The Emerging Role of MMP12 in the Oral Environment
Abstract
1. Introduction
2. Expression of MMP12 in Different Tissues and Cell Types
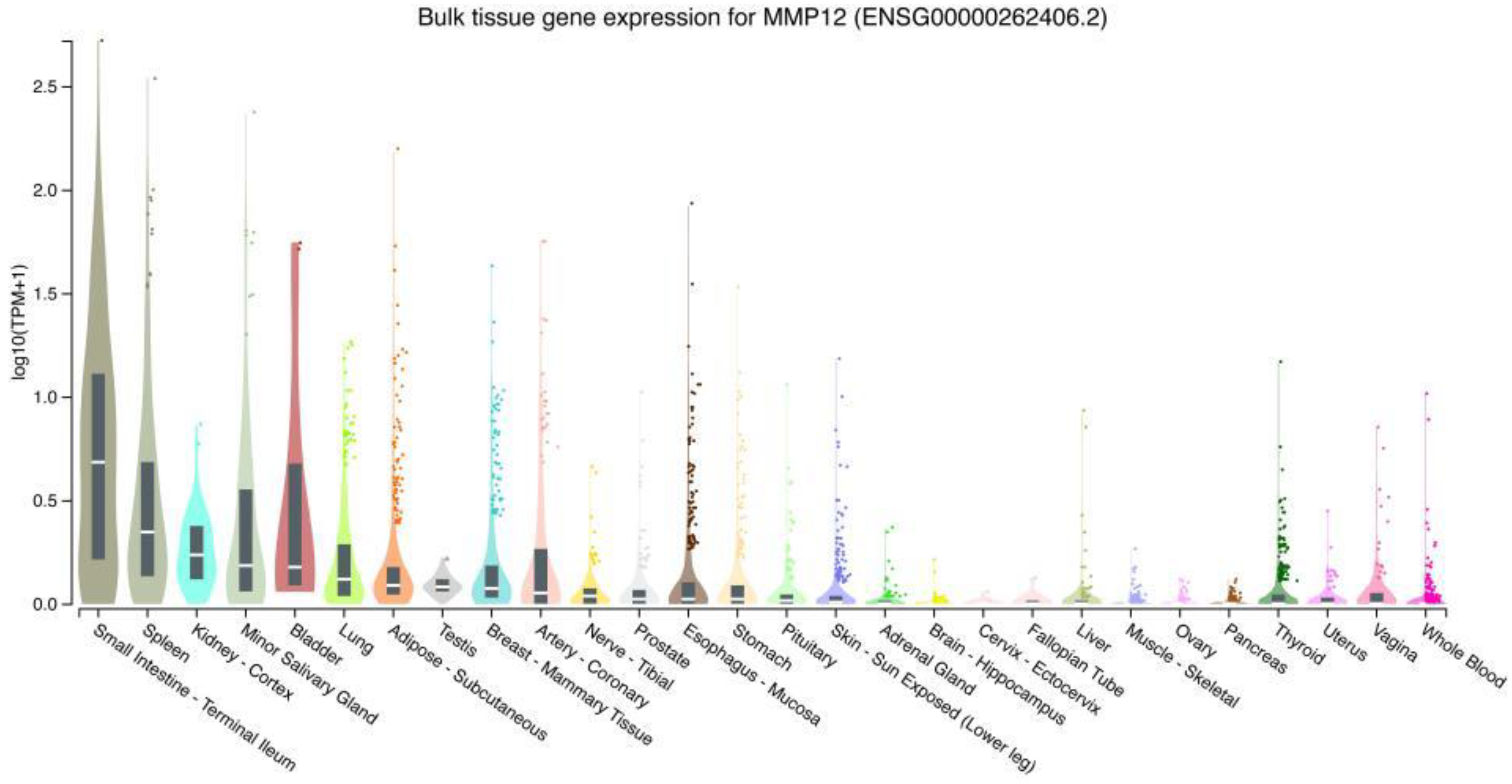
2.1. Expression of MMP12 in Normal Human Tissues
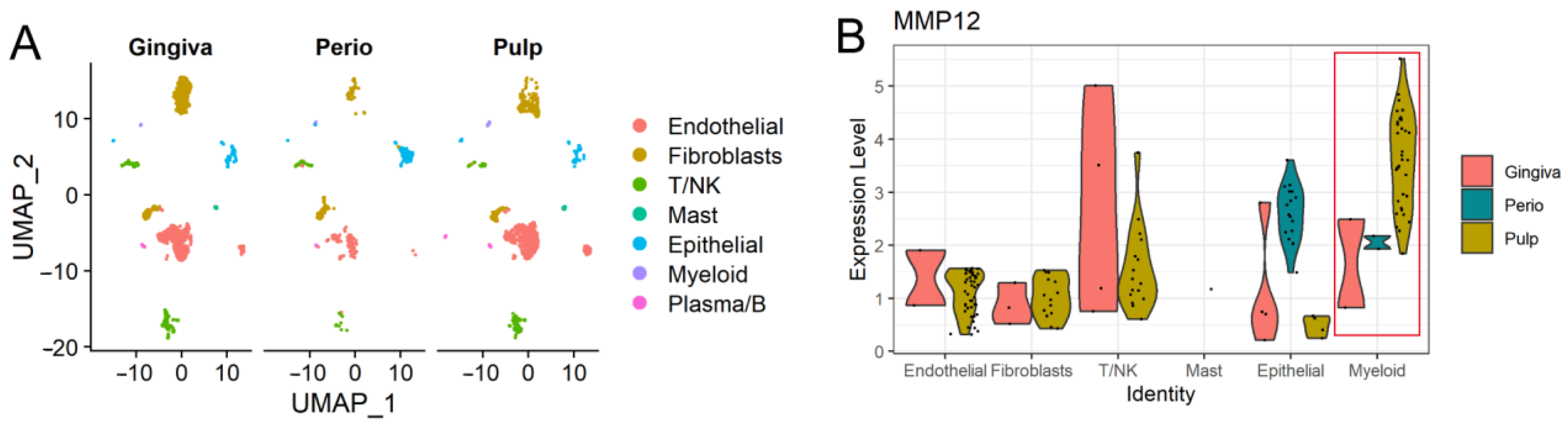
2.2. MMP12 in Oral Tissues
3. Role of MMP12 in the Oral Environment, Diseases, and Cancers
3.1. MMP12 in Periodontal Disease
3.2. MMP12 in Bone Remodelling
3.3. MMP12 in Orthodontic Tooth Movement
3.4. MMP12, the Connection with Oral Squamous Cell Carcinoma (OSCC)
3.5. MMP12 in Temporomandibular Joint Dysfunction (TMD)
3.6. MMP12 in Other Oral Diseases
4. Targeting MMP12 as the Therapeutic Strategy for Oral Diseases
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belaaouaj, A.; Shipley, J.M.; Kobayashi, D.K.; Zimonjic, D.B.; Popescu, N.; Silverman, G.A.; Shapiro, S.D. Human Macrophage Metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J. Biol. Chem. 1995, 270, 14568–14575. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.M.; Hartzell, W.O.; Robbins, C.S.; Gomis-Rüth, F.X.; Shapiro, S.D. Macrophage elastase kills bacteria within murine macrophages. Nature 2009, 460, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Zhang, C.-P.; Li, B.-J.; Jiang, S.-S.; He, W.-H.; Long, S.-Y.; Tian, Y. MMP-12 as a potential biomarker to forecast ischemic stroke in obese patients. Med. Hypotheses 2020, 136, 109524. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Nalamolu, K.R.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. MMP-12, a Promising Therapeutic Target for Neurological Diseases. Mol. Neurobiol. 2018, 55, 1405–1409. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kobayashi, T.; Katoh, M.; Saito, S.; Ikeda, Y.; Kobori, M.; Masuho, Y.; Watanabe, T. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: Relationship to lesion development. Am. J. Pathol. 1998, 153, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Warhekar, A.; Dillard, M.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci. Rep. 2015, 5, 9504. [Google Scholar] [CrossRef]
- Chen, Y.E. MMP-12, An Old Enzyme Plays a New Role in the Pathogenesis of Rheumatoid Arthritis? Am. J. Pathol. 2004, 165, 1069–1070. [Google Scholar] [CrossRef]
- Shipley, J.M.; Wesselschmidt, R.L.; Kobayashi, D.K.; Ley, T.J.; Shapiro, S.D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 3942–3946. [Google Scholar] [CrossRef]
- Nighot, M.; Ganapathy, A.S.; Saha, K.; Suchanec, E.; Castillo, E.F.; Gregory, A.; Shapiro, S.; Ma, T.; Nighot, P. Matrix Metalloproteinase MMP-12 Promotes Macrophage Transmigration Across Intestinal Epithelial Tight Junctions and Increases Severity of Experimental Colitis. J. Crohn’s Colitis 2021, 15, 1751–1765. [Google Scholar] [CrossRef]
- Spix, B.; Butz, E.S.; Chen, C.-C.; Rosato, A.S.; Tang, R.; Jeridi, A.; Kudrina, V.; Plesch, E.; Wartenberg, P.; Arlt, E.; et al. Lung emphysema and impaired macrophage elastase clearance in mucolipin 3 deficient mice. Nat. Commun. 2022, 13, 318. [Google Scholar] [CrossRef]
- Hautamaki, R.D.; Kobayashi Dale, K.; Senior Robert, M.; Shapiro Steven, D. Requirement for Macrophage Elastase for Cigarette Smoke-Induced Emphysema in Mice. Science 1997, 277, 2002–2004. [Google Scholar] [CrossRef] [PubMed]
- Madlener, M.; Parks, W.C.; Werner, S. Matrix Metalloproteinases (MMPs) and Their Physiological Inhibitors (TIMPs) Are Differentially Expressed during Excisional Skin Wound Repair. Exp. Cell Res. 1998, 242, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Sampath, D.; Yu Lin, M.O.; Bilton, M.; Huang, C.-K.; Nai, M.H.; Njah, K.; Goy, P.-A.; Wang, C.-C.; Guccione, E.; et al. Agrin-Matrix Metalloproteinase-12 axis confers a mechanically competent microenvironment in skin wound healing. Nat. Commun. 2021, 12, 6349. [Google Scholar] [CrossRef]
- Houghton, A.M.; Grisolano, J.L.; Baumann, M.L.; Kobayashi, D.K.; Hautamaki, R.D.; Nehring, L.C.; Cornelius, L.A.; Shapiro, S.D. Macrophage Elastase (Matrix Metalloproteinase-12) Suppresses Growth of Lung Metastases. Cancer Res. 2006, 66, 6149–6155. [Google Scholar] [CrossRef]
