Stem Cells in Kidney Ischemia: From Inflammation and Fibrosis to Renal Tissue Regeneration
Abstract
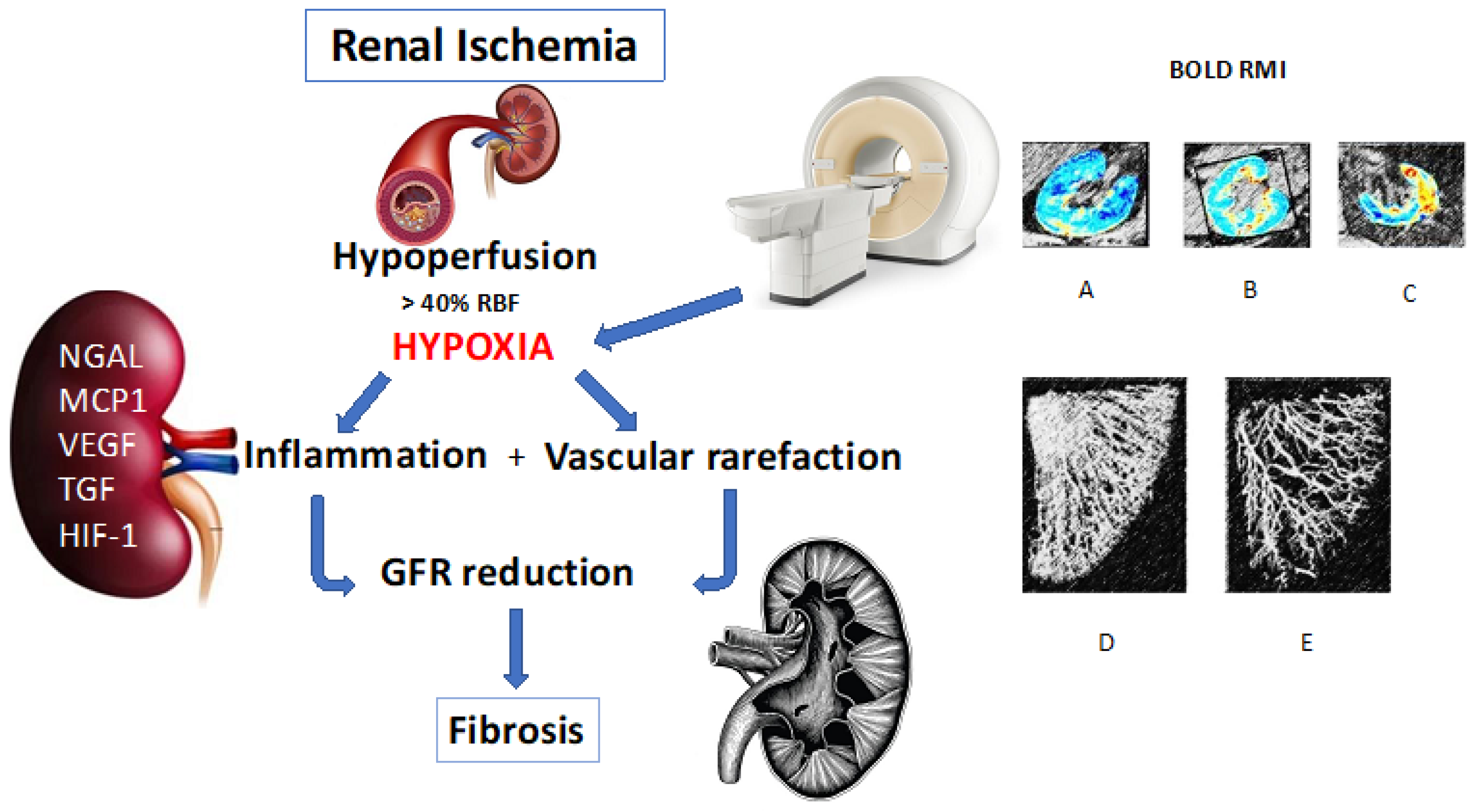
1. Introduction
2. Renal Hypoxia
2.1. How to Evaluate and Quantify the Hypoxic State and Inflammatory Cell Activity
2.2. Role of Mesenchymal and Resident Renal Stem Cells in Kidney Inflammation and Repair
2.3. Mesenchymal and Renal Stem Cell Use in Renal Ischemia
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Onofrio, G.; Simeoni, M.; Rizza, P.; Caroleo, M.; Capria, M.; Mazzitello, G.; Sacco, T.; Mazzuca, E.; Panzino, M.T.; Cerantonio, A.; et al. Quality of life, clinical outcome, personality and coping in chronic hemodialysis patients. Ren. Fail. 2017, 39, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.C.; Lerman, L.O. The Role of Hypoxia in Ischemic Chronic Kidney Disease. Semin. Nephrol. 2019, 39, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.; Textor, S.C.; Beckman, J.A.; Casanegra, A.I.; Cooper, C.J.; Kim, E.S.H.; Luther, J.M.; Misra, S.; Oderich, G.S.; American Heart Association Council on the Kidney in Cardiovascular Disease; et al. Revascularization for Renovascular Disease: A Scientific Statement From the American Heart Association. Hypertension 2022, 79, e128–e143. [Google Scholar] [CrossRef]
- Cianci, R.; Simeoni, M.; Zingaretti, V.; Bagordo, D.; Barbano, B.; Granatelli, A.; Gigante, A.; Lai, S. Resistant hypertension: Drug-eluting balloon for revascularization of bilateral renal fibromuscular dysplasia. QJM Int. J. Med. 2020, 114, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Textor, S.C.; Lerman, L. Renovascular hypertension and ischemic nephropathy. Am. J. Hypertens. 2010, 23, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Rosato, E.; Gigante, A.; Barbano, B.; Gasperini, M.L.; Cianci, R.; Muscaritoli, M. Prognostic Factors of Renal Involvement in Systemic Sclerosis. Kidney Blood Press. Res. 2018, 43, 682–689. [Google Scholar] [CrossRef]
- Abumoawad, A.; Saad, A.; Ferguson, C.M.; Eirin, A.; Woollard, J.R.; Herrmann, S.M.; Hickson, L.J.; Bendel, E.C.; Misra, S.; Glockner, J.; et al. Tissue hypoxia, inflammation, and loss of glomerular filtration rate in human atherosclerotic renovascular disease. Kidney Int. 2019, 95, 948–957. [Google Scholar] [CrossRef]
- Cianci, R.; Simeoni, M.; Gigante, A.; Marco Perrotta, A.; Ronchey, S.; Mangialardi, N.; Schioppa, A.; De Marco, O.; Cianci, E.; Barbati, C.; et al. Renal Stem Cells, Renal Resistive Index, and Neutrophil Gelatinase Associated Lipocalin Changes After Revascularization in Patients With Renovascular Hypertension and Ischemic Nephropathy. Curr. Pharm. Des. 2022, 29, 133–138. [Google Scholar] [CrossRef]
- Hicks, C.W.; Clark, T.W.I.; Cooper, C.J.; de Bhailis, A.M.; De Carlo, M.; Green, D.; Malyszko, J.; Miglinas, M.; Textor, S.C.; Herzog, C.A.; et al. Atherosclerotic Renovascular Disease: A KDIGO (Kidney Disease: Improving Global Outcomes) Controversies Conference. Am. J. Kidney Dis. 2022, 79, 289–301. [Google Scholar] [CrossRef]
- Foley, R.N.; Collins, A.J. End-stage renal disease in the United States: An update from the United States Renal Data System. J. Am. Soc. Nephrol. 2007, 18, 2644–2648. [Google Scholar] [CrossRef]
