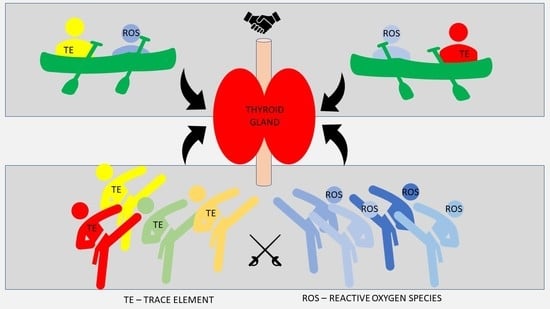
The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases
Abstract
1. Introduction
2. Methods
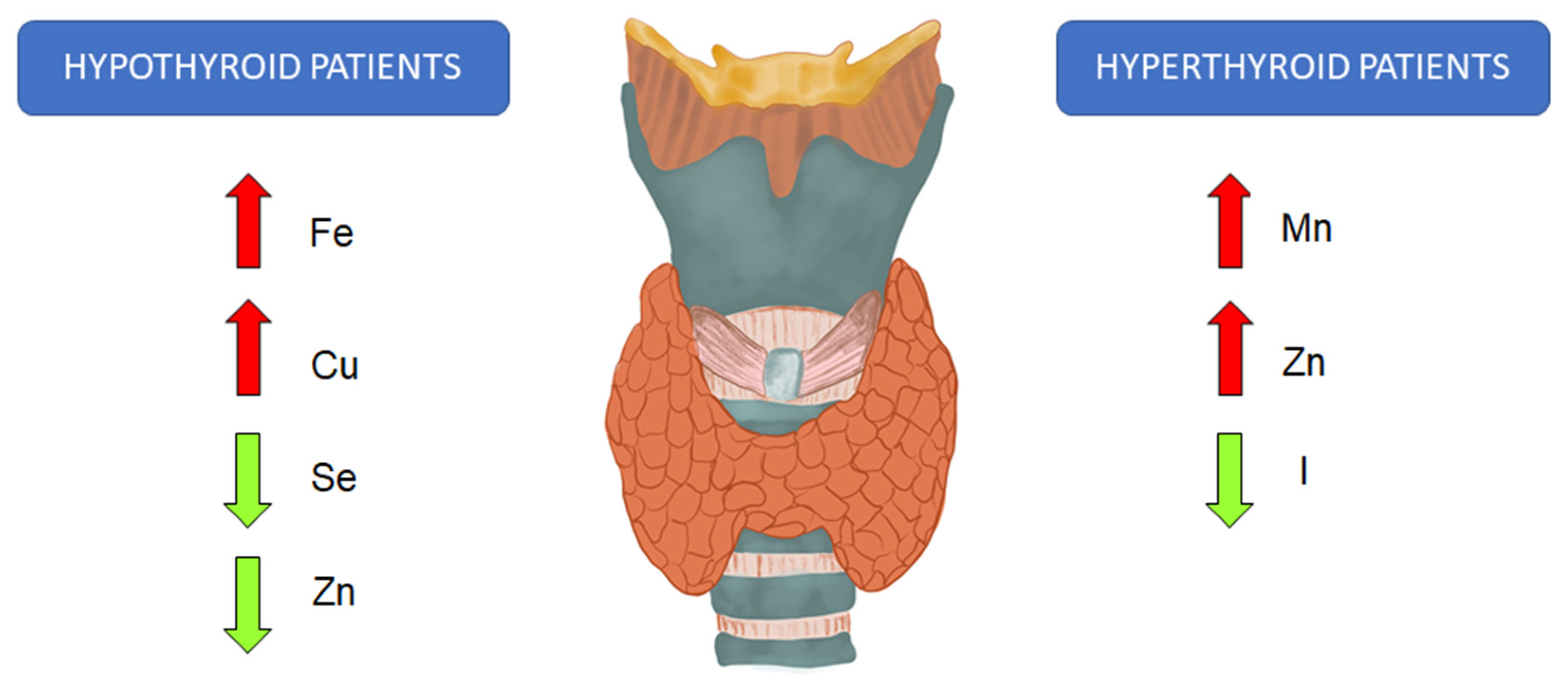
3. Trace Elements and Thyroid Function
4. Reactive Oxygen Species in Thyroid Disorders
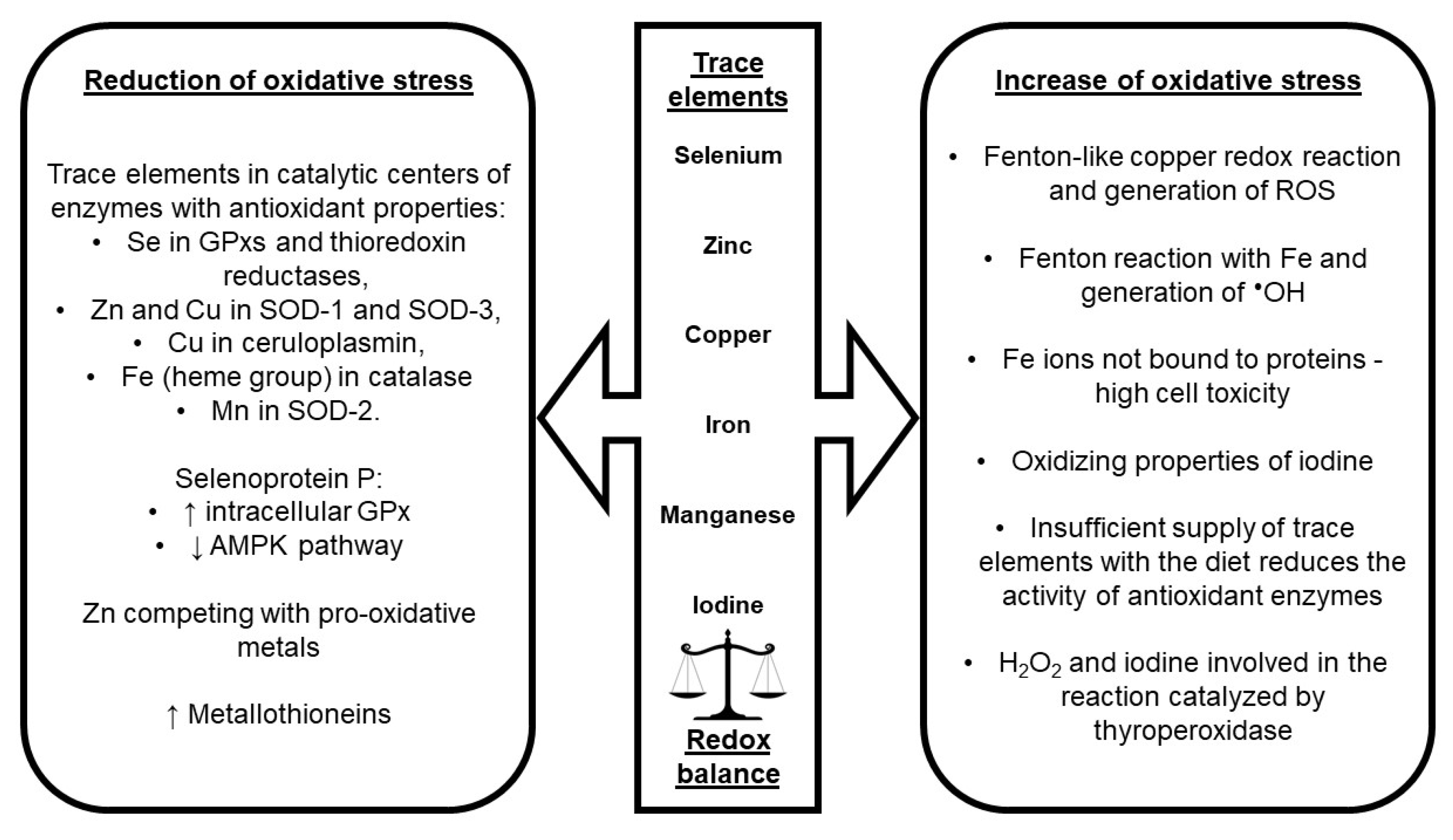
5. Impact of Trace Elements on Oxidative Stress
6. Speciation of Selected Trace Elements in Thyroid Diseases
7. Impact of Trace Elements on Oxidative Stress Markers in Thyroid Diseases
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alyahya, A.; AlNaim, A.; AlBahr, A.W.; Almansour, F.; Elshebiny, A. Knowledge of Thyroid Disease Manifestations and Risk Factors Among Residents of the Eastern Province, Saudi Arabia. Cureus 2021, 13, e13035. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.M.A.; Akhtar, S. Thyroid Disorders, Etiology and Prevalence. J. Med. Sci. 2002, 2, 89–94. [Google Scholar] [CrossRef]
- Garmendia Madariaga, A.; Santos Palacios, S.; Guillén-Grima, F.; Galofré, J.C. The Incidence and Prevalence of Thyroid Dysfunction in Europe: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.J. The epidemiology of thyroid disease. Br. Med. Bull. 2011, 99, 39–51. [Google Scholar] [CrossRef]
- Luster, M. The Thyroid and Its Diseases; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-72100-2. [Google Scholar]
- Al-Bazi, M.M.; Kumosani, T.A.; Al-Malki, A.L.; Kannan, K.; Moselhy, S.S. Association of trace elements abnormalities with thyroid dysfunction. Afr. Health Sci. 2021, 21, 1451–1459. [Google Scholar] [CrossRef]
- Heuck, C.; Kallner, A.; Kanagasabapathy, A.; Riesen, W. WHO Diagnosis and Monitoring of Diseases of The Thyroid. 2000. Available online: https://apps.who.int/iris/handle/10665/66342 (accessed on 11 December 2022).