- Dean, R.A.; Cox, J.H.; Bellac, C.L.; Doucet, A.; Starr, A.E.; Overall, C.M. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: Potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood 2008, 112, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.J.; Bellac, C.L.; Moraes, T.J.; Wadsworth, S.J.; Dufour, A.; Butler, G.S.; Bilawchuk, L.M.; Hendry, R.G.; Robertson, A.G.; Cheung, C.T.; et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014, 20, 493–502. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, K.; Huang, L.; Sun, K.; Zou, Y.; Xiong, Z.; Li, B. Virtual Screening and Discovery of Matrix Metalloproteinase-12 Inhibitors by Swarm Intelligence Optimization Algorithm-Based Machine Learning. ChemistrySelect 2020, 5, 11112–11119. [Google Scholar] [CrossRef]
- Vaalamo, M.; Kariniemi, A.-L.; Saarialho-Kere, U.; Shapiro, S.D. Enhanced Expression of Human Metalloelastase (MMP-12) in Cutaneous Granulomas and Macrophage Migration. J. Investig. Dermatol. 1999, 112, 499–505. [Google Scholar] [CrossRef]
- Liu, M.; Sun, H.; Wang, X.; Koike, T.; Mishima, H.; Ikeda, K.; Watanabe, T.; Ochiai, N.; Fan, J. Association of increased expression of macrophage elastase (matrix metalloproteinase 12) with rheumatoid arthritis. Arthritis Rheum. 2004, 50, 3112–3117. [Google Scholar] [CrossRef]
- Chiba, Y.; Yu, Y.; Sakai, H.; Misawa, M. Increase in the Expression of Matrix Metalloproteinase-12 in the Airways of Rats with Allergic Bronchial Asthma. Biol. Pharm. Bull. 2007, 30, 318–323. [Google Scholar] [CrossRef]
- Nelson, M.P.; Christmann, B.S.; Dunaway, C.W.; Morris, A.; Steele, C. Experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 303, L469–L475. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Nuttall, R.K.; Liu, S.; Edwards, D.R.; Yong, V.W. Tenascin-C Stimulates Glioma Cell Invasion through Matrix Metalloproteinase-12. Cancer Res. 2006, 66, 11771–11780. [Google Scholar] [CrossRef] [PubMed]
- Kerkelä, E.; Ala-aho, R.; Jeskanen, L.; Rechardt, O.; Grénman, R.; Shapiro, S.D.; Kähäri, V.-M.; Saarialho-Kere, U. Expression of Human Macrophage Metalloelastase (MMP-12) by Tumor Cells in Skin Cancer. J. Investig. Dermatol. 2000, 114, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Balaz, P.; Friess, H.; Kondo, Y.; Zhu, Z.; Zimmermann, A.; Büchler, M.W. Human Macrophage Metalloelastase Worsens the Prognosis of Pancreatic Cancer. Ann. Surg. 2002, 235, 519–527. [Google Scholar] [CrossRef]
- Ding, Y.; Shimada, Y.; Gorrin-Rivas, M.J.; Itami, A.; Li, Z.; Hong, T.; Maeda, M.; Komoto, I.; Kawabe, A.; Kaganoi, J.; et al. Clinicopathological Significance of Human Macrophage Metalloelastase Expression in Esophageal Squamous Cell Carcinoma. Oncology 2002, 63, 378–384. [Google Scholar] [CrossRef]
- Liang, J.; Liu, E.; Yu, Y.; Kitajima, S.; Koike, T.; Jin, Y.; Morimoto, M.; Hatakeyama, K.; Asada, Y.; Watanabe, T.; et al. Macrophage Metalloelastase Accelerates the Progression of Atherosclerosis in Transgenic Rabbits. Circulation 2006, 113, 1993–2001. [Google Scholar] [CrossRef]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix Metalloproteinase-12 Induces Blood–Brain Barrier Damage After Focal Cerebral Ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef]
- Wells, J.E.A.; Rice, T.K.; Nuttall, R.K.; Edwards, D.R.; Zekki, H.; Rivest, S.; Yong, V.W. An Adverse Role for Matrix Metalloproteinase 12 after Spinal Cord Injury in Mice. J. Neurosci. 2003, 23, 10107. [Google Scholar] [CrossRef]
- Wells, J.E.A.; Biernaskie, J.; Szymanska, A.; Larsen, P.H.; Yong, V.W.; Corbett, D. Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. Eur. J. Neurosci. 2005, 21, 187–196. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Feinberg, M.W.; Jain, M.K.; Werner, F.; Sibinga, N.E.; Wiesel, P.; Wang, H.; Topper, J.N.; Perrella, M.A.; Lee, M.E. Transforming growth factor-beta 1 inhibits cytokine-mediated induction of human metalloelastase in macrophages. J. Biol. Chem. 2000, 275, 25766–25773. [Google Scholar] [CrossRef] [PubMed]
- Unemori, E.N.; Hibbs, M.S.; Amento, E.P. Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. J. Clin. Investig. 1991, 88, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Pagenstecher, A.; Stalder, A.K.; Kincaid, C.L.; Shapiro, S.D.; Campbell, I.L. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am. J. Pathol. 1998, 152, 729–741. [Google Scholar] [PubMed]
- Kerkelä, E.; Ala-aho, R.; Klemi, P.; Grénman, S.; Shapiro, S.D.; Kähäri, V.-M.; Saarialho-Kere, U. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J. Pathol. 2002, 198, 258–269. [Google Scholar] [CrossRef]
