Study on Anti-Constipation Effects of Hemerocallis citrina Baroni through a Novel Strategy of Network Pharmacology Screening
Abstract
1. Introduction
2. Results
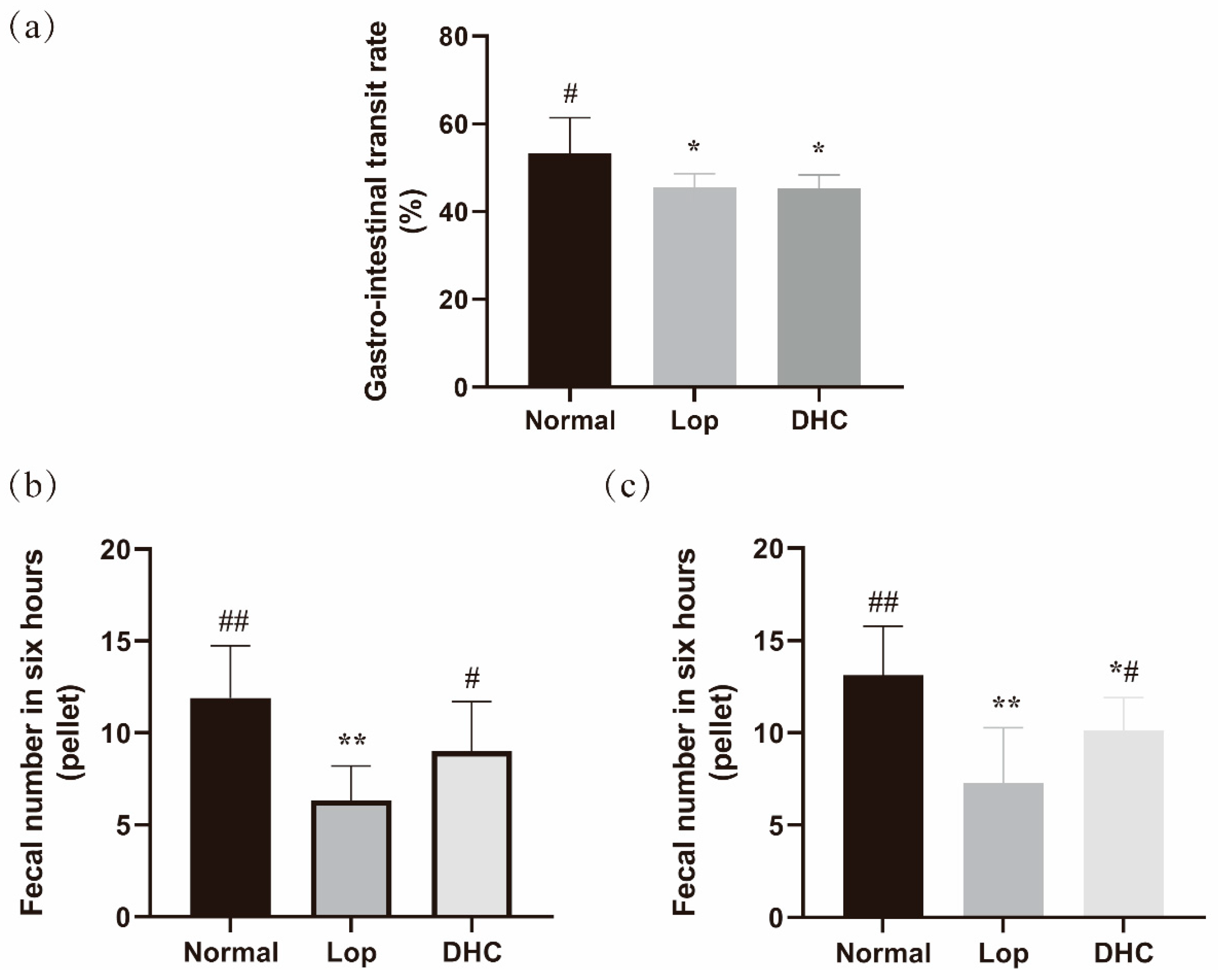
2.1. Anti-Constipation Effect of DHC
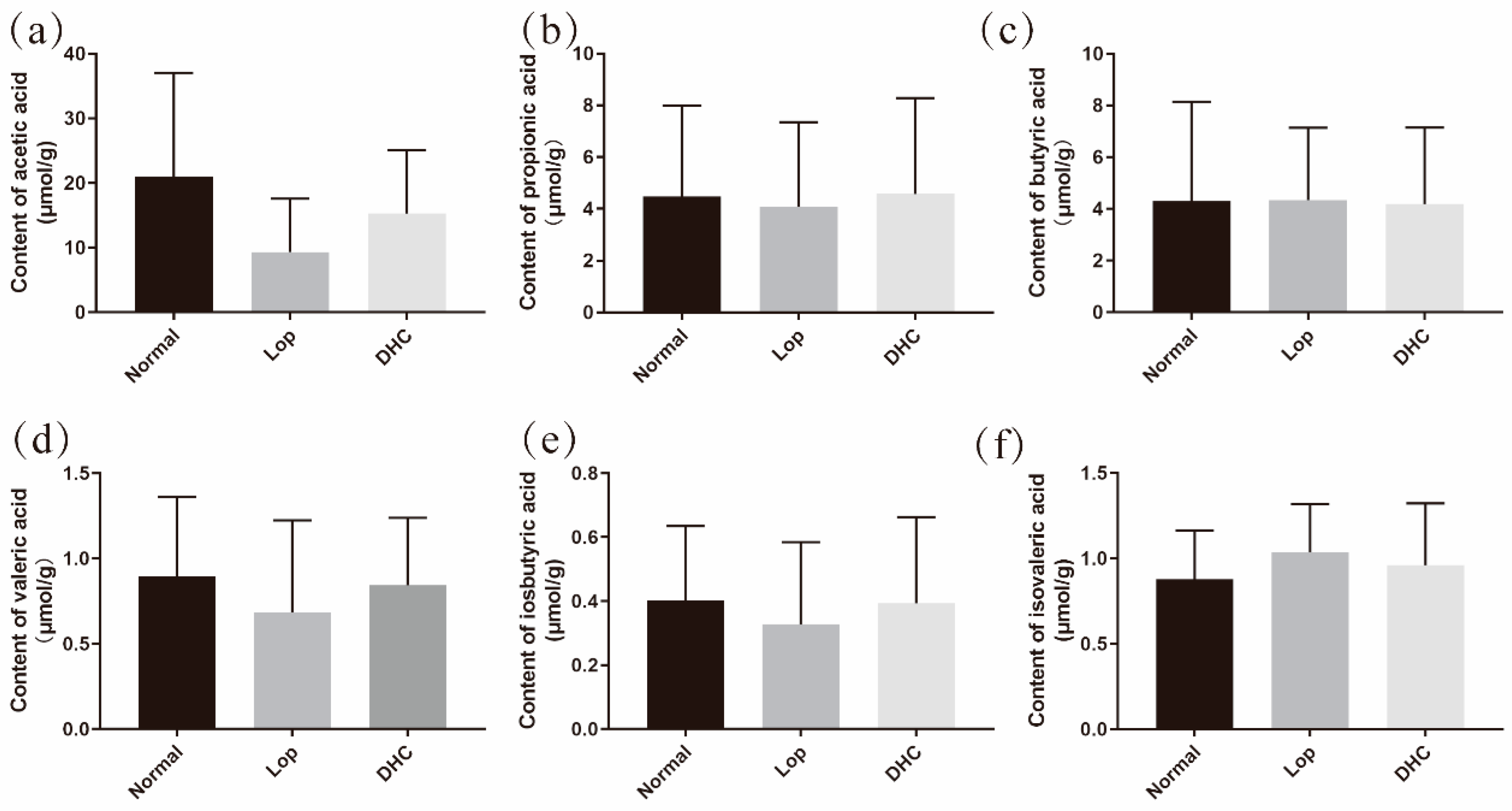
2.2. Effect of DHC on the Content of Short-Chain Organic Acids
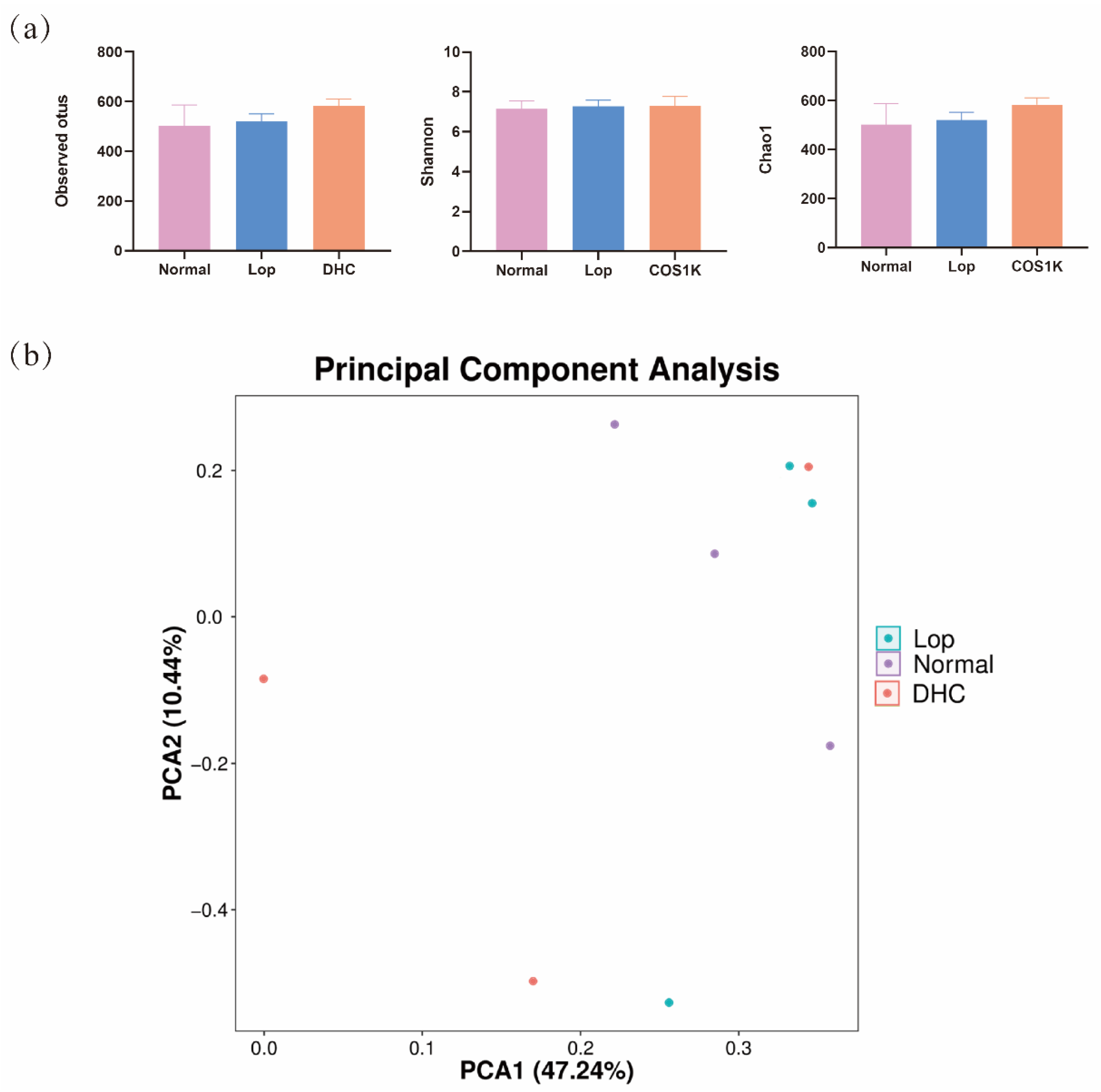
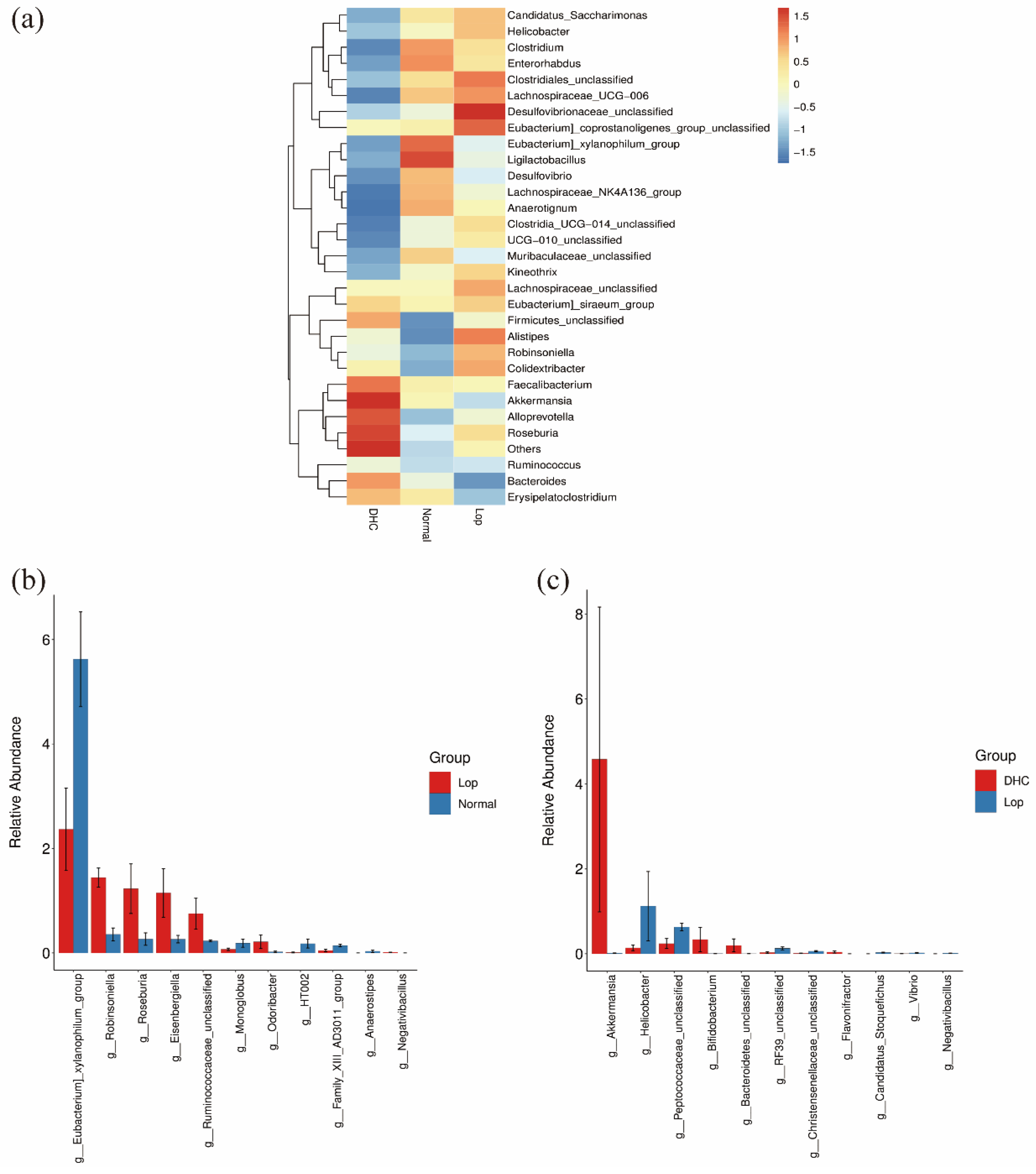
2.3. Effect of DHC on Gut Microbiota
2.4. Network Pharmacology Strategy
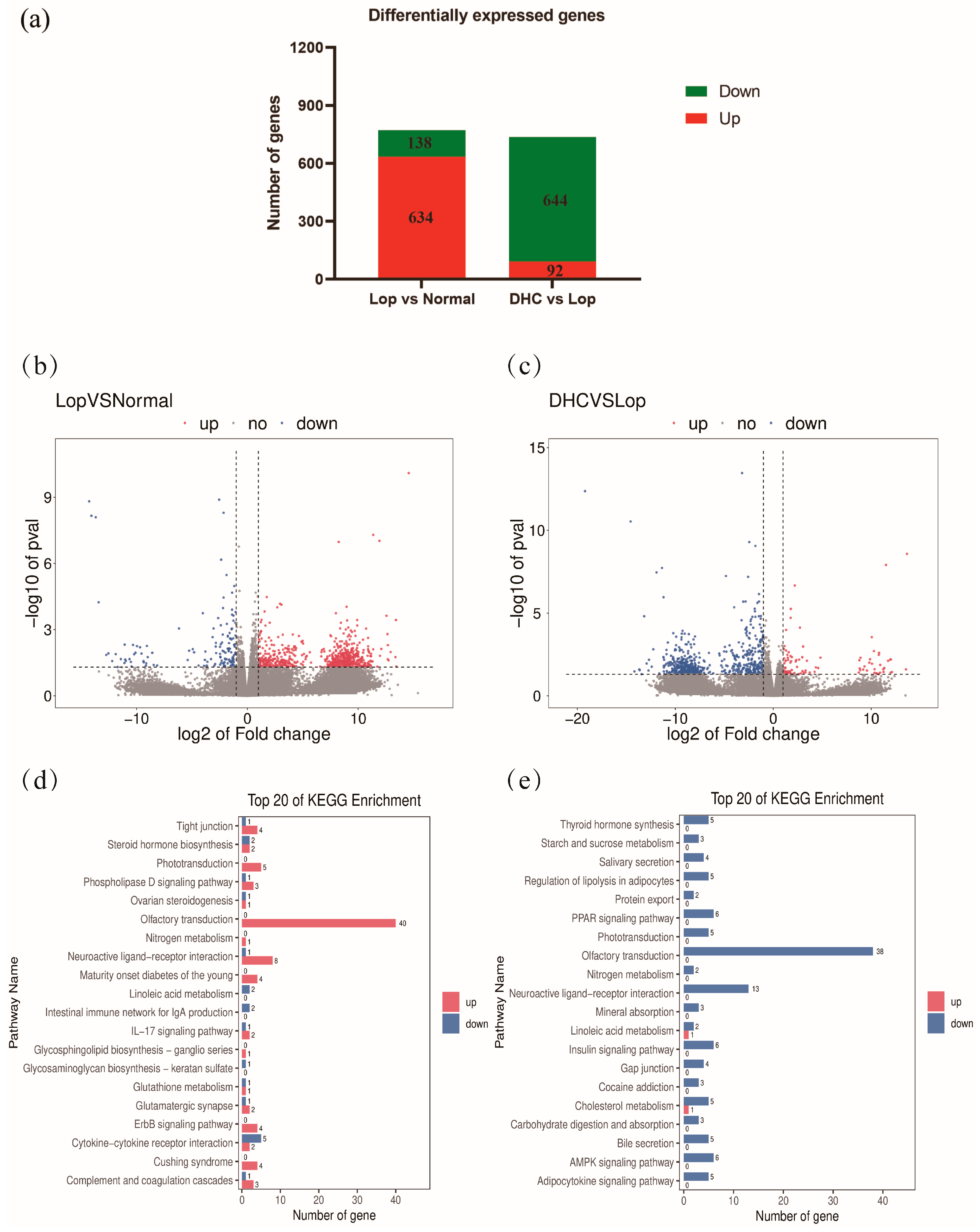
2.5. RNA Sequencing of Colon
2.6. KEGG Pathway Enrichment
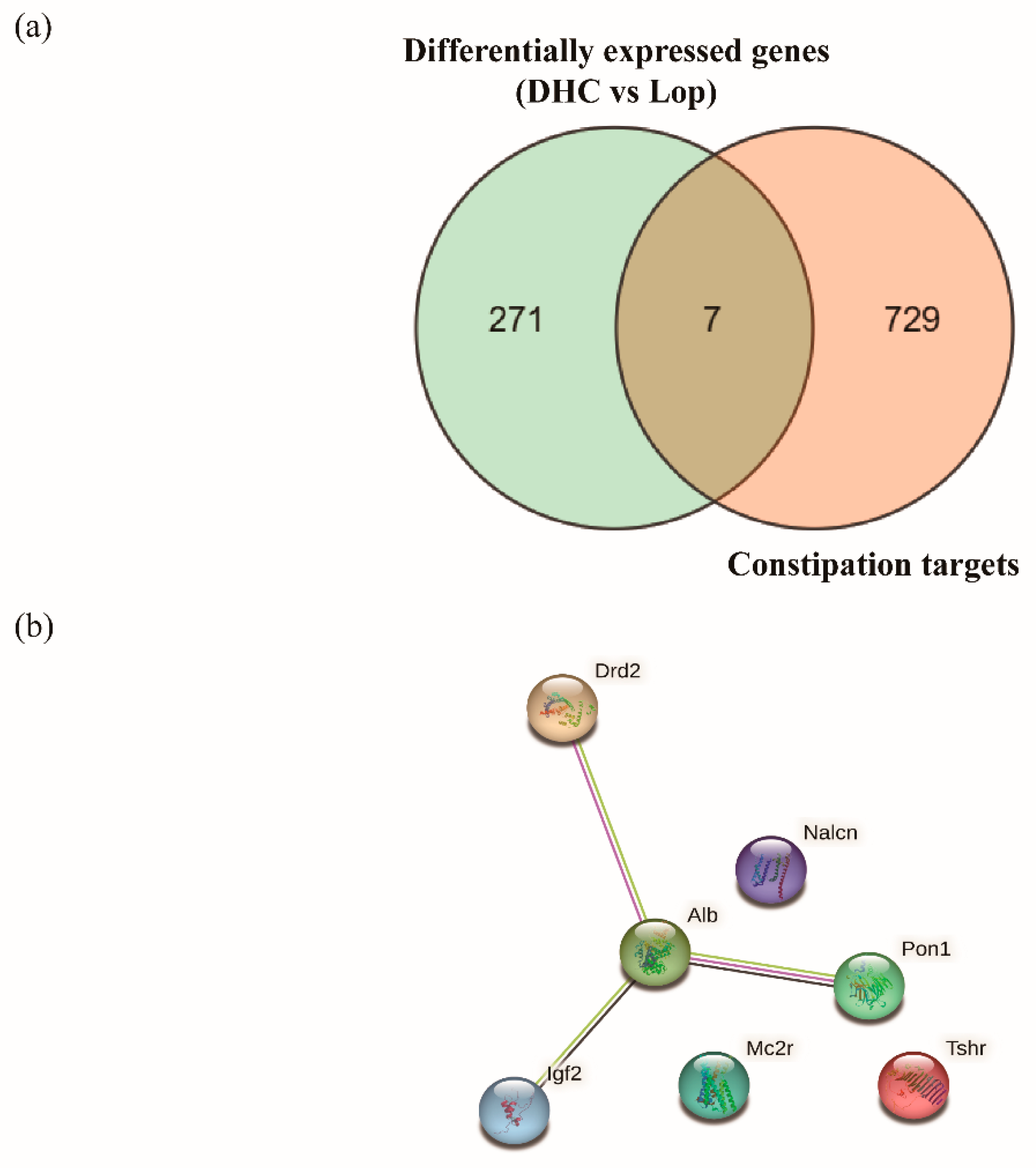
2.7. Joint Analysis of Network Pharmacology and RNA Sequencing
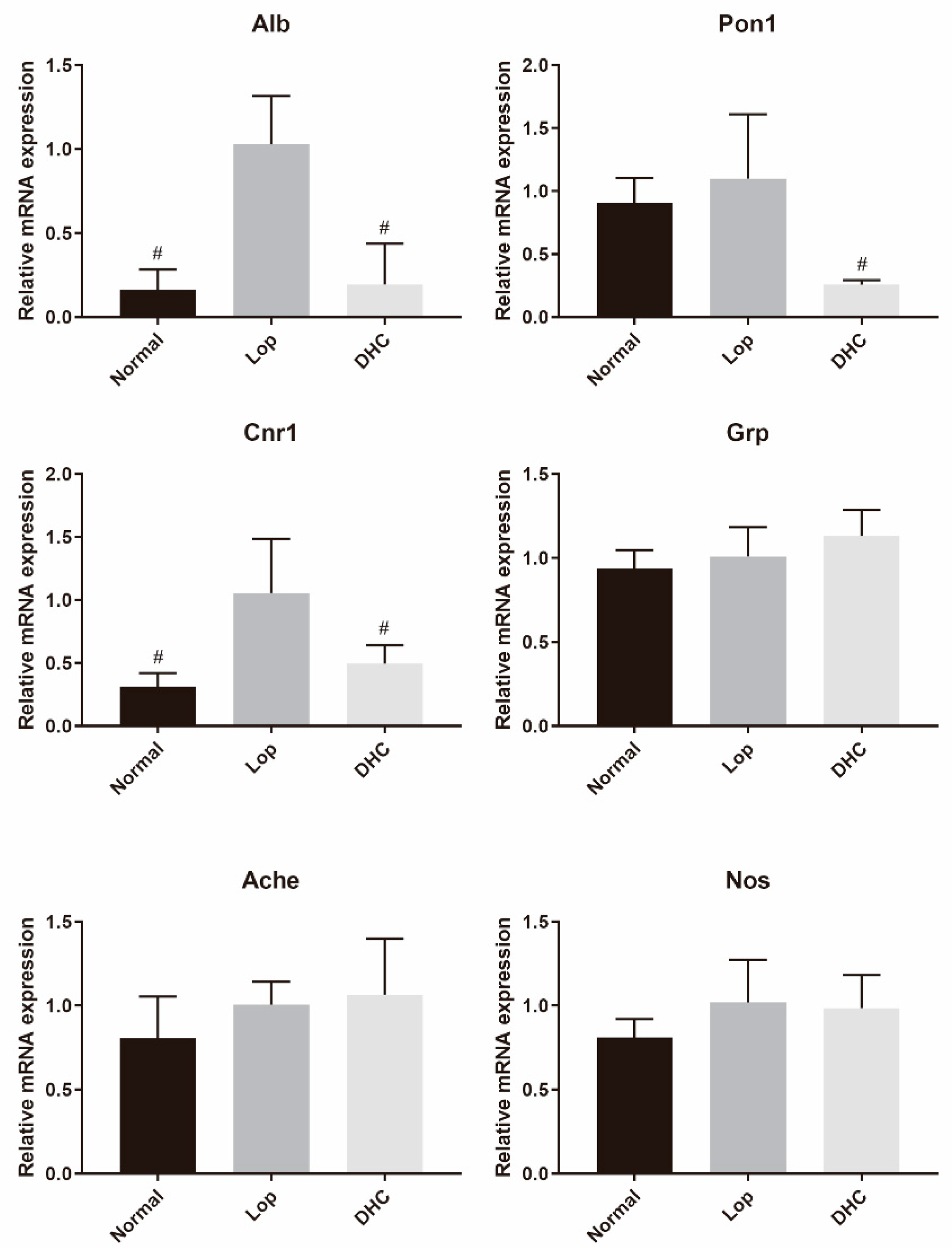
2.8. mRNA Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mice
4.3. Animal Experiment Design
4.4. Short-Chain Organic Acid Determination
4.5. 16S rRNA Sequencing
4.6. Network Pharmacology Strategy
4.7. Transcriptomic Analysis
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation: A review of literature. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.C.; Yu, L.L.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Chen, W.; Zhai, Q.X. Strain-specific effect of Limosilactobacillus fermentum with distinct genetic lineages on loperamide-induced constipation in mice: Attributing effects to certain genes. Food Funct. 2022, 13, 12742–12754. [Google Scholar] [CrossRef]
- Shi, Y.L.; Chen, F.; Wang, Z.Q.; Cao, J.; Li, C. Effect and mechanism of functional compound fruit drink on gut microbiota in constipation mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of gut microbiota in functional constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Ruggeri, E.; Stanghellini, V.; Eusebi, L.H.; Bazzoli, F.; Chiarioni, G. Chronic constipation in the elderly: A primer for the gastroenterologist. BMC Gastroenterol. 2015, 15, 130. [Google Scholar] [CrossRef]
