SARS-CoV-2 Transplacental Transmission: A Rare Occurrence? An Overview of the Protective Role of the Placenta
Abstract
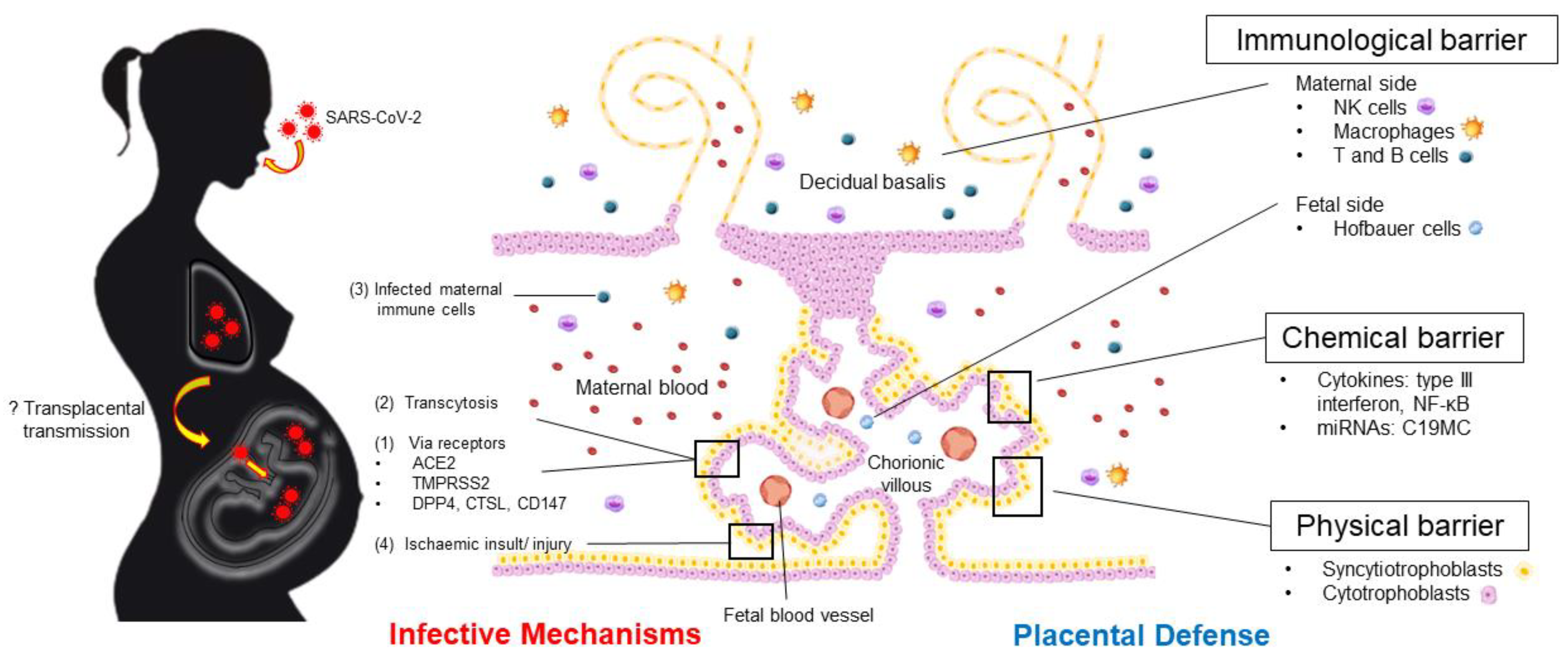
1. Introduction
2. SARS-CoV-2 and Pregnancy
2.1. Evidence of Vertical Transmission of SARS-CoV-2
2.2. SARS-CoV-2 Cell-Entry Pathways
2.3. The Effects of SARS-CoV-2 Infection on the Placenta
3. Placental Structure and Defense: An Overview
3.1. Placental Embryology and Development
3.2. Trophoblast Host Defense
3.2.1. Immunological Barrier
3.2.2. Physical Barrier
3.2.3. Chemical Barrier: Cytokines, Exosomes, and microRNAs
Cytokines
Exosomes and Trophoblastic microRNAs
4. Impact of Maternal SARS-CoV-2 Infection on Pregnancy Outcomes
5. Concluding Remarks and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedford, J.; Enria, D.; Giesecke, J.; Heymann, D.L.; Ihekweazu, C.; Kobinger, G.; Lane, H.C.; Memish, Z.; Oh, M.D.; Sall, A.A.; et al. COVID-19: Towards controlling of a pandemic. Lancet 2020, 395, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 19 January 2023).
