Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis?
Abstract
1. Introduction
2. Leishmania Species and Leishmaniasis
3. FA Profiles of Leishmania Lipids
3.1. FA Profile and Distribution in Lipids, Phospholipids, and Phospholipid Classes
3.2. Interspecies Differences and Similarities in FA Composition
3.3. Changes in FA Composition Relating to Parasite Differentiation and Drug Resistance/Sensitivity
4. FA Acquisition in Leishmania
4.1. De Novo Synthesis of FA and PUFA in Leishmania Parasites
4.2. FA Uptake
5. Significance of FAs in Parasite/Host Interactions and Parasite Survival in Host Cell
5.1. Myristic Acid (14:0) and Myristoylation
5.2. Cyclopropanated Fatty Acids
5.3. PUFAs: LA, AA and DHA
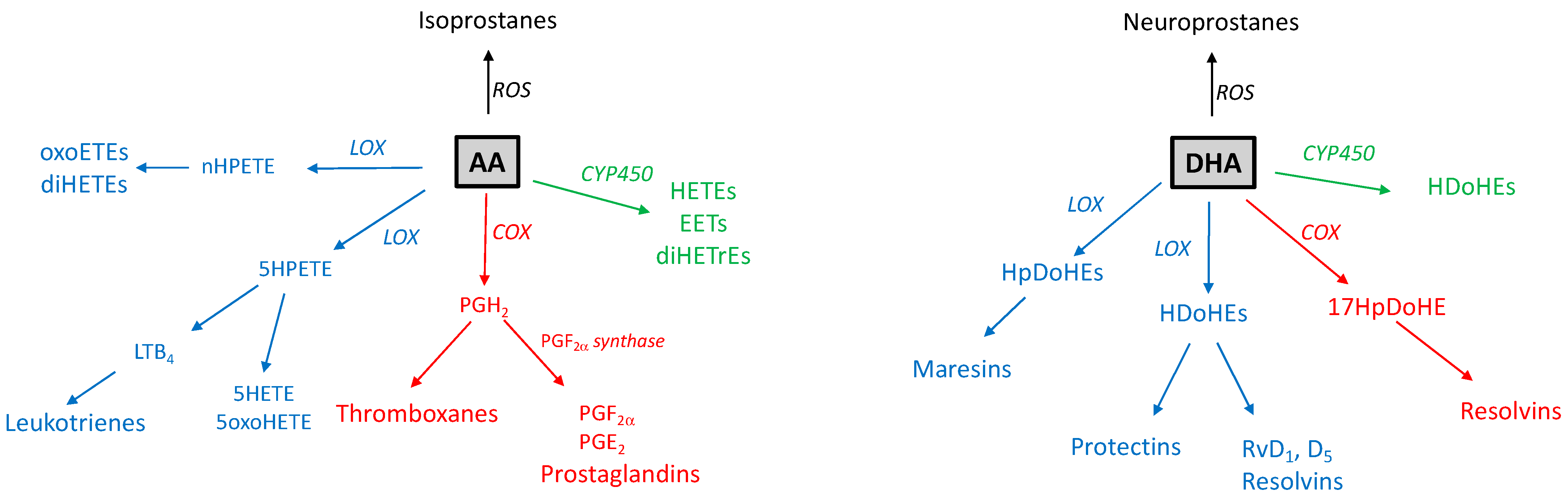
6. PUFA Oxygenated Metabolism
7. Therapeutics Insights: Lipids and Fatty Acids as Druggable Targets in Leishmaniasis
7.1. Current Treatments
7.2. Lipid Status as Prognostic/Diagnostic Biomarker for Leishmaniasis
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Murray, H.W.; Berman, J.D.; Davies, C.R.; Saravia, N.G. Advances in Leishmaniasis. Lancet 2005, 366, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the Host-Pathogen Interface. Nat. Rev. Microbiol. 2011, 9, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Akuffo, R.; Wilson, M.; Sarfo, B.; Attram, N.; Mosore, M.-T.; Yeboah, C.; Cruz, I.; Ruiz-Postigo, J.-A.; Boakye, D.; Moreno, J.; et al. Prevalence of Leishmania Infection in Three Communities of Oti Region, Ghana. PLoS Negl. Trop. Dis. 2021, 15, e0009413. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Petersen, C.A.; Barr, S.C. Canine Leishmaniasis in North America: Emerging or Newly Recognized? Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the Macrophage: A Multifaceted Interaction. Future Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.A.; Jafurulla, M.; Chattopadhyay, A. The Membrane as the Gatekeeper of Infection: Cholesterol in Host-Pathogen Interaction. Chem. Phys. Lipids 2016, 199, 179–185. [Google Scholar] [CrossRef]
- Schaible, U.E.; Schlesinger, P.H.; Steinberg, T.H.; Mangel, W.F.; Kobayashi, T.; Russell, D.G. Parasitophorous Vacuoles of Leishmania mexicana Acquire Macromolecules from the Host Cell Cytosol via Two Independent Routes. J. Cell Sci. 1999, 112 Pt 5, 681–693. [Google Scholar] [CrossRef]
- Semini, G.; Paape, D.; Paterou, A.; Schroeder, J.; Barrios-Llerena, M.; Aebischer, T. Changes to Cholesterol Trafficking in Macrophages by Leishmania Parasites Infection. Microbiologyopen 2017, 6, e00469. [Google Scholar] [CrossRef]
- De Macedo-Silva, S.T.; de Souza, W.; Rodrigues, J.C.F. Sterol Biosynthesis Pathway as an Alternative for the Anti-Protozoan Parasite Chemotherapy. Curr. Med. Chem. 2015, 22, 2186–2198. [Google Scholar] [CrossRef]
- Besteiro, S.; Bertrand-Michel, J.; Lebrun, M.; Vial, H.; Dubremetz, J.-F. Lipidomic Analysis of Toxoplasma Gondii Tachyzoites Rhoptries: Further Insights into the Role of Cholesterol. Biochem. J. 2008, 415, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Biagiotti, M.; Dominguez, S.; Yamout, N.; Zufferey, R. Lipidomics and Anti-Trypanosomatid Chemotherapy. Clin. Transl. Med. 2017, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, N.; Soumya, N.; Singh, S. Antileishmanial Effect of Mevastatin Is Due to Interference with Sterol Metabolism. Parasitol. Res. 2015, 114, 3873–3883. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wilson, M.E. Dynamics of Sterol Synthesis during Development of Leishmania Spp. Parasites to Their Virulent Form. Parasites Vectors 2016, 9, 200. [Google Scholar] [CrossRef]
- McCall, L.-I.; El Aroussi, A.; Choi, J.Y.; Vieira, D.F.; De Muylder, G.; Johnston, J.B.; Chen, S.; Kellar, D.; Siqueira-Neto, J.L.; Roush, W.R.; et al. Targeting Ergosterol Biosynthesis in Leishmania Donovani: Essentiality of Sterol 14 Alpha-Demethylase. PLoS Negl. Trop. Dis. 2015, 9, e0003588. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Dhembla, C.; Makde, R.D.; Sundd, M.; Kundu, S. An Overview of the Fatty Acid Biosynthesis in the Protozoan Parasite Leishmania and Its Relevance as a Drug Target against Leishmaniasis. Mol. Biochem. Parasitol. 2021, 246, 111416. [Google Scholar] [CrossRef] [PubMed]
- Kuhls, K.; Chicharro, C.; Cañavate, C.; Cortes, S.; Campino, L.; Haralambous, C.; Soteriadou, K.; Pratlong, F.; Dedet, J.-P.; Mauricio, I.; et al. Differentiation and Gene Flow among European Populations of Leishmania infantum MON-1. PLoS Negl. Trop. Dis. 2008, 2, e261. [Google Scholar] [CrossRef]
- Aoun, K.; Bouratbine, A.; Harrat, Z.; Guizani, I.; Mokni, M.; Bel Hadj Ali, S.; Ben Osman, A.; Belkaïd, M.; Dellagi, K.; Ben Ismaïl, R. Epidemiologic and parasitologic data concerning sporadic cutaneous leishmaniasis in northern Tunisia. Bull. Soc. Pathol. Exot. 2000, 93, 101–103. [Google Scholar]
- Kallel, K.; Pratlong, F.; Belhadj, S.; Cherif, F.; Hammami, M.; Dedet, J.P.; Chaker, E. Cutaneous Leishmaniasis in Tunisia: Results of the Iso-Enzymatic Characterization of 71 Strains. Ann. Trop. Med. Parasitol. 2005, 99, 11–19. [Google Scholar] [CrossRef]
