1. Introduction
Antimicrobial resistance is a worldwide issue associated with high morbidity and mortality. Conventional antimicrobials are no longer able to overcome bacterial pathogens with a multidrug-resistant profile, resulting in difficult-to-treat or even untreatable infections [
1]. The insufficiency of effective drugs and inefficient prevention measures have engaged prescribers and researchers in the development of novel treatment possibilities and alternative antimicrobial therapies [
1].
In this context, medicinal plants can play an important role in the discovery of new natural bioactive compounds with antimicrobial activity. For thousands of years, plants have been the primary source of compounds with biological activities. Medicinal and aromatic plants have been widely used for the treatment and the prevention of various human diseases, such as cardiovascular, neurodegenerative, and hepatic diseases, skin disorders, and bacterial and viral infections [
2,
3,
4,
5,
6]. In the last few decades, the research on natural products of plant origin has been almost fully replaced by drug design and combinatorial chemistry due to the time- and resource-consuming procedures that are required for the isolation, identification, and development of a product with pharmaceutical applications [
7]. However, while chemical synthesis creates random molecules, plant secondary metabolites are produced as a result of an adaptation process and might be more efficient as therapeutic molecules in the battle against multidrug-resistant pathogens [
7].
These bioactive compounds can also strongly affect the plant-associated microbiome and its physiological traits. It has been shown that the composition and structure of the microbial communities of the roots and the rhizosphere microenvironments change in response to the production of specific root exudates secreted by the plant as a result of microorganisms exposure [
8,
9,
10]. Moreover, there is evidence of the different distribution of bacterial communities inside the internal tissues of the same medicinal plants, suggesting the existence of selective forces able to compartmentalize the microbes into specific microenvironments, which have been attributed to antagonistic interactions between microorganisms and their specific adaptation to the plant’s secondary metabolites [
11,
12,
13,
14,
15]. Large-scale comparative genomic analyses also revealed that plant-associated bacteria have gained specific metabolic attributes which allow their adaptation to plant anatomical parts [
16]. However, how secondary metabolites influence the relative abundance of a particular bacterial genus/species/strain and model the microbial community remains unclear.
A large number of medicinal plants have been characterized for their pharmacological properties and applications, but members of their microbiota are also able to synthesize bioactive molecules, both soluble and volatile ones [
11,
14,
17,
18,
19]. Plants rely on their microbiome for specific traits, including nutrient acquisition, growth promotion, induced systemic resistance, and tolerance to abiotic stress factors [
20]. Endophytes are considered an almost untapped source of natural compounds with potential therapeutical properties. Egamberdieva et al. [
21] showed that plants whose extracts exhibited the highest antibacterial properties also hosted bacterial endophytes able to exert a comparable action toward bacterial pathogens. This confirms the evolutionary adaptation of endophytes to the plant microenvironments and identifies medicinal plants as an excellent starting point to isolate microorganisms able to produce biologically active molecules.
Among these bacteria, strains belonging to the genera
Arthrobacter, Gram-positive bacteria of the family Micrococcaceae, are commonly found associated with the plant’s microenvironments: they are regular inhabitants of the rhizosphere [
22,
23], but they have also been isolated from the surface [
24] and the inner tissues [
25] of plants. Bacteria of the
Arthrobacter genus often establish a beneficial relationship with their host plants through various mechanisms of action. Many
Arthrobacter strains fulfill a plant growth-promoting activity, mostly due to the production of indole-3-acetic acid and siderophores, which is able to increase the solubility of various minerals and enhance nutrient acquisition [
22,
23,
26]. In addition, many works focused on the interesting ability of
Arthrobacter species to degrade organic and inorganic compounds which they utilize as substrates for their metabolism, thus making
Arthrobacter genus a promising tool for plant-based bioremediation [
24,
27]. The
Arthrobacter genus can be defined as a metabolically versatile group of bacteria, but their role as antimicrobial secondary metabolite producers has not been fully explored. Some plant-associated
Arthrobacter strains, however, were able to antagonize both plant and human pathogens [
28,
29,
30]. Taken together, these features highlight the importance of
Arthrobacter strains from an ecological perspective and call attention to the possible biotechnological and therapeutical application of this bacterial genus.