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Vouga, M.; Favre, G.; Martinez-Perez, O.; Pomar, L.; Acebal, L.F.; Abascal-Saiz, A.; Hernandez, M.R.V.; Hcini, N.; Lambert, V.; Carles, G.; et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci. Rep. 2021, 11, 13898. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef]
- Konstantinidou, A.E.; Angelidou, S.; Havaki, S.; Paparizou, K.; Spanakis, N.; Chatzakis, C.; Sotiriadis, A.; Theodora, M.; Donoudis, C.; Daponte, A.; et al. Stillbirth due to SARS-CoV-2 placentitis without evidence of intrauterine transmission to fetus: Association with maternal risk factors. Ultrasound Obstet. Gynecol. 2022, 59, 813–822. [Google Scholar] [CrossRef]
- Cimolai, N. A comprehensive analysis of maternal and newborn disease and related control for COVID-19. SN Compr. Clin. Med. 2021, 3, 1272–1294. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition and Categorization of the Timing of Mother-to-Child Transmission of SARS-CoV-2: Scientific Brief. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1 (accessed on 16 December 2022).
- Shah, P.S.; Diambomba, Y.; Acharya, G.; Morris, S.K.; Bitnun, A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 2020, 99, 565–568. [Google Scholar] [CrossRef]
- Sharps, M.C.; Hayes, D.J.L.; Lee, S.; Zou, Z.; Brady, C.A.; Almoghrabi, Y.; Kerby, A.; Tamber, K.K.; Jones, C.J.; Adams Waldorf, K.M.; et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta 2020, 101, 13–29. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Dhaliwal, A. Infections in pregnancy with COVID-19 and other respiratory RNA virus diseases are rarely, if ever, transmitted to the fetus: Experiences with coronaviruses, HPIV, hMPV RSV and influenza. Arch. Pathol. Lab. Med. 2020, 144, 920–928. [Google Scholar] [CrossRef]
- Algarroba, G.N.; Rekawek, P.; Vahanian, S.A.; Khullar, P.; Palaia, T.; Peltier, M.R.; Chavez, M.R.; Vintzileos, A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020, 223, 275–278. [Google Scholar] [CrossRef]
- Wong, Y.P.; Tan, G.C.; Omar, S.Z.; Mustangin, M.; Singh, Y.; Salker, M.S.; Abd Aziz, N.H.; Shafiee, M.N. SARS-CoV-2 infection in pregnancy: Placental histomorphological patterns, disease severity and perinatal outcomes. Int. J. Environ. Res. Public Health 2022, 19, 9517. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C.; Torous, V.F.; Roberts, D.J. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch. Pathol. Lab. Med. 2021, 145, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P. SARS–CoV-2 infection of the placenta. J. Clin. Invest. 2020, 130, 4947–4953. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, S.; Snijder, P.; Verdijk, R.M.; Kuiken, T.; Kamphuis, S.S.M.; Koopman, L.P.; Krasemann, T.B.; Rousian, M.; Broekhuizen, M.; Steegers, E.A.P.; et al. Severe acute respiratory syndrome Coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J. Pediatric. Infect. Dis. Soc. 2020, 10, 556–561. [Google Scholar] [CrossRef]
- Konstantinidou, A.E.; Skaltsounis, P.; Eleftheriades, M.; Antsaklis, P.; Charitou, A.; Anatolitou, F.; Moutafi, A.; Petropoulos, P.; Daskalakis, G. Pharyngeal sampling for PCR-testing in the investigation of SARS-COV-2 vertical transmission in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 18–21. [Google Scholar] [CrossRef]
- Cai, J.; Tang, M.; Gao, Y.; Zhang, H.; Yang, Y.; Zhang, D.; Wang, H.; Liang, H.; Zhang, R.; Wu, B. Cesarean section or vaginal delivery to prevent possible vertical transmission from a pregnant mother confirmed with COVID-19 to a neonate: A systematic review. Front. Med. 2021, 8, 634949. [Google Scholar] [CrossRef]
- Chi, H.; Chiu, N.-C.; Tai, Y.-L.; Chang, H.-Y.; Lin, C.-H.; Sung, Y.-H.; Tseng, C.-Y.; Liu, L.Y.-M.; Lin, C.-Y. Clinical features of neonates born to mothers with coronavirus disease-2019: A systematic review of 105 neonates. J. Microbiol. Immunol. Infect. 2021, 54, 69–76. [Google Scholar] [CrossRef]