- Higgins, P.D.R.; Johanson, J.E. Epidemiology of constipation in North America: A systematic review. Am. J. Gastroenterol. 2004, 99, 750–759. [Google Scholar] [CrossRef]
- Ding, C.; Ge, X.; Zhang, X.; Tian, H.; Wang, H.; Gu, L.; Gong, J.; Zhu, W.; Li, N. Efficacy of Synbiotics in Patients with Slow Transit Constipation: A Prospective Randomized Trial. Nutrients 2016, 8, 605. [Google Scholar] [CrossRef]
- Szewczyk, K.; Kalemba, D.; Miazga-Karska, M.; Krzemińska, B.; Dąbrowska, A.; Nowak, R. The essential oil composition of selected Hemerocallis cultivars and their biological activity %. J. Open Chem. 2019, 17, 1412–1422. [Google Scholar] [CrossRef]
- International Daylily Groups. Available online: http://oldsite.daylilies.org/ingroups.html (accessed on 14 February 2023).
- Liu, W.; Zhao, Y.; Sun, J.H.; Li, G.Y.; Shan, Y.; Chen, P. Study the effects of drying processes on chemical compositions in daylily flowers using flow injection mass spectrometric fingerprinting method and chemometrics. Food Res. Int. 2017, 102, 493–503. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Nair, M.G. Isolation and characterization of stelladerol, a new antioxidant naphthalene glycoside, and other antioxidant glycosides from edible daylily (Hemerocallis) flowers. J. Agric. Food Chem. 2002, 50, 87–91. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, R.; Chen, Y.; Zhong, J.; Deng, J.; Wang, Z.; Wu, Z.; Li, M.; Wang, H.; Sun, Y. Study on the Sleep-Improvement Effects of Hemerocallis citrina Baroni in Drosophila melanogaster and Targeted Screening to Identify Its Active Components and Mechanism. Foods 2021, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liang, Y.; Chen, Y.; Zhang, J.; Zou, X.; Deng, J.; Wang, D.; Sun, Y.; Li, M. Study and Experimental Validation of