- KerkelÄ, E.; Böhling, T.; Herva, R.; Uria, J.A.; Saarialho-Kere, U. Human macrophage metalloelastase (MMP-12) expression is induced in chondrocytes during fetal development and malignant transformation. Bone 2001, 29, 487–493. [Google Scholar] [CrossRef]
- Björnfot Holmström, S.; Clark, R.; Zwicker, S.; Bureik, D.; Kvedaraite, E.; Bernasconi, E.; Nguyen Hoang, A.T.; Johannsen, G.; Marsland, B.J.; Boström, E.A.; et al. Gingival Tissue Inflammation Promotes Increased Matrix Metalloproteinase-12 Production by CD200; Monocyte-Derived Cells in Periodontitis. J. Immunol. 2017, 199, 4023. [Google Scholar] [CrossRef]
- Wan, Z.; Jiang, D.; Chen, S.; Jiao, J.; Ji, L.; Shah, A.S.; Wei, H.; Yang, X.; Li, X.; Wang, Y.; et al. T-box transcription factor brachyury promotes tumor cell invasion and metastasis in non-small cell lung cancer via upregulation of matrix metalloproteinase 12. Oncol. Rep. 2016, 36, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; E, C.; Yao, Y.; Ren, S.; Wang, G.; Jin, H. Matrix metalloproteinase expression and molecular interaction network analysis in gastric cancer. Oncol. Lett. 2016, 12, 2403–2408. [Google Scholar] [CrossRef]
- Clark, R.; Lira-Junior, R.; Johannsen, G.; Boström, E.A. Colony-stimulating factor-1 receptor blockade attenuates inflammation in inflamed gingival tissue explants. J. Periodontal Res. 2021, 56, 1141–1153. [Google Scholar] [CrossRef]
- Narimiya, T.; Wada, S.; Kanzaki, H.; Ishikawa, M.; Tsuge, A.; Yamaguchi, Y.; Nakamura, Y. Orthodontic tensile strain induces angiogenesis via type IV collagen degradation by matrix metalloproteinase-12. J. Periodontal Res. 2017, 52, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Troen, T.; Ovejero, M.C.; Kirkegaard, T.; Andersen, T.L.; Byrjalsen, I.; Ferreras, M.; Sato, T.; Shapiro, S.D.; Foged, N.T.; et al. Matrix metalloproteinase-12 (MMP-12) in osteoclasts: New lesson on the involvement of MMPs in bone resorption. Bone 2004, 34, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Palosaari, H.; Pennington, C.J.; Larmas, M.; Edwards, D.R.; Tjäderhane, L.; Salo, T. Expression profile of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in mature human odontoblasts and pulp tissue. Eur. J. Oral Sci. 2003, 111, 117–127. [Google Scholar] [CrossRef]
- Kobayashi, K.; Jokaji, R.; Miyazawa-Hira, M.; Takatsuka, S.; Tanaka, A.; Ooi, K.; Nakamura, H.; Kawashiri, S. Elastin-derived peptides are involved in the processes of human temporomandibular disorder by inducing inflammatory responses in synovial cells. Mol. Med. Rep. 2017, 16, 3147–3154. [Google Scholar] [CrossRef]
- Yamashita-Futani, Y.; Jokaji, R.; Ooi, K.; Kobayashi, K.; Kanakis, I.; Liu, K.; Kawashiri, S.; Bou-Gharios, G.; Nakamura, H. Metalloelastase-12 is involved in the temporomandibular joint inflammatory response as well as cartilage degradation by aggrecanases in STR/Ort mice. Biomed. Rep. 2021, 14, 51. [Google Scholar] [CrossRef]
- Almeida, R.C.; Capelli, J., Jr.; Teles, R.P. Levels of gingival crevicular fluid matrix metalloproteinases in periodontally compromised teeth under orthodontic forces. Angle Orthod. 2015, 85, 1009–1014. [Google Scholar] [CrossRef]
- Kim, K.-N.; Kim, J.-Y.; Cha, J.-Y.; Choi, S.-H.; Kim, J.; Cho, S.-W.; Hwang, C.-J. Antifibrotic effects of sulforaphane treatment on gingival elasticity reduces orthodontic relapse after rotational tooth movement in beagle dogs. Korean J. Orthod. 2020, 50, 391–400. [Google Scholar] [CrossRef]
- Canavarro, C.; Teles, R.P.; Capelli Júnior, J. Matrix metalloproteinases -1, -2, -3, -7, -8, -12, and -13 in gingival crevicular fluid during orthodontic tooth movement: A longitudinal randomized split-mouth study. Eur. J. Orthod. 2013, 35, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Suda, N. Comprehensive gene expression analysis in human periodontal ligaments of the mandibular third molars performing vertical movement and the maxillary second premolars with occlusal contact. Orthod. Craniofacial Res. 2008, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Shaikh, A.H.; Zaman, U.; Ahmed, S.; Majeed, M.M.; Kazmi, A.; Farooqui, W.A. Estimation of salivary matrix metalloproteinases- 12 (MMP- 12) levels among patients presenting with oral submucous fibrosis and oral squamous cell carcinoma. BMC Oral Health 2021, 21, 205. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Q.; Chen, J.; Yi, P.; Xu, X.; Fan, Y.; Cui, B.; Yu, Y.; Li, X.; Du, Y.; et al. Salivary protease spectrum biomarkers of oral cancer. Int. J. Oral Sci. 2019, 11, 7. [Google Scholar] [CrossRef]
