Effects of Ascorbic Acid on Physiological Characteristics during Somatic Embryogenesis of Fraxinus mandshurica
Abstract
1. Introduction
2. Results
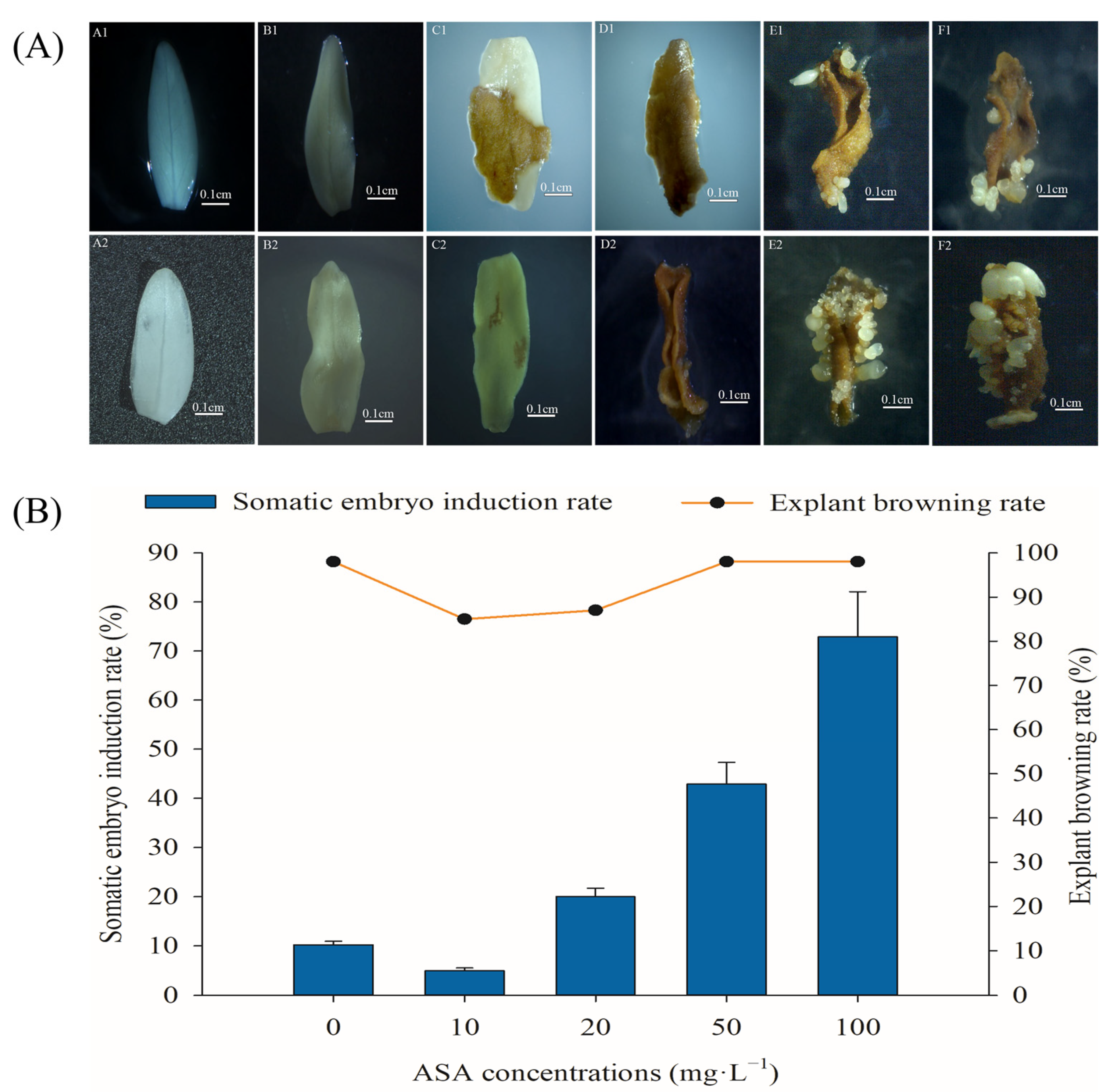
2.1. Effects of Exogenous ASA on Somatic Embryogenesis and Explant Browning of F. mandshurica
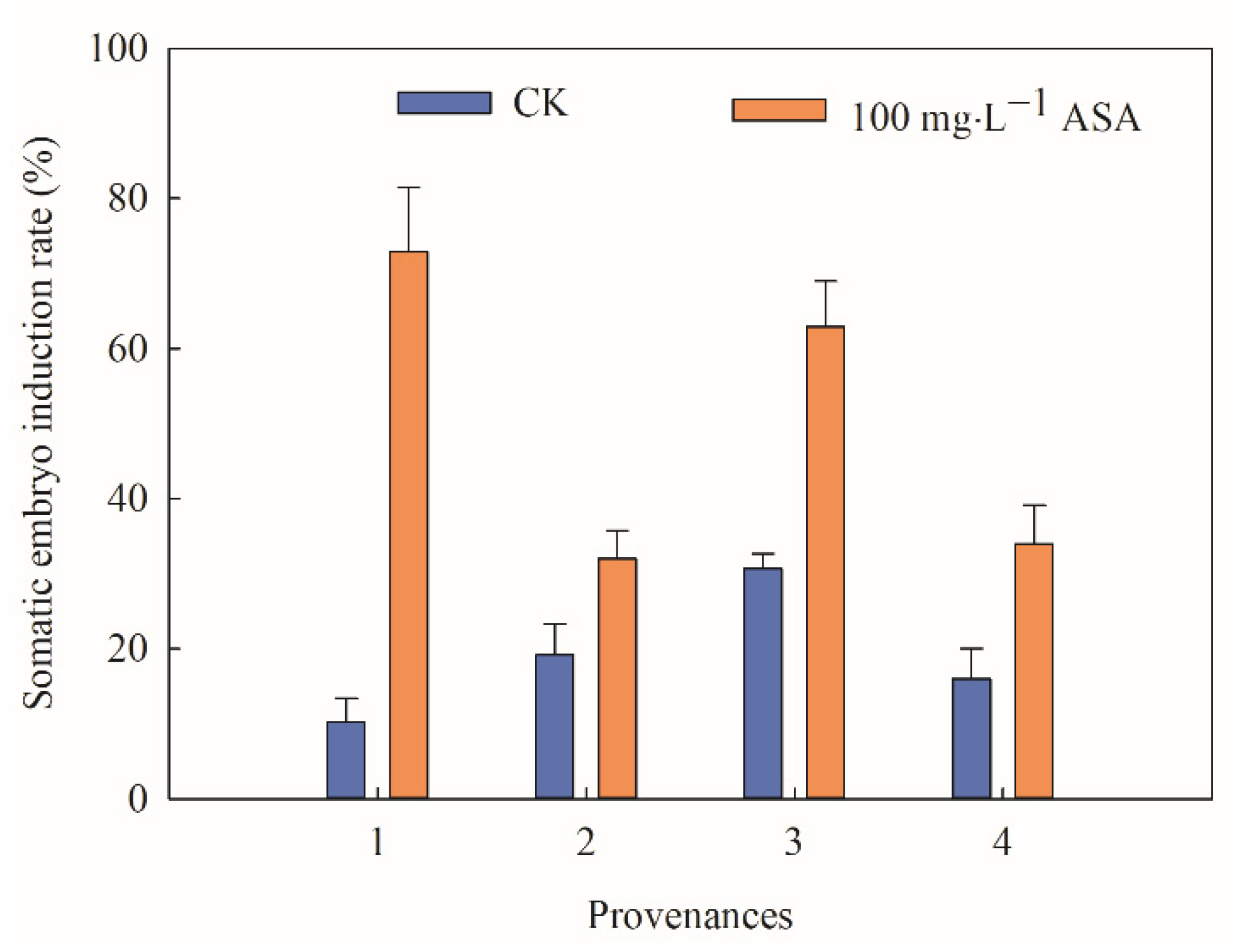
2.2. Effect of 100 mg·L−1 ASA on Somatic Embryogenesis of Heterogenous F. mandshurica
2.3. Effects of Exogenous ASA on Browning of Explants during Somatic Embryogenesis of F. mandshurica
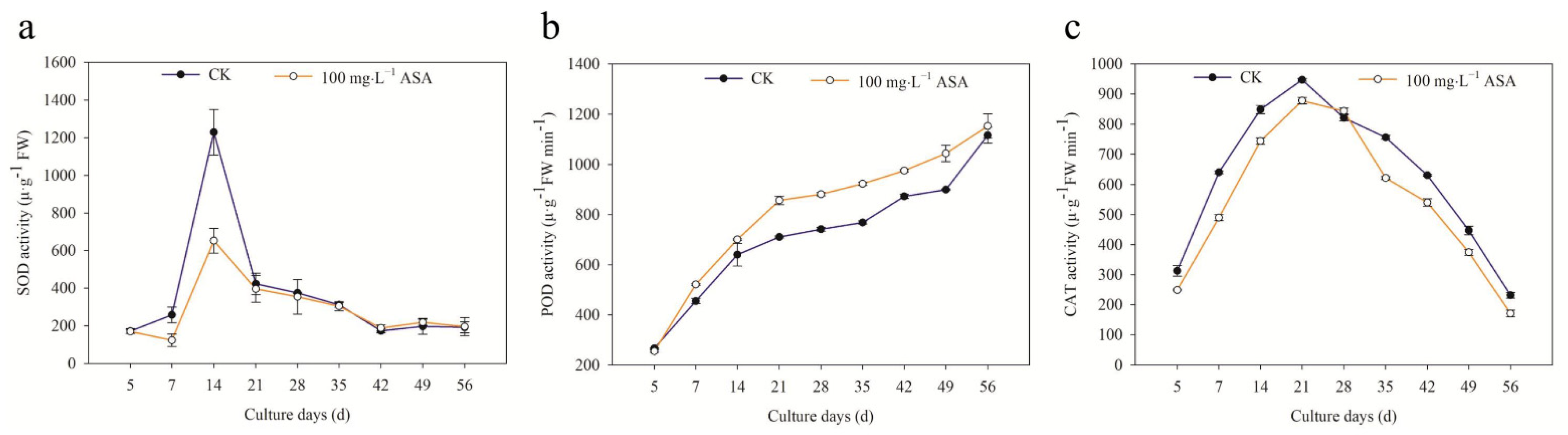
2.4. Effects of Exogenous ASA on Activities of Antioxidant Enzymes during Somatic Embryogenesis of F. mandshurica
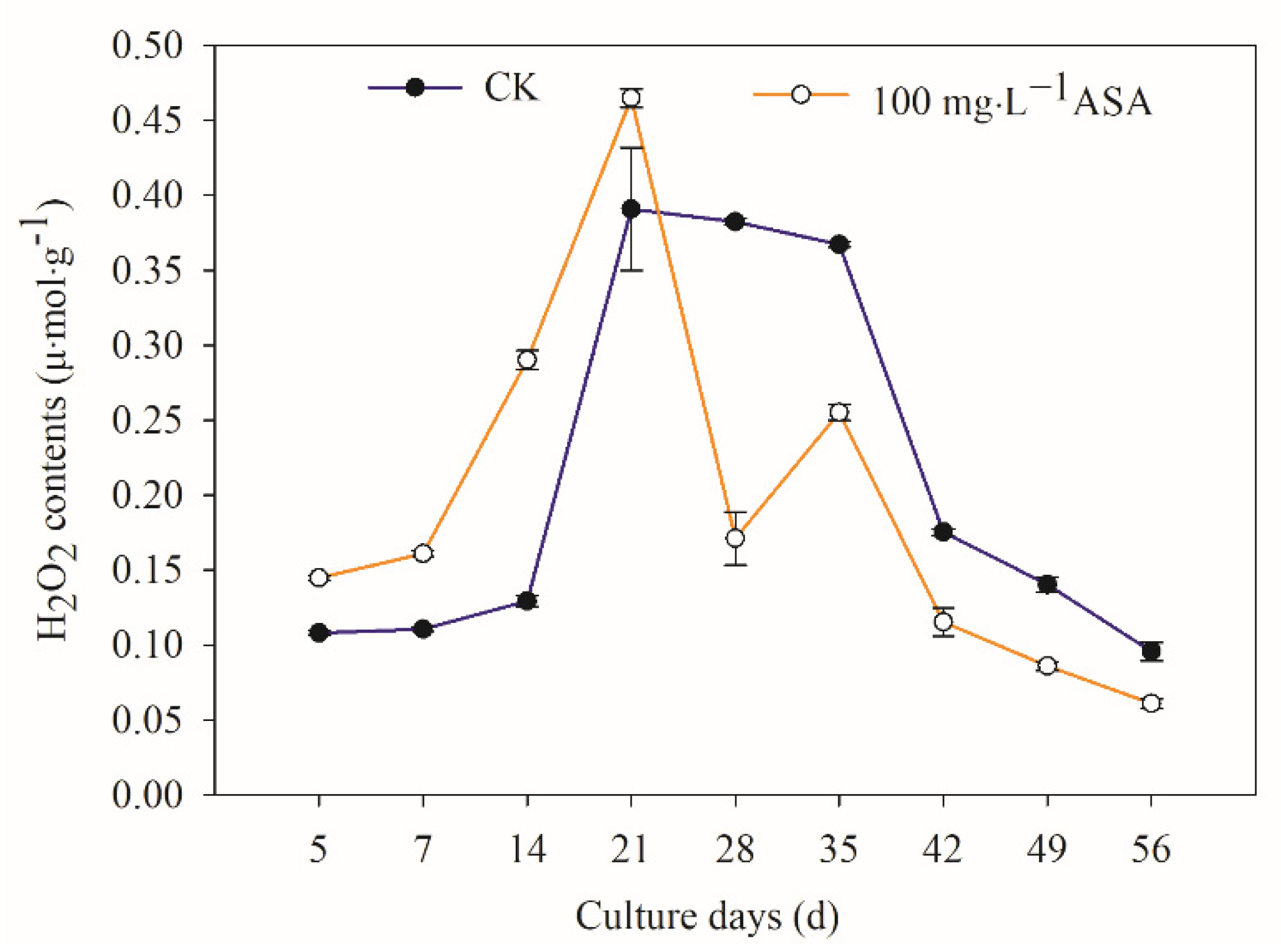
2.5. Effects of Exogenous ASA on Intracellular H2O2 during Somatic Embryogenesis of F. mandshurica
2.6. Effects of Exogenous ASA on Intracellular MDA in Explants during Somatic Embryogenesis of F. mandshurica
2.7. Effects of Exogenous ASA on Intracellular NO Synthesis and Metabolism during Somatic Embryogenesis of F. mandshurica
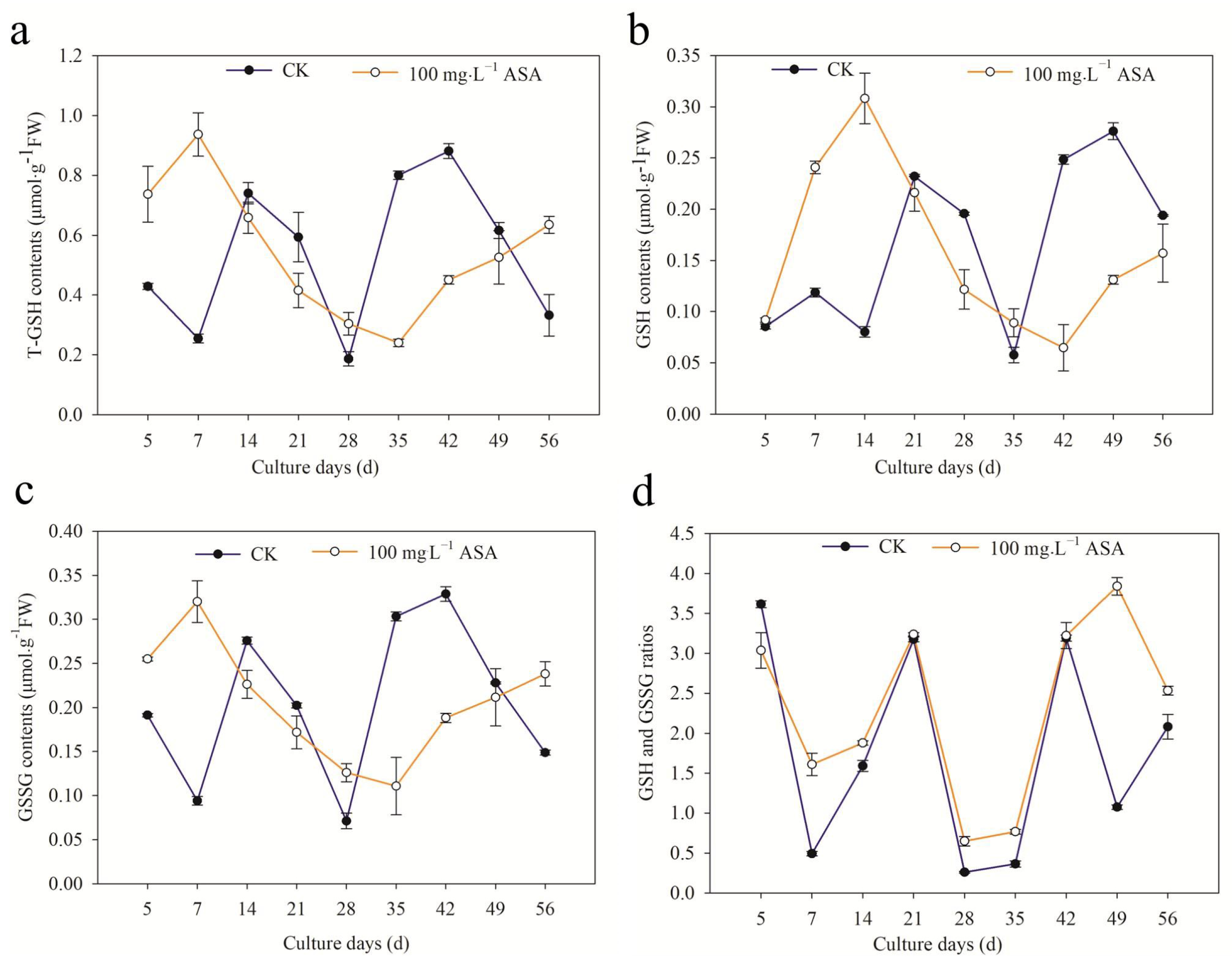
2.8. Effects of Exogenous ASA on ASA and GSH Content during Somatic Embryogenesis of F. mandshurica
2.9. Effects of Exogenous ASA on GSH Content during Somatic Embryogenesis of F. mandshurica
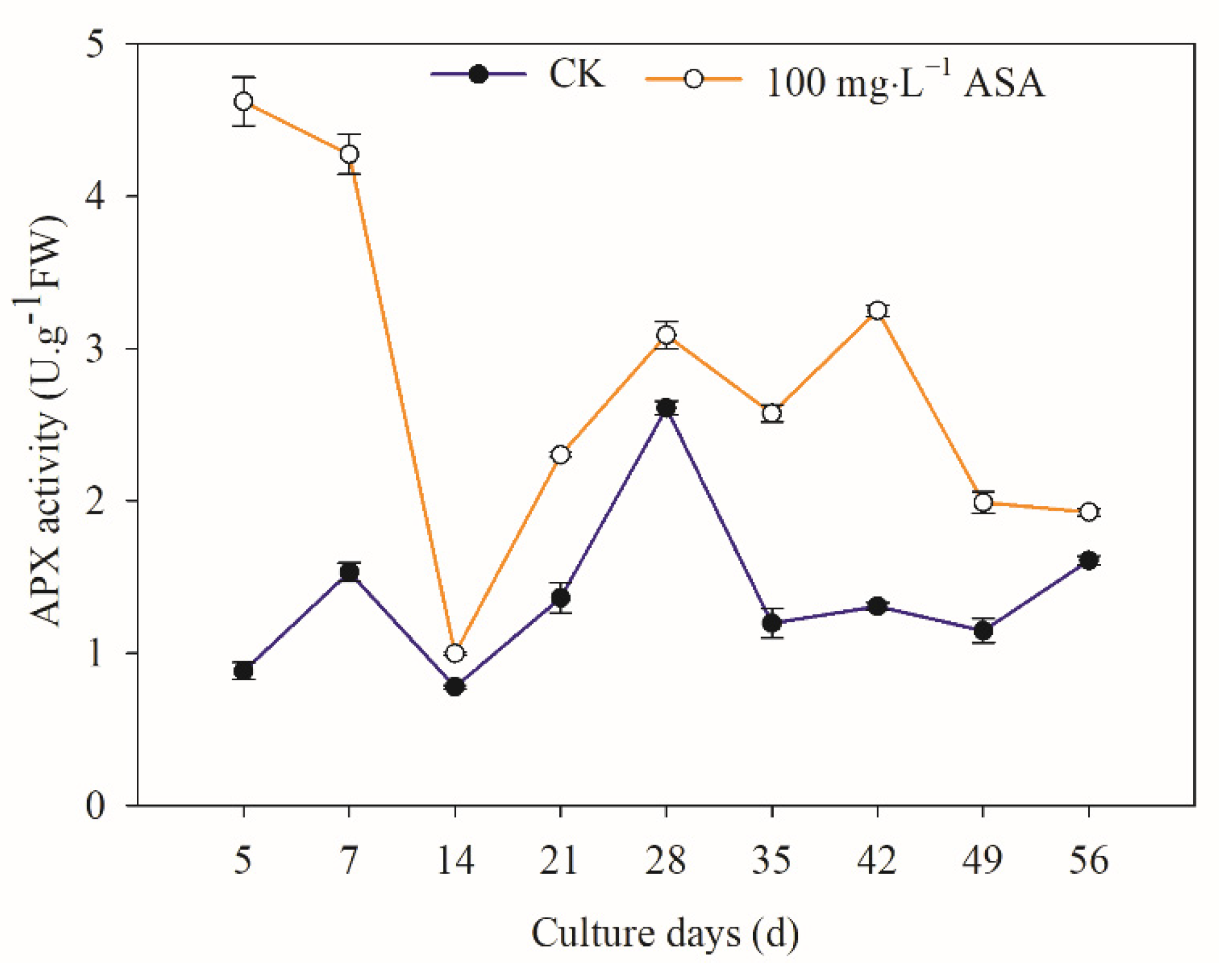
2.10. Effects of Exogenous ASA on APX Activity in Explants during Somatic Embryogenesis of F. mandshurica
