquarium plants and ornamental fish trade [70,78,79], which is supported by the fact that the B. straminea in Hong Kong groups into the Brazilian B. straminea clade in the molecular phylogenetic tree [80]. The original population of B. straminea was later expanded to various agricultural areas in the New Territories, namely Shui Wai, Shek Kong, Sha Kok Mei, and Pak Shek Au, probably due to the interconnected irrigation field ditch system (Figure 4) [81,82,83]. Allozyme frequencies at polymorphic loci Aat-1, Est-1, and Est-2 suggested that the B. straminea population in Hong Kong was caused by multiple introduction events [81,83]. A few decades after its initial invasion, B. straminea seems to have established and can be found in a variety of different places in the New Territories of Hong Kong [84].
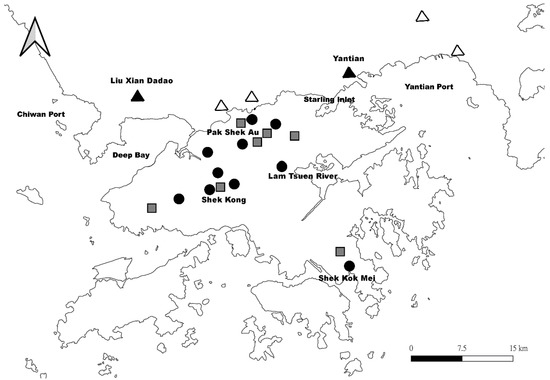
Figure 4.
The geographic distribution of the snail, Biomphalaria in Hong Kong and Southern China. Black circles represent the distribution of B. straminea reported in Yipp [85]. Grey squares represent the distribution of B. straminea reported in Zeng et al. [84]. Black and white triangles represent the distribution of B. straminea and B. kuhniana reported in Attwood et al. [80], respectively.
Across the border in Shenzhen (Guangdong Province, China), the presence of Biomphalaria was reported in 1981 [86,87]. In the molecular phylogenetic analyses, some populations (Yantian and Liu Xian Dadao) clustered with B. straminea from Brazil, while some other populations (Guanlan, Kui Yong, Lianhuashan Park, Shenzhen Reservoir, and Yongzhen) formed a clade with B. kuhniana [80]. Due to the close trading relationship between China and Brazil [88], Attwood et al. [80] proposed that the Yantian population was transported from the Port of Belem (Northeast of Brazil) to the Yantian International Container Terminal with cargo, while the Liu Xian Dadao population could be an expansion of the established Hong Kong population (Figure 4). To date, B. straminea can now be found in different places in southern China including Shenzhen, Dongguan, Huizhou, and Puning [76,87,89], and is predicted to be spreading further, including Taiwan, Southern Guangxi, Fujian and other Pearl River Delta cities such as Zhongshan, Zhuhai, Jiangmen, and Yangjiang in the future [76,90,91].
Tolerance limits to different environmental stresses have been considered an important determinant for predicting the geographic distribution of Biomphalaria and determining their habitat suitability [76,90]. Previous research found that regional temperatures and salinity exert a strong influence on the distribution of Biomphalaria [92,93,94,95,96,97]. Earlier studies on B. pfeifferi reported that the optimal temperature for egg production was between 19 °C and 30 °C, whereas no eggs would hatch at 35 °C, resulting in the absence of this species in the African countries that lie close to the equator [93,98,99]. Interestingly, Yipp [82] reported that B. straminea has the ability to survive and reproduce at temperatures up to 35 °C in Hong Kong, contributing to the successful establishment of this invasive species. In terms of salinity, previous studies on B. arabica showed that 100% mortality occurred at 7.2‰ [97], while B. glabrata has a high survival rate even at 7.7‰ [96]. The greater tolerance of B. glabrata to more hypersaline conditions supports the records of this species in the coastal areas of Brazil [96].
4. Immune System of Biomphalaria
4.1. Inducible Immune Receptors
In the innate immunity of Biomphalaria, pathogen-associated molecular patterns (PAMPs) of invaded parasites have been detected [100]. Parasites would first be recognized by pattern recognition receptors (PRRs), and in Biomphalaria, fibrinogen-related protein (FREPs) is the most well-studied PRR consisting of C-terminal fibrinogen-related (FBG) domain and one or two N-terminal immunoglobulin (IgSF) domains [101,102]. The expression of FREP2, 3, 4, and 6 was reported to be upregulated in both resistant and susceptible Biomphalaria following schistosome infection [103,104,105,106,107], and knockdown of FREP3 will result in one-third of the resistant B. glabrata becoming susceptible to Schistosoma infection [108]. Moreover, knockdown of FREP2, 3, and 4 resulted in approximately 15% of the primo-infected snails being infected during secondary challenges while the immune memory protected all primo-infected snails in the control group, suggesting their roles in innate immune memory [109]. Several FREPs also could combine with the thioester protein (TEP) and biomphalysin to form an immunocomplex that further interacts with the polymorphic mucins antigens of S. mansoni (SmPoMucs) [110,111,112,113,114]. The TEP family is known as a vital element in the phagocytosis of pathogens in insects [115], and in Biomphalaria activated TEP is involved in the opsonization process of pathogens and parasites, which actuates the phagocytosis of foreign cells [116,117].
Another group of PRRs is the Toll-like receptor (TLR), where the upregulation of its expression was observed in resistant snails at 12 and 24 h after schistosome invasion [118]. Previous research described that the parasitic PAMPs attach to the TLRs and activate a series of downstream signal transduction cascades, including the nuclear factor kappa B1 (NF-κB1) that further promotes the expression of defense genes for inflammatory and anti-apoptotic processes [100,105,106,119,120].
4.2. Cell Signaling
Various cytokine sequences were also found in the genome of B. glabrata, including 12 interleukins (ILs), 11 tumour necrosis factors (TNF), and 4 macrophage migration inhibitory factor (MIF) [106]. Cytokines are well-known for their roles in anti-microbial responses [121,122], and in vertebrates, cytokines bind to receptors (e.g., TNFR, IL-17R) and trigger the signaling cascades of NF-κB, mitogen-activated protein kinase (MAPK), and activator protein 1 (AP1) pathways [123]. In the NF-κB pathway, the activated receptor stimulates the enzyme complex IKK and the inhibitory protein IκBα [124], while in the MAPK pathway, the ligand-activated receptor stimulates the activation of either c-Jun N-terminal kinases (JNKs) or p38 MAPK [125,126]. The identification of these signaling components in Biomphalaria suggests that the snail host could also rely on them to combat parasite infection [119,120,124,126].
4.3. Inducible Immune Effectors
In addition to the above factors that are associated with parasite recognition and cell signaling, several molecular components related to immune effectors were also found to be differentially expressed in Biomphalaria after infection. One of these is the oxidative attack strategy [127,128,129]. In the presence of antigens, the NADPH oxidase complex will be activated and subsequently generate reactive oxygen species (ROS) to kill the sporocyst [127,129]. At the same time, Biomphalaria have also developed antioxidant molecules such as Cu/Zn superoxidase dismutase (SOD) which act to avoid the damaging effects of ROS on its own cells. SOD can convert toxic superoxide radicals into oxygen (O2) and hydrogen peroxide (H2O2) molecules [130], and upregulation of Cu/Zn SOD genes are observed in response to the penetration of S. mansoni and other infectious agents [103,104,107,130,131]. Other enzymes which can break down hydrogen peroxide (H2O2) into water were also found to have their expression upregulated upon schistosome infection [132], including peroxidase, glutathione peroxidase, peroxinectin, and dual oxidase [119,133].