- Chen, X.; Lei, H.; Cheng, Y.; Fang, S.; Sun, W.; Zhang, X.; Jin, Z. CXCL8, MMP12, and MMP13 are common Biomarkers of Periodontitis and Oral Squamous Cell Carcinoma. Oral Dis. 2022, 2022, 14419. [Google Scholar] [CrossRef]
- Holmström, S.B.; Lira-Junior, R.; Zwicker, S.; Majster, M.; Gustafsson, A.; Åkerman, S.; Klinge, B.; Svensson, M.; Boström, E.A. MMP-12 and S100s in saliva reflect different aspects of periodontal inflammation. Cytokine 2019, 113, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kubota, T.; Nakasone, N.; Abe, D.; Morozumi, T.; Yoshie, H. Microarray and quantitative RT-PCR analyses in calcium-channel blockers induced gingival overgrowth tissues of periodontitis patients. Arch. Oral Biol. 2011, 56, 277–284. [Google Scholar] [CrossRef]
- Gonçalves, P.F.; Huang, H.; McAninley, S.; Alfant, B.; Harrison, P.; Aukhil, I.; Walker, C.; Shaddox, L.M. Periodontal Treatment Reduces Matrix Metalloproteinase Levels in Localized Aggressive Periodontitis. J. Periodontol. 2013, 84, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Windsor, L.J. Porphyromonas gingivalis affects host collagen degradation by affecting expression, activation, and inhibition of matrix metalloproteinases. J. Periodontal Res. 2006, 41, 47–54. [Google Scholar] [CrossRef]
- Jurairutporn, T.; Suwanwela, J. Whole Genome Gene Expression: Histologic and Immunohistologic Study of Grafted Bone Using Allograft and Xenograft. Int. J. Oral. Maxillofac. Implant. 2021, 36, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-M.; Lee, J.-H.; Jeon, M.; Song, J.S.; Kim, S.-O. The Effect of MMP-13, MMP-12, and AMBN on Gingival Enlargement and Root Deformation In a New Type of Gingival Fibromatosis. J. Clin. Pediatr. Dent. 2018, 42, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ahn, S.H.; Lee, J.S.; Song, J.E.; Cho, S.H.; Jung, S.; Kim, S.K.; Kim, S.H.; Lee, K.P.; Kwon, K.S.; et al. Differential Matrix Metalloprotease (MMP) Expression Profiles Found in Aged Gingiva. PLoS ONE 2016, 11, 0158777. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, J.; Wu, Z.; Chan, K.-G.; Wang, L.; Li, J.; Lee, L.-H.; Law, J.W.-F. Potential Roles of Selectins in Periodontal Diseases and Associated Systemic Diseases: Could They Be Targets for Immunotherapy? Int. J. Mol. Sci. 2022, 23, 14280. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Arce, R.M. Periodontal inflammation: Integrating genes and dysbiosis. Periodontology 2000 2020, 82, 129–142. [Google Scholar] [CrossRef]
- Williams, R.C.; Skelton, A.J.; Todryk, S.M.; Rowan, A.D.; Preshaw, P.M.; Taylor, J.J. Leptin and Pro-Inflammatory Stimuli Synergistically Upregulate MMP-1 and MMP-3 Secretion in Human Gingival Fibroblasts. PLoS ONE 2016, 11, e0148024. [Google Scholar] [CrossRef]
- Wisithphrom, K.; Windsor, L.J. The Effects of Tumor Necrosis Factor-α, Interleukin-1β, Interleukin-6, and Transforming Growth Factor-β1 on Pulp Fibroblast Mediated Collagen Degradation. J. Endod. 2006, 32, 853–861. [Google Scholar] [CrossRef]
- Steenport, M.; Khan, K.M.F.; Du, B.; Barnhard, S.E.; Dannenberg, A.J.; Falcone, D.J. Matrix Metalloproteinase (MMP)-1 and MMP-3 Induce Macrophage MMP-9: Evidence for the Role of TNF-α and Cyclooxygenase-2. J. Immunol. 2009, 183, 8119. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Zheng, T.; Zhu, Z.; Liu, W.; Lee, C.G.; Ma, B.; Chen, Q.; Homer, R.J.; Wang, J.; Rabach, L.A.; et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13–induced inflammation and remodeling. J. Clin. Investig. 2002, 110, 463–474. [Google Scholar] [CrossRef]
- Guan, C.; Xiao, Y.; Li, K.; Wang, T.; Liang, Y.; Liao, G. MMP-12 regulates proliferation of mouse macrophages via the ERK/P38 MAPK pathways during inflammation. Exp. Cell Res. 2019, 378, 182–190. [Google Scholar] [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of oral microbiome in periodontal health and periodontitis: A critical review on prevention and treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Lau, A.W.Y.; Tan, L.T.H.; Ab Mutalib, N.S.; Wong, S.H.; Letchumanan, V.; Lee, L.H. The chemistry of gut microbiome in health and diseases. Prog. Microbes Mol. Biol. 2021, 4, 175. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef] [PubMed]