the Functional Components and Mechanisms of Hemerocallis citrina Baroni in the Treatment of Lactation Deficiency. Foods 2021, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, Y.J.; Wang, Y.B.; Yi, L.T. Role for monoaminergic systems in the antidepressant-like effect of ethanol extracts from Hemerocallis citrina. J. Ethnopharmacol. 2012, 139, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhan, X.; Wei, X.; Zhong, J.; Deng, J.; Chen, Y.; Pan, L.; Zhang, J.; Li, M.; Huang, R.; et al. Integration of omics and targeted screening strategy provide insights into the material basis and mechanism of Hemerocallis citrina Baroni on sleep-improvement. Food Res. Int. 2023, 112562. [Google Scholar] [CrossRef]
- Ti, Y.R.; Wang, W.Z.; Zhang, Y.L.; Wang, X.X.; Wang, P.; Song, Z.H. Polysaccharide from Hemerocallis citrina Borani by subcritical water: Bioactivity, purification, characterization, and anti-diabetic effects in T2DM rats. Int. J. Biol. Macromol. 2022, 215, 169–183. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Zhang, H.N.; Jiang, F.C.; Zhang, J.S.; Wang, W.H.; Li, L.; Yan, J.K. Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review. Int. J. Biol. Macromol. 2022, 204, 169–192. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, M.R.; Park, J.J.; Choi, J.Y.; Song, B.R.; Son, H.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm. Biol. 2018, 56, 309–317. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, N.; Jiang, H.; Li, H.; Cui, X.; Tang, S.; Jin, C.; Tian, J.; Li, B. Comparative study on alleviating effect of kiwi berry (Actinidia arguta) polysaccharide and polyphenol extracts on constipated mice. Food Res. Int. 2022, 162, 112037. [Google Scholar] [CrossRef]
- Lembo, A.; Camilleri, M. Chronic Constipation. N. Engl. J. Med. 2003, 349, 1360–1368. [Google Scholar] [CrossRef]