3. Discussion
3.1. Exogenous ASA Could Synchronously Promote Explant Browning and Somatic Embryogenesis of F. mandshurica
3.2. Exogenous ASA Showed Diverse Patterns on Antioxidant Enzymes in Explants of F. mandshurica
3.3. Exogenous ASA Has Regulation Function for Reducing Oxidative Damage in Explants of F. mandshurica
3.4. Exogenous ASA Increase NO Content and NR Activity of F. mandshurica Explants
3.5. Exogenous ASA on ASA and GSH Content and APX Activity in F. mandshurica Explants
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of Explants of F. mandshurica
4.3. Induction of Somatic Embryogenesis
4.4. Determination Indicators and Methods
4.5. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.F.; Wrobel-Marek, W.; Heidmann, I.; Horstman, A.; Chen, B.J.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol. 2022, 188, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, A.M.; Wójcikowska, B.; Gaj, M.D. Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int. J. Mol. Sci. 2020, 21, 1333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chao, Y.; Han, L. Uncovering a phenomenon of active hormone transcriptional regulation during early somatic embryogenesis in Medicago sativa. Int. J. Mol. Sci. 2022, 23, 8633. [Google Scholar] [CrossRef] [PubMed]
- Pais, M.S. Somatic embryogenesis induction in woody species: The future after OMICs data assessment. Front. Plant Sci. 2019, 10, 240. [Google Scholar] [CrossRef]
- Guan, Y.; Li, S.G.; Fan, X.F.; Su, Z.H. Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016, 7, 938. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.M.; Shen, H.L.; Feng, D.D.; Zhang, L.J. Cytological investigation of somatic and zygotic embryogenesis of Fraxinus mandshurica. Sci. Silvae Sin. 2006, 42, 130–133. (In Chinese) [Google Scholar]
- Kong, D.M.; Preece, J.E.; Shen, H.L. Somatic embryogenesis in immature cotyledons of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tissue Organ Cult. 2012, 108, 485–492. [Google Scholar] [CrossRef]
- Yang, L.; Bian, L.; Shen, H.L.; Li, Y.H. Somatic embryogenesis and plantlet regeneration from mature zygotic embryos of Manchurian ash (Fraxinus mandshurica Rupr.). Plant Cell Tissue Organ Cult. 2013, 115, 115–125. [Google Scholar] [CrossRef]
- Shen, H.L.; Wang, Q.S.; Liu, Y.; Liu, C.P.; Yang, L.; Zhang, P. Effects of BA and NAA on explant browning and SE from immature zygotic cotyledon of Fraxinus mandshurica. Mol. Plant Breed. 2020, 18, 1266–1273. (In Chinese) [Google Scholar]
- Pinto, G.; Silva, S.; Park, Y.S.; Neves, L.; Arau’jo, C.; Santos, C. Factors influencing somatic embryogenesis induction in Eucalyptus globulus Labill.: Basal medium and anti-browning agents. Plant Cell Tissue Organ Cult. 2008, 95, 79–88. [Google Scholar] [CrossRef]