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef]
- Bilek, R.; Dvorakova, M.; Grimmichova, T.; Jiskra, J. Iodine, Thyroglobulin and Thyroid Gland. Physiol. Res. 2020, 69, S225–S236. [Google Scholar] [CrossRef]
- Anagianni, S.; Tuschl, K. Genetic Disorders of Manganese Metabolism. Curr. Neurol. Neurosci. Rep. 2019, 19, 33. [Google Scholar] [CrossRef]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An element of life essential for thyroid function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef]
- Guo, C.; Qian, Y.; Yan, L.; Li, Z.; Liu, H.; Li, X.; Wang, Z.; Zhu, X.; Wang, Z.; Wang, J.; et al. The changes of essential trace elements in residents from an e-waste site and the relationships between elements and hormones of the hypothalamic-pituitary-thyroid (HPT) axis. Ecotoxicol. Environ. Saf. 2021, 222, 112513. [Google Scholar] [CrossRef]
- Khanam, S. Impact of zinc on thyroid metabolism. J. Diabetes. Metab. Disord. Control 2018, 5, 27–28. [Google Scholar] [CrossRef]
- Ratajczak, M.; Gietka-Czernel, M. Rola selenu w organizmie człowieka The influence of selenium to human health. Postępy Nauk Med. 2016, 29, 929–933. [Google Scholar]
- Hanif, S.; Ilyas, A.; Shah, M.H. Statistical Evaluation of Trace Metals, TSH and T4 in Blood Serum of Thyroid Disease Patients in Comparison with Controls. Biol. Trace Elem. Res. 2018, 183, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, P.; Russo, M.; Ronchi, A.; Moretti, F.; Gianì, F.; Vigneri, P.; Masucci, R.; Pellegriti, G.; Belfiore, A.; Vigneri, R. Concentration of Metals and Trace Elements in the Normal Human and Rat Thyroid: Comparison with Muscle and Adipose Tissue and Volcanic Versus Control Areas. Thyroid 2020, 30, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bibi, K.; Shah, M.H. Appraisal of Metal Imbalances in the Blood of Thyroid Cancer Patients in Comparison with Healthy Subjects. Biol. Trace Elem. Res. 2020, 198, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Stojsavljević, A.; Rovčanin, B.; Krstić, Đ.; Borković-Mitić, S.; Paunović, I.; Kodranov, I.; Gavrović-Jankulović, M.; Manojlović, D. Evaluation of trace metals in thyroid tissues: Comparative analysis with benign and malignant thyroid diseases. Ecotoxicol. Environ. Saf. 2019, 183, 109479. [Google Scholar] [CrossRef]
- Maouche, N.; Meskine, D.; Alamir, B.; Koceir, E.A. Trace elements profile is associated with insulin resistance syndrome and oxidative damage in thyroid disorders: Manganese and selenium interest in Algerian participants with dysthyroidism. J. Trace Elem. Med. Biol. 2015, 32, 112–121. [Google Scholar] [CrossRef]
- Vidal, Z.E.O.; Rufino, S.C.; Tlaxcalteco, E.H.; Trejo, C.H.; Campos, R.M.; Meza, M.N.; Rodríguez, R.C.; Arroyo-Helguera, O. Oxidative stress increased in pregnant women with iodine deficiency. Biol. Trace Elem. Res. 2014, 157, 211–217. [Google Scholar] [CrossRef]
- Sur, U.; Erkekoglu, P.; Bulus, A.D.; Andiran, N.; Kocer-Gumusel, B. Oxidative stress markers, trace elements, and endocrine disrupting chemicals in children with Hashimoto’s thyroiditis. Toxicol. Mech. Methods 2019, 29, 633–643. [Google Scholar] [CrossRef]
- Arthur, J.R.; Beckett, G.J. Thyroid Function. Br. Med. Bull. 1999, 55, 658–668. [Google Scholar] [CrossRef]
- Carvalho, D.P.; Dupuy, C. Thyroid hormone biosynthesis and release. Mol. Cell Endocrinol. 2017, 458, 6–15. [Google Scholar] [CrossRef]
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef] [PubMed]
- Szanto, I.; Pusztaszeri, M.; Mavromati, M. H2O2 metabolism in normal thyroid cells and in thyroid tumorigenesis: Focus on NADPH oxidases. Antioxidants 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, J.; Podgórski, T.; Domaszewska, K. The level of zinc, copper and antioxidant status in the blood serum of women with Hashimoto’s thyroiditis. Int. J. Environ. Res. Public Health 2021, 18, 7805. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Lu, Y.; Liu, B.; Girault, H.H. Copper-catalyzed tyrosine nitration. J. Am. Chem. Soc. 2011, 133, 19823–19831. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Aschner, M. Effects of manganese on thyroid hormone homeostasis: Potential links. Neurotoxicology 2007, 28, 951–956. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell Mol. Life Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- And Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, E.; Golgiri, F.; Janani, L.; Moradi, N.; Fallah, S.; Abiri, B.; Vafa, M. Randomized Study of the Effects of Zinc, Vitamin A, and Magnesium Co-supplementation on Thyroid Function, Oxidative Stress, and hs-CRP in Patients with Hypothyroidism. Biol. Trace Elem. Res. 2021, 199, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiu, M.L.; Badiu, C. Selenium involvement in mitochondrial function in thyroid disorders. Hormones 2020, 19, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Seymen, H.O.; Civelek, S.; Seven, A.; Yiǧit, G.; Hatemi, H.; Burçak, G. Iron supplementation in experimental hyperthyroidism: Effects on oxidative stress in skeletal muscle tissue. Yonsei Med. J. 2004, 45, 413–418. [Google Scholar] [CrossRef]