Last but not least, heat shock proteins (HSPs) are another notable family of proteins that are upregulated to manage stressful conditions after schistosome infection in Biomphalaria. In innate immunity, HSPs act similarly to antigens and bind to the immune receptors and activate signaling cascades to facilitate antimicrobial responses [134]. Upon infection, HSPs act as molecular chaperones to support the folding and conformation of proteins that are associated with the inflammatory process [135,136]. Th expression of HSP70 was upregulated in the juveniles of both resistant and susceptible snails upon schistosome penetration, but in the adult stages, upregulation of HSP70 expression was only detected in schistosome-resistant snails [137,138].
4.4. Cellular and Humoral Effectors
Similar to other invertebrates, the immune system of Biomphalaria is comprised of both cellular and humoral responses [139]. Hemocytes are the circulating cells that are involved in the invertebrate cellular defense by encapsulation and phagocytosis of sporocysts and other pathogens [140,141,142]. Previous histological studies have indicated that the migration of hemocytes to the infiltration site is quicker in resistant snail species than in species with higher susceptibility to schistostomes [139,143,144]. Thus, hemocytes have been considered as one of the main effectors of Biomphalaria in antiparasitic responses. In addition, injection of cell-free hemolymph from a resistant snail to a susceptible snail strain significantly reduced schistosome infective rates, suggesting soluble proteins in the hemolymph also play an indispensable role in the immune system [145,146].
4.5. Compatibility Polymorphism between Biomphalaria and Schistosoma
Within a population, it appears that only some individual Biomphalaria snail hosts are successfully infested by the schistosome, while others are incompatible [147]. The reasons for this phenomenon, however, are not yet wholly understood. There are two primary hypotheses that have been suggested, including the “resistance hypothesis” [63] and the “matching hypothesis” [148]. For the resistance hypothesis, Webster and Davies [63] proposed that the resistance and susceptibility status of the snail hosts are the main determinants for successful infection. An experiment that was conducted by Allan et al. [149] showed that there is a low cercarial shedding rate (as an indication of infection of the snail) even when exposing the snail host to an environment with high schistosome density, suggesting that resistance status rather than the encounter rate plays an important role in the lack of successful infection. In support of this hypothesis, previous research has found significant expression differences in immune-related genes between compatible and incompatible snails, such as the putative immune receptor FREP3 [108], Cu/Zn superoxide dismutase (SOD) [130,131], and growth factor granulin (BgGRN) [150]. Vulnerable snails, therefore, usually lack the ability to recognize the parasite or produce effective effector cells [147].
On the other hand, the matching hypothesis suggests that infection success is due to the matched phenotype composition between the infected snail and the schistosome [148]. Theron et al. [151] revealed that all snail hosts are potentially susceptible to infection. Successful infection occurs when increasing the population of miracidia and raising the phenotypic diversity accordingly, in order to enhance the probability of finding a matched phenotype [147,151]. In experimental treatments, the infection rate of snails decreased by more than half after transferring the experiment from the field to the laboratory. This was interpreted to be a result of the presence of a reduced set of phenotypes in the small miracidia population due to genetic drift in the laboratory as compared to the field situation, supporting the hypothesis that successful infection increases with the phenotypic diversity of miracidia [151,152,153,154].
5. Genomics of Biomphalaria—A New Angle to Shed Light on Traditional Knowledge?
The control of schistosomiasis has traditionally relied on therapeutics, but increasing levels of resistance to existing drugs has decreased the efficacy of these treatments. The advancement and feasibility of sequencing technologies can now, however, offer better opportunities to understand the intermediate host Biomphalaria from the genome-wide perspective. The genomic data provide information on the introduction routes and population movement of invasive species via monitoring the changes of single nucleotide polymorphisms (SNPs) among the populations. For instance, the study of population genomic structure on Aedes albopictus, an important vector of numerous viral pathogens, discovered that the population in Italy was caused by multiple recent colonization events [155]. The genetic mixture of various populations generated novel genotypes with higher stress adaptation in Ae. albopictus and resulted in rapid range expansion [155]. This finding exposed the importance of monitoring the new introduction to high-infested areas, in order to prevent the importation of resistant populations. In addition, whole genome sequences contain the sum of all genetic information that can uncover the association between the genotypes and the expressed phenotypes. Analyzing the information on genome allows us to identify novel genes and gene functions which is essential in ecological responses and interactions. As such, it should be possible to develop specific pesticides by altering crucial metabolic or infection pathways. Faucon et al. [156] identified the genes that are associated with insecticide resistance in Ae. albopictus from the genome, which allowed the scientists to track down the metabolic resistance pathways and develop a new insecticide that was specified for resistance mosquitoes. Special interest is also given to the infection resistance and susceptibility of snail hosts. The identification of novel candidate resistance genes enables us to resolve the problem of why only some snail hosts are compatible with the schistosomes. A better understanding of the genomes of Biomphalaria will allows us to recognize unique characteristics in resistant and susceptible snails, which can be selected as new targets for molluscicides that target only the susceptible snails, avoiding detrimental effects on the native ecosystem. Furthermore, modification of the targeted sequence using CRISPR technology can potentially make susceptible snails infection-resistant, reducing or even blocking the spread of schistosomes. For example, Peng et al. [157] identified that the CsLOB1 gene promoter contributes to the susceptibility of orange Citrus sinensis against the bacterium Xanthomonas citri that causes lesions on fruits and leaves. Modification of the CsLOB1 gene promoter using the CRISPR/Cas9 technology-generated transgenic plants that are resistant to the disease citrus canker [157].
To date, the genomes of B. glabrata [106] and B. straminea [158], are publicly available. The genome assembly of B. glabrata is approximately 916 Mb in size with a scaffold N50 length of around 48 kb. This important genomic study that was conducted by Adema et al. [106] identified the immune and stress responses of Biomphalaria, including Toll-like receptors (TLRs), fibrinogen-related proteins (FRPs), and heat-shock proteins (HSPs) [106]. In addition, genes that are involved in the cytokine signaling pathway (i.e., IL17, MIF, TNF) and apoptotic pathway (i.e., BIR) were discovered for the first time in Biomphalaria which could be potential new targets for designing specific molluscicides [106]. Other than that, this study also demonstrated the expression of core cardiac genes in Biomphalaria heart tissues, and the independent divergence of actin genes in molluscs, which provides a better understanding of how Biomphalaria evolves [106].
Recently, the genome of B. straminea, which is now the dominant species spreading schistosomiasis in Asia, has also been obtained and analyzed [158]. The genome assembly size of this species is around 1Gb which is similar to B. glabrata; and yet, it has a scaffold N50 length of more than 25 Mbp [158]. Similar to another study, this recent study also revealed new biological insights, including the identification of the first set of ecdysteroid biosynthetic pathway genes, genes that are involved in cholesterol metabolism, as well as insect sesquitperneoid pathway genes in Biomphalaria straminea [158]. In addition, the authors revealed a sesquiterpenoid hormone responsive system in Biomphalaria [158], which could also provide potential new targets for making specific molluscicides, given the fact that sesquiterpenoid juvenile hormone has been an effective target for insecticides.