- Centeno-Tablante, E.; Medina-Rivera, M.; Finkelstein, J.L.; Rayco-Solon, P.; Garcia-Casal, M.N.; Rogers, L.; Ghezzi-Kopel, K.; Ridwan, P.; Peña-Rosas, J.P.; Mehta, S. Transmission of SARS-CoV-2 through breast milk and breastfeeding: A living systematic review. Ann. N. Y. Acad. Sci. 2021, 1484, 32–54. [Google Scholar] [CrossRef]
- Musa, S.S.; Bello, U.M.; Zhao, S.; Abdullahi, Z.U.; Lawan, M.A.; He, D. Vertical transmission of SARS-CoV-2: A systematic review of systematic reviews. Viruses 2021, 13, 1877. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Dong, L.; Tian, J.; He, S.; Zhu, C.; Wang, J.; Liu, C.; Yang, J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020, 323, 1846–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Y.H.; Yang, H.X.; Poon, L.C. Intrauterine vertical transmission of SARS-CoV-2: What we know so far. Ultrasound Obstet. Gynecol. 2020, 55, 724–725. [Google Scholar] [CrossRef]
- Raschetti, R.; Vivanti, A.J.; Vauloup-Fellous, C.; Loi, B.; Benachi, A.; De Luca, D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020, 11, 5164. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Chatterjee, S.; Kew, T.; Gaetano, A.; Stallings, E.; Fernández-García, S.; Yap, M.; Sheikh, J.; Lawson, H.; Coomar, D.; et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: Living systematic review and meta-analysis. BMJ 2022, 376, e067696. [Google Scholar] [CrossRef]
- Fan, C.; Wu, Y.; Rui, X.; Yang, Y.; Ling, C.; Liu, S.; Liu, S.; Wang, Y. Animal models for COVID-19: Advances, gaps and perspectives. Signal. Transduct. Target Ther. 2022, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Cool, K.; Gaudreault, N.N.; Morozov, I.; Trujillo, J.D.; Meekins, D.A.; McDowell, C.; Carossino, M.; Bold, D.; Mitzel, D.; Kwon, T.; et al. Infection and transmission of ancestral SARS-CoV-2 and its alpha variant in pregnant white-tailed deer. Emerg. Microbes Infect. 2022, 11, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Mor, G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017, 127, 1591–1599. [Google Scholar] [CrossRef]
- Coyne, C.B. The tree(s) of life: The human placenta and my journey to learn more about it. PLOS Pathog. 2016, 12, e1005515. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Sadovsky, Y.; Coyne, C.B. The placenta as a barrier to viral infections. Annu. Rev. Virol. 2014, 1, 133–146. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Greber, U.F. Principles of virus uncoating: Cues and the snooker ball. Traffic 2016, 17, 569–592. [Google Scholar] [CrossRef]
- Zaga-Clavellina, V.; Diaz, L.; Olmos-Ortiz, A.; Godínez-Rubí, M.; Rojas-Mayorquín, A.E.; Ortuño-Sahagún, D. Central role of the placenta during viral infection: Immuno-competences and miRNA defensive responses. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166182. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Gengler, C.; Dubruc, E.; Favre, G.; Greub, G.; de Leval, L.; Baud, D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin. Microbiol. Infect. 2021, 27, 489–490. [Google Scholar] [CrossRef]
- Huang, Z.; Xia, S.; Mei, S.; Wen, Y.; Liu, J.; Dong, C.; Chen, W.; Yu, P.; Qu, L.; Luo, Y.; et al. Integrated analysis reveals the characteristics and effects of SARS-CoV-2 maternal–fetal transmission. Front. Microbiol. 2022, 13, 813187. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Luo, W.; Huang, L.; Xiao, J.; Li, F.; Qin, S.; Song, X.; Wu, Y.; Zeng, Q.; et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int. J. Med. Sci. 2020, 17, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Lu-Culligan, A.; Chavan, A.R.; Vijayakumar, P.; Irshaid, L.; Courchaine, E.M.; Milano, K.M.; Tang, Z.; Pope, S.D.; Song, E.; Vogels, C.B.F.; et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med 2021, 2, 591–610.e510. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Yang, L.; Lian, X.; Xie, Y.; Li, S.; Xin, S.; Cao, P.; Lu, J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience 2020, 23, 101160. [Google Scholar] [CrossRef] [PubMed]