- Xing, J.H.; Soffer, E.E. Adverse effects of laxatives. Dis. Colon Rectum 2001, 44, 1201–1209. [Google Scholar] [CrossRef]
- Na, J.R.; Kim, E.; Na, C.S.; Kim, S. Citric Acid-Enriched Extract of Ripe Prunus mume (Siebold) Siebold & Zucc. Induces Laxative Effects by Regulating the Expression of Aquaporin 3 and Prostaglandin E-2 in Rats with Loperamide-Induced Constipation. J. Med. Food 2022, 25, 12–23. [Google Scholar]
- Lu, Y.Y.; Yu, Z.; Zhang, Z.; Liang, X.; Gong, P.M.; Yi, H.X.; Yang, L.Q.; Liu, T.J.; Shi, H.P.; Zhang, L.W. Bifidobacterium animalis F1-7 in combination with konjac glucomannan improves constipation in mice via humoral transport. Food Funct. 2021, 12, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Liu, Q.J.; Hou, Y.M.; Zhao, Y.Q. Alleviating effects of gut micro-ecologically regulatory treatments on mice with constipation. Front. Microbiol. 2022, 13, 956438. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; de Boeck, G.; Becker, K. Dietary Roles of Non-Starch Polysachharides in Human Nutrition: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Yao, S.K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Cui, S.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Adhesive Bifidobacterium Induced Changes in Cecal Microbiome Alleviated Constipation in Mice. Front. Microbiol. 2019, 10, 1721. [Google Scholar] [CrossRef]
- Wang, L.L.; Hu, L.J.; Xu, Q.; Yin, B.X.; Fang, D.S.; Wang, G.; Zhao, J.X.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, S.W.; Lee, J.H.; Park, S.H.; Lee, J.S. The evolution and competitive strategies of Akkermansia muciniphila in gut. Gut Microbes 2022, 14, 2025017. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The role of the probiotic Akkermansia muciniphila in brain functions: Insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2022, 49, 1–26. [Google Scholar] [CrossRef]