- Gigante, A.; Lai, S.; Pellicano, C.; De Marco, O.; Rosato, E.; Giannakakis, K.; D’Amati, G.; Muscaritoli, M.; Ferri, C.; Cianci, R. Nephroangiosclerosis not related to hypertension: A matter to resolve in the era of precision medicine. J. Hum. Hypertens. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, M.; Borrelli, S.; Garofalo, C.; Fuiano, G.; Esposito, C.; Comi, A.; Provenzano, M. Atherosclerotic-nephropathy: An updated narrative review. J. Nephrol. 2021, 34, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Liu, B.C. Hypoxia and Renal Tubulointerstitial Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Capolongo, G.; Suzumoto, Y.; D’Acierno, M.; Simeoni, M.; Capasso, G.; Zacchia, M. ERK1,2 Signalling Pathway along the Nephron and Its Role in Acid-base and Electrolytes Balance. Int. J. Mol. Sci. 2019, 20, 4153. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.S. Hypertensive nephrosclerosis. Curr. Opin. Nephrol. Hypertens. 2008, 17, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Neusser, M.A.; Lindenmeyer, M.T.; Moll, A.G.; Segerer, S.; Edenhofer, I.; Sen, K.; Stiehl, D.P.; Kretzler, M.; Grone, H.J.; Schlondorff, D.; et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. Am. J. Pathol. 2010, 176, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, X.; Song, Y.; Xie, G.; Jiao, S.; Shi, L.; Cao, X.; Han, X.; Qu, A. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol. Ther. 2022, 238, 108186. [Google Scholar] [CrossRef]
- Palomaki, S.; Pietila, M.; Laitinen, S.; Pesala, J.; Sormunen, R.; Lehenkari, P.; Koivunen, P. HIF-1alpha is upregulated in human mesenchymal stem cells. Stem Cells 2013, 31, 1902–1909. [Google Scholar] [CrossRef]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1alpha overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 373. [Google Scholar] [CrossRef]
- Nensén, O.; Hansell, P.; Palm, F. Intrarenal oxygenation determines kidney function during the recovery from an ischemic insult. Am. J. Physiol. Ren. Physiol. 2020, 319, F1067–F1072. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Perrotta, A.M.; Gigante, A.; Errigo, F.; Ferri, C.; Cianci, E.; Simeoni, M.; Mazzaferro, S.; Lai, S. Ischemic Nephropaty: The Role of the Renal Artery Stenosis Revascularization on Renal Stem Cells. Medicina 2021, 57, 944. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, L.; Friederich-Persson, M.; Fasching, A.; Liss, P.; Shoji, K.; Nangaku, M.; Hansell, P.; Palm, F. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J. Am. Soc. Nephrol. 2015, 26, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Haase, V.H. Hypoxia-inducible factors in the kidney. Am. J. Physiol. Ren. Physiol. 2006, 291, F271–F281. [Google Scholar] [CrossRef]
- Pruijm, M.; Milani, B.; Pivin, E.; Podhajska, A.; Vogt, B.; Stuber, M.; Burnier, M. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018, 93, 932–940. [Google Scholar] [CrossRef]
- Kinsey, G.R. Macrophage dynamics in AKI to CKD progression. J. Am. Soc. Nephrol. 2014, 25, 209–211. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Tanaka, T.; Nangaku, M. Renal Hypoxia in CKD.; Pathophysiology and Detecting Methods. Front. Physiol. 2017, 8, 99. [Google Scholar] [CrossRef]
- Nacu, N.; Luzina, I.G.; Highsmith, K.; Lockatell, V.; Pochetuhen, K.; Cooper, Z.A.; Gillmeister, M.P.; Todd, N.W.; Atamas, S.P. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J. Immunol. 2008, 180, 5036–5044. [Google Scholar] [CrossRef]