In a recent work on
Origanum vulgare L., the culturable endophytic microbiota isolated from different anatomical parts of the plant was characterized through molecular and phenotypic analysis, revealing a high degree of biodiversity at the strain level, different antibiotic resistance phenotypes, and the ability, for some strains, to antagonize the growth of bacteria isolated from the same or different
O. vulgare microenvironments and some human pathogens [
14]. Among others,
Arthrobacter sp. OVS8, isolated from the stem of
O. vulgare L., was able to inhibit the growth of various endophytes isolated from different compartments of the plant. Moreover, it efficiently inhibited the growth of ten members of the
Burkholderia cepacia complex [
14]. The antibacterial activity was attributed to the production of volatile organic compounds (VOCs): it has been demonstrated that some
O. vulgare endophytes, including
Arthrobacter sp. OVS8, synthesize a plethora of VOCs, some of which belong to the content of
O. vulgare essential oil (EO), opening the possibility that (at least) some of the bioactive compounds of the plant EO might be synthesized by the microbiota itself [
19].
A better understanding of the pathways and genes involved in the biosynthesis of endophytic secondary metabolites and of the role of such compounds in the interplay between the plant and its associated microbial communities should be strongly encouraged to unravel the mechanisms implied in the plant–microbiota interactions and so enable the prediction of endophytes’ capacity to synthesize novel bioactive secondary metabolites and potential antibacterial drug candidates [
31]. Thus, this work aims to delineate molecular and phenotypic features of
Arthrobacter sp. OVS8 from different viewpoints in order to evaluate its adaptation and influence on the plant internal microenvironments and its biotechnological potential as a producer of antibacterial volatile molecules.
3. Discussion
The lack of new compounds able to combat the current issues of antibiotic resistance, along with the emergence of multidrug-resistant pathogens and the continued presence of untreatable diseases, currently represent a challenge for the scientific community in the study of new and effective molecules with antibacterial activity. In fact, only a small fraction of the antibiotics approved over the past 40 years represent new compound classes, while the majority are derived from already known chemical structures, with the most recent new class of antibiotics being discovered during the 1980s [
34]. Over the last few decades, and more recently over the SARS-COVID19 pandemic, there has been a remarkable resurgence of interest in natural product research to both prevent the toxicity effects of some medical products and to discover new antibiotic-producing strains [
35]. When speaking about natural products, traditional medicine, also known as alternative or complementary medicine, cannot be ignored. The herbs, plants or formulas used in traditional medicine contain a plethora of phytochemicals that function alone or in combination with one another to produce a pharmacological effect. Indeed, many plant-originated drugs were discovered from traditional medicine knowledge, and it has been demonstrated that many of them were discovered thanks to their application in such practices [
36].
The renewed interest in medicinal plants and their bioactive secondary metabolites has also put the spotlight on the complex and multifaced world of bacterial endophytes and their intimate and intricate relationship with their hosts. There is evidence of endophytes adapting to the plant microenvironments and their direct or indirect contribution to plant secondary metabolites [
37,
38,
39], so one may ask (i) if the phytochemicals obtained from medicinal plants could be a result of the bacterial metabolisms, and (ii) if such bioactive compounds could be directly obtained from endophytes.
In this work, we performed a genomic, molecular, and phenotypic characterization of
Arthrobacter sp. OVS8, a promising bacterial endophyte isolated from the medicinal plant
O. vulgare L. [
14]. In particular, we focused on specific features of the strain: its adaptation to the plant microenvironment and essential oil and its ability to inhibit the growth of some multidrug-resistant bacterial pathogens through the emission of VOCs.