- Constantino, F.B.; Cury, S.S.; Nogueira, C.R.; Carvalho, R.F.; Justulin, L.A. Prediction of non-canonical routes for SARS-CoV-2 infection in human placenta cells. Front. Mol. Biosci. 2021, 8, 614728. [Google Scholar] [CrossRef] [PubMed]
- Ashary, N.; Bhide, A.; Chakraborty, P.; Colaco, S.; Mishra, A.; Chhabria, K.; Jolly, M.K.; Modi, D. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 2020, 8, 783. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bansal, V.; Feschotte, C. A Single-cell RNA expression map of human coronavirus entry factors. Cell Rep. 2020, 32, 108175. [Google Scholar] [CrossRef] [PubMed]
- Argueta, L.B.; Lacko, L.A.; Bram, Y.; Tada, T.; Carrau, L.; Rendeiro, A.F.; Zhang, T.; Uhl, S.; Lubor, B.C.; Chandar, V.; et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience 2022, 25, 104223. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.; Waller, M.A.; Moreno, C.L.; Cole, A.J.; Stella, A.O.; Pop, O.-T.; Jochum, A.-K.; Ali, O.H.; Denes, C.E.; Hamoudi, Z.; et al. Fibroblast-expressed LRRC15 is a receptor for SARS-CoV-2 spike and controls antiviral and antifibrotic transcriptional programs. PLOS Biol. 2023, 21, e3001967. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, J.; Skarp, S.; Savolainen, E.R.; Ryynänen, M.; Järvenpää, J. Intrauterine growth restriction and placental gene expression in severe preeclampsia, comparing early-onset and late-onset forms. J. Perinat. Med. 2017, 45, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Woodward, B.O.; Pastor, L.; George, A.M.; Petrechko, O.; Nouvet, F.J.; Haas, D.W.; Jiang, G.; Hildreth, J.E.K. Endogenous retroviral envelope syncytin induces HIV-1 spreading and establishes HIV reservoirs in placenta. Cell Rep. 2020, 30, 4528–4539.e4. [Google Scholar] [CrossRef] [PubMed]
- Egloff, C.; Vauloup-Fellous, C.; Picone, O.; Mandelbrot, L.; Roques, P. Evidence and possible mechanisms of rare maternal-fetal transmission of SARS-CoV-2. J. Clin. Virol. 2020, 128, 104447. [Google Scholar] [CrossRef] [PubMed]
- Kalampokas, T.; Rapani, A.; Papageorgiou, M.; Grigoriadis, S.; Maziotis, E.; Anifandis, G.; Triantafyllidou, O.; Tzanakaki, D.; Neofytou, S.; Bakas, P.; et al. The current evidence regarding COVID-19 and pregnancy: Where are we now and where should we head to next? Viruses 2021, 13, 2000. [Google Scholar] [CrossRef]
- Beharier, O.; Kajiwara, K.; Sadovsky, Y. Ferroptosis, trophoblast lipotoxic damage, and adverse pregnancy outcome. Placenta 2021, 108, 32–38. [Google Scholar] [CrossRef]
- Upton, J.W.; Chan, F.K. Staying alive: Cell death in antiviral immunity. Mol. Cell 2014, 54, 273–280. [Google Scholar] [CrossRef]
- Deshpande, R.; Zou, C. Programmed cell death in SARS-CoV-2 infection: A short review. J. Respir. 2021, 1, 223–228. [Google Scholar] [CrossRef]
- Parcial, A.L.N.; Salomão, N.G.; Portari, E.A.; Arruda, L.V.; de Carvalho, J.J.; de Matos Guedes, H.L.; Conde, T.C.; Moreira, M.E.; Batista, M.M.; Paes, M.V.; et al. SARS-CoV-2 Is persistent in placenta and causes macroscopic, histopathological, and ultrastructural changes. Viruses 2022, 14, 1885. [Google Scholar] [CrossRef]
- Suhren, J.T.; Meinardus, A.; Hussein, K.; Schaumann, N. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta 2022, 117, 72–77. [Google Scholar] [CrossRef]
- Redline, R.W.; Ravishankar, S.; Bagby, C.; Saab, S.; Zarei, S. Diffuse and localized SARS-CoV-2 placentitis: Prevalence and pathogenesis of an uncommon complication of COVID-19 infection during pregnancy. Am. J. Surg. Pathol. 2022, 46, 1036–1047. [Google Scholar] [CrossRef]