- Kim, M.G.; Jo, K.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.B. Prebiotics/Probiotics Mixture Induced Changes in Cecal Microbiome and Intestinal Morphology Alleviated the Loperamide-Induced Constipation in Rat. Food Sci. Anim. Resour. 2021, 41, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Usui, I.; Nawaz, A.; Igarashi, Y.; Okabe, K.; Furusawa, Y.; Watanabe, S.; Yamamoto, S.; Sasahara, M.; Watanabe, Y.; et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PloS One 2013, 8, e76520. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.F.; Zhao, X.; Zalan, Z.; Hegyi, F.; Takacs, K.; Du, M.Y. Lactobacillus plantarum KFY02 enhances the relieving effect of gardenoside on montmorillonite induced constipation in mice. Rsc Adv. 2020, 10, 10368–10381. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Chai, M.; Wang, J.L.; Yu, Q.Q.; Wang, G.; Zhang, H.; Zhao, J.X.; Chen, W. Bifidobacterium longum relieves constipation by regulating the intestinal barrier of mice. Food Funct. 2022, 13, 5037–5049. [Google Scholar] [CrossRef]
- Gupta, A.; Dhakan, D.B.; Maji, A.; Saxena, R.; PK., V.P.; Mahajan, S.; Pulikkan, J.; Kurian, J.; Gomez, A.M.; Scaria, J.; et al. Association of Flavonifractor plautii, a Flavonoid-Degrading Bacterium, with the Gut Microbiome of Colorectal Cancer Patients in India. mSystems 2019, 4, e00438-19. [Google Scholar] [CrossRef]
- Minamida, K.; Nishimura, M.; Miwa, K.; Nishihira, J. Effects of dietary fiber with Bacillus coagulans lilac-01 on bowel movement and fecal properties of healthy volunteers with a tendency for constipation. Biosci. Biotechnol. Biochem. 2015, 79, 300–306. [Google Scholar] [CrossRef]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X.J.C.P.; Science, P. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Kumemura, M.; Hashimoto, F.; Fujii, C.; Matsuo, K.; Kimura, H.; Miyazoe, R.; Okamatsu, H.; Inokuchi, T.; Ito, H.; Oizumi, K.J.; et al. Effects of administration of 4G-β-D-galactosylsucrose on fecal microflora, putrefactive products, short-chain fatty acids, weight, moisture and pH, and subjective sensation of defecation in the elderly with constipation. J. Clin. Biochem. Nutr. 1992, 13, 199–210. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.; ul Qamar, M.T.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.W.; Dugaiczyk, A. The human serum albumin gene: Structure of a unique locus. Gene 1982, 19, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, D.; Takeda, T.; Inami, Y.; Abe, D.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Komori, H.; Akazawa, Y.; et al. Association between the severity of constipation and sarcopenia in elderly adults: A single-center university hospital-based, cross-sectional study. Biomed. Rep. 2021, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Y.; Wen, P.; Chen, Y.; Ouyang, D.; Wang, D.; Zhang, B.; Deng, J.; Chen, Y.; Sun, Y.; et al. The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening. Molecules 2022, 27, 2235. [Google Scholar] [CrossRef]
- Tsatsakis, A.M.; Zafiropoulos, A.; Tzatzarakis, M.N.; Tzanakakis, G.N.; Kafatos, A. Relation of PON1 and CYP1A1 genetic polymorphisms to clinical findings in a cross-sectional study of a Greek rural population professionally exposed to pesticides. Toxicol. Lett. 2009, 186, 66–72. [Google Scholar] [CrossRef]
- Huang, J.Q.; Li, S.Y.; Wang, Q.; Guan, X.F.; Qian, L.; Li, J.; Zheng, Y.; Lin, B. Pediococcus pentosaceusB49 from human colostrum ameliorates constipation in mice. Food Funct. 2020, 11, 5607–5620. [Google Scholar] [CrossRef]
- Sjolund, K.; Fasth, S.; Ekman, R.; Hulten, L.; Jiborn, H.; Nordgren, S.; Sundler, F. Neuropeptides in idiopathic chronic constipation (slow transit constipation). Neurogastroenterol. Motil. 1997, 9, 143–150. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohydr. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef]