The key remaining question is what else can these genomic resources reveal? Here, we argue that at least two other potential directions in addition to those above, should be explored. First, in both genomes, HSP20, HSP40, HSP60, HSP70, and HSP90, which function in mediating biotic and abiotic stressors have been identified [106,158]. Understanding the effect of different stresses on the HSP family may help us to understand how Biomphalaria species adapt to their environment. Another direction will be carrying out population genomics to reveal the relatedness and connectivity of individuals collected from different places, which will help us to better understand the invasion route and spread pattern of the Biomphalaria population, in order to predict the potential distribution in the future. Recognizing areas with high invasion risk allows the government to formulate specific pathway-based preventive strategies.
Author Contributions
Conceptualization, J.H.L.H. and G.A.W.; writing—original draft preparation, M.F.F.A.; writing—review and editing, J.H.L.H. and G.A.W.; visualization, M.F.F.A.; supervision, J.H.L.H. and G.A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hong Kong Research Grant Council NSFC/RGC Joint Research Scheme (N_CUHK401/21), Collaborative Research Fund (C4015-20EF), and The Chinese University of Hong Kong Direct Grant (4053433, 4053489). MFFA was supported by the Ph.D. studentship of The Chinese University of Hong Kong.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study does not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sturrock, R.F. The schistosomes and their intermediate hosts. Schistosomiasis 2001, 3, 7–83. [Google Scholar]
- World Health Organization (WHO). Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Sundaraneedi, M.K.; Tedla, B.A.; Eichenberger, R.M.; Becker, L.; Pickering, D.; Smout, M.J.; Rajan, S.; Wangchuk, P.; Keene, F.; Loukas, A.; et al. Polypyridylruthenium (II) complexes exert anti-schistosome activity and inhibit parasite acetylcholinesterases. PLoS Negl. Trop. Dis. 2017, 11, e0006134. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Mouahid, G.; Rognon, A.; Augusto, R.D.C.; Driguez, P.; Geyer, K.; Karinshak, S.; Luviano, N.; Mann, V.; Quack, T.; Rawlinson, K.; et al. Transplantation of schistosome sporocysts between host snails: A video guide. Wellcome Open Res. 2018, 3, 3. [Google Scholar] [CrossRef]
- Nelwan, M.L. Schistosomiasis: Life cycle, diagnosis, and control. Curr. Ther. Res. 2019, 91, 5–9. [Google Scholar] [CrossRef]
- Braun, L.; Grimes, J.E.; Templeton, M.R. The effectiveness of water treatment processes against schistosome cercariae: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006364. [Google Scholar] [CrossRef]
- Gurarie, D.; Lo, N.C.; Ndeffo-Mbah, M.L.; Durham, D.P.; King, C.H. The human-snail transmission environment shapes long term schistosomiasis control outcomes: Implications for improving the accuracy of predictive modeling. PLoS Negl. Trop. Dis. 2018, 12, e0006514. [Google Scholar] [CrossRef]
- Abe, E.M.; Guan, W.; Guo, Y.-H.; Kassegne, K.; Qin, Z.-Q.; Xu, J.; Chen, J.-H.; Ekpo, U.F.; Li, S.-Z.; Zhou, X.-N. Differentiating snail intermediate hosts of Schistosoma spp. using molecular approaches: Fundamental to successful integrated control mechanism in Africa. Infect. Dis. Poverty 2018, 7, 6–18. [Google Scholar] [CrossRef]
- Bottieau, E.; Clerinx, J.; De Vega, M.R.; Enden, E.V.D.; Colebunders, R.; Van Esbroeck, M.; Vervoort, T.; Van Gompel, A.; Ende, J.V.D. Imported Katayama fever: Clinical and biological features at presentation and during treatment. J. Infect. 2006, 52, 339–345. [Google Scholar] [CrossRef]
- Ross, A.G.; Vickers, D.; Olds, G.R.; Shah, S.M.; McManus, D.P. Katayama syndrome. Lancet Infect. Dis. 2007, 7, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.R.; Al Karawi, M.; Yasawy, M.I. Schistosomal colonic disease. Gut 1990, 31, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Cheever, A.W.; Hoffmann, K.F.; Wynn, T.A. Immunopathology of schistosomiasis mansoni in mice and men. Immunol. Today 2000, 21, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B. The relevance of schistosomiasis for public health. Trop. Med. Parasitol. Off. Organ Dtsch. Trop. Ges. Dtsch. Ges. Fur Tech. Zs. (GTZ) 1989, 40, 134–142. [Google Scholar]
- Santos, L.L.; Santos, J.; Gouveia, M.J.; Bernardo, C.; Lopes, C.; Rinaldi, G.; Brindley, P.J.; Costa, J.M.C.D. Urogenital schistosomiasis—History, pathogenesis, and bladder cancer. J. Clin. Med. 2021, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis (Technical Report Series, no. 912); Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Gryseels, B.; de Vlas, S.J. Worm burdens in schistosome infections. Parasitol. Today 1996, 12, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Melman, S.D.; Steinauer, M.L.; Cunningham, C.; Kubatko, L.S.; Mwangi, I.N.; Wynn, N.B.; Mutuku, M.W.; Karanja, D.M.S.; Colley, D.G.; Black, C.L.; et al. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009, 3, e504. [Google Scholar] [CrossRef] [PubMed]
- Civitello, D.J.; Angelo, T.; Nguyen, K.H.; Hartman, R.B.; Starkloff, N.C.; Mahalila, M.P.; Charles, J.; Manrique, A.; Delius, B.K.; Bradley, L.M.; et al. Transmission potential of human schistosomes can be driven by resource competition among snail intermediate hosts. Proc. Natl. Acad. Sci. USA 2022, 119, e2116512119. [Google Scholar] [CrossRef]
- Coelho, P.M.Z.; Caldeira, R.L. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect. Dis. Poverty 2016, 5, 57. [Google Scholar] [CrossRef]
- Dai, J.R.; Li, Y.Z.; Wang, W.E.I.; Xing, Y.T.; Qu, G.L.; Liang, Y.S. Resistance to niclosamide in Oncomelania hupensis, the intermediate host of Schistosoma japonicum: Should we be worried? Parasitology 2015, 142, 332–340. [Google Scholar] [CrossRef]
- Abreu, F.C.; Goulart, M.O.; Brett, A.M. Detection of the damage caused to DNA by niclosamide using an electrochemical DNA-biosensor. Biosens. Bioelectron. 2002, 17, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Pieri, O.S.; Gonçalves, J.F.; Sarquis, O. Repeated focal mollusciciding for snail control in a sugar-cane area of northeast Brazil. MEMORIAS-INSTITUTO OSWALDO CRUZ 1995, 90, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.A. Strategic control of schistosome intermediate host. Asian J. Epidemiol. 2010, 3, 123–140. [Google Scholar] [CrossRef]
- Singh, D.K.; Agarwal, R.A. Correlation of the anticholinesterase and molluscicidal activity of the latex of Euphorbia royleana on the snail Lymnaea acuminata. J. Nat. Prod. 1984, 47, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.T.; Kariuki, J.M.; Mailu, B.M.; Muchiri, D.R. Isolation and characterisation of chemical compounds from the plants, Phytolacca octandra (L.), Phytolacca dodecandra (LHerit) and Balanites aegyptiaca (L.) commonly used to control schistosomiasis transmitting snails in Kenya. Afr. J. Pure Appl. Chem. 2018, 12, 38–41. [Google Scholar] [CrossRef]