- Liu, C.P.; Yang, L.; Shen, H.L. Proteomic Analysis of Immature Fraxinus mandshurica Cotyledon Tissues during Somatic Embryogenesis: Effects of Explant Browning on Somatic Embryogenesis. Int. J. Mol. Sci. 2015, 16, 13692–13713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Liu, C.P.; Shen, H.L.; Yang, L.; Liu, Y.; Xie, T.Y.; Chen, X.; Zhang, P. Effects of anti-browning agents on explant browning and somatic embryogenesis from mature zygotic cotyledons of Fraxinus mandshurica Rupr. J. Northeast For. Univ. 2019, 47, 32–37. (In Chinese) [Google Scholar]
- Xie, T.Y.; Chen, X.; Wang, Q.S.; Yang, L.; Shen, H.L. Proper concentration of ascorbic acid for regulating browning and somatic embryogenesis of Fraxinus mandshurica mature zygotic embryo explants from different sources. Mol. Plant Breed. 2021, 1–11. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20210621.1107.004.html (accessed on 3 November 2022). (In Chinese).
- Noctor, G.; Foyer, C.H. ASCORBATE AND GLUTATHIONE: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, X.L.; Niu, J.P. Effects of exogenous ascorbic acid on seed germination and seedling salt-tolerance of alfalfa. PLoS ONE 2021, 16, e0250926. [Google Scholar]
