Framing Heartaches: The Cardiac ECM and the Effects of Age
Abstract
1. Age and the Cardiac Extracellular Matrix—A Relationship That Needs More Attention
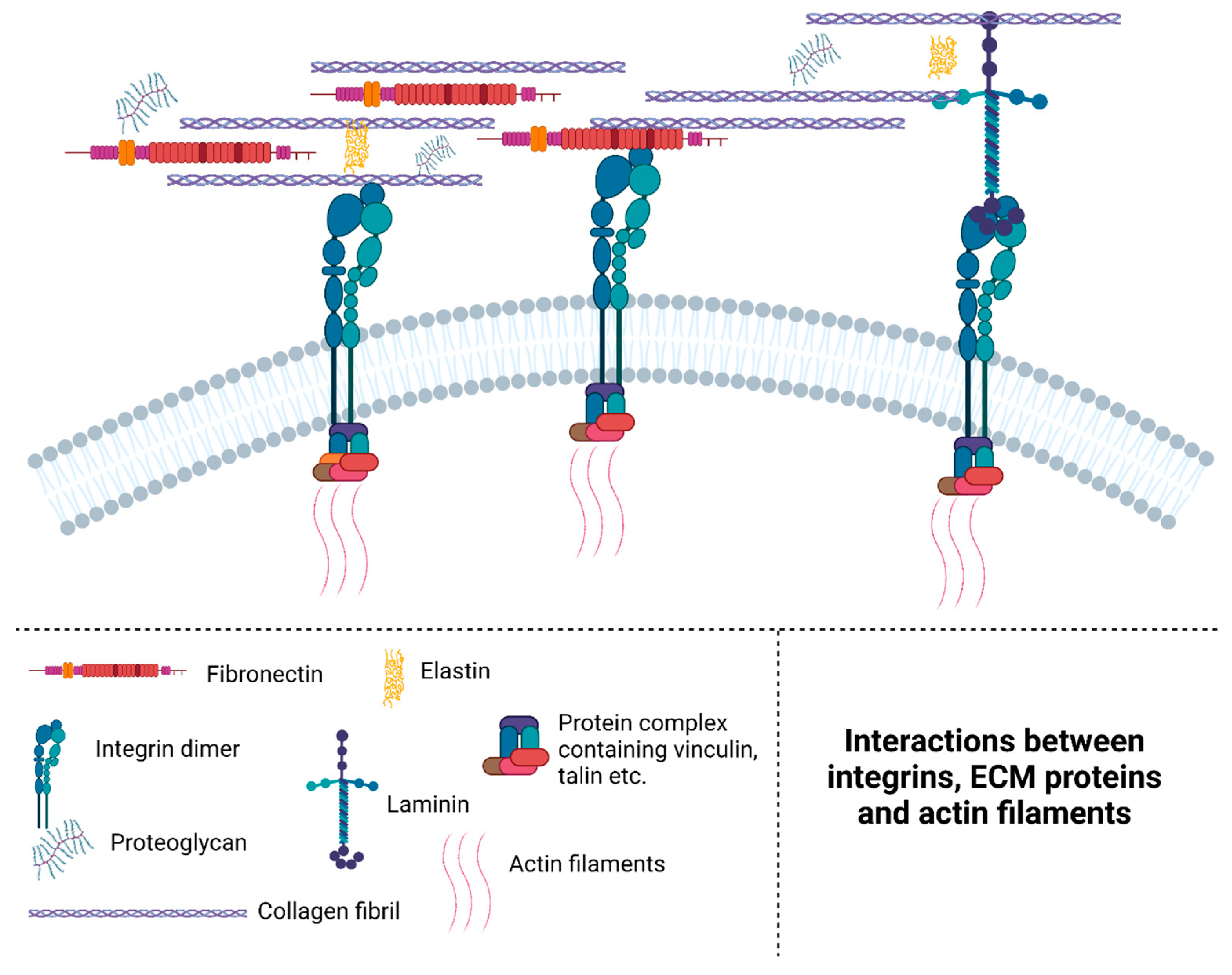
2. Components of the Cardiac ECM
2.1. Epimysium, Perimysium, Endomysium and the Basement Membrane
2.2. Glycoproteins of the Cardiac ECM
2.2.1. Collagen
2.2.2. SPARC
2.2.3. Fibronectin
2.2.4. Elastin
2.2.5. Laminin
2.2.6. Tenascin
2.2.7. Hyaluronan
2.3. Proteoglycans—Decorin, Biglycan and Periostin
2.4. Matrikines
| Structure and Location | Functions | References | |
|---|---|---|---|
| Epimysium, perimysium and endomysium | Thin, fibrous sheets. The epimysium surrounds the whole heart. The perimysium surrounds groups of cardiomyocytes. The endomysium surrounds individual cardiomyocytes. | Protection of the cardiomyocytes. Facilitates supply of nutrients by housing vasculature. | [11] |
| Basement membrane | Lamina lucida: Adjacent to the ECM. Consists mainly of laminins. Lamina densa: Adjacent to the cardiomyocytes. Consists mainly of collagen type IV. | Protection and support of individual cardiomyocytes. Aids cellular alignment, polarity and organisation, adhesion and migration. Enables cells to sense mechanical stimuli. Regulates electrical conduits. Facilitates cellular integrity by forming a semi-permeable membrane. | [12,13,14,15,16] |
| Collagen type I and III | Fibrillar collagens. Crosslinked. | Collagen type I lends strength and resilience, aids isolation of electrical currents. Collagen type III enhances flexible properties. | [19,20,21,22] |
| Collagen type IV and VI | Non-fibrillar collagens. | Collagen type IV is essential for the functions of the BM. Collagen type VI forms a mesh supporting collagen type I and III. | [13,24,26] |
| Fibronectin | Large (440–450 kDa), with several glycosylation sites and isomers. | Cellular adhesion, forms a framework which collagen is deposited upon and aids cellular communication. | [31,32,33,34] |
| Elastin | Disordered form in between contractions. Undergoes rapid crosslinking when stretched. Highly hydrophobic. | Aids elasticity to the heart. | [35,36] |
| Laminin | Large (several hundred kDa), with 3 domains forming a cross shape. | Connects proteins in the ECM and the BM; see Figure 1 for an overview of how laminin interacts with several different proteins. | [37,39] |
| Tenastinc | Large proteins (180–250 kDa) with several isotypes. | Mainly expressed during embryonic development and upregulated during injury. Interferes with the actions of fibronectin. | [40,41,42] |
| Hyaluronan | Anionic, nonsulphated glycosaminoglycan (GAG) | Adds shock absorbing properties to the ECM. Involved in wound healing, immune responses, cellular proliferation and migration. An excess of HA leads to an increased differentiation of myofibroblasts. | [44,45,46,47] |
| Decorin and biglycan | Core proteins surrounded by one chondroitin sulphate or dermatan sulphate. | Decorin modulates collagen type I and III by “decorating” the fibrils. These interactions between the proteins are essential for stability of the ECM. It also interacts with TGF-β through competitive binding, further affecting the ECM. Biglycan has the same functions, but studies have shown they are not interchangeable. | [52,53,54,55,56,57,58,59,60] |
| Periostin | Relatively large protein of ~90 kDa | Regulates collagen type I fibrillogenesis. Associated with differentiation of fibroblasts into myofibroblasts. | [61,62] |
| Matrikines | Varies. The result of incomplete proteolytic cleavage of different ECM proteins such as fibronectin. [63] | Varies. The function is often different from that of the original ECM protein. Glycyl-L-histodyl-L-lysine (GHK) is an important matrikine example. It is involved in stimulation of collagen type I, GAGs and MMP production. | [67,68,69,71] |
3. Remodelling of the Cardiac ECM—The Role of Fibroblasts, MMPs and TIMPs
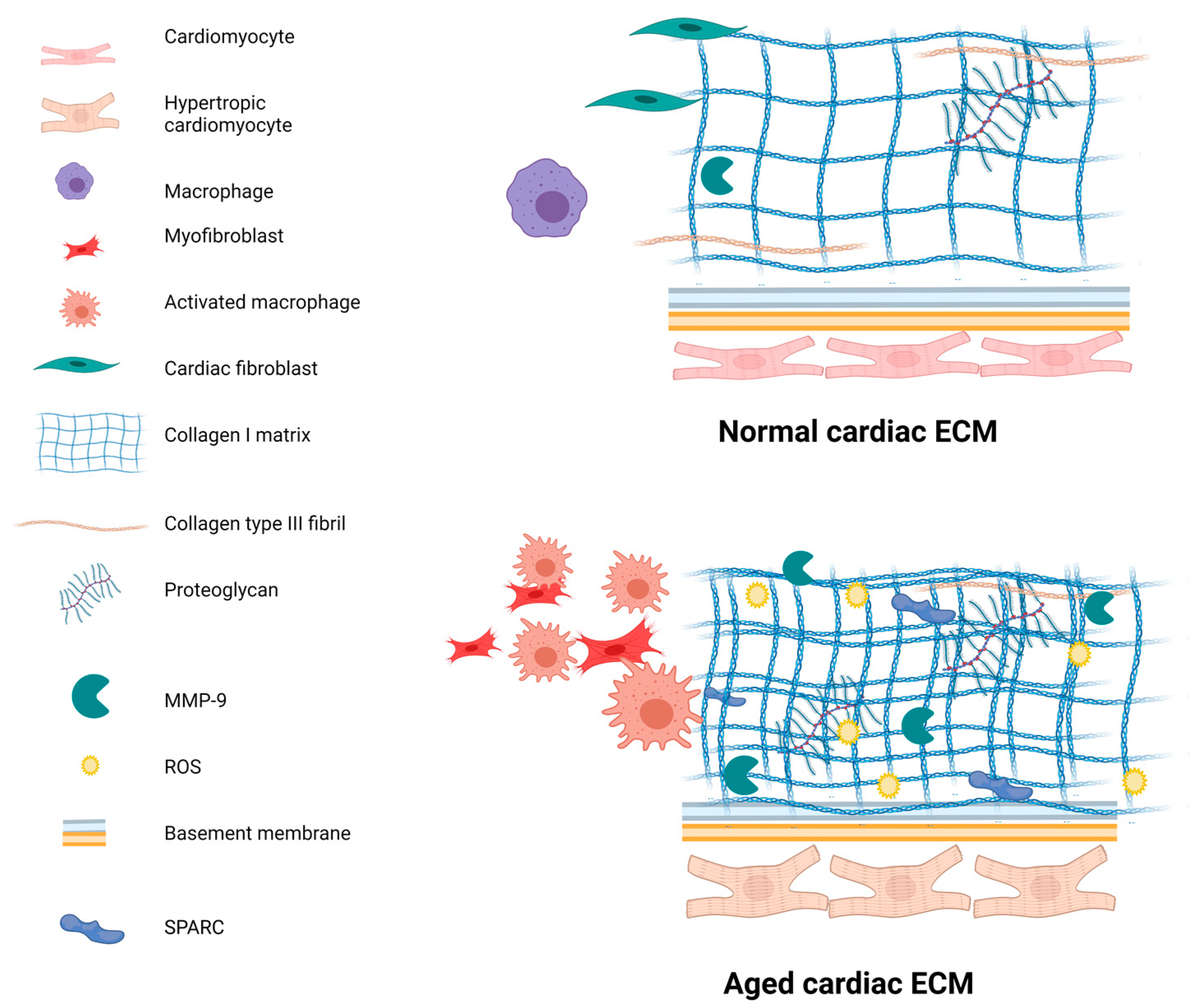