- Kallel, K.; Pratlong, F.; Haouas, N.; Kaouech, E.; Belhadj, S.; Anane, S.; Dedet, J.P.; Babba, H.; Chaker, E. Isoenzymatic Variability of Leishmania infantum in Tunisia Concerning 254 Human Strains. Acta Trop. 2008, 106, 132–136. [Google Scholar] [CrossRef]
- Scarpini, S.; Dondi, A.; Totaro, C.; Biagi, C.; Melchionda, F.; Zama, D.; Pierantoni, L.; Gennari, M.; Campagna, C.; Prete, A.; et al. Visceral Leishmaniasis: Epidemiology, Diagnosis, and Treatment Regimens in Different Geographical Areas with a Focus on Pediatrics. Microorganisms 2022, 10, 1887. [Google Scholar] [CrossRef]
- Goto, H.; Lauletta Lindoso, J.A. Cutaneous and Mucocutaneous Leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 293–307. [Google Scholar] [CrossRef] [PubMed]
- De Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Derm. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Thomaidou, E.; Horev, L.; Jotkowitz, D.; Zamir, M.; Ingber, A.; Enk, C.D.; Molho-Pessach, V. Lymphatic Dissemination in Cutaneous Leishmaniasis Following Local Treatment. Am. J. Trop. Med. Hyg. 2015, 93, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Van Henten, S.; Adriaensen, W.; Fikre, H.; Akuffo, H.; Diro, E.; Hailu, A.; Van der Auwera, G.; van Griensven, J. Cutaneous Leishmaniasis Due to Leishmania aethiopica . EClinicalMedicine 2018, 6, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, A.; Cocuzza, S.; Pinzone, M.R.; Postorino, M.C.; Cosentino, S.; Serra, A.; Cacopardo, B.; Nunnari, G. Mucosal Leishmaniasis: An Underestimated Presentation of a Neglected Disease. Biomed. Res. Int. 2013, 2013, 805108. [Google Scholar] [CrossRef] [PubMed]
- Das, V.N.R.; Siddiqui, N.A.; Pandey, K.; Lal, C.S.; Sinha, S.K.; Bimal, S.; Topno, R.K.; Singh, S.K.; Kumar, S.; Das, P. The Usefulness of Trained Field Workers in Diagnosis of Post-Kala-Azar Dermal Leishmaniasis (PKDL) and Clinico-Epidemiological Profile in Highly Endemic Areas of Bihar. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 332–340. [Google Scholar] [CrossRef]
- Ait Maatallah, I.; Akarid, K.; Lemrani, M. Tissue Tropism: Is It an Intrinsic Characteristic of Leishmania Species? Acta Trop. 2022, 232, 106512. [Google Scholar] [CrossRef]
- Beach, D.H.; Holz, G.G.; Anekwe, G.E. Lipids of Leishmania Promastigotes. J. Parasitol. 1979, 65, 201–216. [Google Scholar] [CrossRef]
- Wassef, M.K.; Fioretti, T.B.; Dwyer, D.M. Lipid Analyses of Isolated Surface Membranes of Leishmania donovani Promastigotes. Lipids 1985, 20, 108–115. [Google Scholar] [CrossRef]
- Adosraku, R.K.; Anderson, M.M.; Anderson, G.J.; Choi, G.; Croft, S.L.; Yardley, V.; Phillipson, J.D.; Gibbons, W.A. Proton NMR Lipid Profile of Leishmania donovani Promastigotes. Mol. Biochem. Parasitol. 1993, 62, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; T’Kind, R.; Decuypere, S.; von Freyend, S.J.; Coombs, G.H.; Watson, D.G. Profiling of Lipids in Leishmania donovani Using Hydrophilic Interaction Chromatography in Combination with Fourier Transform Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, A.F.; Dutra, J.L.; Santos, M.L.; Santos Dde, A.; Alves, P.B.; de Moura, T.R.; de Almeida, R.P.; Fernandes, M.F.; Scher, R.; Fernandes, R.P. Fatty Acid Profiles in Leishmania Spp. Isolates with Natural Resistance to Nitric Oxide and Trivalent Antimony. Parasitol. Res. 2014, 113, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Das, R.P.; Ranjan, A.; Shaha, C. Elevated Ergosterol Protects Leishmania Parasites against Antimony-Generated Stress. FASEB J. 2015, 29, 4201–4213. [Google Scholar] [CrossRef]
- Bouazizi-Ben Messaoud, H.; Guichard, M.; Lawton, P.; Delton, I.; Azzouz-Maache, S. Changes in Lipid and Fatty Acid Composition During Intramacrophagic Transformation of Leishmania donovani Complex Promastigotes into Amastigotes. Lipids 2017, 52, 433–441. [Google Scholar] [CrossRef]
- Bouabid, C.; Yamaryo-Botté, Y.; Rabhi, S.; Bichiou, H.; Hkimi, C.; Bouglita, W.; Chaouach, M.; Eddaikra, N.; Ghedira, K.; Guizani-Tabbane, L.; et al. Fatty Acid Profiles of Leishmania major Derived from Human and Rodent Hosts in Endemic Cutaneous Leishmaniasis Areas of Tunisia and Algeria. Pathogens 2022, 11, 92. [Google Scholar] [CrossRef]
- Oyola, S.O.; Evans, K.J.; Smith, T.K.; Smith, B.A.; Hilley, J.D.; Mottram, J.C.; Kaye, P.M.; Smith, D.F. Functional Analysis of Leishmania Cyclopropane Fatty Acid Synthetase. PLoS ONE 2012, 7, e51300. [Google Scholar] [CrossRef]
- Hsu, F.-F.; Kuhlmann, F.M.; Turk, J.; Beverley, S.M. Multiple-Stage Linear Ion-Trap with High Resolution Mass Spectrometry towards Complete Structural Characterization of Phosphatidylethanolamines Containing Cyclopropane Fatty Acyl Chain in Leishmania infantum . J. Mass Spectrom. 2014, 49, 201–209. [Google Scholar] [CrossRef]