- Restini, L.A.O.; Dessordi, R.; Ferreira, S.M.S.; Magalhães, P.K.R.; Maciel, L.M.Z.; Júnior, F.B.; Costa, T.M.B.; Júnior, A.A.J.; Navarro, A.M. Assessment of thyroid function, ioduria and oxidative stress in women in the first trimester of pregnancy. Nutr. Hosp. 2018, 35, 1387–1393. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Rovčanin, B. Impact of Essential and Toxic Trace Metals on Thyroid Health and Cancer: A Review. Expo. Health 2021, 13, 613–627. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Rovčanin, B.; Jagodić, J.; Krstić, Đ.; Paunović, I.; Gavrović-Jankulović, M.; Manojlović, D. Alteration of Trace Elements in Multinodular Goiter, Thyroid Adenoma, and Thyroid Cancer. Biol. Trace Elem. Res. 2021, 199, 4055–4065. [Google Scholar] [CrossRef]
- Giray, B.; Arnaud, J.; Sayek, I.; Favier, A.; Hincal, F. Trace elements status in multinodular goiter. J. Trace Elem. Med. Biol. 2010, 24, 106–110. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.C.; Chung, S.; Kim, S.; Yoon, J.W.; Park, Y.J. Exploring the role of copper and selenium in the maintenance of normal thyroid function among healthy Koreans. J. Trace Elem. Med. Biol. 2020, 61, 126558. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Arora, M. Study of Trace Elements in Patients of Hypothyroidism with Special Reference to Zinc and Copper. Biomed. J. Sci. Tech. Res. 2018, 6, 11–16. [Google Scholar] [CrossRef]
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, A.R.S.; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Turksoy, V.A. Selenium, Zinc, and Copper Status in Euthyroid Nodular Goiter: A Cross-Sectional Study. Int. J. Prev. Med. 2021, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.M.; Al-Ardhi, G.H.; Abd-Alameer, A.M. Estimation of Serum Trace metals (Zn and Cu) and Thyroid Hormones in Hypothyroidism. Int. J. Drug Deliv. Technol. 2022, 12, 252–255. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef]
- Starchl, C.; Scherkl, M.; Amrein, K. Celiac disease and the thyroid: Highlighting the roles of vitamin d and iron. Nutrients 2021, 13, 1755. [Google Scholar] [CrossRef]
- Li, S.; Gao, X.; Wei, Y.; Zhu, G.; Yang, C. The relationship between iron deficiency and thyroid function in chinese women during early pregnancy. J. Nutr. Sci. Vitaminol. 2016, 62, 397–401. [Google Scholar] [CrossRef]
- Saeed, A.A.; Ataitalla, H.A.; Taha, S.; Elmukashfi, A. Evaluation of serum Manganese and Zinc in non-cancerous thyroid disorders in Khartoum State-Sudan. J. Drug Deliv. Ther. 2019, 9, 497–499. [Google Scholar] [CrossRef]
- Andersson, M.; Braegger, C.P. The Role of Iodine for Thyroid Function in Lactating Women and Infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef]
- Kravchenko, V.I.; Andrusyshyna, I.M.; Luzanchuk, I.A.; Polumbryk, M.O.; Tarashchenko, Y.M. Association Between Thyroid Hormone Status and Trace Elements in Serum of Patients with Nodular Goiter. Biol. Trace Elem. Res. 2020, 196, 393–399. [Google Scholar] [CrossRef]
- Barysheva, E.S. Experimental Simulation of the Effects of Essential and Toxic Trace Elements on Thyroid Function. Bull. Exp. Biol. Med. 2018, 164, 439–441. [Google Scholar] [CrossRef]
- Pop, V.; Krabbe, J.; Maret, W.; Rayman, M. Plasma mineral (selenium, zinc or copper) concentrations in the general pregnant population, adjusted for supplement intake, in relation to thyroid function. Br. J. Nutr. 2021, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V. Comparison of trace element contents in normal thyroid and thyroid with Hashimoto’s thyroiditis using neutron activation analysis. Saudi J. Biomed. Res. 2021, 6, 246–255. [Google Scholar] [CrossRef]