- Ito, Y.; Goldschmeding, R.; Kasuga, H.; Claessen, N.; Nakayama, M.; Yuzawa, Y.; Sawai, A.; Matsuo, S.; Weening, J.J.; Aten, J. Expression patterns of connective tissue growth factor and of TGF-beta isoforms during glomerular injury recapitulate glomerulogenesis. Am. J. Physiol. Ren. Physiol. 2010, 299, F545–F558. [Google Scholar] [CrossRef]
- Cianci, R.; Martina, P.; Cianci, M.; Lavini, R.; Stivali, G.; Di Donato, D.; Polidori, L.; Lai, S.; Renzulli, R.; Gigante, A.; et al. Ischemic nephropathy: Proteinuria and renal resistance index could suggest if revascularization is recommended. Ren. Fail. 2010, 32, 1167–1171. [Google Scholar] [CrossRef]
- Cianfrone, P.; Simeoni, M.; Comi, N.; Piraina, V.; Talarico, R.; Cerantonio, A.; Gentile, I.; Fabiano, F.F.; Lucisano, G.; Foti, D.; et al. How to improve duration and efficiency of the antiproteinuric response to Ramipril: RamiPROT-a prospective cohort study. J. Nephrol. 2017, 30, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, M.; Cianfrone, P.; Comi, N.; Gentile, I.; Fabiano, F.F.; Piraina, V.; Talarico, R.; Lucisano, G.; Rivoli, L.; Andreucci, M.; et al. Is it feasible to improve the duration and the efficiency of Ramipril anti-proteinuric response? G. Ital. Nefrol. 2015, 32, gin-32. [Google Scholar]
- Herrmann, S.M.; Textor, S.C. Current Concepts in the Treatment of Renovascular Hypertension. Am. J. Hypertens. 2018, 31, 139–149. [Google Scholar] [CrossRef]
- Aukland, K.; Krog, J. Renal oxygen tension. Nature 1960, 188, 671. [Google Scholar] [CrossRef] [PubMed]
- Brezis, M.; Heyman, S.N.; Dinour, D.; Epstein, F.H.; Rosen, S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J. Clin. Investig. 1991, 88, 390–395. [Google Scholar] [CrossRef]
- Gullichsen, E.; Nelimarkka, O.; Halkola, L.; Niinikoski, J. Renal oxygenation in endotoxin shock in dogs. Crit. Care Med. 1989, 17, 547–550. [Google Scholar] [CrossRef]
- Evans, R.G.; Iguchi, N.; Cochrane, A.D.; Marino, B.; Hood, S.G.; Bellomo, R.; McCall, P.R.; May, C.N.; Lankadeva, Y.R. Renal hemodynamics and oxygenation during experimental cardiopulmonary bypass in sheep under total intravenous anesthesia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R206–R213. [Google Scholar] [CrossRef]
- Sugahara, M.; Tanaka, T.; Nangaku, M. Hypoxia-Inducible Factor and Oxygen Biology in the Kidney. Kidney360 2020, 1, 1021–1031. [Google Scholar] [CrossRef]
- Evans, R.G.; Gardiner, B.S.; Smith, D.W.; O’Connor, P.M. Methods for studying the physiology of kidney oxygenation. Clin. Exp. Pharm. Physiol. 2008, 35, 1405–1412. [Google Scholar] [CrossRef]
- Nangaku, M.; Eckardt, K.U. Hypoxia and the HIF system in kidney disease. J. Mol. Med. 2007, 85, 1325–1330. [Google Scholar] [CrossRef]
- Rosenberger, C.; Mandriota, S.; Jurgensen, J.S.; Wiesener, M.S.; Horstrup, J.H.; Frei, U.; Ratcliffe, P.J.; Maxwell, P.H.; Bachmann, S.; Eckardt, K.U. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J. Am. Soc. Nephrol. 2002, 13, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lu, X.; Gao, F.; Zhang, X.; Xia, X.; Sun, H. Assessment of neutrophil gelatinase-associated lipocalin as an early biomarker for canine renal ischemia-reperfusion injury. Ann. Transl. Med. 2020, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, A.; Coppolino, G.; Barreca, G.S.; Gentile, I.; Rivoli, L.; Postorino, M.C.; Mazzitelli, M.; Greco, G.; Costa, C.; Pisani, V.; et al. Evolution of glomerular filtration rates and neutrophil gelatinase-associated lipocalin during treatment with direct acting antivirals. Clin. Mol. Hepatol. 2018, 24, 151–162. [Google Scholar] [CrossRef]