- Nelson, D.M.; Crouch, E.C.; Curran, E.M.; Farmer, D.R. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. Am. J. Pathol. 1990, 136, 855–865. [Google Scholar] [PubMed]
- Wong, Y.P.; Khong, T.Y.; Tan, G.C. The Effects of COVID-19 on placenta and pregnancy: What do we know so far? Diagnostics 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Shook, L.L.; Brigida, S.; Regan, J.; Flynn, J.P.; Mohammadi, A.; Etemad, B.; Siegel, M.R.; Clapp, M.A.; Li, J.Z.; Roberts, D.J.; et al. SARS-CoV-2 placentitis associated with B.1.617.2 (Delta) variant and fetal distress or demise. J. Infect. Dis. 2022, 225, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A.; Mulkey, S.B.; Roberts, D.J. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: Clinical-pathologic correlations. Am. J. Obstet. Gynecol. 2022, 228, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, B.; O’Donoghue, K.; McEntagart, N.; Gillan, J.E.; Kelehan, P.; O’Leary, J.; Downey, P.; Dean, J.; De Gascun, C.F.; Bermingham, J.; et al. Fetal deaths in Ireland due to SARS-CoV-2 placentitis caused by SARS-CoV-2 Alpha. Arch. Pathol. Lab. Med. 2022, 146, 529–537. [Google Scholar] [CrossRef]
- Gesaka, S.R.; Obimbo, M.M.; Wanyoro, A. Coronavirus disease 2019 and the placenta: A literature review. Placenta 2022, 126, 209–223. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Khalil, A.; Alameddine, S.; D’Angelo, E.; Galliani, C.; Matarrelli, B.; Buca, D.; Liberati, M.; Rizzo, G.; D’Antonio, F. Placental histopathology after SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100468. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1, S6–S8. [Google Scholar] [CrossRef]
- Roberts, V.H.J.; Morgan, T.K.; Bednarek, P.; Morita, M.; Burton, G.J.; Lo, J.O.; Frias, A.E. Early first trimester uteroplacental flow and the progressive disintegration of spiral artery plugs: New insights from contrast-enhanced ultrasound and tissue histopathology. Hum. Reprod. 2017, 32, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Invest. 2004, 114, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Frank, H. Placental development. In Fetal and Neonatal Physiology, 5th ed.; Polin, R.A., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 101–113. [Google Scholar]
- Zeldovich, V.B.; Clausen, C.H.; Bradford, E.; Fletcher, D.A.; Maltepe, E.; Robbins, J.R.; Bakardjiev, A.I. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 2013, 9, e1003821. [Google Scholar] [CrossRef] [PubMed]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140070. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; De Wolf, F.; Robertson, W.B.; Brosens, I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br. J. Obstet. Gynaecol. 1986, 93, 1049–1059. [Google Scholar] [CrossRef]
- Burton, G.J.; Charnock-Jones, D.S.; Jauniaux, E. Regulation of vascular growth and function in the human placenta. Reproduction 2009, 138, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Mihu, C.M.; Suşman, S.; Rus Ciucă, D.; Mihu, D.; Costin, N. Aspects of placental morphogenesis and angiogenesis. Rom. J. Morphol. Embryol. 2009, 50, 549–557. [Google Scholar]
- Chew, B.S.; Ghazali, R.; Othman, H.; Ismail, N.A.M.; Othman, A.S.; Laim, N.; Wong, Y.P.; Tan, G.C. Endocan expression in placenta of women with hypertension. J. Obstet. Gynaecol. Res. 2019, 45, 345–351. [Google Scholar] [CrossRef]
- Wong, Y.P.; Cheah, F.C.; Wong, K.K.; Shah, S.A.; Phon, S.E.; Ng, B.K.; Lim, P.S.; Khong, T.Y.; Tan, G.C. Gardnerella vaginalis infection in pregnancy: Effects on placental development and neonatal outcomes. Placenta 2022, 120, 79–87. [Google Scholar] [CrossRef]