- Kazi Tani, L.S.; Gourlan, A.T.; Dennouni-Medjati, N.; Telouk, P.; Dali-Sahi, M.; Harek, Y.; Sun, Q.; Hackler, J.; Belhadj, M.; Schomburg, L.; et al. Copper Isotopes and Copper to Zinc Ratio as Possible Biomarkers for Thyroid Cancer. Front. Med. 2021, 8, 698167. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Ameziane El Hassani, R.; Buffet, C.; Leboulleux, S.; Dupuy, C. Oxidative stress in thyroid carcinomas: Biological and clinical significance. Endocr. Relat. Cancer 2019, 26, R131–R143. [Google Scholar] [CrossRef] [PubMed]
- Paprocki, J.; Sutkowy, P.; Piechocki, J.; Woźniak, A. Markers of oxidant-antioxidant equilibrium in patients with sudden sensorineural hearing loss treated with hyperbaric oxygen therapy. Oxid. Med. Cell Longev. 2019, 2019, 8472346. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Coelho, R.G.; Fortunato, R.S.; Carvalho, D.P. Metabolic reprogramming in thyroid carcinoma. Front. Oncol. 2018, 8, 82. [Google Scholar] [CrossRef]
- Raad, H.; Eskalli, Z.; Corvilain, B.; Miot, F.; De Deken, X. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2and its maturation factorDUOXA2. Free Radic. Biol. Med. 2013, 56, 216–225. [Google Scholar] [CrossRef]
- Ohye, H.; Sugawara, M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp. Biol. Med. 2010, 235, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Driessens, N.; Costa, M.; De Deken, X.; Detours, V.; Corvilain, B.; Maenhaut, C.; Miot, F.; Van Sande, J.; Many, M.C.; et al. Review: Roles of hydrogen peroxide in thyroid physiology and disease. J. Clin. Endocrinol. Metab. 2007, 92, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Bann, D.V.; Jin, Q.; Sheldon, K.E.; Houser, K.R.; Nguyen, L.; Warrick, J.I.; Baker, M.J.; Broach, J.R.; Gerhard, G.S.; Goldenberg, D. Genetic variants implicate dual oxidase-2 in familial and sporadic nonmedullary thyroid cancer. Cancer Res. 2019, 79, 5490–5499. [Google Scholar] [CrossRef] [PubMed]
- Weyemi, U.; Caillou, B.; Talbot, M.; Ameziane-El-Hassani, R.; Lacroix, L.; Lagent-Chevallier, O.; Al Ghuzlan, A.; Roos, D.; Bidart, J.M.; Virion, A.; et al. Intracellular expression of reactive oxygen species-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr. Relat. Cancer 2010, 17, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sutkowy, P.; Wróblewska, J.; Wróblewski, M.; Nuszkiewicz, J.; Modrzejewska, M.; Woźniak, A. The Impact of Exercise on Redox Equilibrium in Cardiovascular Diseases. J. Clin. Med. 2022, 11, 4833. [Google Scholar] [CrossRef] [PubMed]
- Nuszkiewicz, J.; Czuczejko, J.; Maruszak, M.; Pawłowska, M.; Woźniak, A.; Małkowski, B.; Szewczyk-Golec, K. Parameters of Oxidative Stress, Vitamin D, Osteopontin, and Melatonin in Patients with Lip, Oral Cavity, and Pharyngeal Cancer. Oxid. Med. Cell Longev. 2021, 2021, 2364931. [Google Scholar] [CrossRef]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef] [PubMed]
- Limón-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 674, 137–147. [Google Scholar] [CrossRef]
- Olsen, L.F.; Issinger, O.G.; Guerra, B. The Yin and Yang of redox regulation. Redox Rep. 2013, 18, 245–252. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Kim, H.; Lee, T.H.; Park, E.S.; Suh, J.M.; Park, S.J.; Chung, H.K.; Kwon, O.Y.; Kim, Y.K.; Ro, H.K.; Shong, M. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J. Biol. Chem. 2000, 275, 18266–18270. [Google Scholar] [CrossRef] [PubMed]
- Nadolnik, L.I.; Valentyukevich, O.I. Peculiarities of the antioxidant status of the thyroid gland. Bull. Exp. Biol. Med. 2007, 144, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, C.; Mentrup, B.; Schomburg, L.; Hoang-Vu, C.; Herzog, V.; Köhrle, J. Selenoproteins of the thyroid gland: Expression, localization and possible function of glutathione peroxidase 3. Biol. Chem. 2007, 388, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Gérard, A.C.; Many, M.C.; Daumerie, C.; Knoops, B.; Colin, I.M. Peroxiredoxin 5 expression in the human thyroid gland. Thyroid 2005, 15, 205–209. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef]
- Cornejo, P.; Tapia, G.; Puntarulo, S.; Galleano, M.; Videla, L.A.; Fernández, V. Iron-induced changes in nitric oxide and superoxide radical generation in rat liver after lindane or thyroid hormone treatment. Toxicol. Lett. 2001, 119, 87–93. [Google Scholar] [CrossRef]
- Villanueva, I.; Alva-Sánchez, C.; Pacheco-Rosado, J. The role of thyroid hormones as inductors of oxidative stress and neurodegeneration. Oxid. Med. Cell Longev. 2013, 2013, 218145. [Google Scholar] [CrossRef]
- Oziol, L.; Faure, P.; Vergely, C.; Rochette, L.; Artur, Y.; Chomard, P. In vitro free radical scavenging capacity of thyroid hormones and structural analogues. J. Endocrinol. 2001, 170, 197–206. [Google Scholar] [CrossRef]