- Textor, S.C.; Abumoawad, A.; Saad, A.; Ferguson, C.; Dietz, A. Stem Cell Therapy for Microvascular Injury Associated with Ischemic Nephropathy. Cells 2021, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Pruijm, M.; Mendichovszky, I.A.; Liss, P.; Van der Niepen, P.; Textor, S.C.; Lerman, L.O.; Krediet, C.T.P.; Caroli, A.; Burnier, M.; Prasad, P.V. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: A statement paper and systematic review. Nephrol. Dial. Transplant. 2018, 33, ii22–ii28. [Google Scholar] [CrossRef]
- Gloviczki, M.L.; Saad, A.; Textor, S.C. Blood oxygen level-dependent (BOLD) MRI analysis in atherosclerotic renal artery stenosis. Curr. Opin. Nephrol. Hypertens. 2013, 22, 519–524. [Google Scholar] [CrossRef]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The role of oxidative stress and hypoxia in renal disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef]
- Karopadi, A.N. Analysis of Costs in Renal Replacement Therapy. Perit Dial. Int. 2017, 37, 497–499. [Google Scholar] [CrossRef]
- Grange, C.; Skovronova, R.; Marabese, F.; Bussolati, B. Stem Cell-Derived Extracellular Vesicles and Kidney Regeneration. Cells 2019, 8, 1240. [Google Scholar] [CrossRef]
- Purwaningrum, M.; Jamilah, N.S.; Purbantoro, S.D.; Sawangmake, C.; Nantavisai, S. Comparative characteristic study from bone marrow-derived mesenchymal stem cells. J. Vet. Sci. 2021, 22, e74. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Birtwistle, L.; Chen, X.M.; Pollock, C. Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury. Int. J. Mol. Sci. 2021, 22, 6596. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Roufosse, C.; Cook, H.T. Stem cells and renal regeneration. Nephron Exp. Nephrol. 2008, 109, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.F.; Ricardo, S.D. Mesenchymal stem cells in kidney inflammation and repair. Nephrology 2012, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Canales, D.M.; Li, J.Y.; Makuei, L.; Gleadle, J.M. Compensatory renal hypertrophy following nephrectomy: When and how? Nephrology 2019, 24, 1225–1232. [Google Scholar] [CrossRef]
- Simeoni, M.; Armeni, A.; Summaria, C.; Cerantonio, A.; Fuiano, G. Current evidence on the use of anti-RAAS agents in congenital or acquired solitary kidney. Ren. Fail. 2017, 39, 660–670. [Google Scholar] [CrossRef]
- Fortrie, G.; de Geus, H.R.H.; Betjes, M.G.H. The aftermath of acute kidney injury: A narrative review of long-term mortality and renal function. Crit. Care 2019, 23, 24. [Google Scholar] [CrossRef]
- Romagnani, P. Toward the identification of a “renopoietic system”? Stem Cells 2009, 27, 2247–2253. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Valerius, M.T.; Kobayashi, A.; Mugford, J.W.; Soeung, S.; Duffield, J.S.; McMahon, A.P.; Bonventre, J.V. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008, 2, 284–291. [Google Scholar] [CrossRef]