- Xu, W.; Mukherjee, S.; Ning, Y.; Hsu, F.-F.; Zhang, K. Cyclopropane Fatty Acid Synthesis Affects Cell Shape and Acid Resistance in Leishmania mexicana . Int. J. Parasitol. 2018, 48, 245–256. [Google Scholar] [CrossRef]
- Rakotomanga, M.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Alteration of Fatty Acid and Sterol Metabolism in Miltefosine-Resistant Leishmania donovani Promastigotes and Consequences for Drug-Membrane Interactions. Antimicrob. Agents Chemother. 2005, 49, 2677–2686. [Google Scholar] [CrossRef]
- Mbongo, N.; Loiseau, P.M.; Billion, M.A.; Robert-Gero, M. Mechanism of Amphotericin B Resistance in Leishmania donovani Promastigotes. Antimicrob. Agents Chemother. 1998, 42, 352–357. [Google Scholar] [CrossRef]
- t’Kindt, R.; Scheltema, R.A.; Jankevics, A.; Brunker, K.; Rijal, S.; Dujardin, J.-C.; Breitling, R.; Watson, D.G.; Coombs, G.H.; Decuypere, S. Metabolomics to Unveil and Understand Phenotypic Diversity between Pathogen Populations. PLoS Negl. Trop. Dis. 2010, 4, e904. [Google Scholar] [CrossRef] [PubMed]
- Barratt, G.; Saint-Pierre-Chazalet, M.; Loiseau, P.M. Cellular Transport and Lipid Interactions of Miltefosine. Curr. Drug. Metab. 2009, 10, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Prada, C.; Vincent, I.M.; Brotherton, M.-C.; Roberts, M.; Roy, G.; Rivas, L.; Leprohon, P.; Smith, T.K.; Ouellette, M. Different Mutations in a P-Type ATPase Transporter in Leishmania Parasites Are Associated with Cross-Resistance to Two Leading Drugs by Distinct Mechanisms. PLoS Negl. Trop. Dis. 2016, 10, e0005171. [Google Scholar] [CrossRef] [PubMed]
- Imbert, L.; Gaudin, M.; Libong, D.; Touboul, D.; Abreu, S.; Loiseau, P.M.; Laprévote, O.; Chaminade, P. Comparison of Electrospray Ionization, Atmospheric Pressure Chemical Ionization and Atmospheric Pressure Photoionization for a Lipidomic Analysis of Leishmania donovani . J. Chromatogr. A 2012, 1242, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Guarnizo, S.A.; Tikhonova, E.B.; Zabet-Moghaddam, M.; Zhang, K.; Muskus, C.; Karamyshev, A.L.; Karamysheva, Z.N. Drug-Induced Lipid Remodeling in Leishmania Parasites. Microorganisms 2021, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Uttaro, A.D. Acquisition and Biosynthesis of Saturated and Unsaturated Fatty Acids by Trypanosomatids. Mol. Biochem. Parasit. 2014, 196, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Parreira de Aquino, G.; Mendes Gomes, M.A.; Köpke Salinas, R.; Laranjeira-Silva, M.F. Lipid and Fatty Acid Metabolism in Trypanosomatids. Microb. Cell. 2021, 8, 262–275. [Google Scholar] [CrossRef]
- Lee, S.H.; Stephens, J.L.; Englund, P.T. A Fatty-Acid Synthesis Mechanism Specialized for Parasitism. Nat. Rev. Microbiol. 2007, 5, 287–297. [Google Scholar] [CrossRef]
- Alloatti, A.; Uttaro, A.D. Highly Specific Methyl-End Fatty-Acid Desaturases of Trypanosomatids. Mol. Biochem. Parasitol. 2011, 175, 126–132. [Google Scholar] [CrossRef]
- Maache, M.; Azzouz, S.; Diaz de la Guardia, R.; Alvarez, P.; Gil, R.; de Pablos, L.M.; Osuna, A. Host Humoral Immune Response to Leishmania Lipid-Binding Protein. Parasite Immunol. 2005, 27, 227–234. [Google Scholar] [CrossRef]
- Berman, J.D.; Gallalee, J.V.; Best, J.M.; Hill, T. Uptake, Distribution, and Oxidation of Fatty Acids by Leishmania mexicana Amastigotes. J. Parasitol. 1987, 73, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Leroux, M.; Bouazizi-Ben Messaoud, H.; Luquain-Costaz, C.; Jordheim, L.P.; Le Faouder, P.; Gustin, M.-P.; Aoun, K.; Lawton, P.; Azzouz-Maache, S.; Delton, I. Enriched PUFA Environment of Leishmania infantum Promastigotes Promotes the Accumulation of Lipid Mediators and Favors Parasite Infectivity towards J774 Murine Macrophages. Lipids 2022. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, N.N.; Pereira, M.G.; Correa, J.R.; Andrade-Neto, V.V.; Saraiva, F.B.; Chagas-Lima, A.C.; Gondim, K.C.; Torres-Santos, E.C.; Folly, E.; Saraiva, E.M.; et al. LDL Uptake by Leishmania Amazonensis: Involvement of Membrane Lipid Microdomains. Exp. Parasitol. 2012, 130, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Neto, V.V.; Cicco, N.N.T.; Cunha-Junior, E.F.; Canto-Cavalheiro, M.M.; Atella, G.C.; Torres-Santos, E.C. The Pharmacological Inhibition of Sterol Biosynthesis in Leishmania Is Counteracted by Enhancement of LDL Endocytosis. Acta Trop. 2011, 119, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Courtoy, P.J. The Adaptative Mechanisms of Trypanosoma Brucei for Sterol Homeostasis in Its Different Life-Cycle Environments. Annu. Rev. Microbiol. 2000, 54, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, J.A.; Smith, B.A.; Yu, Z.; Brzozowski, A.M.; Hodgkinson, M.R.; Maroof, A.; Price, H.P.; Meier, F.; Leatherbarrow, R.J.; Tate, E.W.; et al. N-Myristoyltransferase from Leishmania donovani: Structural and Functional Characterisation of a Potential Drug Target for Visceral Leishmaniasis. J. Mol. Biol. 2010, 396, 985–999. [Google Scholar] [CrossRef]