- Lopes, N.M.D.; Lens, H.H.M.; da Silva Brito, W.A.; Bianchi, J.K.; Marinello, P.C.; Cecchini, R.; Armani, A.; Cecchini, A.L. Role of papillary thyroid carcinoma patients with Hashimoto thyroiditis: Evaluation of oxidative stress and inflammatory markers. Clin. Transl. Oncol. 2022, 24, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Trace Elements and Healthcare: A Bioinformatics Perspective. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; Volume 1005, pp. 63–98. ISBN 978-981-10-5716-8. [Google Scholar]
- Squadrone, S.; Brizio, P.; Mancini, C.; Abete, M.C.; Brusco, A. Altered homeostasis of trace elements in the blood of SCA2 patients. J. Trace Elem. Med. Biol. 2018, 47, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Soares de Oliveira, A.R.; Jayanne Clímaco Cruz, K.; Beatriz Silva Morais, J.; Rocha Dos Santos, L.; Rodrigues de Sousa Melo, S.; Fontenelle, L.C.; Santos de Sousa, G.; Costa Maia, C.S.; Oliveira Duarte de Araújo, C.; Leal Mendes, I.; et al. Selenium status and oxidative stress in obese: Influence of adiposity. Eur. J. Clin. Investig. 2021, 51, e13538. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; Campos, M.M.; Bogo, M.R. Copper toxicology, oxidative stress and inflammation using zebrafish as experimental model. J. Appl. Toxicol. 2016, 36, 876–885. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Karbownik-Lewińska, M.; Stępniak, J.; Iwan, P.; Lewiński, A. Iodine as a potential endocrine disruptor—A role of oxidative stress. Endocrine 2022, 78, 219–240. [Google Scholar] [CrossRef]
- Gunizi, H.; Savas, H.B.; Genc, S. Trace Elements (Zn and Cu) and Oxidative Stress in Pediatric Patients with Persistent Allergic Rhinitis. J. Coll. Physicians Surg. Pak. 2022, 32, 324–328. [Google Scholar] [CrossRef]
- Culhuac, E.B.; Elghandour, M.M.M.Y.; Adegbeye, M.J.; Barbabosa-Pliego, A.; Salem, A.Z.M. Influence of Dietary Selenium on the Oxidative Stress in Horses. Biol. Trace Elem. Res. 2022, 201, 1695–1703. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Acute human toxicity and mortality after selenium ingestion: A review. J. Trace Elem. Med. Biol. 2020, 58, 126435. [Google Scholar] [CrossRef]
- Pons, D.G.; Moran, C.; Alorda-Clara, M.; Oliver, J.; Roca, P.; Sastre-Serra, J. Micronutrients Selenomethionine and Selenocysteine Modulate the Redox Status of MCF-7 Breast Cancer Cells. Nutrients 2020, 12, 865. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Al-Mubarak, A.A.; van der Meer, P.; Bomer, N. Selenium, Selenoproteins, and Heart Failure: Current Knowledge and Future Perspective. Curr. Heart Fail. Rep. 2021, 18, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ma, H.; Butler, M.R.; Ding, X.Q. Deficiency of type 2 iodothyronine deiodinase reduces necroptosis activity and oxidative stress responses in retinas of Leber congenital amaurosis model mice. FASEB J. 2018, 32, 6316–6329. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Andrade, I.G.A.; Suano-Souza, F.I.; Fonseca, F.L.A.; Lago, C.S.A.; Sarni, R.O.S. Selenium levels and glutathione peroxidase activity in patients with ataxia-telangiectasia: Association with oxidative stress and lipid status biomarkers. Orphanet J. Rare Dis. 2021, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiao, T.; Wang, Q.; Liang, B.; Zhang, A. Effects of Essential Trace Elements and Oxidative Stress on Endemic Arsenism Caused by Coal Burning in PR China. Biol. Trace Elem. Res. 2020, 198, 25–36. [Google Scholar] [CrossRef]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019, 140, 28–35. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T. Hepatokine Selenoprotein P-Mediated Reductive Stress Causes Resistance to Intracellular Signal Transduction. Antioxid. Redox Signal. 2020, 33, 517–524. [Google Scholar] [CrossRef]
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.; Steinberg, S.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J. Copper and Zinc, Biological Role and Significance of Copper/Zinc Imbalance. J. Clin. Toxicol. 2011, 3, 0495. [Google Scholar] [CrossRef]