- Brooke, G.; Cook, M.; Blair, C.; Han, R.; Heazlewood, C.; Jones, B.; Kambouris, M.; Kollar, K.; McTaggart, S.; Pelekanos, R.; et al. Therapeutic applications of mesenchymal stromal cells. Semin. Cell Dev. Biol. 2007, 18, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.M.; Chamberlain, G.; Ashton, B.A.; Middleton, J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br. J. Haematol. 2007, 137, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Hua, T.; Li, F.; Han, J.; Fang, J.; Xu, L.; Sun, C.; Zhang, Z.; Feng, Z.; Jiang, X. Hypoxia-inducible factor 1 alpha protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am. J. Transl. Res. 2017, 9, 2492–2499. [Google Scholar] [PubMed]
- Togel, F.; Isaac, J.; Hu, Z.; Weiss, K.; Westenfelder, C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005, 67, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Pochampally, R.R.; Hsu, S.C.; Sanchez, C.; Chen, S.C.; Spees, J.; Prockop, D.J. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE 2007, 2, e416. [Google Scholar] [CrossRef]
- Ji, J.F.; He, B.P.; Dheen, S.T.; Tay, S.S. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004, 22, 415–427. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonne, A.; Delorme, B.; Herault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Little, M.H.; Bertram, J.F. Is there such a thing as a renal stem cell? J. Am. Soc. Nephrol. 2009, 20, 2112–2117. [Google Scholar] [CrossRef]
- Appel, D.; Kershaw, D.B.; Smeets, B.; Yuan, G.; Fuss, A.; Frye, B.; Elger, M.; Kriz, W.; Floege, J.; Moeller, M.J. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 2009, 20, 333–343. [Google Scholar] [CrossRef]
- Ronconi, E.; Sagrinati, C.; Angelotti, M.L.; Lazzeri, E.; Mazzinghi, B.; Ballerini, L.; Parente, E.; Becherucci, F.; Gacci, M.; Carini, M.; et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol. 2009, 20, 322–332. [Google Scholar] [CrossRef]
- Sagrinati, C.; Netti, G.S.; Mazzinghi, B.; Lazzeri, E.; Liotta, F.; Frosali, F.; Ronconi, E.; Meini, C.; Gacci, M.; Squecco, R.; et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J. Am. Soc. Nephrol. 2006, 17, 2443–2456. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.J.; Rea, R.W.; Steptoe, A.L.; Busslinger, M.; Bertram, J.F.; Perkins, A.C. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation 2007, 75, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, K.; Li, Y.; He, Q.H.; Li, G.Q.; Zheng, Q.Y.; Zhang, K.Q. Mesenchymal stem cells alleviate acute kidney injury by down-regulating C5a/C5aR pathway activation. Int. Urol. Nephrol. 2018, 50, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, L.; Wang, L.; Lin, L.; Yu, L.; Zhang, L.; Shang, T. Therapeutic benefit of mesenchymal stem cells in pregnant rats with angiotensin receptor agonistic autoantibody-induced hypertension: Implications for immunomodulation and cytoprotection. Hypertens. Pregnancy 2017, 36, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Reinders, M.E.; Fibbe, W.E.; Rabelink, T.J. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol. Dial. Transplant. 2010, 25, 17–24. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef]
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal cells protect against acute tubular injury via an endocrine effect. J. Am. Soc. Nephrol. 2007, 18, 2486–2496. [Google Scholar] [CrossRef]
- Imberti, B.; Morigi, M.; Tomasoni, S.; Rota, C.; Corna, D.; Longaretti, L.; Rottoli, D.; Valsecchi, F.; Benigni, A.; Wang, J.; et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J. Am. Soc. Nephrol. 2007, 18, 2921–2928. [Google Scholar] [CrossRef]