- Azliana, A.F.; Zainul-Rashid, M.R.; Chandramaya, S.F.; Farouk, W.I.; Nurwardah, A.; Wong, Y.P.; Tan, G.C. Vascular endothelial growth factor expression in placenta of hypertensive disorder in pregnancy. Indian J. Pathol. Microbiol. 2017, 60, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Shaaya, E.S.; Yahaya, A.; Mustangin, M.; Alfian, N.; Noor Aizuddin, A.; Wong, Y.P.; Tan, G.C. Placental cyclophilin A expression in pregnancies complicated with hypertension. Int. J. Environ. Res. Public Health 2022, 19, 5448. [Google Scholar] [CrossRef]
- Castellucci, M.; Zaccheo, D.; Pescetto, G. A three-dimensional study of the normal human placental villous core. I. The Hofbauer cells. Cell Tissue Res. 1980, 210, 235–247. [Google Scholar] [CrossRef]
- Thomas, J.R.; Naidu, P.; Appios, A.; McGovern, N. The ontogeny and function of placental macrophages. Front. Immunol. 2021, 12, 771054. [Google Scholar] [CrossRef]
- Yagel, S. The developmental role of natural killer cells at the fetal-maternal interface. Am. J. Obstet. Gynecol. 2009, 201, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.; Wolfe, B.; Golos, T. Hofbauer cells: Placental macrophages of fetal origin. Results Probl. Cell Differ. 2017, 62, 45–60. [Google Scholar] [CrossRef]
- Holmlund, U.; Cebers, G.; Dahlfors, A.R.; Sandstedt, B.; Bremme, K.; Ekström, E.S.; Scheynius, A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology 2002, 107, 145–151. [Google Scholar] [CrossRef]
- Koga, K.; Mor, G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am. J. Reprod. Immunol. 2010, 63, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Celik, O.; Saglam, A.; Baysal, B.; Derwig, I.E.; Celik, N.; Ak, M.; Aslan, S.N.; Ulas, M.; Ersahin, A.; Tayyar, A.T.; et al. Factors preventing materno-fetal transmission of SARS-CoV-2. Placenta 2020, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.R.; Skrzypczynska, K.M.; Zeldovich, V.B.; Kapidzic, M.; Bakardjiev, A.I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010, 6, e1000732. [Google Scholar] [CrossRef]
- Kreis, N.N.; Ritter, A.; Louwen, F.; Yuan, J. A message from the human placenta: Structural and immunomodulatory defense against SARS-CoV-2. Cells 2020, 9, 1777. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nelemans, T.; Kikkert, M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses 2019, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.I.; Coyne, C.B. Type III interferons in antiviral defenses at barrier surfaces. Trends Immunol. 2018, 39, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T., Jr.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Jagger, B.W.; Miner, J.J.; Cao, B.; Arora, N.; Smith, A.M.; Kovacs, A.; Mysorekar, I.U.; Coyne, C.B.; Diamond, M.S. Gestational stage and IFN-λ signaling regulate ZIKV infection in utero. Cell Host Microbe 2017, 22, 366–376.e363. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.S.; Cambou, M.C.; Mok, T.; Fajardo, V.M.; Jung, K.L.; Fuller, T.; Chen, W.; Kerin, T.; Mei, J.; Bhattacharya, D.; et al. The systemic inflammatory landscape of COVID-19 in pregnancy: Extensive serum proteomic profiling of mother-infant dyads with in utero SARS-CoV-2. Cell Rep. Med. 2021, 2, 100453. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Lindström, T.M.; Bennett, P.R. The role of nuclear factor kappa B in human labour. Reproduction 2005, 130, 569–581. [Google Scholar] [CrossRef]
- Hariharan, A.; Hakeem, A.R.; Radhakrishnan, S.; Reddy, M.S.; Rela, M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology 2021, 29, 91–100. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Xu, Q.; Li, J.; Xu, S.; Tang, C. Micro-RNAs in human placenta: Tiny molecules, immense power. Molecules 2022, 27, 5943. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.C.; Chan, E.; Molnar, A.; Sarkar, R.; Alexieva, D.; Isa, I.M.; Robinson, S.; Zhang, S.; Ellis, P.; Langford, C.F.; et al. 5′ isomiR variation is of functional and evolutionary importance. Nucleic Acids Res. 2014, 42, 9424–9435. [Google Scholar] [CrossRef]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Noguer-Dance, M.; Abu-Amero, S.; Al-Khtib, M.; Lefèvre, A.; Coullin, P.; Moore, G.E.; Cavaillé, J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum. Mol. Genet. 