- Gao, X.Y.; Hu, Y.F.; Tao, Y.F.; Liu, S.F.; Chen, H.W.; Li, J.Y.; Zhao, Y.; Sheng, J.; Tian, Y.; Fan, Y.H. Cymbopogon citratus (DC.) Stapf aqueous extract ameliorates loperamide-induced constipation in mice by promoting gastrointestinal motility and regulating the gut microbiota. Front. Microbiol. 2022, 13, 1017804. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Bisogno, T.; Guarino, M.; Cocca, S.; Maccarrone, M.; Cicala, M. OC.03.3 TYPE-1 Cannabinoid Receptor Effect on Human Colonic Motility in Patients With Slow Transit Constipation and Controls. Dig. Liver Dis. 2018, 50, e75. [Google Scholar] [CrossRef]
- Fichna, J.; Sibaev, A.; Salaga, M.; Sobczak, M.; Storr, M. The cannabinoid-1 receptor inverse agonist taranabant reduces abdominal pain and increases intestinal transit in mice. Neurogastroenterol. Motil. 2013, 25, E550–E559. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.-G.; Wen, P.; Fu, H.-Z.; Lin, G.-Y.; Liao, S.-T.; Zou, Y.-X.J.F. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef]
- Liang, Y.-X.; Wen, P.; Wang, Y.; OuYang, D.-M.; Wang, D.; Chen, Y.-Z.; Song, Y.; Deng, J.; Sun, Y.-M.; Wang, H. The Constipation-Relieving Property of d-Tagatose by Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5721. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids. Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids. Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Xiang, X.; Liu, C.; Cai, T.Y.; Li, T.X.; Chen, Y.F.; Bai, J.J.; Shi, H.; Zheng, T.X.; Huang, M.Z.; et al. Transcriptomic Analysis Reveals Lactobacillus reuteri Alleviating Alcohol-Induced Liver Injury in Mice by Enhancing the Farnesoid X Receptor Signaling Pathway. J. Agric. Food Chem. 2022, 70, 12550–12564. [Google Scholar] [CrossRef]