- Azizi, F.; Farsaraei, S.; Moghaddam, M. Application of exogenous ascorbic acid modifies growth and pigment content of Calendula officinalis L. flower heads of plants exposed to NaCl stress. J. Soil Sci. Plant Nutr. 2021, 21, 2803–2814. [Google Scholar] [CrossRef]
- Zhou, X.H.; Li, K.Z.; Zhao, Z.; Zhang, X.L.; Cheng, X.; Feng, Q. Effects of exogenous ascorbic acid on physiological indexes in rice under Aluminum stress. Chin. J. Trop. Crops 2021, 42, 769–776. (In Chinese) [Google Scholar]
- Irshad, M.; Rizwan, H.M.; Debnath, B.; Anwar, M.; Li, M.; Liu, S.; He, B.Z.; Qiu, D.L. Ascorbic acid controls lethal browning and pluronic F-68 promotes high-frequency multiple shoot regeneration from cotyledonary node explant of Okra (Abelmoschus esculentus L.). HortScience 2018, 53, 183–190. [Google Scholar] [CrossRef]
- Song, M.B.; Wu, S.J.; Shuai, L.; Duan, Z.H.; Chen, Z.L.; Shang, F.F.; Fang, F. Effects of exogenous ascorbic acid and ferulic acid on the yellowing of fresh-cut Chinese water chestnut. Postharvest Biol. Technol. 2019, 148, 15–21. [Google Scholar] [CrossRef]
- Vanden Abeele, C.; Raes, K.; Sampers, I. Effect of mild heat treatment on browning-related parameters in fresh-cut Iceberg lettuce. J. Food Biochem. 2019, 43, e12906. [Google Scholar] [CrossRef] [PubMed]
- Massolo, J.F.; Concellón, A.; Chaves, A.R.; Vicente, A.R. 1-Methylcyclopropene (1-MCP) delays senescence, maintains quality and reduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharvest Biol. Technol. 2011, 59, 10–15. [Google Scholar] [CrossRef]