- Marston, A.; Hecker, E. On the active principles of the Euphorbiaceae. Planta Med. 1983, 47, 141–147. [Google Scholar] [CrossRef]
- Yadav, S.C.; Jagannadham, M.V. Physiological changes and molluscicidal effects of crude latex and Milin on Biomphalaria glabrata. Chemosphere 2008, 71, 1295–1300. [Google Scholar] [CrossRef]
- Oliveira-Filho, E.C.; Paumgartten, F.J. Toxicity of Euphorbia milii latex and niclosamide to snails and nontarget aquatic species. Ecotoxicol. Environ. Saf. 2000, 46, 342–350. [Google Scholar] [CrossRef]
- Augusto, R.D.C.; de Mello-Silva, C.C.C. Phytochemical molluscicides and schistosomiasis: What we know and what we still need to learn. Vet. Sci. 2018, 5, 94. [Google Scholar] [CrossRef]
- Yang, F.; Long, E.; Wen, J.; Cao, L.; Zhu, C.; Hu, H.; Ruan, Y.; Okanurak, K.; Hu, H.; Wei, X.; et al. Linalool, derived from Cinnamomum camphora (L.) Presl leaf extracts, possesses molluscicidal activity against Oncomelania hupensis and inhibits infection of Schistosoma japonicum. Parasites Vectors 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Majoros, G.; Fehér, Z.; Deli, T.; Földvári, G. Establishment of Biomphalaria tenagophila snails in Europe. Emerg. Infect. Dis. 2008, 14, 1812. [Google Scholar] [CrossRef]
- Brown, D.S. Freshwater Snails of Africa and Their Medical Importance; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Malek, E.A. Snail Hosts of Schistosomiasis and Other Snail-Transmitted Diseases in Tropical America: A Manual; Pan American Health Organization: Washington, DC, USA, 1985. [Google Scholar]
- Corrêa, L.R.; Paraense, W.L. Susceptibility of Biomphalaria amazonica to infection with two strains of Schistosoma mansoni. Rev. Do Inst. De Med. Trop. De São Paulo 1971, 13, 387–390. [Google Scholar]
- Paraense, W.L.; Corrêa, L.R. Susceptibility of Biomphalaria peregrina from Brazil and Ecuador to two strains of Schistasoma mansoni. Rev. Do Inst. De Med. Trop. De São Paulo 1973, 15, 127–130. [Google Scholar]
- Teodoro, T.M.; Janotti-Passos, L.K.; dos Santos Carvalho, O.; Caldeira, R.L. Occurrence of Biomphalaria cousini (Mollusca: Gastropoda) in Brazil and its susceptibility to Schistosoma mansoni (Platyhelminths: Trematoda). Mol. Phylogenetics Evol. 2010, 57, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Scholte, R.G.; Carvalho, O.S.; Malone, J.B.; Utzinger, J.; Vounatsou, P. Spatial distribution of Biomphalaria spp., the intermediate host snails of Schistosoma mansoni, in Brazil. Geospat. Health 2012, 6, S95–S101. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.M. Snail hosts of Asian Schistosoma infecting man: Evolution and coevolution. Snail Hosts Asian Schistosoma Infect. Man Evol. Coevol. 1980, 128, 195–238. [Google Scholar]
- Pilsbry, H.A. Non-marine mollusca of Patagonia. In Princeton University Expeditions to Patagonia, 1896-1899; Forgotten Books: London, UK, 1911. [Google Scholar]
- Davis, G.M. Evolution of prosobranch snails transmitting Asian Schistosoma; coevolution with Schistosoma: A review. Prog. Clin. Parasitol. 1993, 3, 145–204. [Google Scholar]
- Meier-Brook, C. A preliminary biogeography of freshwater pulmonate gastropods. World-Wide Snails 1984, 1, 23–27. [Google Scholar]
- Thorpe, J.P. Enzyme variation, genetic distance, and evolutionary divergence in relation to levels of taxonomic separation. Protein Polymorph. Adapt. Taxon. Significance 1983, 24, 131–152. [Google Scholar]
- Woodruff, D.S.; Staub, K.C.; Suchart Upatham, E.; Viyanant, V.; Suchart, H.C.Y. Genetic variation in Oncomelania hupensis: Schistosoma japonicum transmitting snails in China and the Philippines are distinct species. Malacologia 1988, 29, 347–361. [Google Scholar]
- Woodruff, D.S.; Mulvey, M. Neotropical schistosomiasis: African affinities of the host snail Biomphalaria glabrata (Gastropoda: Planorbidae). Biol. J. Linn. Soc. 1997, 60, 505–516. [Google Scholar] [CrossRef]
- Van Damme, D. The freshwater Mollusca of northern Africa: Distribution, biogeography and palaeoecology. In Developments in hydrobiology; Dr W. Junk Publishers: Dordrecht, Germany, 1984; Volume 25, p. 164. [Google Scholar]
- Parodiz, J.J. The Tertiary non-marine mollusca of South America. Carnegie Mus. 1969, 40, 1–242. [Google Scholar] [CrossRef]
- Morgan, J.A.T.; Dejong, R.J.; Snyder, S.D.; Mkoji, G.M.; Loker, E.S. Schistosoma mansoni and Biomphalaria: Past history and future trends. Parasitology 2001, 123, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Jones, C.S.; Lockyer, A.E.; Hughes, S.; Brown, D.; Noble, L.R.; Rollinson, D. Molecular evidence supports an African affinity of the Neotropical freshwater gastropod, Biomphalaria glabrata, Say 1818, an intermediate host for Schistosoma mansoni. Proc. R. Soc. London. Ser. B Biol. Sci. 2000, 267, 2351–2358. [Google Scholar] [CrossRef]
- DeJong, R.J.; Morgan, J.A.; Paraense, W.L.; Pointier, J.P.; Amarista, M.; Ayeh-Kumi, P.F.; Babiker, A.; Barbosa, C.; Brémond, P.; Canese, A.; et al. Evolutionary relationships and biogeography of Biomphalaria (Gastropoda: Planorbidae) with implications regarding its role as host of the human bloodfluke, Schistosoma mansoni. Mol. Biol. Evol. 2001, 18, 2225–2239. [Google Scholar] [CrossRef]
- Colinvaux, P. Amazon diversity in light of the paleoecological record. Quat. Sci. Rev. 1987, 6, 93–114. [Google Scholar] [CrossRef]
- Lowe, J.J.; Walker, M. Reconstructing Quaternary Environments, 3rd ed.; Routledge: Harlow, UK, 2014. [Google Scholar]
- Jørgensen, A.; Kristensen, T.K.; Stothard, J.R. Phylogeny and biogeography of African Biomphalaria (Gastropoda: Planorbidae), with emphasis on endemic species of the great East African lakes. Zool. J. Linn. Soc. 2007, 151, 337–349. [Google Scholar] [CrossRef]
- DeJong, R.J.; Morgan, J.A.T.; Wilson, W.D.; Al-Jaser, M.H.; Appleton, C.C.; Coulibaly, G.; D’Andrea, P.S.; Doenhoff, M.J.; Haas, W.; Idris, M.A.; et al. Phylogeography of Biomphalaria glabrata and B. pfeifferi, important intermediate hosts of Schistosoma mansoni in the New and Old World tropics. Mol. Ecol. 2003, 12, 3041–3056. [Google Scholar] [CrossRef]
- Van Andel, T.H.; Tzedakis, P.C. Palaeolithic landscapes of Europe and environs, 150,000-25,000 years ago: An overview. Quat. Sci. Rev. 1996, 15, 481–500. [Google Scholar] [CrossRef]
- Reader, J. Africa: A Biography of the Continent; Penguin: London, UK, 1998. [Google Scholar]