- Cao, P.Q.; Li, X.P.; Jian, O.Y.; Jiang, R.G.; Huang, F.F.; Wen, B.B.; Zhang, X.N.; Huang, J.A.; Liu, Z.H. The protective effects of yellow tea extract against loperamide-induced constipation in mice. Food Funct. 2021, 12, 5621–5636. [Google Scholar] [CrossRef] [PubMed]
| Node 1 | Node 2 | Combined Score |
|---|---|---|
| Alb | Pon1 | 0.614 |
| Alb | Igf2 | 0.573 |
| Alb | Drd2 | 0.466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Wei, X.; Ren, R.; Zhang, X.; Tang, X.; Yang, J.; Wei, X.; Huang, R.; Hardiman, G.; Sun, Y.; et al. Study on Anti-Constipation Effects of Hemerocallis citrina Baroni through a Novel Strategy of Network Pharmacology Screening. Int. J. Mol. Sci. 2023, 24, 4844. https://doi.org/10.3390/ijms24054844
Liang Y, Wei X, Ren R, Zhang X, Tang X, Yang J, Wei X, Huang R, Hardiman G, Sun Y, et al. Study on Anti-Constipation Effects of Hemerocallis citrina Baroni through a Novel Strategy of Network Pharmacology Screening. International Journal of Molecular Sciences. 2023; 24(5):4844. https://doi.org/10.3390/ijms24054844
Chicago/Turabian StyleLiang, Yuxuan, Xiaoyi Wei, Rui Ren, Xuebin Zhang, Xiyao Tang, Jinglan Yang, Xiaoqun Wei, Riming Huang, Gary Hardiman, Yuanming Sun, and et al. 2023. "Study on Anti-Constipation Effects of Hemerocallis citrina Baroni through a Novel Strategy of Network Pharmacology Screening" International Journal of Molecular Sciences 24, no. 5: 4844. https://doi.org/10.3390/ijms24054844
APA StyleLiang, Y., Wei, X., Ren, R., Zhang, X., Tang, X., Yang, J., Wei, X., Huang, R., Hardiman, G., Sun, Y., & Wang, H. (2023). Study on Anti-Constipation Effects of Hemerocallis citrina Baroni through a Novel Strategy of Network Pharmacology Screening. International Journal of Molecular Sciences, 24(5), 4844. https://doi.org/10.3390/ijms24054844