- Liao, L.Y.; Ling, Y.S.; Liu, Y.F.; Duan, Z.H.; Song, M.B.; Shuai, L. Effects of different anti-browning agents treatments on browning of fresh-cut arrowhead during cold storage. Food Ind. 2020, 41, 195–199. (In Chinese) [Google Scholar]
- Chen, T.F.; Zheng, W.J.; Wong, Y.S.; Yang, F. Selenium-induced changes in activities of antioxidant enzymes and content of photosynthetic pigments in Spirulina platensis. J. Integr. Plant Biol. 2008, 50, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Dong, L. Effect of exogenous ascorbic acid on longan fresh fruit preservation. J. Zhejiang Agri. Sci. 2012, 11, 1557–1562. (In Chinese) [Google Scholar]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Wang, R.L.; Liu, S.H.; Zhou, F.; Ding, C.X.; Hua, C. Exogenous ascorbic acid and glutathione alleviate oxidative stress induced by salt stress in the chloroplasts of Oryza sativa L. J. Biosci. 2014, 69, 226–236. [Google Scholar] [CrossRef]
- Lamotte, O.; Courtois, C.; Barnavon, L.; Pugin, A.; Wendehenne, D. Nitric oxide in plants: The biosynthesis and cell signalling properties of a fascinating molecule. Planta 2005, 221, 1–4. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.N.; Zhang, D.Y.; Wei, C.; Shen, H.L. Effect of plant growth regulators and osmoticums on somatic embryogenesis of Fraxinus mandshurica Rupr. Bull. Bot. Res. 2017, 37, 682–689. (In Chinese) [Google Scholar]