- Price, H.P.; Menon, M.R.; Panethymitaki, C.; Goulding, D.; McKean, P.G.; Smith, D.F. Myristoyl-CoA:Protein N-Myristoyltransferase, an Essential Enzyme and Potential Drug Target in Kinetoplastid Parasites. J. Biol. Chem. 2003, 278, 7206–7214. [Google Scholar] [CrossRef]
- Goldston, A.M.; Sharma, A.I.; Paul, K.S.; Engman, D.M. Acylation in Trypanosomatids: An Essential Process and Potential Drug Target. Trends Parasitol. 2014, 30, 350–360. [Google Scholar] [CrossRef]
- Wright, M.H.; Paape, D.; Storck, E.M.; Serwa, R.A.; Smith, D.F.; Tate, E.W. Global Analysis of Protein N-Myristoylation and Exploration of N-Myristoyltransferase as a Drug Target in the Neglected Human Pathogen Leishmania donovani . Chem. Biol. 2015, 22, 342–354. [Google Scholar] [CrossRef]
- Paape, D.; Prendergast, C.T.; Price, H.P.; Doehl, J.S.P.; Smith, D.F. Genetic Validation of Leishmania Genes Essential for Amastigote Survival in Vivo Using N-Myristoyltransferase as a Model. Parasites Vectors 2020, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.S.; Yu, Z.; Hutton, J.A.; Wright, M.H.; Brannigan, J.A.; Paape, D.; Roberts, S.M.; Sutherell, C.L.; Ritzefeld, M.; Wilkinson, A.J.; et al. Novel Thienopyrimidine Inhibitors of Leishmania N-Myristoyltransferase with On-Target Activity in Intracellular Amastigotes. J. Med. Chem. 2020, 63, 7740–7765. [Google Scholar] [CrossRef] [PubMed]
- Corpas-Lopez, V.; Moniz, S.; Thomas, M.; Wall, R.J.; Torrie, L.S.; Zander-Dinse, D.; Tinti, M.; Brand, S.; Stojanovski, L.; Manthri, S.; et al. Pharmacological Validation of N-Myristoyltransferase as a Drug Target in Leishmania donovani . ACS Infect. Dis. 2019, 5, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Rai, A.K. Linoleic Acid Inhibits the Release of Leishmania donovani Derived Microvesicles and Decreases Its Survival in Macrophages. Front. Cell. Infect. Microbiol. 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Reverte, M.; Snäkä, T.; Fasel, N. The Dangerous Liaisons in the Oxidative Stress Response to Leishmania Infection. Pathogens 2022, 11, 409. [Google Scholar] [CrossRef]
- Carneiro, P.P.; Conceição, J.; Macedo, M.; Magalhães, V.; Carvalho, E.M.; Bacellar, O. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. PLoS ONE 2016, 11, e0148084. [Google Scholar] [CrossRef]
- Deschacht, M.; Van Assche, T.; Hendrickx, S.; Bult, H.; Maes, L.; Cos, P. Role of Oxidative Stress and Apoptosis in the Cellular Response of Murine Macrophages upon Leishmania Infection. Parasitology 2012, 139, 1429–1437. [Google Scholar] [CrossRef]
- Gantt, K.R.; Goldman, T.L.; McCormick, M.L.; Miller, M.A.; Jeronimo, S.M.; Nascimento, E.T.; Britigan, B.E.; Wilson, M.E. Oxidative Responses of Human and Murine Macrophages during Phagocytosis of Leishmania Chagasi. J. Immunol. 2001, 167, 893–901. [Google Scholar] [CrossRef]
- Roma, E.H.; Macedo, J.P.; Goes, G.R.; Gonçalves, J.L.; De Castro, W.; Cisalpino, D.; Vieira, L.Q. Impact of Reactive Oxygen Species (ROS) on the Control of Parasite Loads and Inflammation in Leishmania amazonensis Infection. Parasites Vectors 2016, 9, 193. [Google Scholar] [CrossRef]
- Andrade, Y.M.F.d.S.; de Castro, M.V.; Tavares, V.d.S.; Souza, R.d.S.O.; Faccioli, L.H.; Lima, J.B.; Sorgi, C.A.; Borges, V.d.M.; Araújo-Santos, T. Polyunsaturated Fatty Acids Alter the Formation of Lipid Droplets and Eicosanoid Production in Leishmania Promatigotes. bioRxiv 2022. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W.; Harwood, J.L. Oxidation of Polyunsaturated Fatty Acids to Produce Lipid Mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.S.; Galano, J.-M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.-Y.; Oger, C.; Durand, T. Moving Forward with Isoprostanes, Neuroprostanes and Phytoprostanes: Where Are We Now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Panis, C.; Mazzuco, T.L.; Costa, C.Z.F.; Victorino, V.J.; Tatakihara, V.L.H.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Cecchini, R.; Rizzo, L.V.; Pinge-Filho, P. Trypanosoma Cruzi: Effect of the Absence of 5-Lipoxygenase (5-LO)-Derived Leukotrienes on Levels of Cytokines, Nitric Oxide and INOS Expression in Cardiac Tissue in the Acute Phase of Infection in Mice. Exp. Parasitol. 2011, 127, 58–65. [Google Scholar] [CrossRef]
- Panis, C.; Victorino, V.J.; Tatakihara, V.L.H.; Cecchini, R.; Rizzo, L.V.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Martins-Pinge, M.C.; Pinge-Filho, P. Differences in CNOS/INOS Activity during Resistance to Trypanosoma Cruzi Infection in 5-Lipoxygenase Knockout Mice. Mediat. Inflamm. 2019, 2019, 5091630. [Google Scholar] [CrossRef]
- Saini, S.; Singh, B.; Prakash, S.; Kumari, S.; Kureel, A.K.; Dube, A.; Sahasrabuddhe, A.A.; Rai, A.K. Parasitic Load Determination by Differential Expressions of 5-Lipoxygenase and PGE2 Synthases in Visceral Leishmaniasis. Prostaglandins Other Lipid Mediat. 2020, 147, 106390. [Google Scholar] [CrossRef]
- Sacramento, L.A.; Cunha, F.Q.; de Almeida, R.P.; da Silva, J.S.; Carregaro, V. Protective Role of 5-Lipoxigenase during Leishmania infantum Infection Is Associated with Th17 Subset. Biomed. Res. Int. 2014, 2014, 264270. [Google Scholar] [CrossRef]