- Semedo, P.; Palasio, C.G.; Oliveira, C.D.; Feitoza, C.Q.; Goncalves, G.M.; Cenedeze, M.A.; Wang, P.M.; Teixeira, V.P.; Reis, M.A.; Pacheco-Silva, A.; et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int. Immunopharmacol. 2009, 9, 677–682. [Google Scholar] [CrossRef]
- Togel, F.; Cohen, A.; Zhang, P.; Yang, Y.; Hu, Z.; Westenfelder, C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009, 18, 475–485. [Google Scholar] [CrossRef]
- Togel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed]
- Togel, F.; Weiss, K.; Yang, Y.; Hu, Z.; Zhang, P.; Westenfelder, C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Ren. Physiol. 2007, 292, F1626–F1635. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, M.J.; Sun, H.Y.; Xiong, J.; Zhang, Y.; Liu, C.Y.; Fu, L.L.; Liu, D.M.; Liu, H.Q.; Mei, C.L. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2011, 300, F207–F218. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M.; Walbaum, D.; Rees, A.J. Macrophages and the kidney. Curr. Opin. Nephrol. Hypertens. 2004, 13, 285–290. [Google Scholar] [CrossRef]
- Zhang, M.; Johnson-Stephenson, T.K.; Wang, W.; Wang, Y.; Li, J.; Li, L.; Zen, K.; Chen, X.; Zhu, D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res. Ther. 2022, 13, 484. [Google Scholar] [CrossRef]
- Stout, R.D.; Jiang, C.; Matta, B.; Tietzel, I.; Watkins, S.K.; Suttles, J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005, 175, 342–349. [Google Scholar] [CrossRef]
- Simeoni, M.; Nicotera, R.; Colao, M.; Citraro, M.L.; Pelagi, E.; Cerantonio, A.; Comi, N.; Coppolino, G.; Fuiano, G. Direct inhibition of plasmatic renin activity with aliskiren: A promising but under-investigated therapeutic option for non-diabetic glomerulonephritis. Int. Urol. Nephrol. 2016, 48, 229–237. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diabetes Rep. 2022, 22, 39–52. [Google Scholar] [CrossRef]
- Kohan, D.E.; Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014, 86, 896–904. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Toson, E.A.; El-Mezayen, H.A.; Rashed, L.A.; Elsherbiny, E.S. Role of mesenchymal stem cells versus angiotensin converting enzyme inhibitor in kidney repair. Nephrology 2017, 22, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Kitamura, S.; Wada, J. Potential Strategies for Kidney Regeneration With Stem Cells: An Overview. Front. Cell Dev. Biol. 2022, 10, 892356. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Strazar, K.; Kocijan, R.; Nau, T.; Grillari, J.; Marolt Presen, D. Age-related alterations and senescence of mesenchymal stromal cells: Implications for regenerative treatments of bones and joints. Mech. Ageing Dev. 2021, 198, 111539. [Google Scholar] [CrossRef] [PubMed]
- Mitalipov, S.; Wolf, D. Totipotency, pluripotency and nuclear reprogramming. Adv. Biochem. Eng. Biotechnol. 2009, 114, 185–199. [Google Scholar] [CrossRef]
- Bochon, B.; Kozubska, M.; Surygala, G.; Witkowska, A.; Kuzniewicz, R.; Grzeszczak, W.; Wystrychowski, G. Mesenchymal Stem Cells-Potential Applications in Kidney Diseases. Int. J. Mol. Sci. 2019, 20, 2462. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng. Part B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef]