2010, 19, 3566–3582. [Google Scholar] [CrossRef] [PubMed]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Sany, M.R.U.; Islam, M.S.; Islam, A. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front. Genet. 2020, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Saulle, I.; Garziano, M.; Fenizia, C.; Cappelletti, G.; Parisi, F.; Clerici, M.; Cetin, I.; Savasi, V.; Biasin, M. MiRNA profiling in plasma and placenta of SARS-CoV-2-infected pregnant women. Cells 2021, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Ding, Y. Association of microRNA-155, interleukin 17A, and proteinuria in preeclampsia. Medicine 2017, 96, e6509. [Google Scholar] [CrossRef]
- Hardin, L.T.; Xiao, N. miRNAs: The key regulator of COVID-19 disease. Int. J. Cell Biol. 2022, 2022, 1645366. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, Y.H.; Liu, P.J.; Hu, W.C.; Lu, K.C.; Tsai, K.W. The emerging role of miRNAs in the pathogenesis of COVID-19: Protective effects of nutraceutical polyphenolic compounds against SARS-CoV-2 infection. Int. J. Med. Sci. 2022, 19, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.; Garcia-Ruiz, I.; Maiz, N.; Rodo, C.; Garcia-Manau, P.; Serrano, B.; Lopez-Martinez, R.M.; Balcells, J.; Fernandez-Hidalgo, N.; Carreras, E.; et al. Pre-eclampsia-like syndrome induced by severe COVID-19: A prospective observational study. BJOG 2020, 127, 1374–1380. [Google Scholar] [CrossRef]
- Ahlberg, M.; Neovius, M.; Saltvedt, S.; Söderling, J.; Pettersson, K.; Brandkvist, C.; Stephansson, O. Association of SARS-CoV-2 test status and pregnancy outcomes. JAMA 2020, 324, 1782–1785. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.H.; Dollinger, C.Y.; VanderWeele, T.J.; Wyszynski, D.F.; Hernández-Díaz, S. Timing and severity of COVID-19 during pregnancy and risk of preterm birth in the International Registry of Coronavirus Exposure in Pregnancy. BMC Pregnancy Childbirth 2022, 22, 775. [Google Scholar] [CrossRef]
- Jering, K.S.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Vardeny, O.; Greene, M.F.; Solomon, S.D. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern. Med. 2021, 181, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Karasek, D.; Baer, R.J.; McLemore, M.R.; Bell, A.J.; Blebu, B.E.; Casey, J.A.; Coleman-Phox, K.; Costello, J.M.; Felder, J.N.; Flowers, E.; et al. The association of COVID-19 infection in pregnancy with preterm birth: A retrospective cohort study in California. Lancet Reg. Health Am. 2021, 2, 100027. [Google Scholar]
- Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 2020, 150, 105187. [Google Scholar] [CrossRef] [PubMed]
- Hoyert, D.L.; Gregory, E.C.W. Cause-of-death data from the fetal death file, 2015–2017. Natl. Vital Stat Rep. 2020, 69, 1–20. [Google Scholar]
- DeSisto, C.L.; Wallace, B.; Simeone, R.M.; Polen, K.; Ko, J.Y.; Meaney-Delman, D.; Ellington, S.R. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization–United States, March 2020–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1640–1645. [Google Scholar] [CrossRef]
- Stenton, S.; McPartland, J.; Shukla, R.; Turner, K.; Marton, T.; Hargitai, B.; Bamber, A.; Pryce, J.; Peres, C.L.; Burguess, N.; et al. SARS-COV-2 placentitis and pregnancy outcome: A multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine 2022, 47, 101389. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.; Johnstone, E.D.; Simcox, L.E.; Myers, J.E. The impact of COVID-19 on pregnancy outcomes in a diverse cohort in England. Sci. Rep. 2022, 12, 942. [Google Scholar] [CrossRef]