4. Changes with Age
4.1. Fibrosis
4.2. Increased Inflammation—The Involvement of Immune Cells and ROS
4.3. Altered Protein Levels in the ECM
4.4. Collagens and SPARC Changes with Age
| Changes with Age | Species | Anatomical Structure | References | |
|---|---|---|---|---|
| Collagen, overall | Increases | Mice, sheep, rats, humans, dogs | Left ventricle, atria, left atria | [27,106,107,108,109,115,116,117] |
| Collagen type I | Increases | Humans, sheep | Left ventricle | [27,111,112,113,114,115,116,117,118] |
| Collagen type III | Decreases in humans | Humans | Left ventricle | [118] |
| Collagen type IV and VI | Increases | Mice | Left ventricle | [116] |
| Fibronectin | Increases in mice, decreases in hamsters and rats | Mice, hamsters, rats | Left ventricle, left ventricle and atria in rats | [104,106,109] |
| Lminin | β1: Increases. Β2: Decreases | Mice | Left ventricle | [108] |
| Integrin | α1, α5: Increases. β1: Decreases | Mice | Left ventricle | [106] |
| Mimecan | Decreases | Mice | Whole hearts and left ventricle from females | [105,109] |
| SPARC | Increases | Sheep, mice | Left ventricle | [27,111] |
| Change with Age | Species | Anatomical Structure | References | |
|---|---|---|---|---|
| Collagen type I and III | Decreases in rats and hamsters. No change in sheep. No change in murine atria. | Rats, sheep and Syrian hamsters | Atria and ventricles. | [107,110,111,113] |
| Fibronectin | Decreases | Rats, hamsters, mice | Atria and ventricles. | [106,107,110] |
4.5. Changes in MMPs and TIMPs with Age
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karamichalakis, N.; Letsas, K.P.; Vlachos, K.; Georgopoulos, S.; Bakalakos, A.; Efremidis, M.; Sideris, A. Managing atrial fibrillation in the very elderly patient: Challenges and solutions. Vasc. Health Risk Manag. 2015, 11, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Rask-Madsen, C.; Jensen, G.; Kober, L.; Melchior, T.; Torp-Pedersen, C.; Hildebrand, P. Age-related mortality, clinical heart failure, and ventricular fibrillation in 4259 Danish patients after acute myocardial infarction. Eur. Heart J. 1997, 18, 1426–1431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Z. Aging, Arterial Stiffness, and Hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Pellman, J.; Lyon, R.C.; Sheikh, F. Extracellular matrix remodeling in atrial fibrosis: Mechanisms and implications in atrial fibrillation. J. Mol. Cell Cardiol. 2010, 48, 461–467. [Google Scholar] [CrossRef]
- Miner, E.C.; Miller, W.L. A look between the cardiomyocytes: The extracellular matrix in heart failure. Mayo Clin. Proc. 2006, 81, 71–76. [Google Scholar] [CrossRef]
- Wu, L.; Han, D.K. Overcoming the dynamic range problem in mass spectrometry-based shotgun proteomics. Expert Rev. Proteomics 2006, 3, 611–619. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Yan, Q.; Sage, E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 1999, 47, 1495–1505. [Google Scholar] [CrossRef]
- Jürgensen, H.J.; Madsen, D.H.; Ingvarsen, S.; Melander, M.C.; Gårdsvoll, H.; Patthy, L.; Engelholm, L.H.; Behrendt, N. A novel functional role of collagen glycosylation: Interaction with the endocytic collagen receptor uPARAP/ENDO180. J. Biol. Chem. 2011, 286, 32736–32748. [Google Scholar] [CrossRef]
- Hsiao, C.T.; Cheng, H.W.; Huang, C.M.; Li, H.R.; Ou, M.H.; Huang, J.R.; Khoo, K.H.; Yu, H.W.; Chen, Y.Q.; Wang, Y.K.; et al. Fibronectin in cell adhesion and migration via N-glycosylation. Oncotarget 2017, 8, 70653–70668. [Google Scholar] [CrossRef]
- Robinson, T.F.; Cohen-Gould, L.; Factor, S.M.; Eghbali, M.; Blumenfeld, O.O. Structure and function of connective tissue in cardiac muscle: Collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 1988, 2, 1005–1015. [Google Scholar] [PubMed]
- Battig, C.G.; Low, F.N. The ultrastructure of human cardiac muscle and its associated tissue space. Am. J. Anat. 1961, 108, 199–229. [Google Scholar] [CrossRef]