O. vulgare essential oil is widely known for its antimicrobial potential [
40]; thus, it can be imagined that bacterial endophytes inhabiting the inner microenvironment of such plants might be adapted to the bioactive volatile molecules of which the plant tissues are rich in [
12], becoming resistant to them or using them as a carbon and energy source. Shimasaki et al. revealed that tobacco roots, a microenvironment abounding in nicotine and santhopine, were enriched in
Arthrobacter strains with the catabolic capacity to detoxify or utilize them as nutrients, further supporting the relevance of plant secondary metabolites in the shaping of the plant’s microbial community with specific metabolic competences [
41]. The diesel fuel-degrading potential test revealed the ability of
Arthrobacter sp. OVS8 to grow in the presence of diesel fuel as the only carbon and energy source. The GC/MS analysis of the essential oil hydro-distilled from the same
O. vulgare plant from which the endophyte was isolated revealed that the major chemical groups consisted of sesquiterpene hydrocarbons (73.5%), monoterpene hydrocarbons (17.6%), oxygenated sesquiterpenes (4.8%), and 3.7% oxygenated monoterpenes (4.8%) [
14]. The ability of the endophyte to grow in the presence of diesel fuel could suggest its adaptation to the hydrocarbon components of
O. vulgare essential oil, leading to the hypothesis that its composition might represent one of the factors involved in the plant–endophytes symbiosis [
13]. For this reason, we set up another experiment using β-caryophyllene, a bicyclic sesquiterpene representing 19.2% of
O. vulgare essential oil, as the only carbon and energy source. Sesquiterpenes are a class of terpenes that consist of three isoprene units.
Arthrobacter strains isolated from the under-canopy soil of the isoprene-emitting
Tectona grandis and
Madhuca latifolia trees had a high isoprene tolerance and a great degrading potential toward the compound [
