Metabolomics Analysis Reveals Novel Targets of Chemosensitizing Polyphenols and Omega-3 Polyunsaturated Fatty Acids in Triple Negative Breast Cancer Cells
Abstract
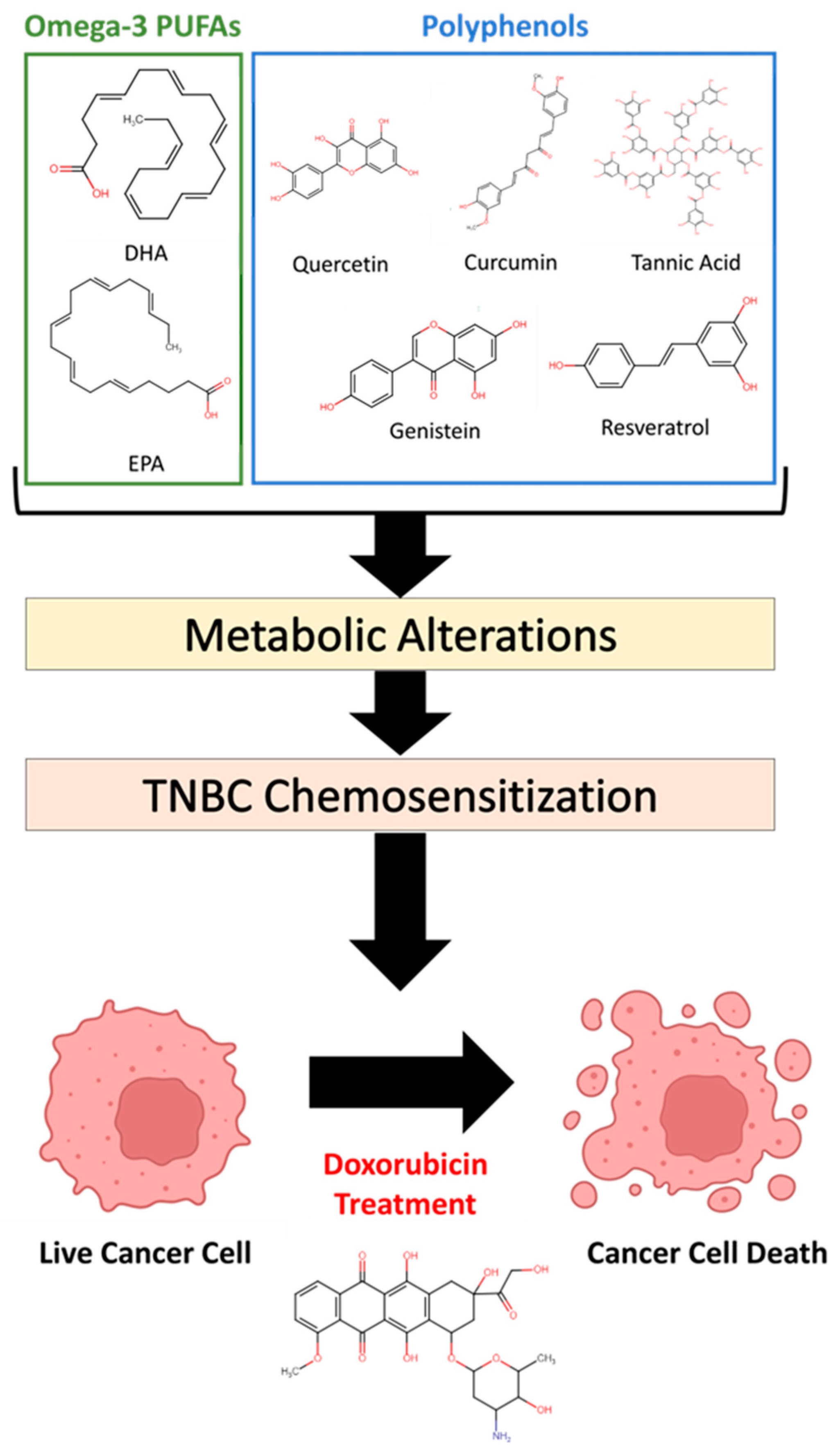
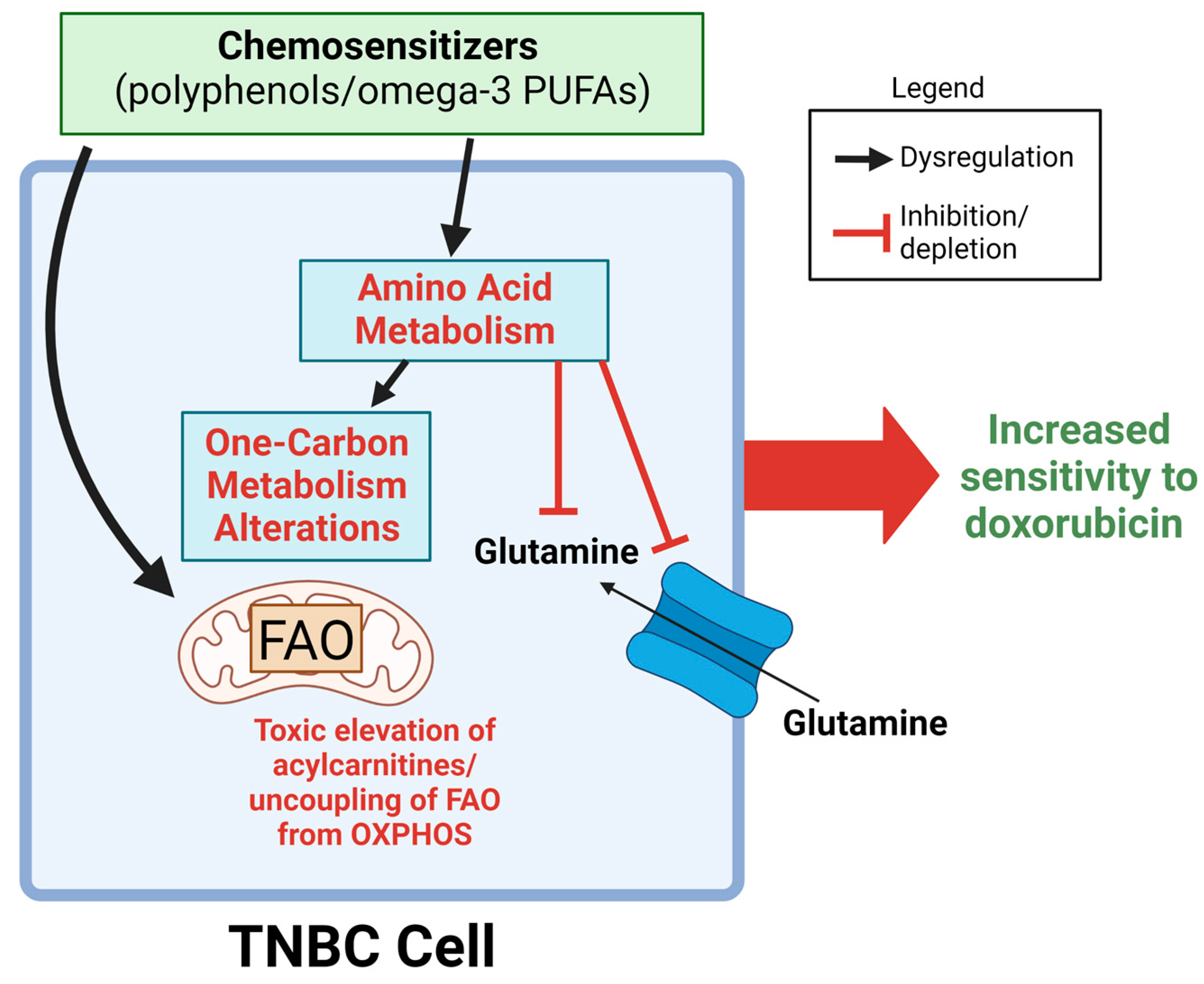
1. Introduction
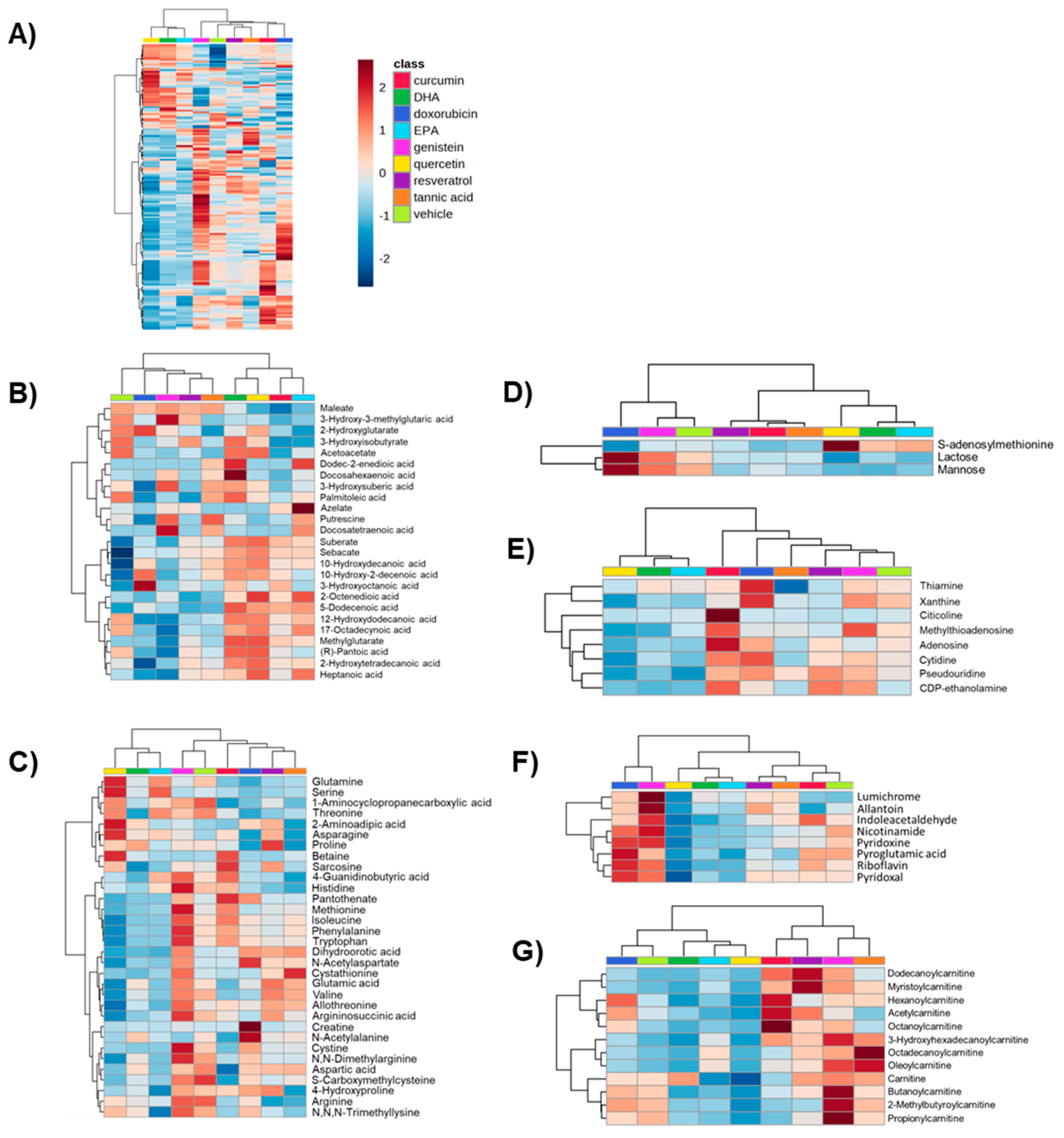
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Cell Culture
4.3. Metabolite Extraction
4.4. UHPLC-HRMS Metabolomics Data Acquisition, Preprocessing, and Multivariate Analysis
4.5. Multivariate and Univariate Statistical Analysis
4.6. Compound Identification/Annotation
4.7. Pathway Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2015, 66, 43–73. [Google Scholar] [CrossRef]