- Sage, H. Collagens of basement membranes. J. Investig. Dermatol. 1982, 79, 51–59. [Google Scholar] [CrossRef][Green Version]
- Paulson, M. Basement membrane proteins: Structure, assembly, and cellular interactions. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 93–127. [Google Scholar] [CrossRef]
- Boland, E.; Quondamatteo, F.; Van Agtmael, T. The role of basement membranes in cardiac biology and disease. Biosci. Rep. 2021, 41, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Borg, T.K.; Liu, H.; Gao, B.Z. Interactive relationship between basement-membrane development and sarcomerogenesis in single cardiomyocytes. Exp. Cell Res. 2015, 330, 222–232. [Google Scholar] [CrossRef]
- Frank, J.S.; Langer, G.A.; Nudd, L.M.; Seraydarian, K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its structure and cellular ionic exchange. Circ. Res. 1977, 41, 702–714. [Google Scholar] [CrossRef]
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Duffield, M. Dynamic Views. Pract. App. Dev. Aurelia 2018, 341, 185–194. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Qu, Z.; Weiss, J.N. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J. Mol. Cell Cardiol. 2014, 70, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Collier, P.; Watson, C.J.; van Es, M.H.; Phelan, D.; McGorrian, C.; Tolan, M.; Ledwidge, M.T.; McDonald, K.M.; Baugh, J.A. Getting to the heart of cardiac remodeling; how collagen subtypes may contribute to phenotype. J. Mol. Cell Cardiol. 2012, 52, 148–153. [Google Scholar] [CrossRef]
- Naugle, J.E.; Olson, E.R.; Zhang, X.; Mase, S.E.; Pilati, C.F.; Maron, M.B.; Folkesson, H.G.; Horne, W.I.; Doane, K.J.; Meszaros, J.G. Type VI collagen induces cardiac myofibroblast differentiation: Implications for postinfarction remodeling. Am. J. Physiol. Hear. Circ. Physiol. 2006, 290, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Levine, M. Topics in Dental Biochemistry. Top Dent. Biochem. 2011. [Google Scholar] [CrossRef]
- Olsen, B.R.; LuValle, P.A.; Jacenko, O. Role of Non-Fibrillar Collagens in Matrix Assemblies. In Tissue Engineering: Current Perspectives; Bell, E., Ed.; Birkhäuser Boston: Boston, MA, USA, 1993; pp. 19–25. [Google Scholar] [CrossRef]
- Mollnau, H.; Münkel, B.; Schaper, J. Collagen VI in the extracellular matrix of normal and failing human myocardium. Herz 1995, 20, 89–94. [Google Scholar]
- Bradshaw, A.D.; Baicu, C.F.; Rentz, T.J.; Van Laer, A.O.; Bonnema, D.D.; Zile, M.R. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: Role of SPARC in post-synthetic procollagen processing. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H614–H622. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Lane, T.F.; Pallero, M.A.; Sage, E.H. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J. Cell Biochem. 1995, 57, 341–350. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Xi, B.; Xue, J.; He, D.; Zhang, J.; Zhao, Y. SPARC is a key regulator of proliferation, apoptosis and invasion in human ovarian cancer. PLoS ONE 2012, 7, 1–15. [Google Scholar] [CrossRef]
- Muro, A.F.; Chauhan, A.K.; Gajovic, S.; Iaconcig, A.; Porro, F.; Stanta, G.; Baralle, F.E. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J. Cell Biol. 2003, 162, 149–160. [Google Scholar] [CrossRef]
- Johnson, C.M.; Helgeson, S.C. Fibronectin biosynthesis and cell-surface expression by cardiac and non- cardiac endothelial cells. Am. J. Pathol. 1993, 142, 1401–1408. [Google Scholar]
- Lin, J.; Wang, Y.; Qian, J. Effects of domain unfolding and catch-like dissociation on the collective behavior of integrin–fibronectin bond clusters. Acta Mech. Sin Xuebao 2021, 37, 229–243. [Google Scholar] [CrossRef]
- Barry, E.L.R.; Mosher, D.F. Factor XIIIa-mediated cross-linking of fibronectin in fibroblast cell layers. Cross-linking of cellular and plasma fibronectin and of amino-terminal fibronectin fragments. J. Biol. Chem. 1989, 264, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Cary, L.A.; Guan, J.L. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 1999, 4, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Suyama, K. Mechanism of formation of elastin crosslinks. Connect Tissue Res. 2000, 41, 131–141. [Google Scholar] [CrossRef]