- Snyder, S.D.; Loker, E.S. Evolutionary relationships among the Schistosomatidae (Platyhelminthes: Digenea) and an Asian origin for Schistosoma. J. Parasitol. 2000, 86, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Rollinson, D.; Kaukas, A.; Johnston, D.A.; Simpson, A.J.; Tanaka, M. Some molecular insights into schistosome evolution. Int. J. Parasitol. 1997, 27, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Müller-Graf, C.D.M. Muscles or testes? Comparative evidence for sexual competition among dioecious blood parasites (Schistosomatidae) of vertebrates. Parasitology 2000, 120, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Despres, L.; Imbert-Establet, D.; Combes, C.; Bonhomme, F. Molecular evidence linking hominid evolution to recent radiation of schistosomes (Platyhelminthes: Trematoda). Mol. Phylogenetics Evol. 1992, 1, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Manning, S.D.; Woolhouse, M.E.J. Parasite―host coevolution and geographic patterns of parasite infectivity and host susceptibility. Proc. R. Soc. London Ser. B Biol. Sci. 1996, 263, 119–128. [Google Scholar]
- Webster, J.P.; Davies, C.M. Coevolution and compatibility in the snail–schistosome system. Parasitology 2001, 123, 41–56. [Google Scholar] [CrossRef]
- Files, V.S. A study of the vector-parasite relationships in Schistosoma mansoni. Parasitology 1951, 41, 264–269. [Google Scholar] [CrossRef]
- Fletcher, M.; LoVerde, P.T.; Woodruff, D.S. Genetic variation in Schistosoma mansoni: Enzyme polymorphisms in populations from Africa, Southwest Asia, South America, and the West Indies. Am. J. Trop. Med. Hyg. 1981, 30, 406–421. [Google Scholar] [CrossRef]
- Vidigal, T.H.D.A.; Kissinger, J.C.; Caldeira, R.L.; Pires, E.C.R.; Monteiro, E.; Simpson, A.J.G.; Carvalho, O.S. Phylogenetic relationships among Brazilian Biomphalaria species (Mollusca: Planorbidae) based upon analysis of ribosomal ITS2 sequences. Parasitology 2000, 121, 611–620. [Google Scholar] [CrossRef]
- Carvalho, O.S.; Caldeira, R.L.; Simpson, A.J.G.; Vidigal, T.H.D.A. Genetic variability and molecular identification of Brazilian Biomphalaria species (Mollusca: Planorbidae). Parasitology 2001, 123, 197–209. [Google Scholar] [CrossRef]
- Wright, W.H.; Ayad, N.; De Azevedo, J.F.; Barbosa, F.S.; Clarke, V.D.V.; Farooq, M.; Gear, J.H.S.; Gillet, J.; Jordan, P.; Komiya, Y.; et al. Geographical distribution of schistosomes and their intermediate hosts. In Epidemiology and Control of Schistosomiasis (Bilharziasis); Karger Publishers: Basel, Switzerland, 1973; pp. 32–249. [Google Scholar]
- Paraense, W.L. The schistosome vectors in the Americas. Memórias Do Inst. Oswaldo Cruz 2001, 96, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Pointier, J.P.; David, P.; Jarne, P. Biological invasions: The case of planorbid snails. J. Helminthol. 2005, 79, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.S. Biomphalaria straminea (Dunker) en Colombia. Antioquia Med. 1968, 18, 753–758. [Google Scholar]
- Paraense, W.L.; Zeledón-Araya, R.; Rojas-Herrera, G. Biomphalaria straminea and other planorbid molluscs in Costa Rica. Biomphalaria straminea y otros moluscos (Planorbidae) en Costa Rica. J. Parasitol. 1981, 67, 282–283. [Google Scholar] [CrossRef]
- Paraense, W.L. Planorbídeos hospedeiros intermediários do Schistosoma mansoni. In Esquistossomose Mansoni; Sarvier/Edusp Universidade de São Paulo: São Paulo, Spain, 1970; Volume 19, pp. 13–30. [Google Scholar]
- Paraense, W.L.; Corrêa, L.R. A potential vector of Schistosoma mansoni in Uruguay. Memórias Do Inst. Oswaldo Cruz 1989, 84, 281–288. [Google Scholar] [CrossRef]
- Barboza, D.M.; Zhang, C.; Santos, N.C.; Silva, M.M.B.L.; Rollemberg, C.V.V.; de Amorim, F.J.R. Biomphalaria species distribution and its effect on human Schistosoma mansoni infection in an irrigated area used for rice cultivation in northeast Brazil. Geospat. Health 2012, 6, S103–S109. [Google Scholar] [CrossRef]
- Habib, M.R.; Guo, Y.H.; Lv, S.; Gu, W.B.; Li, X.H.; Zhou, X.N. Predicting the spatial distribution of Biomphalaria straminea, a potential intermediate host for Schistosoma mansoni, in China. Geospat. Health 2016, 11, 453. [Google Scholar] [CrossRef]
- Meier-Brook, C. A snail intermediate host of Schistosoma mansoni introduced into Hong Kong. Bull. World Health Organ. 1974, 51, 661. [Google Scholar]
- Han, Z.; Lai, L.W.; Fan, J. The ornamental fish retail market in Hong Kong: Its evolution and evaluation. Aquac. Econ. Manag. 2002, 6, 231–247. [Google Scholar] [CrossRef]
- Livengood, E.J.; Chapman, F.A. The ornamental fish trade: An introduction with perspectives for responsible aquarium fish ownership. IFAS Ext.—Fla. Univ. 2020, 124, 1–7. [Google Scholar] [CrossRef]
- Attwood, S.W.; Huo, G.N.; Qiu, J.W. Update on the distribution and phylogenetics of Biomphalaria (Gastropoda: Planorbidae) populations in Guangdong Province, China. Acta Trop. 2015, 141, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Yipp, M.W. A report on the gastropod fauna of aquarium fish farms in Hong Kong, with special reference to an introduced human schistosome host species, Biomphalaria straminea (Pulmonata: Planorbidae). Malacol. Rev. 1983, 16, 93–94. [Google Scholar]
- Yipp, W.M. The ecology of Biomphalaria straminea (Dunker, 1848) (Gastropoda: Pulmonata) introduced into Hong Kong. HKU Theses Online (HKUTO) 1983. [Google Scholar]
- Woodruff, D.S.; Mulvey, M.; Yipp, M.W. Population genetics of Biomphalaria straminea in Hong Kong: A neotropical schistosome-transmitting snail recently introduced into China. J. Hered. 1985, 76, 355–360. [Google Scholar] [PubMed]
- Zeng, X.; Yiu, W.C.; Cheung, K.H.; Yip, H.Y.; Nong, W.; He, P.; Yuan, D.; Rollinson, D.; Qiu, J.-W.; Fung, M.; et al. Distribution and current infection status of Biomphalaria straminea in Hong Kong. Parasites Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yipp, M.W. Distribution of the schistosome vector snail, Biomphalaria straminea (Pulmonata: Planorbidae) in Hong Kong. J. Molluscan Stud. 1990, 56, 47–55. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.X.; Zhang, W.Z. The discovery of Biomphalaria straminea (Dunker), an intermediate host of Schistosoma mansoni, from China. Acta Zootaxon Sin. 1982, 7, 256. [Google Scholar]
- Gao, S.T.; Li, X.H.; Huang, S.Y.; Xie, X.; Mei, S.J.; Ruan, C.W.; Huang, D.N. Primary investigation of distribution and ecological environment of Biomphalaria straminea in Dasha and Guanlan Rivers in Shenzhen areas. Chin. Trop Med. 2013, 13, 313–317. [Google Scholar]
- Miller, L.S.; Downie, A. In Brazil, Hu Jintao aims for bigger piece of Latin America trade. Christ. Sci. Monit. 2010, pp. 5–6. Available online: https://www.csmonitor.com/Business/2010/0415/In-Brazil-Hu-Jintao-aims-for-bigger-piece-of-Latin-America-trade (accessed on 6 February 2023).