42]. Our results suggest that β-caryophyllene does not represent a carbon or energy source for
Arthrobacter sp. OVS8. Moreover, according to Ponce et al., the strain can be classified as extremely sensitive (inhibition zone diameter ≥ 20 mm) to β-caryophyllene (100%) [
43]. As the strain was isolated from the stem of
O. vulgare, it can be hypothesized that the content and composition of essential oil from flowers, leaves and stems differ among anatomical parts [
44] or that the endophyte is not intimately associated with plant organs and cells responsible for the production of the essential oil [
45]. The hydrocarbon-degrading potential of the endophytes could be then associated with an adaptive mechanism through which the endophyte takes advantage of the abundant long chain aliphatic compounds that constitute the plant tissues, as hypothesized for epiphytic bacteria [
46].
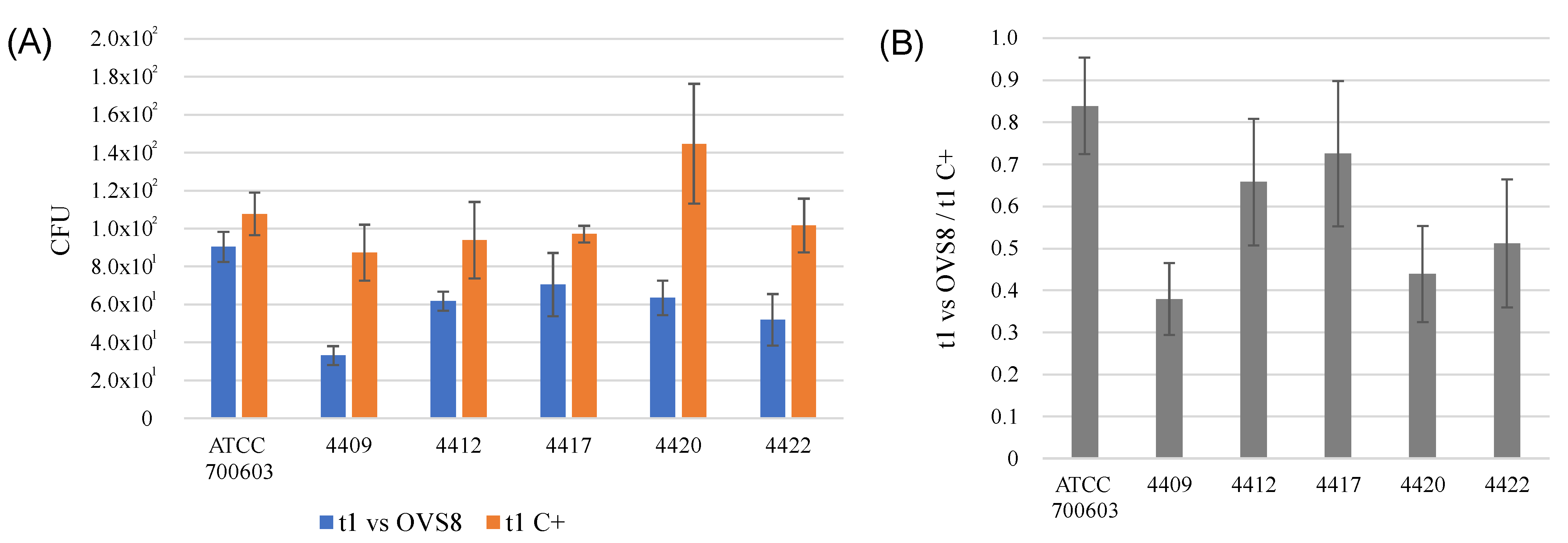
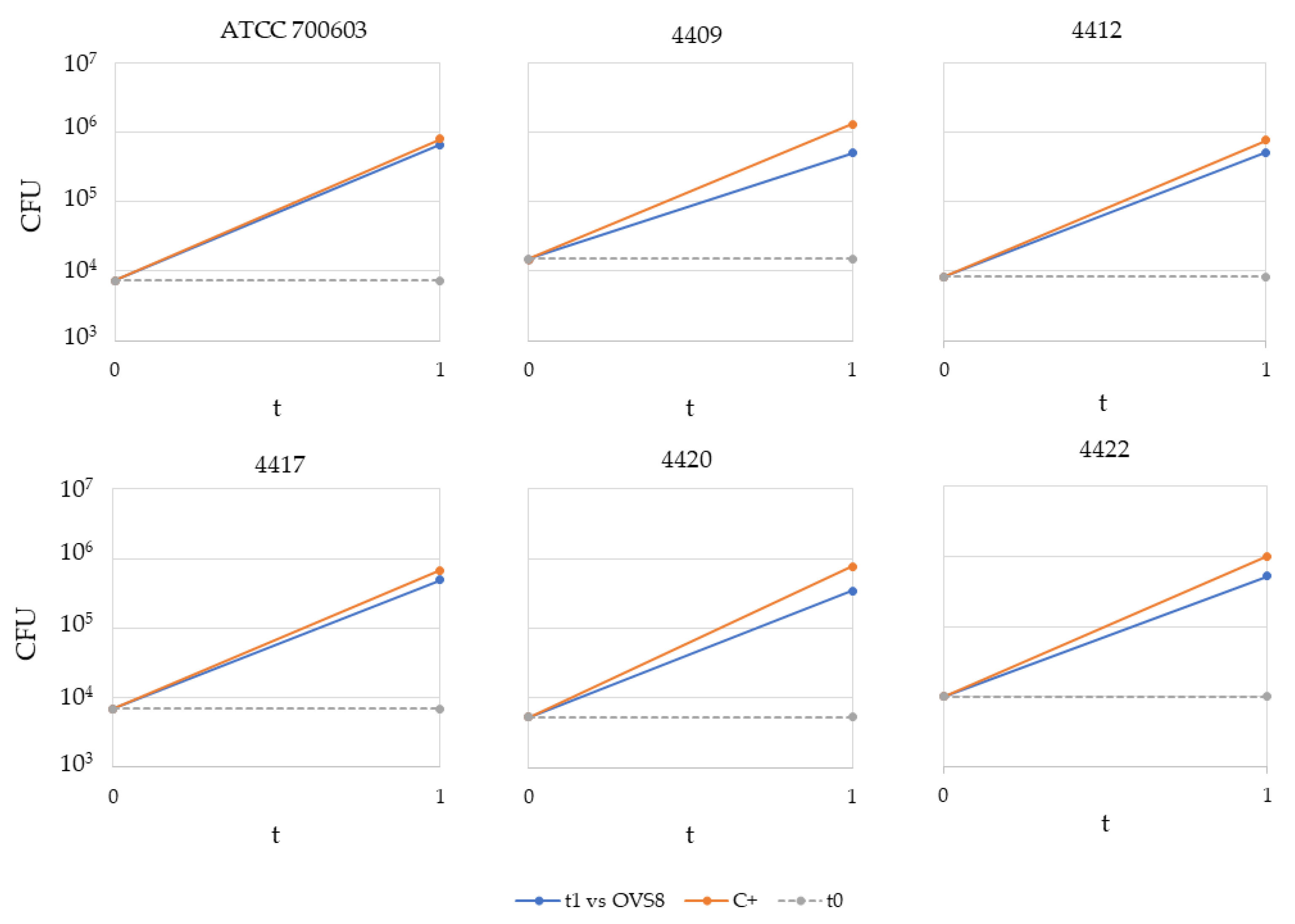
Arthrobacter sp. OVS8 was selected amongst the collection of endophytes isolated from
O. vulgare because of its antibacterial activity against 10 members of the
Burkholderia cepacia complex (Bcc), opportunistic pathogens able to cause severe infections in immunocompromised patients, such as cystic fibrosis patients. This activity was attributed also to the production of VOCs, which induced a bactericidal effect toward 7 out of 10 Bcc target strains, most of which were of clinical origin [
19]. The endophyte’s emitted VOCs were tested against a panel of 36 multidrug-resistant human pathogens through the cross-streaking method. Data obtained revealed that target strains used in this work were much more resistant than Bcc strains, and only
K. pneumoniae strains’ growth seemed to be affected by
Arthrobacter sp. OVS8 VOCs. There is still little evidence on the antibacterial metabolites produced by
Arthrobacter species, and most of them refer to strains isolated from the Antarctic or marine environments [