- Rauscher, S.; Pomès, R. The liquid structure of elastin. Elife 2017, 6, 1–21. [Google Scholar] [CrossRef]
- Hunter, I.; Engel, J. Anatomy of a Multidomain Glycoprotein. FASEB J 2016, 4, 148–160. [Google Scholar]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.R.; et al. A simplified laminin nomenclature. Matrix. Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef]
- Mak, K.M.; Mei, R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. 2017, 300, 1371–1390. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in cardiovascular tissue remodeling—From development to inflammation and repair. Circ. J. 2012, 76, 2513–2520. [Google Scholar] [CrossRef]
- Chiquet-Ehrismann, R.; Kalla, P.; Pearson, C.A.; Beck, K.; Chiquet, M. Tenascin interferes with fibronectin action. Cell 1988, 53, 383–390. [Google Scholar] [CrossRef]
- De Laporte, L.; Rice, J.J.; Tortelli, F.; Hubbell, J.A. Tenascin C Promiscuously Binds Growth Factors via Its Fifth Fibronectin Type III-Like Domain. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Hunger, J.; Bernecker, A.; Bakker, H.J.; Bonn, M.; Richter, R.P. Hydration dynamics of hyaluronan and dextran. Biophys. J. 2012, 103, L10–L12. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Yoshizaki, A.; Komura, K.; Shimizu, K.; Ogawa, F.; Hara, T.; Muroi, E.; Bae, S.; Takenaka, M.; Yukami, T.; et al. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am. J. Pathol. 2009, 175, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wang, X.; Qin, L.; Guo, Z.; Li, D. Effect of molecular weight and concentration of hyaluronan on cell proliferation and osteogenic differentiation in vitro. Biochem. Biophys. Res. Commun. 2015, 465, 569–574. [Google Scholar] [CrossRef]
- Meran, S.; Thomas, D.W.; Stephens, P.; Enoch, S.; Martin, J.; Steadman, R.; Phillips, A.O. Hyaluronan facilitates transforming growth factor-β1- mediated fibroblast proliferation. J. Biol. Chem. 2008, 283, 6530–6545. [Google Scholar] [CrossRef]
- Midgley, A.C.; Rogers, M.; Hallett, M.B.; Clayton, A.; Bowen, T.; Phillips, A.O.; Steadman, R. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 2013, 288, 14824–14838. [Google Scholar] [CrossRef]
- Koch, C.D.; Lee, C.M.; Apte, S.S. Aggrecan in Cardiovascular Development and Disease. J. Histochem. Cytochem. 2020, 68, 777–795. [Google Scholar] [CrossRef]
- Mittal, N.; Yoon, S.H.; Enomoto, H.; Hiroshi, M.; Shimizu, A.; Kawakami, A.; Fujita, M.; Watanabe, H.; Fukuda, K.; Makino, S. Versican is crucial for the initiation of cardiovascular lumen development in medaka (Oryzias latipes). Sci. Rep. 2019, 9, 9475. [Google Scholar] [CrossRef]
- Nandadasa, S.; Foulcer, S.; Apte, S.S. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix. Biol. 2014, 35, 34–41. [Google Scholar] [CrossRef]
- Handler, M.; Yurchenco, P.D.; Iozzo, R.V. Developmental expression of perlecan during murine embryogenesis. Dev. Dyn. 1997, 210, 130–145. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Noble, N.A.; Stecker, K.K. Role of transforming growth factor beta and decorin in controlling fibrosis. Methods Enzym. 1994, 245, 241–254. [Google Scholar]
- Schmidt, G.; Hausser, H.; Kresse, H. Interaction of the small proteoglycan decorin with fibronectin. Involvement of the sequence NKISK of the core protein. Biochem. J. 1991, 280, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, E.; Hausser, H.; Beavan, L.; Kresse, H. Decorin-type I collagen interaction: Presence of separate core protein-binding domains. J. Biol. Chem. 1995, 270, 8877–8883. [Google Scholar] [CrossRef]
- Douglas, T.; Heinemann, S.; Bierbaum, S.; Scharnweber, D.; Worch, H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules 2006, 7, 2388–2393. [Google Scholar] [CrossRef]