- Marreiro, D.; Cruz, K.; Morais, J.; Beserra, J.; Severo, J.; de Oliveira, A. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- Gonzalez-Iglesias, H.; Alvarez, L.; García, M.; Petrash, C.; Sanz-Medel, A.; Coca-Prados, M. Metallothioneins (MTs) in the human eye: A perspective article on the zinc–MT redox cycle. Metallomics 2014, 6, 201–208. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out what you put in: Copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118867. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Fu, P.P.; Lutterodt, H.; Zhou, Y.T.; Antholine, W.E.; Wamer, W. Dual role of selected antioxidants found in dietary supplements: Crossover between anti- and pro-oxidant activities in the presence of copper. J. Agric. Food Chem. 2012, 60, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Lui, E.M.K. Prooxidative effect of copper-metallothionein in the acute cytotoxicity of hydrogen peroxide in Ehrlich ascites tumour cells. Toxicology 2006, 217, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Altobelli, G.G.; Van Noorden, S.; Balato, A.; Cimini, V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front. Med. 2020, 7, 183. [Google Scholar] [CrossRef]
- Inoue, K.; Sakano, N.; Ogino, K.; Sato, Y.; Wang, D.-H.; Kubo, M.; Takahashi, H.; Kanbara, S.; Miyatake, N. Relationship between ceruloplasmin and oxidative biomarkers including ferritin among healthy Japanese. J. Clin. Biochem. Nutr. 2013, 52, 160–166. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.-P. Does Ceruloplasmin Defend Against Neurodegenerative Diseases? Curr. Neuropharmacol. 2018, 17, 539–549. [Google Scholar] [CrossRef]
- Wan, J.; Ren, H.; Wang, J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc. Neurol. 2019, 4, 93–95. [Google Scholar] [CrossRef]
- Isidori, A.; Loscocco, F.; Visani, G.; Chiarucci, M.; Musto, P.; Kubasch, A.-S.; Platzbecker, U.; Vinchi, F. Iron Toxicity and Chelation Therapy in Hematopoietic Stem Cell Transplant. Transplant. Cell Ther. 2021, 27, 371–379. [Google Scholar] [CrossRef]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef]
- Kajarabille; Latunde-Dada Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a remarkable enzyme: Targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. Landmark 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Godlewska, M.; Gawel, D.; Buckle, A.M.; Banga, J.P. Thyroid Peroxidase Revisited—What’s New? Horm. Metab. Res. 2019, 51, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.M. The importance of trace element speciation in biomedical science. Anal. Bioanal. Chem. 2003, 375, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- De Farias, C.R.; Cardoso, B.R.; De Oliveira, G.M.B.; De Mello Guazzelli, I.C.; Catarino, R.M.; Chammas, M.C.; Cozzolino, S.M.F.; Knobel, M. A randomized-controlled, double-blind study of the impact of selenium supplementation on thyroid autoimmunity and inflammation with focus on the GPx1 genotypes. J. Endocrinol. Investig. 2015, 38, 1065–1074. [Google Scholar] [CrossRef]
- Jafari, E.; Alavi, M.; Zal, F. The evaluation of protective and mitigating effects of vitamin c against side effects induced by radioiodine therapy. Radiat. Environ. Biophys. 2018, 57, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Konukoǧlu, D.; Hüsrev Hatemi, H.; Arikan, S.; Demir, M.; Akçay, T. Radioiodine treatment and oxidative stress in thyroidectomised patients for differentiated thyroid cancers. Pharmacol. Res. 1998, 38, 311–315. [Google Scholar] [CrossRef]
- Tian, X.; Li, N.; Su, R.; Dai, C.; Zhang, R. Selenium Supplementation May Decrease Thyroid Peroxidase Antibody Titer via Reducing Oxidative Stress in Euthyroid Patients with Autoimmune Thyroiditis. Int. J. Endocrinol. 2020, 2020, 3–6. [Google Scholar] [CrossRef]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.D. Physiology of iron metabolism. Transfus. Med. Hemotherapy 2014, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Stern, B.R.; Solioz, M.; Krewski, D.; Aggett, P.; Aw, T.; Baker, S.; Crump, K.; Dourson, M.; Haber, L.; Hertzberg, R.; et al. Copper and Human Health: Biochemistry, Genetics, and Strategies for Modeling Dose-response Relationships. J. Toxicol. Environ. Health Part B 2007, 10, 157–222. [Google Scholar] [CrossRef] [PubMed]
- Daniel, K.G.; Harbach, H.R.; Guida, W.C.; Dou, Q.P. Copper storage diseases: Menkes, Wilson’s, and Cancer. Front. Biosci. 2004, 9, 2652–2662. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Hajo, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, R.; Moncayo, H. Applying a systems approach to thyroid physiology: Looking at the whole with a mitochondrial perspective instead of judging single TSH values or why we should know more about mitochondria to understand metabolism. BBA Clin. 2017, 7, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, S.J.; Hegedüs, L. Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Endocr. Rev. 2012, 33, 920–980. [Google Scholar] [CrossRef]