47,
48]. Further strategies for the identification of new antibiotics produced by unexplored targets (such as bacterial endophytes associated with medicinal plants) are required to ensure the availability of effective drugs and overcome the issue of antimicrobial resistance.
The genome of
Arthrobacter sp. OVS8 has a total length of 4,175,013 bp and a GC content of 67.14%, which reflects the characteristic high GC content of the genus. Secondary metabolite biosynthetic gene clusters (BGCs) analysis predicted a desferrioxamine BGC, a NRPS-independent siderophore, with a 66% similarity to the desferrioxamine BGC from
Streptomyces argillaceus [
49]. The production of desferrioxamine is quite common for species belonging to the
Streptomyces genus, and it benefits the plant–microorganism interaction by being able to promote plant growth, alleviate oxidative stress, and promote the solubilization of iron and other metals [
50,
51,
52]. Siderophore-producing bacteria play a crucial role in plants’ survival and growth, enhancing metal bioavailability in the rhizosphere [
53]. Siderophore production has also been reported for the genus
Arthrobacter. A high siderophore-yielding
Arthrobacter strain isolated from the wild grass
Dichanthium annulatum colonizing an abandoned mine efficiently solubilized iron and increased iron-stress resilience in iron-deficiency-sensitive maize [
26]. Moreover, the naturally occurring rhizosphere bacteria
Arthrobacter oxydans releases a variety of desferrioxamine-like compounds that induce direct plant growth-promoting effects and increase the mobility and solubility of a great variety of metals and minerals through the formation of soluble element-organic complexes that can move toward the plant roots [
23]. Hence, the presence of the desferrioxamine BGC in the
Arthrobacter sp. OVS8 genome could suggest a putative plant-growth promoting role of the endophyte in facilitating