- Robinson, K.A.; Sun, M.; Barnum, C.E.; Weiss, S.N.; Huegel, J.; Shetye, S.S.; Lin, L.; Saez, D.; Adams, S.M.; Iozzo, R.V.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix. Biol. 2017, 64, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, A.; Romarís, M.; Rasmussen, L.M.; Heinegård, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 1994, 302, 527–534. [Google Scholar] [CrossRef]
- Droguett, R.; Cabello-Verrugio, C.; Riquelme, C.; Brandan, E. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix. Biol. 2006, 25, 332–341. [Google Scholar] [CrossRef]
- Reed, C.C.; Iozzo, R.V. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002, 19, 249–255. [Google Scholar] [CrossRef]
- Norris, R.A.; Damon, B.; Mironov, V.; Kasyanov, V.; Moreno-rodriguez, R.; Trusk, T.; Potts, J.D.; Richard, L.; Davis, J.; Hoffman, S.; et al. Periostin Regulates Collagen Fibrillogenesis. J. Cell. Biochem. 2007, 101, 695–711. [Google Scholar] [CrossRef]
- Crawford, J.; Nygard, K.; Gan, B.S.; O’Gorman, D.B. Periostin induces fibroblast proliferation and myofibroblast persistence in hypertrophic scarring. Exp. Dermatol. 2015, 24, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity—Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Ambesi, A.; McKeown-Longo, P.J. Anastellin, the angiostatic fibronectin peptide, is a selective inhibitor of lysophospholipid signaling. Mol. Cancer Res. 2009, 7, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Neskey, D.M.; Ambesi, A.; Pumiglia, K.M.; McKeown-Longo, P.J. Endostatin and anastellin inhibit distinct aspects of the angiogenic process. J. Exp. Clin. Cancer Res. 2008, 27, 61. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.C.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Cangul, I.T.; Gul, N.Y.; Topal, A.; Yilmaz, R. Evaluation of the effects of topical tripeptide-copper complex and zinc oxide on open-wound healing in rabbits. Vet. Dermatol. 2006, 17, 417–423. [Google Scholar] [CrossRef]
- Canapp, S.O.; Farese, J.P.; Schultz, G.S.; Gowda, S.; Ishak, A.M.; Swaim, S.F.; Vangilder, J.; Lee-Ambrose, L.; Martin, F.G. The effect of topical tripeptide-copper complex on healing of ischemic open wounds. Vet. Surg. 2003, 32, 515–523. [Google Scholar] [CrossRef]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK and DNA: Resetting the Human Genome to Health. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Siméon, A.; Wegrowski, Y.; Bontemps, Y.; Maquart, F.X. Expression of glycosaminoglycans and small proteoglycans in wounds: Modulation by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. J. Investig. Dermatol. 2000, 115, 962–968. [Google Scholar] [CrossRef]
- Maquart, F.X.; Bellon, G.; Pasco, S.; Monboisse, J.C. Matrikines in the regulation of extracellular matrix degradation. Biochimie 2005, 87, 353–360. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Li, N.; Teng, W.; Wang, M.; Zhang, Y.; Xiao, Z. TGF-β1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011, 123, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Chen, W.; Su, Y.; Rai, V.; Uche, O.U.; Li, N.; Frangogiannis, N.G. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J. Immunol. 2013, 191, 4838–4848. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Michael, L.H.; Entman, M.L.; Frangogiannis, N.G. Morphological characteristics of the microvasculature in healing myocardial infarcts. J. Histochem. Cytochem. 2002, 50, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Hawkes, W.; Hu, J.; Megone, W.V.; Gautrot, J.; Anilkumar, N.; Zhang, M.; Hirvonen, L.; Cox, S.; Ehler, E.; et al. Cardiomyocytes Sense Matrix Rigidity through a Combination of Muscle and Non-muscle Myosin Contractions. Dev. Cell 2018, 44, 326–336.e3. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.; Kim, M.-S.; van Rooij, E.; Plato, C.; Papst, P.; Vega, R.; Mcanally, J.; Richardson, J.; Bassel-Duby, R.; Olson, E.; et al. Regulation of Cardiac Stress Signaling by Protein Kinase D1. Mol. Cell Biol. 2006, 26, 3875–3888. [Google Scholar] [CrossRef]