- Rosário, P.W.; Batista, K.C.S.; Calsolari, M.R. Radioiodine-induced oxidative stress in patients with differentiated thyroid carcinoma and effect of supplementation with vitamins C and E and selenium (Antioxidants). Arch. Endocrinol. Metab. 2016, 60, 328–332. [Google Scholar] [CrossRef]
- Wolfram, R.M.; Palumbo, B.; Chehne, F.; Palumbo, R.; Budinsky, A.C.; Sinzinger, H. (ISO) Prostaglandins in saliva indicate oxidation injury after radioiodine therapy. Rev. Española Med. Nucl. 2004, 23, 183–188. [Google Scholar] [CrossRef]
- Duntas, L.H.; Mantzou, E.; Koutras, D.A. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur. J. Endocrinol. 2003, 148, 389–393. [Google Scholar] [CrossRef]
- Wajner, S.M.; Rohenkohl, H.C.; Serrano, T.; Maia, A.L. Sodium selenite supplementation does not fully restore oxidative stress-induced deiodinase dysfunction: Implications for the nonthyroidal illness syndrome. Redox Biol. 2015, 6, 436–445. [Google Scholar] [CrossRef]
- Kryczyk-Kozioł, J.; Zagrodzki, P.; Paśko, P. Wpływ suplementacji diety selenem na przebieg autoimmunologicznego zapalenia tarczycy—Przegląd badań klinicznych przeprowadzonych w populacji europejskiej. Postepy Hig. Med. Dosw. 2021, 75, 683–695. [Google Scholar] [CrossRef]
- O’Grady, T.J.; Kitahara, C.M.; DiRienzo, A.G.; Gates, M.A. The association between selenium and other micronutrients and thyroid cancer incidence in the NIHAARP diet and health study. PLoS ONE 2014, 9, e110886. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Vanderpas, J.; Chanoine, J.; Ne, J.; Bourdoux, P.; Reine, H.; Brussels, B. Does Not Affect the Increased Thyroxine-to- Triiodothyronine Ratio in Children with Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2001, 86, 1160–1163. [Google Scholar]
- Falfushynska, H.I. Manifestations of oxidative stress and molecular damages in ovarian cancer tissue. Ukr. Biochem. J. 2015, 87, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Lu, F.J.; Gau, R.J.; Yang, M.L.; Huang, T.S. 15-Deoxy-δ12,14-prostaglandin J2 induces apoptosis of a thyroid papillary cancer cell line (CG3 cells) through increasing intracellular iron and oxidative stress. Anticancer. Drugs 2002, 13, 759–765. [Google Scholar] [CrossRef]
| Disease | Patients | Parameters | Refs |
|---|---|---|---|
| Hypothyroidism | n = 43, age (20–65) | Lower level of MDA and CRP after supplementation zinc gluconate, magnesium oxide and vitamin A; no significant change in level TAC | [34] |
| Autoimmune thyroiditis | n = 18, age (40.2 ± 10.9) | Lover level of MDA, higher level of TAC and SOD after supplementation selenious yeast | [133] |
| n = 28, age (20–58) | Higher level of GPx after supplementation sodium selenite or selenomethionine vs. placebo group | [130] | |
| Dysthyroidism | n = 170, age (30–50) | Lower concentration of Se and Cu and higher concentration of Mn vs. control group Lower level of GPx, mitochondrial SOD and TAS vs. control group | [21] |
| Hashiomoto’s thyroiditis | n = 42, age (45–60) | Higher level of TBARS; no significant differences in the concentration of Cu and Zn vs. control group | [29] |
| Thyroid cancer | n = 30, age (43 ± 7) | Higher level of MDA, lower level of GR and GPx vs. control group Higher level of MDA, GR and GPx after radioiodine therapy vs. control group | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewski, M.; Wróblewska, J.; Nuszkiewicz, J.; Pawłowska, M.; Wesołowski, R.; Woźniak, A. The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. Int. J. Mol. Sci. 2023, 24, 4840. https://doi.org/10.3390/ijms24054840
Wróblewski M, Wróblewska J, Nuszkiewicz J, Pawłowska M, Wesołowski R, Woźniak A. The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. International Journal of Molecular Sciences. 2023; 24(5):4840. https://doi.org/10.3390/ijms24054840
Chicago/Turabian StyleWróblewski, Marcin, Joanna Wróblewska, Jarosław Nuszkiewicz, Marta Pawłowska, Roland Wesołowski, and Alina Woźniak. 2023. "The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases" International Journal of Molecular Sciences 24, no. 5: 4840. https://doi.org/10.3390/ijms24054840
APA StyleWróblewski, M., Wróblewska, J., Nuszkiewicz, J., Pawłowska, M., Wesołowski, R., & Woźniak, A. (2023). The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. International Journal of Molecular Sciences, 24(5), 4840. https://doi.org/10.3390/ijms24054840