O. vulgare nutrient acquisition.
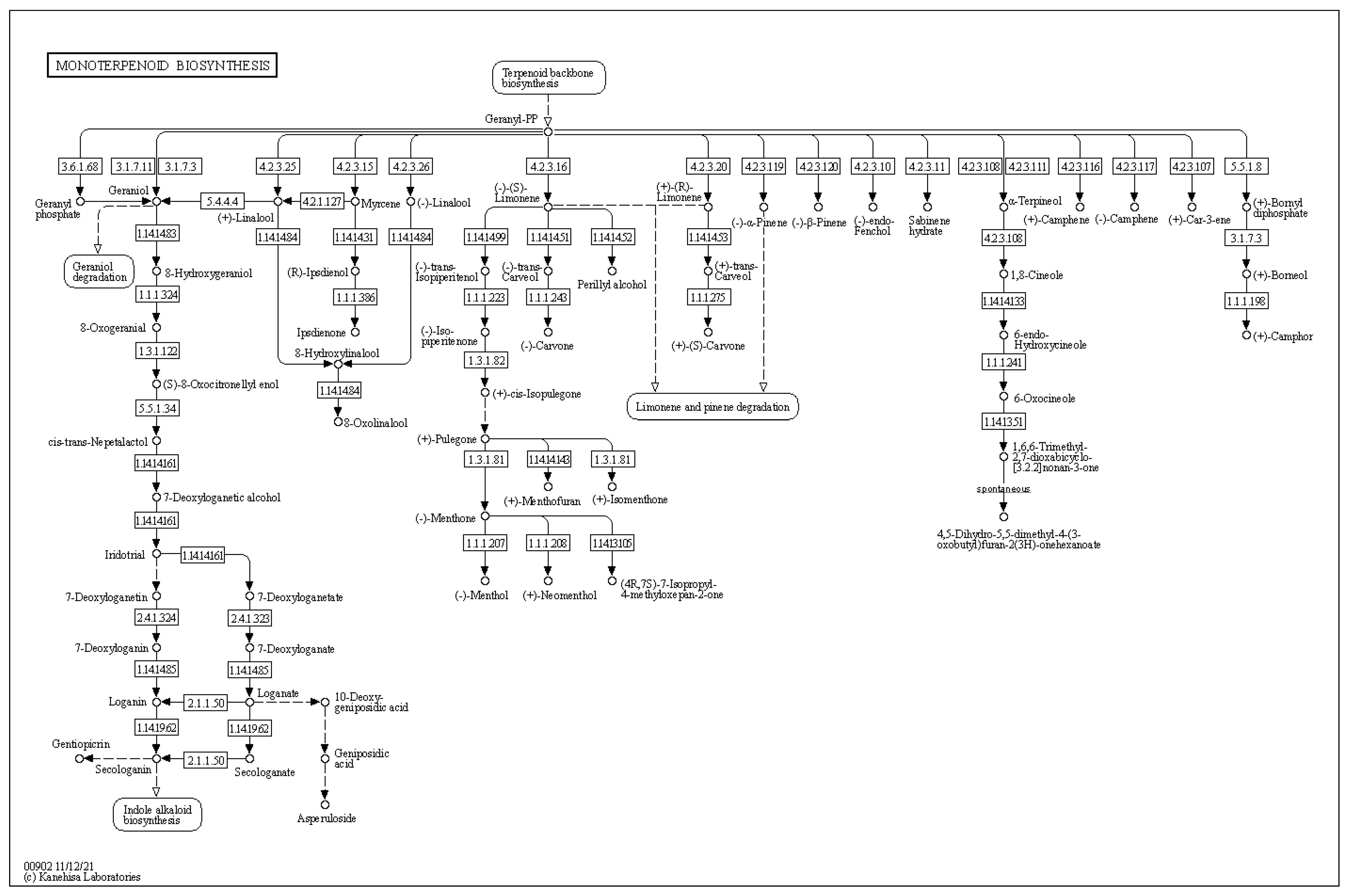
Given the previous data regarding the ability of
Arthrobacter sp. OVS8-emitted VOCs to induce a bactericidal or bacteriostatic effect against some clinical and environmental Bcc isolates, we focused on the metabolic pathways involved in the production of such VOCs; in particular, to three monoterpenes also found in
O. vulgare essential oil composition, i.e., α-pinene, p-cymene, and γ-terpinene [
19]. In nature, we can find different terpene carbon skeletons. Such abundance is attributed to the large number of the enzyme class of terpene synthases and to their ability to convert the same substrate to multiple products [
33]. Even though there is a solid degree of amino acid sequence similarity among plant and fungi monoterpene synthases, this similarity is based more on taxonomic affinities of the plant species rather than the type of compound formed. The best recognized structural motif of the terpene synthase family is an aspartate-rich region, [D/N)DXX(D/E) or DDXXXE], located within 80–120 (bacteria and fungi) or 230–270 aa (plants) of the N terminus [
33,
54]. However, the discovery and biochemical characterization of bacterial terpene synthases represent a great challenge because, unlike plant and fungal enzymes, bacterial terpene synthases do not exhibit an overall amino acid sequence similarity to those from plants and fungi and display a relatively low degree of sequence similarity to other known bacterial terpene synthases [
54]. Through the use of hidden Markov models and protein family database searching methods, various structurally diverse bacterial terpene synthases were identified; most of these were sesquiterpene synthases widely distributed amongst Gram-negative bacteria and the