- Uitto, V.J.; Baillie, D.; Wu, Q.; Gendron, R.; Grenier, D.; Putnins, E.E.; Kanervo, A.; Firth, J.D. Fusobacterium nucleatum increases collagenase 3 production and migration of epithelial cells. Infect Immun. 2005, 73, 1171–1179. [Google Scholar] [CrossRef]
- Signat, B.; Roques, C.; Poulet, P.; Duffaut, D. Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 2011, 13, 25–36. [Google Scholar]
- Ganther, S.; Radaic, A.; Malone, E.; Kamarajan, P.; Chang, N.-Y.N.; Tafolla, C.; Zhan, L.; Fenno, J.C.; Kapila, Y.L. Treponema denticola dentilisin triggered TLR2/MyD88 activation upregulates a tissue destructive program involving MMPs via Sp1 in human oral cells. PLoS Pathog. 2021, 17, e1009311. [Google Scholar] [CrossRef]
- Li, Z.; Gurung, M.; Rodrigues, R.R.; Padiadpu, J.; Newman, N.K.; Manes, N.P.; Pederson, J.W.; Greer, R.L.; Vasquez-Perez, S.; You, H.; et al. Microbiota and adipocyte mitochondrial damage in type 2 diabetes are linked by Mmp12+ macrophages. J. Exp. Med. 2022, 219, 17. [Google Scholar] [CrossRef]
- Umeda, J.E.; Demuth, D.R.; Ando, E.S.; Faveri, M.; Mayer, M.P. Signaling transduction analysis in gingival epithelial cells after infection with Aggregatibacter actinomycetemcomitans. Mol. Oral. Microbiol. 2012, 27, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.; Cossins, J.; Lury, J.; Wells, G. Macrophage Metalloelastase Degrades Matrix and Myelin Proteins and Processes a Tumour Necrosis Factor-α Fusion Protein. Biochem. Biophys. Res. Commun. 1996, 228, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Ikeda, Y.; Nara, H.; Kato, M.; Kumegawa, M.; Nojima, H.; Kawashima, H. Large scale isolation of osteoclast-specific genes by an improved method involving the preparation of a subtracted cDNA library. Genes Cells 1998, 3, 459–475. [Google Scholar] [CrossRef]
- Husmann, K.; Arlt, M.J.E.; Muff, R.; Langsam, B.; Bertz, J.; Born, W.; Fuchs, B. Matrix Metalloproteinase 1 promotes tumor formation and lung metastasis in an intratibial injection osteosarcoma mouse model. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 347–354. [Google Scholar] [CrossRef]
- Vidal, C.M.P.; Tjäderhane, L.; Scaffa, P.M.; Tersariol, I.L.; Pashley, D.; Nader, H.B.; Nascimento, F.D.; Carrilho, M.R. Abundance of MMPs and Cysteine Cathepsins in Caries-affected Dentin. J. Dent. Res. 2013, 93, 269–274. [Google Scholar] [CrossRef]
- Wang, X.; Liang, J.; Koike, T.; Sun, H.; Ichikawa, T.; Kitajima, S.; Morimoto, M.; Shikama, H.; Watanabe, T.; Sasaguri, Y.; et al. Overexpression of Human Matrix Metalloproteinase-12 Enhances the Development of Inflammatory Arthritis in Transgenic Rabbits. Am. J. Pathol. 2004, 165, 1375–1383. [Google Scholar] [CrossRef]
- Janusz, M.J.; Hare, M.; Durham, S.L.; Potempa, J.; McGraw, W.; Pike, R.; Travis, J.; Shapiro, S.D. Cartilage proteoglycan degradation by a mouse transformed macrophage cell line is mediated by macrophage metalloelastase. Inflamm. Res. 1999, 48, 280–288. [Google Scholar] [CrossRef]
- Takei, I.; Takagi, M.; Santavirta, S.; Ida, H.; Ishii, M.; Ogino, T.; Ainola, M.; Konttinen, Y.T. Messenger ribonucleic acid expression of 16 matrix metalloproteinases in bone–implant interface tissues of loose artificial hip joints. J. Biomed. Mater. Res. 2000, 52, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.S.; Meng, X.; Li, F.; Venardos, N.; Fullerton, D.; Jaggers, J. MMP-12-Induced Pro-osteogenic Responses in Human Aortic Valve Interstitial Cells. J. Surg Res. 2019, 235, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, M.; Liu, C.-J.; Ilalov, K.; Egol, K.A. Matrix Metalloproteinases That Associate With and Cleave Bone Morphogenetic Protein-2 In Vitro Are Elevated in Hypertrophic Fracture Nonunion Tissue. J. Orthop. Trauma 2010, 24, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.R.; Zhang, X.; Dumpit, R.; Coleman, I.; Lakely, B.; Roudier, M.; Higano, C.S.; True, L.D.; Lange, P.H.; Montgomery, B.; et al. Characterization of osteoblastic and osteolytic proteins in prostate cancer bone metastases. Prostate 2013, 73, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wu, P.; Xiao, S.; Zhang, W.; Li, Y.; Ren, B.; Li, Z.; Xia, K.; Wang, B. Matrix Metalloproteinases in Relation to Bone Mineral Density: A Two-Sample Mendelian Randomization Study. Front. Genet. 2021, 12, 754795. [Google Scholar] [CrossRef]