- Plagge, M.; Laskay, T. Early Production of the Neutrophil-Derived Lipid Mediators LTB4 and LXA4 Is Modulated by Intracellular Infection with Leishmania major . Biomed. Res. Int. 2017, 2017, 2014583. [Google Scholar] [CrossRef]
- Lefèvre, L.; Lugo-Villarino, G.; Meunier, E.; Valentin, A.; Olagnier, D.; Authier, H.; Duval, C.; Dardenne, C.; Bernad, J.; Lemesre, J.L.; et al. The C-Type Lectin Receptors Dectin-1, MR, and SIGNR3 Contribute Both Positively and Negatively to the Macrophage Response to Leishmania infantum . Immunity 2013, 38, 1038–1049. [Google Scholar] [CrossRef]
- Serezani, C.H.; Perrela, J.H.; Russo, M.; Peters-Golden, M.; Jancar, S. Leukotrienes Are Essential for the Control of Leishmania amazonensis Infection and Contribute to Strain Variation in Susceptibility. J. Immunol. 2006, 177, 3201–3208. [Google Scholar] [CrossRef]
- Tavares, N.; Afonso, L.; Suarez, M.; Ampuero, M.; Prates, D.B.; Araújo-Santos, T.; Barral-Netto, M.; DosReis, G.A.; Borges, V.M.; Brodskyn, C. Degranulating Neutrophils Promote Leukotriene B4 Production by Infected Macrophages To Kill Leishmania amazonensis Parasites. J. Immunol. 2016, 196, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Tavares, N.M.; Araújo-Santos, T.; Afonso, L.; Nogueira, P.M.; Lopes, U.G.; Soares, R.P.; Bozza, P.T.; Bandeira-Melo, C.; Borges, V.M.; Brodskyn, C. Understanding the Mechanisms Controlling Leishmania amazonensis Infection in Vitro: The Role of LTB4 Derived from Human Neutrophils. J. Infect. Dis. 2014, 210, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.; Savio, L.E.; Coutinho-Silva, R. Purinergic Signaling: A New Front-Line Determinant of Resistance and Susceptibility in Leishmaniasis. Biomed. J. 2022, 45, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Majumder, S.; Das, S.; Ghosh, S.; Biswas, S.; Majumdar, S. Leishmania donovani-Induced Prostaglandin E2 Generation Is Critically Dependent on Host Toll-Like Receptor 2-Cytosolic Phospholipase A2 Signaling. Infect. Immun. 2016, 84, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Biswas, A.; Srivastav, S.; Mukherjee, M.; Das, P.K.; Ukil, A. Prostaglandin E2 Negatively Regulates the Production of Inflammatory Cytokines/Chemokines and IL-17 in Visceral Leishmaniasis. J. Immunol. 2014, 193, 2330–2339. [Google Scholar] [CrossRef]
- López-Muñoz, R.A.; Molina-Berríos, A.; Campos-Estrada, C.; Abarca-Sanhueza, P.; Urrutia-Llancaqueo, L.; Peña-Espinoza, M.; Maya, J.D. Inflammatory and Pro-Resolving Lipids in Trypanosomatid Infections: A Key to Understanding Parasite Control. Front. Microbiol. 2018, 9, 1961. [Google Scholar] [CrossRef]
- Penke, L.R.; Sudan, R.; Sathishkumar, S.; Saha, B. Prostaglandin E₂ Receptors Have Differential Effects on Leishmania major Infection. Parasite Immunol. 2013, 35, 51–54. [Google Scholar] [CrossRef]
- Guimarães, E.T.; Santos, L.A.; Ribeiro dos Santos, R.; Teixeira, M.M.; dos Santos, W.L.C.; Soares, M.B.P. Role of Interleukin-4 and Prostaglandin E2 in Leishmania amazonensis Infection of BALB/c Mice. Microbes Infect. 2006, 8, 1219–1226. [Google Scholar] [CrossRef]
- Lima, J.B.; Araújo-Santos, T.; Lázaro-Souza, M.; Carneiro, A.B.; Ibraim, I.C.; Jesus-Santos, F.H.; Luz, N.F.; Pontes, S.d.M.; Entringer, P.F.; Descoteaux, A.; et al. Leishmania infantum Lipophosphoglycan Induced-Prostaglandin E2 Production in Association with PPAR-γ Expression via Activation of Toll like Receptors-1 and 2. Sci. Rep. 2017, 7, 14321. [Google Scholar] [CrossRef]
- Gregory, D.J.; Sladek, R.; Olivier, M.; Matlashewski, G. Comparison of the Effects of Leishmania major or Leishmania donovani Infection on Macrophage Gene Expression. Infect. Immun. 2008, 76, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Colas, R.A.; Ashton, A.W.; Mukherjee, S.; Dalli, J.; Akide-Ndunge, O.B.; Huang, H.; Desruisseaux, M.S.; Guan, F.; Jelicks, L.A.; Matos Dos Santos, F.; et al. Trypanosoma Cruzi Produces the Specialized Proresolving Mediators Resolvin D1, Resolvin D5, and Resolvin E2. Infect. Immun. 2018, 86, e00688-17. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.; Mukherjee, S.; Weiss, L.M.; Tanowitz, H.B.; Ashton, A.W. Bioactive Lipids in Trypanosoma Cruzi Infection. Adv. Parasitol. 2011, 76, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Paloque, L.; Perez-Berezo, T.; Abot, A.; Dalloux-Chioccioli, J.; Bourgeade-Delmas, S.; Le Faouder, P.; Pujo, J.; Teste, M.-A.; François, J.-M.; Schebb, N.H.; et al. Polyunsaturated Fatty Acid Metabolites: Biosynthesis in Leishmania and Role in Parasite/Host Interaction. J. Lipid Res. 2019, 60, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Tavares, V.d.S.; de Castro, M.V.; Souza, R.d.S.O.; Gonçalves, I.K.A.; Lima, J.B.; Borges, V.d.M.; Araújo-Santos, T. Lipid Droplets of Protozoan Parasites: Survival and Pathogenicity. Memórias Do Inst. Oswaldo Cruz 2022, 116, e210270. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Keller, N.P. Co-Opting Oxylipin Signals in Microbial Disease. Cell. Microbiol. 2019, 21, e13025. [Google Scholar] [CrossRef]