- Huang, S.Y.; Zhang, Q.M.; Li, X.H.; Deng, Z.H. The distribution of B. straminea in China and risk of transmission. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2014, 3, 235–237. [Google Scholar]
- Yang, Y.; Cheng, W.; Wu, X.; Huang, S.; Deng, Z.; Zeng, X.; Yuan, D.; Yang, Y.; Wu, Z.; Chen, Y.; et al. Prediction of the potential global distribution for Biomphalaria straminea, an intermediate host for Schistosoma mansoni. PLoS Negl. Trop. Dis. 2018, 12, e0006548. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, X.; Sanogo, B.; He, P.; Xiang, S.; Du, S.; Zhang, Y.; Wang, L.; Wan, S.; Zeng, X.; et al. The potential risk of Schistosoma mansoni transmission by the invasive freshwater snail Biomphalaria straminea in South China. PLoS Negl. Trop. Dis. 2020, 14, e0008310. [Google Scholar] [CrossRef] [PubMed]
- Chernin, E. Behavior of Biomphalaria glabrata and of other snails in a thermal gradient. J. Parasitol. 1967, 53, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.C. The influence of temperature on the life-cycle and distribution of Biomphalaria pfeifferi (Krauss, 1948) in South-Eastern Africa. Int. J. Parasitol. 1977, 7, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P.H.; Pretorius, S.J.; De Kock, K.N.; Van Eeden, J.A. The effect of constant low temperatures on the survival of Bulinus africanus (Krauss), Bulinus globosus (Morelet) and Biomphalaria pfeifferi (Krauss). South Afr. J. Zool. 1984, 19, 314–316. [Google Scholar] [CrossRef]
- Pimentel-Souza, F.; Barbosa, N.D.; Resende, D.F. Effect of temperature on the reproduction of the snail Biomphalaria glabrata. Braz. J. Med. Biol. Res.=Rev. Bras. De Pesqui. Med. E Biol. 1990, 23, 441–449. [Google Scholar]
- Silva, P.B.D.; Barbosa, C.S.; Pieri, O.; Travassos, A.; Florencio, L. Physico-chemical and biological aspects related to the occurrence of Biomphalaria glabrata in foci of schistosomiasis in coastal areas of the state of Pernambuco, Brazil. Química Nova 2006, 29, 901–906. [Google Scholar] [CrossRef]
- Mostafa, O. Effect of salinity and drought on the survival of Biomphalaria arabica, the intermediate host of Schistosoma mansoni in Saudi Arabia. Egypt. Acad. J. Biol. Sci. B. Zool. 2009, 1, 1–6. [Google Scholar] [CrossRef]
- Sturrock, R.F. The influence of temperature on the biology of Biomphalaria pfeifferi (Krauss), an intermediate host of Schistosoma mansoni. Ann. Trop. Med. Parasitol. 1966, 60, 100–105. [Google Scholar] [CrossRef]
- Stensgaard, A.S.; Utzinger, J.; Vounatsou, P.; Hürlimann, E.; Schur, N.; Saarnak, C.F.; Simoonga, C.; Mubita, P.; Kabatereine, N.; Tchuenté, L.-A.; et al. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: Does climate matter? Acta Trop. 2013, 128, 378–390. [Google Scholar] [CrossRef]
- Castillo, M.G.; Humphries, J.E.; Mourão, M.M.; Marquez, J.; Gonzalez, A.; Montelongo, C.E. Biomphalaria glabrata immunity: Post-genome advances. Dev. Comp. Immunol. 2020, 104, 103557. [Google Scholar] [CrossRef]
- Adema, C.M. Fibrinogen-related proteins (FREPs) in mollusks. Pathog. -Host Interact. Antigen. Var. v. Somat. Adapt. 2015, 57, 111–129. [Google Scholar]
- Portet, A.; Pinaud, S.; Tetreau, G.; Galinier, R.; Cosseau, C.; Duval, D.; Grunau, C.; Mitta, G.; Gourbal, B. Integrated multi-omic analyses in Biomphalaria-Schistosoma dialogue reveal the immunobiological significance of FREP-SmPoMuc interaction. Dev. Comp. Immunol. 2017, 75, 16–27. [Google Scholar] [CrossRef]
- Adema, C.M.; Hanington, P.C.; Lun, C.M.; Rosenberg, G.H.; Aragon, A.D.; Stout, B.A.; Richard, M.L.L.; Gross, P.; Loker, E.S. Differential transcriptomic responses of Biomphalaria glabrata (Gastropoda, Mollusca) to bacteria and metazoan parasites, Schistosoma mansoni and Echinostoma paraensei (Digenea, Platyhelminthes). Mol. Immunol. 2010, 47, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Hanington, P.C.; Lun, C.M.; Adema, C.M.; Loker, E.S. Time series analysis of the transcriptional responses of Biomphalaria glabrata throughout the course of intramolluscan development of Schistosoma mansoni and Echinostoma paraensei. Int. J. Parasitol. 2010, 40, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Kenny, N.J.; Truchado-García, M.; Grande, C. Deep, multi-stage transcriptome of the schistosomiasis vector Biomphalaria glabrata provides platform for understanding molluscan disease-related pathways. BMC Infect. Dis. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Adema, C.M.; Hillier, L.W.; Jones, C.S.; Loker, E.S.; Knight, M.; Minx, P.; Oliveira, G.; Raghavan, N.; Shedlock, A.; do Amaral, L.R.; et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Buddenborg, S.K.; Bu, L.; Zhang, S.M.; Schilkey, F.D.; Mkoji, G.M.; Loker, E.S. Transcriptomic responses of Biomphalaria pfeifferi to Schistosoma mansoni: Investigation of a neglected African snail that supports more S. mansoni transmission than any other snail species. PLoS Negl. Trop. Dis. 2017, 11, e0005984. [Google Scholar] [CrossRef]
- Hanington, P.C.; Forys, M.A.; Loker, E.S. A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection. PLoS Negl. Trop. Dis. 2012, 6, e1591. [Google Scholar] [CrossRef]
- Pinaud, S.; Portela, J.; Duval, D.; Nowacki, F.C.; Olive, M.A.; Allienne, J.F.; Galinier, R.; Dheilly, N.; Kieffer-Jaquinod, S.; Mitta, G.; et al. A shift from cellular to humoral responses contributes to innate immune memory in the vector snail Biomphalaria glabrata. PLoS Pathog. 2016, 12, e1005361. [Google Scholar] [CrossRef]
- Moné, Y.; Gourbal, B.; Duval, D.; Du Pasquier, L.; Kieffer-Jaquinod, S.; Mitta, G. A large repertoire of parasite epitopes matched by a large repertoire of host immune receptors in an invertebrate host/parasite model. PLOS Negl. Trop. Dis. 2010, 4, e813. [Google Scholar] [CrossRef]