- Vasiliadis, E.S.; Kaspiris, A.; Grivas, T.B.; Khaldi, L.; Lamprou, M.; Pneumaticos, S.G.; Nikolopoulos, K.; Korres, D.S.; Papadimitriou, E. Expression of macrophage elastase (MMP12) in rat tail intervertebral disc and growth plate after asymmetric loading. Bone Jt. Res. 2014, 3, 273–279. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.H.; Hung, W.C.; Paul, C.; Zhu, F.; Guan, P.P.; Huso, D.L.; Kontrogianni-Konstantopoulos, A.; Konstantopoulos, K. Fluid shear promotes chondrosarcoma cell invasion by activating matrix metalloproteinase 12 via IGF-2 and VEGF signaling pathways. Oncogene 2015, 34, 4558–4569. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, X.; Li, H.; Liu, F.; Zhu, H.; Song, X.; Niu, Z.; Ni, Q.; Yang, C.; Lu, J. Role of Matrix Metallopeptidase 12 in the Development of Hepatocellular Carcinoma. J. Investig. Surg. 2021, 34, 366–372. [Google Scholar] [CrossRef]
- Hung, W.-Y.; Lee, W.-J.; Cheng, G.-Z.; Tsai, C.-H.; Yang, Y.-C.; Lai, T.-C.; Chen, J.-Q.; Chung, C.-L.; Chang, J.-H.; Chien, M.-H. Blocking MMP-12-modulated epithelial-mesenchymal transition by repurposing penfluridol restrains lung adenocarcinoma metastasis via uPA/uPAR/TGF-β/Akt pathway. Cell. Oncol. 2021, 44, 1087–1103. [Google Scholar] [CrossRef]
- Nabha, S.M.; dos Santos, E.B.; Yamamoto, H.A.; Belizi, A.; Dong, Z.; Meng, H.; Saliganan, A.; Sabbota, A.; Bonfil, R.D.; Cher, M.L. Bone marrow stromal cells enhance prostate cancer cell invasion through type I collagen in an MMP-12 dependent manner. Int. J. Cancer 2008, 122, 2482–2490. [Google Scholar] [CrossRef]
- Lv, F.Z.; Wang, J.L.; Wu, Y.; Chen, H.F.; Shen, X.Y. Knockdown of MMP12 inhibits the growth and invasion of lung adenocarcinoma cells. Int. J. Immunopathol. Pharmacol. 2015, 28, 77–84. [Google Scholar] [CrossRef]
- Kahlert, C.; Pecqueux, M.; Halama, N.; Dienemann, H.; Muley, T.; Pfannschmidt, J.; Lasitschka, F.; Klupp, F.; Schmidt, T.; Rahbari, N.; et al. Tumour-site-dependent expression profile of angiogenic factors in tumour-associated stroma of primary colorectal cancer and metastases. Br. J. Cancer 2014, 110, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.C.; Chen, L.-C.; Chung, A.-K.; Chao, M.; Huang, H.-Y.; Hsueh, C.; Tsang, N.-M.; Chang, K.-P.; Liang, Y.; Li, H.-P.; et al. Matrix metalloproteinase 12 is induced by heterogeneous nuclear ribonucleoprotein K and promotes migration and invasion in nasopharyngeal carcinoma. BMC Cancer 2014, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.-H.; Wu, K.; Yang, R.; Liu, Z.-Q.; Cao, W. Differential expression of matrix metalloproteinases and miRNAs in the metastasis of oral squamous cell carcinoma. BMC Oral. Health 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Impola, U.; Uitto, V.J.; Hietanen, J.; Hakkinen, L.; Zhang, L.; Larjava, H.; Isaka, K.; Saarialho-Kere, U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J. Pathol. 2004, 202, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Gynther, G.W.; Holmlund, A.B.; Reinholt, F.P.; Lindblad, S. Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: A clinical, arthroscopic, histologic, and immunohistochemical study. Int. J. Oral Maxillofac. Surg. 1997, 26, 10–16. [Google Scholar] [CrossRef]
- Dijkgraaf, L.C.; Liem, R.S.B.; de Bont, L.G.M. Synovial membrane involvement in osteoarthritic temporomandibular joints: A light microscopic study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 373–386. [Google Scholar] [CrossRef]
- Pereira, F.J.; Lundh, H.; Eriksson, L.; Westesson, P.-L. Microscopic changes in the retrodiscal tissues of painful temporomandibular joints. J. Oral Maxillofac. Surg. 1996, 54, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kaspiris, A.; Khaldi, L.; Chronopoulos, E.; Vasiliadis, E.; Grivas, T.B.; Kouvaras, I.; Dagkas, S.; Papadimitriou, E. Macrophage-specific metalloelastase (MMP-12) immunoexpression in the osteochondral unit in osteoarthritis correlates with BMI and disease severity. Pathophysiology 2015, 22, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Yamasaki, M.; Nakata, K.; Tsuji, M.; Nakamura, H. The Expression of Macrophage and Neutrophil Elastases in Rat Periradicular Lesions. J. Endod. 2008, 34, 1072–1076. [Google Scholar] [CrossRef]