- Song, R.; Zhang, L. Cardiac ECM: Its Epigenetic Regulation and Role in Heart Development and Repair. Int. J. Mol. Sci. 2020, 21, 8610. [Google Scholar] [CrossRef]
- Hohn, J.; Tan, W.; Carver, A.; Barrett, H.; Carver, W. Roles of Exosomes in Cardiac Fibroblast Activation and Fibrosis. Cells 2021, 10, 2933. [Google Scholar] [CrossRef]
- Felisbino, M.B.; McKinsey, T.A. Epigenetics in Cardiac Fibrosis: Emphasis on Inflammation and Fibroblast Activation. JACC Basic to Transl. Sci. 2018, 3, 704–715. [Google Scholar] [CrossRef]
- Fanjul-Fernández, M.; Folgueras, A.R.; Cabrera, S.; López-Otín, C. Matrix metalloproteinases: Evolution, gene regulation and functional analysis in mouse models. Biochim. Biophys. Acta Mol. Cell Res. 2010, 1803, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Lovett, D.H.; Mahimkar, R.; Raffai, R.L.; Cape, L.; Maklashina, E.; Cecchini, G.; Karliner, J.S. A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Escobar, G.P.; Mukherjee, R.; Goshorn, D.K.; Sheats, N.J.; Bruce, J.A.; Mains, I.M.; Hendrick, J.K.; Hewett, K.W.; Gourdie, R.G.; et al. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 2006, 113, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- DeLeon-Pennell, K.Y.; de Castro Brás, L.E.; Iyer, R.P.; Bratton, D.R.; Jin, Y.F.; Ripplinger, C.M.; Lindsey, M.L.P. gingivalis lipopolysaccharide intensifies inflammation post-myocardial infarction through matrix metalloproteinase-9. J. Mol. Cell Cardiol. 2014, 76, 218–226. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Heymans, S.; Schroen, B.; Vermeersch, P.; Milting, H.; Gao, F.; Kassner, A.; Gillijns, H.; Herijgers, P.; Flameng, W.; Carmeliet, P.; et al. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 2005, 112, 1136–1144. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction—From repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Rai, R.; Sun, T.; Ramirez, V.; Lux, E.; Eren, M.; Vaughan, D.E.; Ghosh, A.K. Acetyltransferase p300 inhibitor reverses hypertension-induced cardiac fibrosis. J. Cell Mol. Med. 2019, 23, 3026–3031. [Google Scholar] [CrossRef]
- Hinderer, S.; Schenke-Layland, K. Cardiac fibrosis—A short review of causes and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 77–82. [Google Scholar] [CrossRef]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef]
- de Jong, S.; van Veen, T.A.B.; van Rijen, H.V.M.; de Bakker, J.M.T. Fibrosis and Cardiac Arrhythmias. J. Cardiovasc. Pharmacol. 2011, 57. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.C.; Persi, A.; Tung, M.; Masi, R.; Canitano, S.; Kol, A. Bradyarrhythmias in patients with SARS-CoV-2 infection: A narrative review and a clinical report. Pacing Clin. Electrophysiol. 2021, 44, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Kassiri, Z.; Patel, M.P.; Chappell, M.; Butany, J.; Backx, P.H.; Tsushima, R.G.; Scholey, J.W.; Khokha, R.; Penninger, J.M. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007, 75, 29–39. [Google Scholar] [CrossRef]
- Esfahani, N.S.; Wu, Q.; Kumar, N.; Ganesan, L.P.; Lafuse, W.P.; Rajaram, M.V.S. Aging influences the cardiac macrophage phenotype and function during steady state and during inflammation. Aging Cell 2021, 20, e13438. [Google Scholar] [CrossRef] [PubMed]
- Kuka, S.; Tatarkova, Z.; Racay, P.; Lehotsky, J.; Dobrota, D.; Kaplan, P. Effect of aging on formation of reactive oxygen species by mitochondria of rat heart. Gen. Physiol. Biophys. 2013, 32, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, F.; Preston, C.C.; Emelyanova, L.; Yousufuddin, M.; Viqar, M.; Dakwar, O.; Ross, G.R.; Faustino, R.S.; Holmuhamedov, E.L.; Jahangir, A. Effects of aging on cardiac oxidative stress and transcriptional changes in pathways of reactive oxygen species generation and clearance. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef]
- Jaskova, K.; Pavlovicova, M.; Jurkovicova, D. Electrophysiological variability in the SH-SY5Y cellular line. Gen. Physiol. Biophys. 2014, 31, 375–382. [Google Scholar] [CrossRef]