- Isik, B.; Thaler, R.; Goksu, B.B.; Conley, S.M.; Al-Khafaji, H.; Mohan, A.; Afarideh, M.; Abumoawad, A.M.; Zhu, X.Y.; Krier, J.D.; et al. Hypoxic preconditioning induces epigenetic changes and modifies swine mesenchymal stem cell angiogenesis and senescence in experimental atherosclerotic renal artery stenosis. Stem Cell Res. Ther. 2021, 12, 240. [Google Scholar] [CrossRef]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res. Ther. 2020, 11, 130. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.; Jiang, K.; Krier, J.D.; Zhu, X.; Conley, S.; Lerman, A.; Lerman, L.O. Adjunctive mesenchymal stem/stromal cells augment microvascular function in poststenotic kidneys treated with low-energy shockwave therapy. J. Cell Physiol. 2020, 235, 9806–9818. [Google Scholar] [CrossRef]
- Zhang, X.; Krier, J.D.; Amador Carrascal, C.; Greenleaf, J.F.; Ebrahimi, B.; Hedayat, A.F.; Textor, S.C.; Lerman, A.; Lerman, L.O. Low-Energy Shockwave Therapy Improves Ischemic Kidney Microcirculation. J. Am. Soc. Nephrol. 2016, 27, 3715–3724. [Google Scholar] [CrossRef]
- Zhao, Y.; Santelli, A.; Zhu, X.Y.; Zhang, X.; Woollard, J.R.; Chen, X.J.; Jordan, K.L.; Krier, J.; Tang, H.; Saadiq, I.; et al. Low-Energy Shockwave Treatment Promotes Endothelial Progenitor Cell Homing to the Stenotic Pig Kidney. Cell Transplant. 2020, 29, 963689720917342. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tian, X.; Bai, S.; Fan, J.; Hou, W.; Tong, H.; Li, D. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int. J. Mol. Med. 2012, 30, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Fang, Y.; Xie, L.; Liu, X.; Shan, W.; Zeng, R.; Liu, J.; Liu, X. Adipose-Derived Mesenchymal Stem Cells Transplantation Alleviates Renal Injury in Streptozotocin-Induced Diabetic Nephropathy. J. Histochem. Cytochem. 2015, 63, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Luo, Q.; Wang, Y.; Ma, Y.; Chen, F.; Zhu, X.; Shi, J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020, 295, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Bruno, S.; Grange, C.; Buttiglieri, S.; Deregibus, M.C.; Cantino, D.; Camussi, G. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005, 166, 545–555. [Google Scholar] [CrossRef]
- Dekel, B.; Zangi, L.; Shezen, E.; Reich-Zeliger, S.; Eventov-Friedman, S.; Katchman, H.; Jacob-Hirsch, J.; Amariglio, N.; Rechavi, G.; Margalit, R.; et al. Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 2006, 17, 3300–3314. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cianci, R.; Simeoni, M.; Cianci, E.; De Marco, O.; Pisani, A.; Ferri, C.; Gigante, A. Stem Cells in Kidney Ischemia: From Inflammation and Fibrosis to Renal Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 4631. https://doi.org/10.3390/ijms24054631
Cianci R, Simeoni M, Cianci E, De Marco O, Pisani A, Ferri C, Gigante A. Stem Cells in Kidney Ischemia: From Inflammation and Fibrosis to Renal Tissue Regeneration. International Journal of Molecular Sciences. 2023; 24(5):4631. https://doi.org/10.3390/ijms24054631
Chicago/Turabian StyleCianci, Rosario, Mariadelina Simeoni, Eleonora Cianci, Oriana De Marco, Antonio Pisani, Claudio Ferri, and Antonietta Gigante. 2023. "Stem Cells in Kidney Ischemia: From Inflammation and Fibrosis to Renal Tissue Regeneration" International Journal of Molecular Sciences 24, no. 5: 4631. https://doi.org/10.3390/ijms24054631
APA StyleCianci, R., Simeoni, M., Cianci, E., De Marco, O., Pisani, A., Ferri, C., & Gigante, A. (2023). Stem Cells in Kidney Ischemia: From Inflammation and Fibrosis to Renal Tissue Regeneration. International Journal of Molecular Sciences, 24(5), 4631. https://doi.org/10.3390/ijms24054631