- Hernández, J.A.; Jiménez, A.; Mullineaux, P.; Sevilia, F. Tolerance of pea (Pisum sativum L. ) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous Spermidine Alleviates Low Temperature Injury in Mung Bean (Vigna radiata L.) Seedlings by Modulating Ascorbate-Glutathione and Glyoxalase Pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Shigeoka, S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Zhang, M.; Li, M.J.; Shao, J.H.; Ma, F.W. Influence of exogenous ascorbic acid on postharvest ripening and AsA-GSH cycles in apple fruit. J. Yunnan Agri. Univ. 2012, 27, 384–390. (In Chinese) [Google Scholar]
- Liu, C.P. Physiology Mechanism and Differential Protein of Fraxinus mandshurica Somatic Embrygenesis Acompanied Explant Browning. Master’s Thesis, Northeast Forestry University, Harbin, China, 2009. (In Chinese). [Google Scholar]
- Cui, X.M. Physiological and Biochemical Status of Browned Explants in Somatic Embryogenesis of Fraxinus mandshurica and Syringare reticulata var. mandshurica. Master’s Thesis, Northeast Forestry University, Harbin, China, 2015. (In Chinese). [Google Scholar]
- Wang, J. Optimization of Technology and Function Analysis of H2O2 and NO in Fraxinus mandshurica Somatic Embryogenesis. Master’s Thesis, Northeast Forestry University, Harbin, China, 2015. (In Chinese). [Google Scholar]