- Ozcebe, S.G.; Bahcecioglu, G.; Yue, X.S.; Zorlutuna, P. Effect of cellular and ECM aging on human iPSC-derived cardiomyocyte performance, maturity and senescence. Biomaterials 2021, 268. [Google Scholar] [CrossRef]
- Stegen, S.; Laperre, K.; Eelen, G.; Rinaldi, G.; Fraisl, P.; Torrekens, S.; Van Looveren, R.; Loopmans, S.; Bultynck, G.; Vinckier, S.; et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature 2019, 565, 511–515. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Bajpai, S.; Chaturvedi, P.; Wirtz, D.; Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 2013, 288, 10819–10829. [Google Scholar] [CrossRef]
- Brereton, C.J.; Ridley, R.; Conforti, F.; Yao, L.; Alzetani, A.; Marshall, B.; Fletcher, S.V.; Richeldi, L.; Wang, Y.; Davies, D.E.; et al. HIF pathway activation is a core regulator of collagen structure-function in lung fibrosis. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef]
- Schietke, R.; Warnecke, C.; Wacker, I.; Schödel, J.; Mole, D.R.; Campean, V.; Amann, K.; Goppelt-Struebe, M.; Behrens, J.; Eckardt, K.U.; et al. The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress E-cadherin in Hypoxia: Insights into cellular transformation processes mediated by HIF-1. J. Biol. Chem. 2010, 285, 6658–6669. [Google Scholar] [CrossRef] [PubMed]
- Mamuya, W.; Chobanian, A.; Brecher, P. Age-related changes in fibronectin expression in spontaneously hypertensive, Wistar-Kyoto, and Wistar rat hearts. Circ. Res. 1992, 71, 1341–1350. [Google Scholar] [CrossRef]
- Jazbutyte, V.; Fiedler, J.; Kneitz, S.; Galuppo, P.; Just, A.; Holzmann, A.; Bauersachs, J.; Thum, T. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age 2013, 35, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.L.; McCrea, J.C.; Hedrick, H.L. Age-associated changes in cardiac matrix and integrins. Mech. Ageing Dev. 2001, 122, 1739–1756. [Google Scholar] [CrossRef]
- Masutomo, K.; Makino, N.; Sugano, M.; Miyamoto, S.; Hata, T.; Yanaga, T. Extracellular matrix regulation in the development of Syrian cardiomyopathic Bio 14.6 and Bio 53.58 hamsters. J. Mol. Cell Cardiol. 1999, 31, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.U.G.; Chavakis, E.; Rogg, E.-M.; Muhly-Reinholz, M.; Glaser, S.F.; Günther, S.; John, D.; Bonini, F.; Zeiher, A.M.; Schaefer, L.; et al. Switch in Laminin β2 to Laminin β1 Isoforms During Aging Controls Endothelial Cell Functions—Brief Report. Arterioscler Thromb. Vasc. Biol. 2018, 38, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Deckx, S.; Heggermont, W.; Carai, P.; Rienks, M.; Dresselaers, T.; Himmelreich, U.; van Leeuwen, R.; Lommen, W.; van der Velden, J.; Gonzalez, A.; et al. Osteoglycin prevents the development of age-related diastolic dysfunction during pressure overload by reducing cardiac fibrosis and inflammation. Matrix. Biol. 2018, 66, 110–124. [Google Scholar] [CrossRef]
- Besse, S.; Robert, V.; Assayag, P.; Delcayre, C.; Swynghedauw, B. Nonsynchronous changes in myocardial collagen mRNA and protein during aging: Effect of DOCA-salt hypertension. Am. J. Physiol. 1994, 267, H2237–H2244. [Google Scholar] [CrossRef]
- Horn, M.A.; Graham, H.K.; Richards, M.A.; Clarke, J.D.; Greensmith, D.J.; Briston, S.J.; Hall, M.C.S.; Dibb, K.M.; Trafford, A.W. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: Collagen accumulation in the young and loss in the aged. J. Mol. Cell Cardiol. 2012, 53, 82–90. [Google Scholar] [CrossRef]
- Thomas, D.P.; McCormick, R.J.; Zimmerman, S.D.; Vadlamudi, R.K.; Gosselin, L.E. Aging- and training-induced alterations in collagen characteristics of rat left ventricle and papillary muscle. Am. J. Physiol. Circ. Physiol. 1992, 263, H778–H783. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.J.; Moghtadaei, M.; Mackasey, M.; Rafferty, S.A.; Bogachev, O.; Sapp, J.L.; Howlett, S.E.; Rose, R.A. Atrial structure, function and arrhythmogenesis in aged and frail mice. Sci. Rep. 2017, 7, 44336. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Chang, C.-X.; Zhou, X.; Gu, S.-K.; Jiang, T.-M.; Li, Y.-M. Characterization of atrial histopathological and electrophysiological changes in a mouse model of aging. Int. J. Mol. Med. 2013, 31, 138–146. [Google Scholar] [CrossRef]