- Kubata, B.K.; Duszenko, M.; Kabututu, Z.; Rawer, M.; Szallies, A.; Fujimori, K.; Inui, T.; Nozaki, T.; Yamashita, K.; Horii, T.; et al. Identification of a Novel Prostaglandin f(2alpha) Synthase in Trypanosoma Brucei. J. Exp. Med. 2000, 192, 1327–1338. [Google Scholar] [CrossRef]
- Díaz-Viraqué, F.; Chiribao, M.L.; Trochine, A.; González-Herrera, F.; Castillo, C.; Liempi, A.; Kemmerling, U.; Maya, J.D.; Robello, C. Old Yellow Enzyme from Trypanosoma Cruzi Exhibits In Vivo Prostaglandin F2α Synthase Activity and Has a Key Role in Parasite Infection and Drug Susceptibility. Front. Immunol. 2018, 9, 456. [Google Scholar] [CrossRef]
- Araujo-Santos, T.; Prates, D.B.; Franca-Costa, J.; Luz, N.F.; Andrade, B.B.; Miranda, J.C.; Brodskyn, C.I.; Barral, A.; Bozza, P.T.; Borges, V.M. Prostaglandin E2/Leukotriene B4 Balance Induced by Lutzomyia Longipalpis Saliva Favors Leishmania infantum Infection. Parasites Vectors 2014, 7, 601. [Google Scholar] [CrossRef]
- Alves-Ferreira, E.V.C.; Ferreira, T.R.; Walrad, P.; Kaye, P.M.; Cruz, A.K. Leishmania braziliensis Prostaglandin F2α Synthase Impacts Host Infection. Parasit Vectors 2020, 13, 9. [Google Scholar] [CrossRef]
- Estrada-Figueroa, L.A.; Díaz-Gandarilla, J.A.; Hernández-Ramírez, V.I.; Arrieta-González, M.M.; Osorio-Trujillo, C.; Rosales-Encina, J.L.; Toledo-Leyva, A.; Talamás-Rohana, P. Leishmania mexicana Gp63 Is the Enzyme Responsible for Cyclooxygenase (COX) Activity in This Parasitic Protozoa. Biochimie 2018, 151, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kabututu, Z.; Martin, S.K.; Nozaki, T.; Kawazu, S.; Okada, T.; Munday, C.J.; Duszenko, M.; Lazarus, M.; Thuita, L.W.; Urade, Y.; et al. Prostaglandin Production from Arachidonic Acid and Evidence for a 9,11-Endoperoxide Prostaglandin H2 Reductase in Leishmania . Int. J. Parasitol. 2003, 33, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kubata, B.K.; Duszenko, M.; Martin, K.S.; Urade, Y. Molecular Basis for Prostaglandin Production in Hosts and Parasites. Trends Parasitol. 2007, 23, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Malta-Santos, H.; Andrade, B.B.; Zanette, D.L.; Costa, J.M.; Bozza, P.T.; Bandeira-Melo, C.; Barral, A.; França-Costa, J.; Borges, V.M. Resolvin D1 Drives Establishment of Leishmania amazonensis Infection. Sci. Rep. 2017, 7, 46363. [Google Scholar] [CrossRef]
- Esmaeeli, S.; Hoseinirad, S.M.; Rajabian, M.; Taheri, A.R.; Berenji, F.; Hashemy, S.I. Evaluation of the Oxidant-Antioxidant Balance, Isoprostane and Quantitative CRP in Patients with Cutaneous Leishmaniasis. Microb. Pathog. 2019, 137, 103738. [Google Scholar] [CrossRef]
- Roberts, A.J.; Dunne, J.; Scullion, P.; Norval, S.; Fairlamb, A.H. A Role for Trypanosomatid Aldo-Keto Reductases in Methylglyoxal, Prostaglandin and Isoprostane Metabolism. Biochem. J. 2018, 475, 2593–2610. [Google Scholar] [CrossRef]
- Wyllie, S.; Vickers, T.J.; Fairlamb, A.H. Roles of Trypanothione S-Transferase and Tryparedoxin Peroxidase in Resistance to Antimonials. Antimicrob. Agents Chemother. 2008, 52, 1359–1365. [Google Scholar] [CrossRef]
- Pavli, A.; Maltezou, H.C. Leishmaniasis, an Emerging Infection in Travelers. Int. J. Infect. Dis. 2010, 14, e1032–e1039. [Google Scholar] [CrossRef]
- Polonio, T.; Efferth, T. Leishmaniasis: Drug Resistance and Natural Products (Review). Int. J. Mol. Med. 2008, 22, 277–286. [Google Scholar] [CrossRef]
- Ríos-Marco, P.; Marco, C.; Gálvez, X.; Jiménez-López, J.M.; Carrasco, M.P. Alkylphospholipids: An Update on Molecular Mechanisms and Clinical Relevance. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1657–1667. [Google Scholar] [CrossRef]
- Carnielli, J.B.T.; Crouch, K.; Forrester, S.; Silva, V.C.; Carvalho, S.F.G.; Damasceno, J.D.; Brown, E.; Dickens, N.J.; Costa, D.L.; Costa, C.H.N.; et al. A Leishmania infantum Genetic Marker Associated with Miltefosine Treatment Failure for Visceral Leishmaniasis. EBioMedicine 2018, 36, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Tavangar, P.; Keighobadi, M. An Overview of Azoles Targeting Sterol 14α-Demethylase for Antileishmanial Therapy. Eur. J. Med. Chem. 2017, 135, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulos, P.; Konidas, P.; Durkin-Konidas, M. New World Cutaneous Leishmaniasis: Updated Review of Current and Future Diagnosis and Treatment. J. Am. Acad. Derm. 2010, 63, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.H.; Naidu, T.G.; Drew, J.S.; de Alencar, J.E.; Pearson, R.D. Reciprocal Relationships between Undernutrition and the Parasitic Disease Visceral Leishmaniasis. Rev. Infect. Dis. 1986, 8, 447–453. [Google Scholar] [CrossRef]