Actinomycetales order, as the geosmin producer
Streptomyces genus [
54,
55]. The genome analysis of
Arthrobacter sp. OVS8 revealed the presence of the metabolic pathway leading to the synthesis of geranyl diphosphate, the common precursor of most of the cyclic monoterpenes; however, no monoterpene biosynthetic pathways were highlighted. Given the fact that the endophyte actually produces monoterpenes [
19], the absence of the identification of the latter pathway could be attributed to the high diversity existing between the amino acid sequence of plant and bacteria terpene synthases and the lack of bacterial terpene synthase sequences from the
Arthrobacter genus.
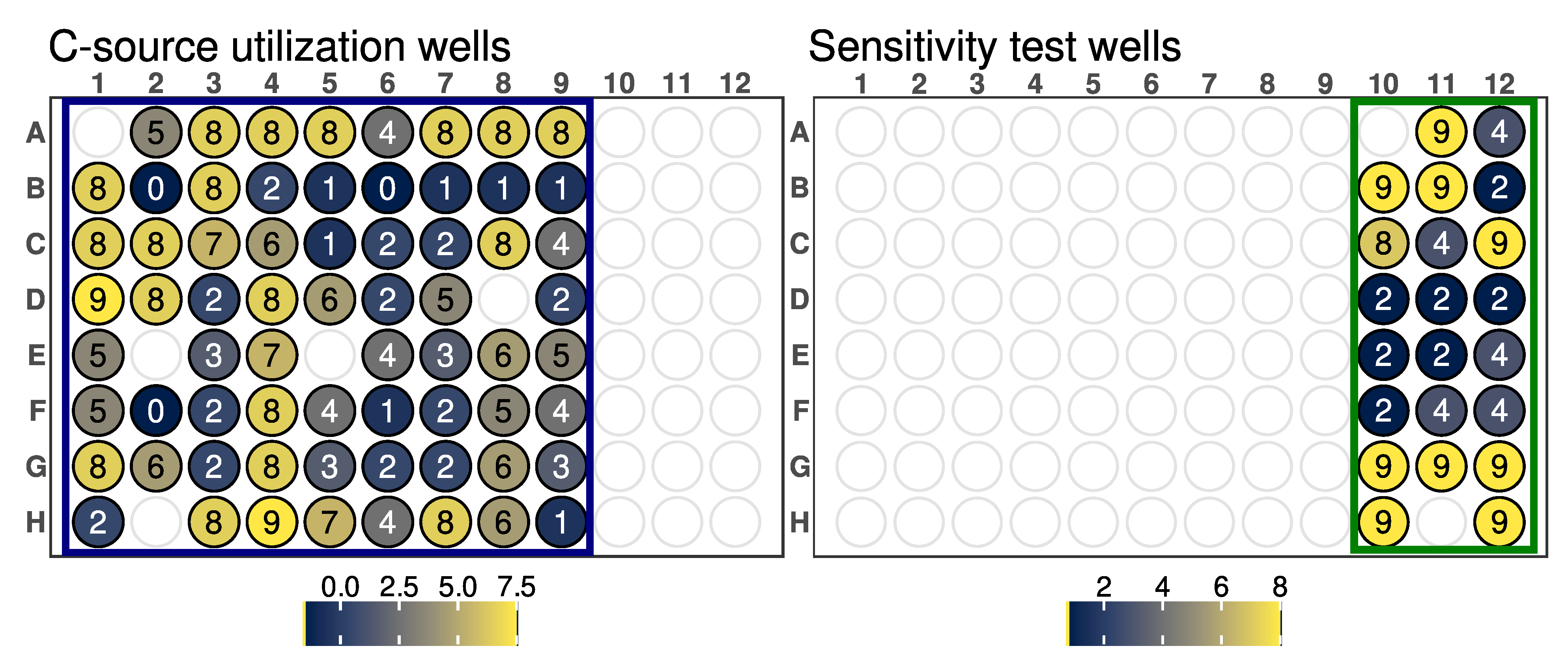
GEN III microplates dissect and analyze the ability of strains to metabolize all major classes of compounds and determine other important physiological properties such as pH, salt, and lactic acid tolerance, reducing power, and chemical sensitivity. It could be assumed that strains metabolizing a wide range of substrates and showing tolerance to high salinity concentrations and acidic pH could be more adapted to changing environmental conditions.
Arthrobacter sp. OVS8 was able to grow in a medium containing up to 4% NaCl and a pH of 6, suggesting its ability to tolerate mild changes in abiotic stress factors. The strain showed a high resistance toward 10 out of 23 available chemical sensitivity assay compounds such as D-serine, which exerts its antimicrobial activity by replacing D-alanine residues of the peptidoglycan rigid envelope surrounding the cytoplasmic membrane of bacterial species [