- Kim, S.-G. Poster 095: Multiple Unerupted Teeth Related to the Under-Expression of MMP-3, MMP-12, and the Over-Expression of FGF-5. J. Oral Maxillofac. Surg. 2007, 65, 43.e56. [Google Scholar] [CrossRef]
- Kreiborg, S.; Jensen, B.L. Tooth formation and eruption—lessons learnt from cleidocranial dysplasia. Eur. J. Oral Sci. 2018, 126, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; Houari, S.; Loiodice, S.; Thuy, T.T.; Le Normand, M.; Berdal, A.; Babajko, S. Chronic Exposure to Bisphenol A Exacerbates Dental Fluorosis in Growing Rats. J. Bone Miner. Res. 2016, 31, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjäderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Pietrzykowska, A.; Zalewska, A.; Knaś, M.; Daniszewska, I. The Significance of Matrix Metalloproteinases in Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 383–390. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.-O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wu, Y.; Wu, J.; Hotchandani, R.; Cunningham, K.; McFadyen, I.; Bard, J.; Morgan, P.; Schlerman, F.; et al. A Selective Matrix Metalloprotease 12 Inhibitor for Potential Treatment of Chronic Obstructive Pulmonary Disease (COPD): Discovery of (S)-2-(8-(Methoxycarbonylamino)dibenzo[b,d]furan-3-sulfonamido)-3-methylbutanoic acid (MMP408). J. Med. Chem. 2009, 52, 1799–1802. [Google Scholar] [CrossRef]
- Baggio, C.; Velazquez, J.V.; Fragai, M.; Nordgren, T.M.; Pellecchia, M. Therapeutic Targeting of MMP-12 for the Treatment of Chronic Obstructive Pulmonary Disease. J. Med. Chem. 2020, 63, 12911–12920. [Google Scholar] [CrossRef]
- Le Quément, C.; Guénon, I.; Gillon, J.Y.; Valença, S.; Cayron-Elizondo, V.; Lagente, V.; Boichot, E. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br. J. Pharmacol. 2008, 154, 1206–1215. [Google Scholar] [CrossRef]
- Devel, L.; Rogakos, V.; David, A.; Makaritis, A.; Beau, F.; Cuniasse, P.; Yiotakis, A.; Dive, V. Development of Selective Inhibitors and Substrate of Matrix Metalloproteinase-12. J. Biol. Chem. 2006, 281, 11152–11160. [Google Scholar] [CrossRef]
- Johnson, J.L.; Devel, L.; Czarny, B.; George, S.J.; Jackson, C.L.; Rogakos, V.; Beau, F.; Yiotakis, A.; Newby, A.C.; Dive, V. A Selective Matrix Metalloproteinase-12 Inhibitor Retards Atherosclerotic Plaque Development in Apolipoprotein E–Knockout Mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 528–535. [Google Scholar] [CrossRef]
- Amar, S.; Fields, G.B. Potential clinical implications of recent matrix metalloproteinase inhibitor design strategies. Expert Rev. Proteom. 2015, 12, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ezra, D.G.; Burton, M.J.; Bailly, M. Doxycycline Prevents Matrix Remodeling and Contraction by Trichiasis-Derived Conjunctival Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4675–4682. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Ryan, M.E. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol. Res. 2011, 63, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.Y.E.; Letchumanan, V.; Tan, L.T.H.; Law, J.W.F. Gut Microbiome in Obsessive Compulsive Disorder: Potential of Probiotics as an Adjuvant Therapy. Prog. Microbes Mol. Biol. 2022, 5, 272. [Google Scholar] [CrossRef]
- Matsubara, V.H.; Bandara, H.M.H.N.; Ishikawa, K.H.; Mayer, M.P.A.; Samaranayake, L.P. The role of probiotic bacteria in managing periodontal disease: A systematic review. Expert Rev. Anti-Infect. Ther. 2016, 14, 643–655. [Google Scholar] [CrossRef]
- Alshareef, A.; Attia, A.; Almalki, M.; Alsharif, F.; Melibari, A.; Mirdad, B.; Azab, E.; Youssef, A.-R.; Dardir, A. Effectiveness of Probiotic Lozenges in Periodontal Management of Chronic Periodontitis Patients: Clinical and Immunological Study. Eur. J. Dent. 2020, 14, 281–287. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.T.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Aimbire, F.; Carvalho, J.L.; Fialho, A.K.; Miranda, M.; Albertini, R.; Keller, A. Role of probiotics Bfidobacterium breve and Lactobacillus rhmanosus on lung inflammation and airway remodeling in an experimental model of chronic obstructive pulmonary disease. Eur. Respir. J. 2019, 54, PA2452. [Google Scholar]

| Year | Disease Type | Diagnostic Method | Findings | Reference |
|---|---|---|---|---|