- Liu, H.N. Mechanism of Hydrogen Peroxide and Nitric Oxide Regulating Somatic Embryogenesis in Fraxinus mandshurica. Master’s Thesis, Northeast Forestry University, Harbin, China, 2017. (In Chinese). [Google Scholar]
- Kampfenkel, K.; Montagu, M.V.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Xie, T.; Yang, L.; Shen, H. Effects of Ascorbic Acid on Physiological Characteristics during Somatic Embryogenesis of Fraxinus mandshurica. Int. J. Mol. Sci. 2023, 24, 289. https://doi.org/10.3390/ijms24010289
Cheng X, Xie T, Yang L, Shen H. Effects of Ascorbic Acid on Physiological Characteristics during Somatic Embryogenesis of Fraxinus mandshurica. International Journal of Molecular Sciences. 2023; 24(1):289. https://doi.org/10.3390/ijms24010289
Chicago/Turabian StyleCheng, Xue, Tianyi Xie, Ling Yang, and Hailong Shen. 2023. "Effects of Ascorbic Acid on Physiological Characteristics during Somatic Embryogenesis of Fraxinus mandshurica" International Journal of Molecular Sciences 24, no. 1: 289. https://doi.org/10.3390/ijms24010289
APA StyleCheng, X., Xie, T., Yang, L., & Shen, H. (2023). Effects of Ascorbic Acid on Physiological Characteristics during Somatic Embryogenesis of Fraxinus mandshurica. International Journal of Molecular Sciences, 24(1), 289. https://doi.org/10.3390/ijms24010289