- Andrade-Neto, V.V.; Manso, P.P.d.A.; Pereira, M.G.; de Cicco, N.N.T.; Atella, G.C.; Pelajo-Machado, M.; Menna-Barreto, R.F.S.; Torres-Santos, E.C. Host Cholesterol Influences the Activity of Sterol Biosynthesis Inhibitors in Leishmania amazonensis . Memórias Do Inst. Oswaldo Cruz 2022, 117, e220407. [Google Scholar] [CrossRef]
- Pucadyil, T.J.; Chattopadhyay, A. Cholesterol: A Potential Therapeutic Target in Leishmania Infection? Trends Parasitol. 2007, 23, 49–53. [Google Scholar] [CrossRef]
- Pessoa, C.C.; Reis, L.C.; Ramos-Sanchez, E.M.; Orikaza, C.M.; Cortez, C.; de Castro Levatti, E.V.; Badaró, A.C.B.; Yamamoto, J.U.d.S.; D’Almeida, V.; Goto, H.; et al. ATP6V0d2 Controls Leishmania Parasitophorous Vacuole Biogenesis via Cholesterol Homeostasis. PLoS Pathog. 2019, 15, e1007834. [Google Scholar] [CrossRef]
- Rodríguez, N.E.; Lockard, R.D.; Turcotte, E.A.; Araújo-Santos, T.; Bozza, P.T.; Borges, V.M.; Wilson, M.E. Lipid Bodies Accumulation in Leishmania infantum-Infected C57BL/6 Macrophages. Parasite Immunol. 2017, 39, e12443. [Google Scholar] [CrossRef]
- Banerjee, S.; Bose, D.; Das, S.; Chatterjee, N.; Mishra, S.; Das Saha, K. Leishmania donovani Infection Induce Extracellular Signal-Regulated Kinase ½ (ERK½) Mediated Lipid Droplet Generation in Macrophages. Mol. Immunol. 2022, 141, 328–337. [Google Scholar] [CrossRef]
- Rabhi, I.; Rabhi, S.; Ben-Othman, R.; Rasche, A.; Daskalaki, A.; Trentin, B.; Piquemal, D.; Regnault, B.; Descoteaux, A.; Guizani-Tabbane, L.; et al. Transcriptomic Signature of Leishmania Infected Mice Macrophages: A Metabolic Point of View. PLoS Negl. Trop. Dis. 2012, 6, e1763. [Google Scholar] [CrossRef]
- Rabhi, S.; Rabhi, I.; Trentin, B.; Piquemal, D.; Regnault, B.; Goyard, S.; Lang, T.; Descoteaux, A.; Enninga, J.; Guizani-Tabbane, L. Lipid Droplet Formation, Their Localization and Dynamics during Leishmania major Macrophage Infection. PLoS ONE 2016, 11, e0148640. [Google Scholar] [CrossRef]
- Liberopoulos, E.N.; Apostolou, F.; Gazi, I.F.; Kostara, C.; Bairaktari, E.T.; Tselepis, A.D.; Elisaf, M. Visceral Leishmaniasis Is Associated with Marked Changes in Serum Lipid Profile. Eur. J. Clin. Investig. 2014, 44, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.M.; Leal, T.F.; Fiúza, M.C.; Reis, E.a.G.; Souza, M.a.L.; Dos-Santos, W.L.; Pontes-de-Carvalho, L. Plasma Lipoproteins in Visceral Leishmaniasis and Their Effect on Leishmania-Infected Macrophages. Parasite Immunol. 2010, 32, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.S.; Verma, N.; Rabidas, V.N.; Ranjan, A.; Pandey, K.; Verma, R.B.; Singh, D.; Kumar, S.; Das, P. Total Serum Cholesterol Determination Can Provide Understanding of Parasite Burden in Patients with Visceral Leishmaniasis Infection. Clin. Chim. Acta 2010, 411, 2112–2113. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Lal, C.S.; Pandey, K.; Das, V.N.R.; Das, P.; Roychoudhury, K.; Roy, S. Human Visceral Leishmaniasis: Decrease in Serum Cholesterol as a Function of Splenic Parasite Load. Ann. Trop. Med. Parasitol. 2011, 105, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Das, S.; Guha, R.; Ghosh, D.; Naskar, K.; Das, A.; Roy, S. Hyperlipidemia Offers Protection against Leishmania donovani Infection: Role of Membrane Cholesterol. J. Lipid Res. 2012, 53, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, J.; Sen, S.; Guha, R.; Dhar, R.; Ghosh, M.; Datta, S.; Raychaudhury, B.; Naskar, K.; Haldar, A.K.; et al. Designing Therapies against Experimental Visceral Leishmaniasis by Modulating the Membrane Fluidity of Antigen-Presenting Cells. Infect. Immun. 2009, 77, 2330–2342. [Google Scholar] [CrossRef]
- Ghosh, J.; Guha, R.; Das, S.; Roy, S. Liposomal Cholesterol Delivery Activates the Macrophage Innate Immune Arm to Facilitate Intracellular Leishmania donovani Killing. Infect. Immun. 2014, 82, 607–617. [Google Scholar] [CrossRef]
- Lal, C.S.; Verma, R.B.; Verma, N.; Siddiqui, N.A.; Rabidas, V.N.; Pandey, K.; Singh, D.; Kumar, S.; Paswan, R.K.; Kumari, A.; et al. Hypertriglyceridemia: A Possible Diagnostic Marker of Disease Severity in Visceral Leishmaniasis. Infection 2016, 44, 39–45. [Google Scholar] [CrossRef]
- Varela, M.G.; de Oliveira Bezerra, M.; Santana, F.V.; Gomes, M.C.; de Jesus Almeida, P.R.; Silveira da Cruz, G.; de Melo, E.V.; de Oliveira Costa, P.R.; de Oliveira, F.A.; de Jesus, A.R.; et al. Association between Hypertriglyceridemia and Disease Severity in Visceral Leishmaniasis. Am. J. Trop. Med. Hyg. 2021, 106, 643–647. [Google Scholar] [CrossRef]
- Carvalho, M.D.T.; Alonso, D.P.; Vendrame, C.M.V.; Costa, D.L.; Costa, C.H.N.; Werneck, G.L.; Ribolla, P.E.M.; Goto, H. Lipoprotein Lipase and PPAR Alpha Gene Polymorphisms, Increased Very-Low-Density Lipoprotein Levels, and Decreased High-Density Lipoprotein Levels as Risk Markers for the Development of Visceral Leishmaniasis by Leishmania infantum . Mediat. Inflamm. 2014, 2014, 230129. [Google Scholar] [CrossRef] [PubMed]