- Wang, F.-F.; Han, Y.-F.; Liang, X.-Y.; Zhang, G.-G.; Lu, Y.-M.; Li, Y.-D.; Tang, B.-P. Aging-induced atrial fibrosis in I(f) current change and its effect on atrial fibrillation in dogs. Ann. Noninvasive Electrocardiol. 2022, 27, e12951. [Google Scholar] [CrossRef] [PubMed]
- De Castro Brás, L.E.; Toba, H.; Baicu, C.F.; Zile, M.R.; Weintraub, S.T.; Lindsey, M.L.; Bradshaw, A.D. Age and SPARC change the extracellular matrix composition of the left ventricle. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Eghbali, M.; Eghbali, M.; Robinson, T.F.; Seifter, S.; Blumenfeld, O.O. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc. Res. 1989, 23, 723–729. [Google Scholar] [CrossRef]
- Souza, M.E.; Araujo, R.C.; Souza, R.R.D.E. Original article quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Results Child Hearts Sect. Stain. Picrosirius 2012, 73, 216–220. [Google Scholar]
- Chiao, Y.A.; Dai, Q.; Zhang, J.; Lin, J.; Lopez, E.F.; Ahuja, S.S.; Chou, Y.-M.; Lindsey, M.L.; Jin, Y.-F. Multi-analyte profiling reveals matrix metalloproteinase-9 and monocyte chemotactic protein-1 as plasma biomarkers of cardiac aging. Circ. Cardiovasc. Genet 2011, 4, 455–462. [Google Scholar] [CrossRef]
- Ma, Y.; Chiao, Y.A.; Zhang, J.; Manicone, A.M.; Jin, Y.-F.; Lindsey, M.L. Matrix metalloproteinase-28 deletion amplifies inflammatory and extracellular matrix responses to cardiac aging. Microsc. Microanal. 2012, 18, 81–90. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Goshorn, D.K.; Squires, C.E.; Escobar, G.P.; Hendrick, J.W.; Mingoia, J.T.; Sweterlitsch, S.E.; Spinale, F.G. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc. Res. 2005, 66, 410–419. [Google Scholar] [CrossRef]
- Huet, E.; Huet, E.; Gabison, E.; Vallee, B.; Mougenot, N.; Linguet, G.; Riou, B.; Jarosz, C.; Menashi, S.; Besse, S.; et al. Deletion of extracellular matrix metalloproteinase inducer/cd147 induces altered cardiac extracellular matrix remodeling in aging mice. J. Physiol. Pharmacol. 2015, 66, 355–366. [Google Scholar] [PubMed]
- Bonnema, D.D.; Webb, C.S.; Pennington, W.R.; Stroud, R.E.; Leonardi, A.E.; Clark, L.L.; McClure, C.D.; Finklea, L.; Spinale, F.G.; Zile, M.R. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J. Card Fail 2007, 13, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Sundström, J.; Evans, J.C.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Sawyer, D.B.; Siwik, D.A.; Colucci, W.S.; Wilson, P.W.F.; Vasan, R.S. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: The Framingham heart study. Eur. Heart J. 2004, 25, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Tayebjee, M.H.; Lip, G.Y.H.; Blann, A.D.; MacFadyen, R.J. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb. Res. 2005, 115, 205–210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringström, N.; Edling, C.; Nalesso, G.; Jeevaratnam, K. Framing Heartaches: The Cardiac ECM and the Effects of Age. Int. J. Mol. Sci. 2023, 24, 4713. https://doi.org/10.3390/ijms24054713
Ringström N, Edling C, Nalesso G, Jeevaratnam K. Framing Heartaches: The Cardiac ECM and the Effects of Age. International Journal of Molecular Sciences. 2023; 24(5):4713. https://doi.org/10.3390/ijms24054713
Chicago/Turabian StyleRingström, Nathalie, Charlotte Edling, Giovanna Nalesso, and Kamalan Jeevaratnam. 2023. "Framing Heartaches: The Cardiac ECM and the Effects of Age" International Journal of Molecular Sciences 24, no. 5: 4713. https://doi.org/10.3390/ijms24054713
APA StyleRingström, N., Edling, C., Nalesso, G., & Jeevaratnam, K. (2023). Framing Heartaches: The Cardiac ECM and the Effects of Age. International Journal of Molecular Sciences, 24(5), 4713. https://doi.org/10.3390/ijms24054713