- Mullins, E.; Hudak, M.L.; Banerjee, J.; Getzlaff, T.; Townson, J.; Barnette, K.; Playle, R.; Perry, A.; Bourne, T.; Lees, C.C. Pregnancy and neonatal outcomes of COVID-19: Coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021, 57, 573–581. [Google Scholar] [CrossRef]
- Narang, K.; Miller, M.; Trinidad, C.; Wick, M.; Theiler, R.; Weaver, A.L.; Mehta, R.A.; Schenone, M. Impact of asymptomatic and mild COVID-19 infection on fetal growth during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 281, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Piekos, S.N.; Roper, R.T.; Hwang, Y.M.; Sorensen, T.; Price, N.D.; Hood, L.; Hadlock, J.J. The effect of maternal SARS-CoV-2 infection timing on birth outcomes: A retrospective multicentre cohort study. Lancet Digit. Health 2022, 4, e95–e104. [Google Scholar] [CrossRef] [PubMed]
- Patberg, E.T.; Adams, T.; Rekawek, P.; Vahanian, S.A.; Akerman, M.; Hernandez, A.; Rapkiewicz, A.V.; Ragolia, L.; Sicuranza, G.; Chavez, M.R.; et al. Coronavirus disease 2019 infection and placental histopathology in women delivering at term. Am. J. Obstet. Gynecol. 2021, 224, 382.e1–382.e18. [Google Scholar] [CrossRef]
- Cosma, S.; Carosso, A.R.; Cusato, J.; Borella, F.; Bertero, L.; Bovetti, M.; Bevilacqua, F.; Mengozzi, G.; Mazzone, R.; Ghisetti, V.; et al. Obstetric and neonatal outcomes after SARS-CoV-2 infection in the first trimester of pregnancy: A prospective comparative study. J. Obstet. Gynaecol. Res. 2022, 48, 393–401. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M.; Tang, S. Effect of initial COVID-19 outbreak during first trimester on pregnancy outcome in Wuxi, China. BMC Pregnancy Childbirth 2022, 22, 54. [Google Scholar] [CrossRef]
- Hessami, K.; Norooznezhad, A.H.; Monteiro, S.; Barrozo, E.R.; Abdolmaleki, A.S.; Arian, S.E.; Zargarzadeh, N.; Shekerdemian, L.S.; Aagaard, K.M.; Shamshirsaz, A.A. COVID-19 pandemic and infant neurodevelopmental impairment: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e2238941. [Google Scholar] [CrossRef]
- Edlow, A.G.; Castro, V.M.; Shook, L.L.; Kaimal, A.J.; Perlis, R.H. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for SARS-CoV-2 during pregnancy. JAMA Netw. Open 2022, 5, e2215787. [Google Scholar] [CrossRef] [PubMed]
- Ayed, M.; Embaireeg, A.; Kartam, M.; More, K.; Alqallaf, M.; AlNafisi, A.; Alsaffar, Z.; Bahzad, Z.; Buhamad, Y.; Alsayegh, H.; et al. Neurodevelopmental outcomes of infants born to mothers with SARS-CoV-2 infections during pregnancy: A national prospective study in Kuwait. BMC Pediatr. 2022, 22, 319. [Google Scholar] [CrossRef] [PubMed]
- Boulanger-Bertolus, J.; Pancaro, C.; Mashour, G.A. Increasing role of maternal immune activation in neurodevelopmental disorders. Front. Behav. Neurosci. 2018, 12, 230. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, Y.P.; Tan, G.C.; Khong, T.Y. SARS-CoV-2 Transplacental Transmission: A Rare Occurrence? An Overview of the Protective Role of the Placenta. Int. J. Mol. Sci. 2023, 24, 4550. https://doi.org/10.3390/ijms24054550
Wong YP, Tan GC, Khong TY. SARS-CoV-2 Transplacental Transmission: A Rare Occurrence? An Overview of the Protective Role of the Placenta. International Journal of Molecular Sciences. 2023; 24(5):4550. https://doi.org/10.3390/ijms24054550
Chicago/Turabian StyleWong, Yin Ping, Geok Chin Tan, and T. Yee Khong. 2023. "SARS-CoV-2 Transplacental Transmission: A Rare Occurrence? An Overview of the Protective Role of the Placenta" International Journal of Molecular Sciences 24, no. 5: 4550. https://doi.org/10.3390/ijms24054550
APA StyleWong, Y. P., Tan, G. C., & Khong, T. Y. (2023). SARS-CoV-2 Transplacental Transmission: A Rare Occurrence? An Overview of the Protective Role of the Placenta. International Journal of Molecular Sciences, 24(5), 4550. https://doi.org/10.3390/ijms24054550