56]. Resistance to sodium lactate, a salt of lactic acid widely used as a food preservative able to lower water activity [
57], and to lithium chloride, reported to induce hyperosmotic stress [
58], could indicate the endophyte’s potential to adapt to changes in water availability and high saline concentration. Potassium tellurite toxicity is due to its ability to act as a strong oxidizing agent over a variety of cell components [
59].
Arthrobacter sp. OVS8 growth in the presence of such compounds could hint at the endophyte’s ability to remove toxic tellurite from polluted environments [
60]. Lastly,
Arthrobacter sp. OVS8 resistance to sodium bromate, which mainly originates when ozonation is adopted to treat bromide (Br−)-containing water, could suggest its role as a potential candidate for bioremediation processes [
61]. On the contrary, the strain did not grow in the presence of the disinfectant polyhexamethylene guanidine hydrochloride, which disrupts the cellular envelope causing leakage of the cytoplasmic content [
62], and the surfactant Niaproof 4.
Arthrobacter sp. OVS8 was sensitive to most of the antibiotics present in GEN III plates: the protein synthesis inhibitors fusidic acid, troleandomycin, rifamycin, minocycline, and lincomicyn, as well as vancomycin, which hampers proper cell wall synthesis in Gram-positive bacteria, did not permit the growth of the endophytic strain. As a Gram-positive bacterium,
Arthrobacter sp. OVS8 showed resistance in the presence of nalidixic acid and aztreonam, antimicrobial molecules more effective toward Gram-negative strains [
63,
64].