- Galinier, R.; Portela, J.; Moné, Y.; Allienne, J.F.; Henri, H.; Delbecq, S.; Mitta, G.; Gourbal, B.; Duval, D. Biomphalysin, a new β pore-forming toxin involved in Biomphalaria glabrata immune defense against Schistosoma mansoni. PLoS Pathog. 2013, 9, e1003216. [Google Scholar] [CrossRef] [PubMed]
- Gordy, M.A.; Pila, E.A.; Hanington, P.C. The role of fibrinogen-related proteins in the gastropod immune response. Fish Shellfish. Immunol. 2015, 46, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Dheilly, N.M.; Duval, D.; Mouahid, G.; Emans, R.; Allienne, J.F.; Galinier, R.; Genthon, C.; Dubois, E.; Pasquier, L.; Adema, C.; et al. A family of variable immunoglobulin and lectin domain containing molecules in the snail Biomphalaria glabrata. Dev. Comp. Immunol. 2015, 48, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hambrook, J.R.; Pila, E.A.; Gharamah, A.A.; Fang, J.; Wu, X.; Hanington, P. Coordination of humoral immune factors dictates compatibility between Schistosoma mansoni and Biomphalaria glabrata. Elife 2020, 9, e51708. [Google Scholar] [CrossRef]
- Fraiture, M.; Baxter, R.H.; Steinert, S.; Chelliah, Y.; Frolet, C.; Quispe-Tintaya, W.; Hoffmann, J.; Blandin, S.; Levashina, E.A. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 2009, 5, 273–284. [Google Scholar] [CrossRef]
- Tetreau, G.; Pinaud, S.; Portet, A.; Galinier, R.; Gourbal, B.; Duval, D. Specific pathogen recognition by multiple innate immune sensors in an invertebrate. Front. Immunol. 2017, 8, 1249. [Google Scholar] [CrossRef]
- Wu, X.-J.; Dinguirard, N.; Sabat, G.; Lui, H.-D.; Gonzalez, L.; Gehring, M.; Bickham-Wright, U.; Yoshino, T.P. Proteomic analysis of Biomphalaria glabrata plasma proteins with binding affinity to those expressed by early developing larval Schistosoma mansoni. PLoS Pathog. 2017, 13, e1006081. [Google Scholar] [CrossRef]
- Pila, E.A.; Tarrabain, M.; Kabore, A.L.; Hanington, P.C. A novel Toll-like receptor (TLR) influences compatibility between the gastropod Biomphalaria glabrata, and the digenean trematode Schistosoma mansoni. PLoS Pathog. 2016, 12, e1005513. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Spinks, J.N.; Walker, A.J.; Kane, R.A.; Noble, L.R.; Rollinson, D.; Dias-Neto, E.; Jones, C.S. Biomphalaria glabrata transcriptome: Identification of cell-signalling, transcriptional control and immune-related genes from open reading frame expressed sequence tags (ORESTES). Dev. Comp. Immunol. 2007, 31, 763–782. [Google Scholar] [CrossRef]
- Zhang, S.M.; Coultas, K.A.A. Identification and characterization of five transcription factors that are associated with evolutionarily conserved immune signaling pathways in the schistosome-transmitting snail Biomphalaria glabrata. Mol. Immunol. 2011, 48, 1868–1881. [Google Scholar] [CrossRef]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood J. Am. Soc. Hematol. 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Humphries, J.; Harter, B. Identification of nuclear factor kappaB (NF-κB) binding motifs in Biomphalaria glabrata. Dev. Comp. Immunol. 2015, 53, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.K.; Meighan, T.; Bowman, L. Role of mitogen-activated protein kinase activation in the production of inflammatory mediators: Differences between primary rat alveolar macrophages and macrophage cell lines. J. Toxicol. Environ. Health Part A 2002, 65, 757–768. [Google Scholar] [CrossRef]
- Humphries, J.E.; Yoshino, T.P. Schistosoma mansoni excretory–secretory products stimulate a p38 signalling pathway in Biomphalaria glabrata embryonic cells. Int. J. Parasitol. 2006, 36, 37–46. [Google Scholar] [CrossRef]
- Bayne, C.J.; Hahn, U.K.; Bender, R.C. Mechanisms of molluscan host resistance and of parasite strategies for survival. Parasitology 2001, 123, 159–167. [Google Scholar] [CrossRef]
- Hahn, U.K.; Bender, R.C.; Bayne, C.J. Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: Carbohydrate-specific stimulation. Dev. Comp. Immunol. 2000, 24, 531–541. [Google Scholar] [CrossRef]
- Hahn, U.K.; Bender, R.C.; Bayne, C.J. Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: Role of reactive oxygen species. J. Parasitol. 2001, 87, 292–299. [Google Scholar] [CrossRef]
- Goodall, C.P.; Bender, R.C.; Broderick, E.J.; Bayne, C.J. Constitutive differences in Cu/Zn superoxide dismutase mRNA levels and activity in hemocytes of Biomphalaria glabrata (Mollusca) that are either susceptible or resistant to Schistosoma mansoni (Trematoda). Mol. Biochem. Parasitol. 2004, 137, 321–328. [Google Scholar] [CrossRef]
- Bender, R.C.; Goodall, C.P.; Blouin, M.S.; Bayne, C.J. Variation in expression of Biomphalaria glabrata SOD1: A potential controlling factor in susceptibility/resistance to Schistosoma mansoni. Dev. Comp. Immunol. 2007, 31, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.N.; Kausar, S.; Cui, H. The biological role of peroxiredoxins in innate immune responses of aquatic invertebrates. Fish Shellfish. Immunol. 2019, 89, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Junior, N.C.P.; de Melo, E.S.; de Lima, I.L.; da Rocha, R.E.T.; Batista, M.; da Silva, R.A.; Feitosa, A.P.S.; Filho, J.L.d.L.; Brayner, F.A.; Alves, L.C. A proteomics evaluation of the primary and secondary immune response of Biomphalaria straminea challenged by Schistosoma mansoni. Parasitol. Res. 2021, 120, 4023–4035. [Google Scholar] [CrossRef] [PubMed]
- Zügel, U.; Kaufmann, S.H. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 1999, 12, 19–39. [Google Scholar] [CrossRef]
- Murshid, A.; Gong, J.; Calderwood, S.K. The role of heat shock proteins in antigen cross presentation. Front. Immunol. 2012, 3, 63. [Google Scholar] [CrossRef]