| 2017 | Closed lock disc disease in the temporomandibular joint | ELISA (Human TMJ synovial fluid) | Increasing MMP12 expression was found in the inflamed TMJ models | [43] |
| 2021 | Temporomandibular joint dysfunction | IHC | MMP12 was expressed in the chondrocytes in the superficial zones of the cartilage | [44] |
| 2015 | Periodontally compromised teeth | Multiplex bead immunoassay | Significant increase of MMP12, 24 h after activation of periodontitis teeth subjected to orthodontic treatment | [45] |
| 2020 | Orthodontic relapse | Quantitative RT-PCR, HE, Masson’s Trichrome staining | Sulforaphane (SFN) reduced the amount of relapse of orthodontic rotation via upregulated MMP12 by increasing gingival elasticity | [46] |
| 2013 | Orthodontic tooth movement | GCF multiplexed bead immunoassay | The mean levels of MMP12 were not significantly different between the test and control groups at each time shown | [47] |
| 2017 | Orthodontic tooth movement | IHC, Quantitative RT-PCR, micro-CT | Orthodontic tensile strain upregulates MMP12 expression in the tension zone of PDL and induces angiogenesis | [40] |
| 2008 | Healthy orthodontic treatment | Quantitative RT-PCR | MMP12 showed more than 100-fold higher expression in PDLs of the second premolars with occlusal contact than the third molars performing a vertical movement | [48] |
| 2021 | OSCC, oral submucous fibrosis (OSF) | ELISA (Saliva) | Increased expression of MMP12 appears as the healthy patient advances to OSF and OSCC | [49] |
| 2019 | OSCC, CPD, JBO, healthy | ELISA (Saliva), WB, IHF | The levels of MMP12 in the saliva of patients with OSCC were significantly increased compared with those of other groups | [50] |
| 2022 | Periodontitis and OSCC | Bioinformatic analysis | MMP12 was identified as one of the hub genes between PD and OSCC | [51] |
| 2017 | Periodontitis | Quantitative RT-PCR, IHF, ELISA, WB | The connection of MMP12 production by monocyte-derived cells with CD200/CD200R pathway may play a crucial role in PD progression | [36] |
| 2019 | Periodontal disease | ELISA (Saliva) | MMP12 level in saliva reflect different aspects of periodontal inflammation | [52] |
| 2011 | Gingival overgrowth periodontitis | Microarray, Quantitative RT-PCR | MMP12 was significantly upregulated in gingival overgrowth tissues of periodontitis patients compared with the healthy control gingival tissues | [53] |
| 2013 | Localized aggressive periodontitis | MMP Enzymatic Assay | MMP12 in GCF was reduced significantly up to 6 months, comparable to healthy sites at the same point | [54] |
| 2003 | Young healthy patients | Quantitative RT-PCR, WB | No MMP12 expression in odontoblasts or pulp tissue | [42] |
| 2006 | Periodontal disease | Quantitative RT-PCR, WB | MMP12 mRNA was induced in the P. gingivalis-treated human gingiva fibroblasts | [55] |
| 2021 | Mouse calvaria defects | Microarray, Quantitative RT-PCR, IHC | DFDBA and DBBM groups showed a higher mRNA and protein level of MMP12 compared with the control | [56] |
| 2018 | Idiopathic gingival fibromatosis | Microarray Quantitative RT-PCR IHC | Low MMP12 expression in the idiopathic gingival fibromatosis | [57] |
| 2016 | Aged gingiva | Quantitative RT-PCR, RNA sequencing | MMP12 was upregulated in old gingival tissues, concomitantly with interleukin-1 beta expression | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Ser, H.L.; Wang, L.; Li, J.; Chan, K.-G.; Lee, L.-H.; Tan, L.T.-H. The Emerging Role of MMP12 in the Oral Environment. Int. J. Mol. Sci. 2023, 24, 4648. https://doi.org/10.3390/ijms24054648
Lin B, Ser HL, Wang L, Li J, Chan K-G, Lee L-H, Tan LT-H. The Emerging Role of MMP12 in the Oral Environment. International Journal of Molecular Sciences. 2023; 24(5):4648. https://doi.org/10.3390/ijms24054648
Chicago/Turabian StyleLin, Bingpeng, Hooi Leng Ser, Lijing Wang, Jiang Li, Kok-Gan Chan, Learn-Han Lee, and Loh Teng-Hern Tan. 2023. "The Emerging Role of MMP12 in the Oral Environment" International Journal of Molecular Sciences 24, no. 5: 4648. https://doi.org/10.3390/ijms24054648
APA StyleLin, B., Ser, H. L., Wang, L., Li, J., Chan, K.-G., Lee, L.-H., & Tan, L. T.-H. (2023). The Emerging Role of MMP12 in the Oral Environment. International Journal of Molecular Sciences, 24(5), 4648. https://doi.org/10.3390/ijms24054648