- Sarnáglia, G.D.; Covre, L.P.; Pereira, F.E.L.; DE Matos Guedes, H.L.; Faria, A.M.C.; Dietze, R.; Rodrigues, R.R.; Maioli, T.U.; Gomes, D.C.O. Diet-Induced Obesity Promotes Systemic Inflammation and Increased Susceptibility to Murine Visceral Leishmaniasis. Parasitology 2016, 143, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.G.; Barrera, R.; Habela, M.A.; Navarrete, I.; Molina, C.; Jiménez, A.; Serrera, J.L. Changes in the Plasma Concentrations of Lipids and Lipoprotein Fractions in Dogs Infected with Leishmania infantum . Vet. Parasitol. 1992, 44, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi Einakchi, M.; Sedaghat Sharifi, N.; Khoshnegah, J.; Heidarpour, M. Canine Visceral Leishmaniosis: The Relationship of Blood Serum Thyroid Hormones, Lipids, and Lipoproteins with Clinical Status. Parasitol. Res. 2018, 117, 3761–3765. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Kottarath, S.K.; Dinda, A.K.; Dube, A.; Sahasrabuddhe, A.A.; Thakur, C.P.; Bhat, M.; Rai, A.K. Preventive as Well as Therapeutic Significances of Linoleic Acid in the Containment of Leishmania donovani Infection. Biochimie 2020, 175, 13–22. [Google Scholar] [CrossRef]
- Schlotz, N.; Ebert, D.; Martin-Creuzburg, D. Dietary Supply with Polyunsaturated Fatty Acids and Resulting Maternal Effects Influence Host--Parasite Interactions. BMC Ecol. 2013, 13, 41. [Google Scholar] [CrossRef]
| L. infantum | L. tropica | L. major | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MON-1 | MON-24 | MON-8 | MON-25 | ||||||
| VL | CanL | CL | CL | CL | |||||
| A | B | C | D | E | F | G | H | I | |
| 14:0 | 1.8 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.6 | 2.6 ± 0.3 | 2.1 ± 0.1 | 1.9 ± 0.2 | 1.7 ± 0.4 | 6.2 ± 0.9a | 6.1 ± 1.0a |
| 16:0 | 5.2 ± 1.9 | 6.4 ± 1.2 | 5.7 ± 1.2 | 5.8 ± 0.6 | 7.2 ± 0.9 | 5.9 ± 2.9 | 6.7 ± 0.4 | 7.0 ± 0.7 | 6.6 ± 0.6 |
| 18:0 | 20.0 ± 1.5 | 20.1 ± 3.9 | 21.5 ± 0.9 | 21.8 ± 1.5 | 25.3 ± 1.6 | 17.9 ± 1.8 | 21.6 ± 4.1 | 18.9 ± 0.6 | 18.8 ± 1.7 |
| 20:0 | 0.5 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1 | 1.2 ± 0.3 | 1.9 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.4 |
| SFAs | 27.5 | 29.3 | 29.9 | 30.9 | 35.0 | 26.9 | 31.9 | 32.5 | 31.9 |
| 16:1n-9 | nd | nd | nd | nd | nd | nd | nd | 0.3 ± 0.1 | 0.3 ± 0.1 |
| 16:1n-7 | 0.5 ± 0.1 | 0.5 ± 0.4 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.4 | 0.8 ± 0.1 | 1.3 ± 0.1 a | 1.2 ± 0.1 a |
| 18:1 n-9 | 23.4 ± 3.3 | 15.3 ± 2.9 | 21.5 ± 1.5 | 15.1 ± 0.6 | 15.8 ± 1.6 | 20.4 ± 1.2 | 22.6 ± 1.2 | 22.6 ± 0.7 | 22.7 ± 1.0 |
| 18:1n-7 | 1.5 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.1 | 2.4 ± 0.2 a | 2.3 ± 0.2 a |
| MUFAs | 25.4 | 17.5 | 23.9 | 17.2 | 18.1 | 22.4 | 25.1 | 26.6 | 26.5 |
| 18:2n-6 | 21.2 ± 1.7 | 20.8 ± 3.4 | 19.1 ± 1.1 | 22.9 ± 1.2 | 19.5 ± 0.6 | 26.5 ± 3.4 | 14.6 ± 4.0 | 25.7 ± 0.4 | 27.9 ± 1.8 |
| 18:3n-6 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.2 | 1.3 ± 0.3 a | 1.2 ± 0.2 a |
| 20:2n-6 | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.2 ± 0.1 | 1.7 ± 0.1 | 1.2 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.5 ± 0.1 a | 0.6 ± 0.1 a |
| 20:3n-6 | 0.9 ± 0.3 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.4 ± 0.1 | 0.8 ± 0.3 | 1.0 ± 0.2 | 0.7 ± 0.1 | 1.5 ± 0.3 | 1.3 ± 0.2 |
| 20:4 n-6 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.1 |
| Tot n-6 | 24.2 | 25.0 | 23.0 | 26.8 | 22.5 | 30.1 | 17.6 | 29.6 | 31.6 |
| 18:3n-3 | 11.2 ± 2.3 | 11.1 ± 0.8 | 9.4 ± 0.8 | 11.2 ± 0.7 | 7.6 ± 1.5 | 9.5 ± 1.4 | 10.0 ± 1.9 | 4.5 ± 1.0 a | 3.9 ± 0.6 a |
| 20:3n-3 | 1.7 ± 0.3 | 2.3 ± 0.2 | 1.4 ± 0.1 | 2.2 ± 0.2 | 1.5 ± 0.3 | 1.0 ± 0.4 | 1.9 ± 0.3 | 0.2 ± 0.1 a | 0.2 ± 0.1 a |
| 20:5n-3 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 22:5n-3 | 2.1 ± 0.4 | 2.7 ± 0.1 | 2.7 ± 0.3 | 2.1 ± 0.1 | 5.1 ± 0.9 | 1.3 ± 0.3 | 2.5 ± 0.9 | 2.3 ± 0.4 | 2.0 ± 0.3 |
| 22:6n-3 | 7.8 ± 0.7 | 12.0 ± 1.0 | 9.5 ± 1.6 | 9.4 ± 0.6 | 10.1 ± 1.0 | 8.6 ± 2.6 | 11.1 ± 1.6 | 4.2 ± 0.3 a | 3.8 ± 0.5 a |
| Tot n-3 | 22.8 | 28.1 | 23.0 | 24.9 | 24.3 | 20.4 | 25.5 | 11.2 | 9.9 |
| n-3/n-6 | 0.9 | 1.1 | 1.0 | 0.9 | 1.1 | 0.7 | 1.4 | 0.4 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroux, M.; Luquain-Costaz, C.; Lawton, P.; Azzouz-Maache, S.; Delton, I. Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis? Int. J. Mol. Sci. 2023, 24, 4702. https://doi.org/10.3390/ijms24054702
Leroux M, Luquain-Costaz C, Lawton P, Azzouz-Maache S, Delton I. Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis? International Journal of Molecular Sciences. 2023; 24(5):4702. https://doi.org/10.3390/ijms24054702
Chicago/Turabian StyleLeroux, Marine, Céline Luquain-Costaz, Philippe Lawton, Samira Azzouz-Maache, and Isabelle Delton. 2023. "Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis?" International Journal of Molecular Sciences 24, no. 5: 4702. https://doi.org/10.3390/ijms24054702
APA StyleLeroux, M., Luquain-Costaz, C., Lawton, P., Azzouz-Maache, S., & Delton, I. (2023). Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis? International Journal of Molecular Sciences, 24(5), 4702. https://doi.org/10.3390/ijms24054702