- Tukaj, S.; Węgrzyn, G. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: A review of preclinical studies. Cell Stress Chaperones 2016, 21, 213–218. [Google Scholar] [CrossRef]
- Lockyer, A.E.; Spinks, J.; Kane, R.A.; Hoffmann, K.F.; Fitzpatrick, J.M.; Rollinson, D.; Noble, L.R.; Jones, C.S. Biomphalaria glabrata transcriptome: cDNA microarray profiling identifies resistant-and susceptible-specific gene expression in haemocytes from snail strains exposed to Schistosoma mansoni. BMC Genom. 2008, 9, 1–17. [Google Scholar] [CrossRef]
- Ittiprasert, W.; Nene, R.; Miller, A.; Raghavan, N.; Lewis, F.; Hodgson, J.; Knight, M. Schistosoma mansoni infection of juvenile Biomphalaria glabrata induces a differential stress response between resistant and susceptible snails. Exp. Parasitol. 2009, 123, 203–211. [Google Scholar] [CrossRef]
- Negrão-Corrêa, D.; Mattos, A.C.A.; Pereira, C.A.J.; Martins-Souza, R.L.; Coelho, P.M.Z. Interaction of Schistosoma mansoni sporocysts and hemocytes of Biomphalaria. J. Parasitol. Res. 2012, 2012, 743920. [Google Scholar] [CrossRef]
- Bayne, C.J.; Buckley, P.M.; DeWan, P.C. Schistosoma mansoni: Cytotoxicity of hemocytes from susceptible snail hosts for sporocysts in plasma from resistant Biomphalaria glabrata. Exp. Parasitol. 1980, 50, 409–416. [Google Scholar] [CrossRef]
- Negrão-Corrêa, D.; Pereira, C.A.J.; Rosa, F.M.; Martins-Souza, R.L.; Andrade, Z.D.A.; Coelho, P.M.Z. Molluscan response to parasite: Biomphalaria and Schistosoma mansoni interaction. Invertebr. Surviv. J. 2007, 4, 101–111. [Google Scholar]
- Pila, E.A.; Li, H.; Hambrook, J.R.; Wu, X.; Hanington, P.C. Schistosomiasis from a snail’s perspective: Advances in snail immunity. Trends Parasitol. 2017, 33, 845–857. [Google Scholar] [CrossRef]
- Souza, C.P.D.; Borges, C.C.; Santana, A.G.; Andrade, Z.A. Comparative histopathology of Biomphalaria glabrata, B. tenagophila and B. straminea with variable degrees of resistance to Schistosoma mansoni miracidia. Memórias Do Inst. Oswaldo Cruz 1997, 92, 517–522. [Google Scholar] [CrossRef]
- De Melo, E.S.; Brayner, F.A.; Junior, N.C.P.; França, I.R.S.; Alves, L.C. Investigation of defense response and immune priming in Biomphalaria glabrata and Biomphalaria straminea, two species with different susceptibility to Schistosoma mansoni. Parasitol. Res. 2020, 119, 189–201. [Google Scholar] [CrossRef]
- Granath, W.O., Jr.; Yoshino, T.P. Schistosoma mansoni: Passive transfer of resistance by serum in the vector snail, Biomphalaria glabrata. Exp. Parasitol. 1984, 58, 188–193. [Google Scholar] [CrossRef]
- Coelho, J.R.; Bezerra, F.S. The effects of temperature change on the infection rate of Biomphalaria glabrata with Schistosoma mansoni. Memórias Do Inst. Oswaldo Cruz 2006, 101, 223–224. [Google Scholar] [CrossRef]
- Mitta, G.; Gourbal, B.; Grunau, C.; Knight, M.; Bridger, J.M.; Théron, A. The compatibility between Biomphalaria glabrata snails and Schistosoma mansoni: An increasingly complex puzzle. Adv. Parasitol. 2017, 97, 111–145. [Google Scholar]
- Theron, A.; Coustau, C. Are Biomphalaria snails resistant to Schistosoma mansoni? J. Helminthol. 2005, 79, 187–191. [Google Scholar] [CrossRef]
- Allan, F.; Dunn, A.M.; Emery, A.M.; Stothard, J.R.; Johnston, D.A.; Kane, R.A.; Khamis, A.N.; Mohammed, K.A.; Rollinson, D. Use of sentinel snails for the detection of Schistosoma haematobium transmission on Zanzibar and observations on transmission patterns. Acta Trop. 2013, 128, 234–240. [Google Scholar] [CrossRef]
- Pila, E.A.; Gordy, M.A.; Phillips, V.K.; Kabore, A.L.; Rudko, S.P.; Hanington, P.C. Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host. Proc. Natl. Acad. Sci. USA 2016, 113, 5305–5310. [Google Scholar] [CrossRef]
- Theron, A.; Coustau, C.; Rognon, A.; Gourbiere, S.; Blouin, M.S. Effects of laboratory culture on compatibility between snails and schistosomes. Parasitology 2008, 135, 1179–1188. [Google Scholar] [CrossRef]
- Stohler, R.A.; Curtis, J.; Minchella, D.J. A comparison of microsatellite polymorphism and heterozygosity among field and laboratory populations of Schistosoma mansoni. Int. J. Parasitol. 2004, 34, 595–601. [Google Scholar] [CrossRef]
- Bech, N.; Beltran, S.; Portela, J.; Rognon, A.; Allienne, J.F.; Boissier, J.; Théron, A. Follow-up of the genetic diversity and snail infectivity of a Schistosoma mansoni strain from field to laboratory. Infect. Genet. Evol. 2010, 10, 1039–1045. [Google Scholar] [CrossRef]
- Mitta, G.; Adema, C.M.; Gourbal, B.; Loker, E.S.; Theron, A. Compatibility polymorphism in snail/schistosome interactions: From field to theory to molecular mechanisms. Dev. Comp. Immunol. 2012, 37, 1–8. [Google Scholar] [CrossRef]
- Pichler, V.; Kotsakiozi, P.; Caputo, B.; Serini, P.; Caccone, A.; Della Torre, A. Complex interplay of evolutionary forces shaping population genomic structure of invasive Aedes albopictus in southern Europe. PLoS Negl. Trop. Dis. 2019, 13, e0007554. [Google Scholar] [CrossRef]
- Faucon, F.; Dusfour, I.; Gaude, T.; Navratil, V.; Boyer, F.; Chandre, F.; Sirisopa, P.; Thanispong, K.; Juntarajumnong, W.; Poupardin, R.; et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res. 2015, 25, 1347–1359. [Google Scholar] [CrossRef]
- Peng, A.; Chen, S.; Lei, T.; Xu, L.; He, Y.; Wu, L.; Yao, L.; Zou, X. Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene Cs LOB 1 promoter in citrus. Plant Biotechnol. J. 2017, 15, 1509–1519. [Google Scholar] [CrossRef]
- Nong, W.; Yu, Y.; Aase-Remedios, M.E.; Xie, Y.; So, W.L.; Li, Y.; Wong, C.; Baril, T.; Law, S.; Lai, S.; et al. Genome of the ramshorn snail Biomphalaria straminea—An obligate intermediate host of schistosomiasis. GigaScience 2022, 11, giac012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).