- Zeichner, S.B.; Terawaki, H.; Gogineni, K. A Review of Systemic Treatment in Metastatic Triple-Negative Breast Cancer. Breast Cancer: Basic Clin. Res. 2016, 10, 25–36. [Google Scholar] [CrossRef]
- Cardoso, F.; Harbeck, N.; Barrios, C.H.; Bergh, J.; Cortes, J.; El Saghir, N.; Francis, P.A.; Hudis, C.A.; Ohno, S.; Partridge, A.H.; et al. Research needs in breast cancer. Ann. Oncol. 2016, 28, 208–217. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In Vitro and in Vivo Antitumoral Effects of Combinations of Polyphenols, or Polyphenols and Anticancer Drugs: Perspectives on Cancer Treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemo-therapeutics and phenolic compounds and anticancer synergy between polyphenols. Adv. Hyg. Exp. Med. 2014, 68, 528–540. [Google Scholar]
- Sarkar, F.H.; Li, Y. Using Chemopreventive Agents to Enhance the Efficacy of Cancer Therapy. Cancer Res. 2006, 66, 3347–3350. [Google Scholar] [CrossRef]
- Tikoo, K.; Sane, M.S.; Gupta, C. Tannic acid ameliorates doxorubicin-induced cardiotoxicity and potentiates its anti-cancer activity: Potential role of tannins in cancer chemotherapy. Toxicol. Appl. Pharmacol. 2011, 251, 191–200. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, Y.J.; Won, A.J.; Lee, B.M.; Choi, W.S.; Jung, J.H.; Chung, H.Y.; Kim, H.S. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.A.; Kim, J.H.; Kim, J.H.; Sung, M.-K.; Kim, M.K.; Park, J.H.Y.; Kim, J.-S. Genistein Induces Glucose-Regulated Protein 78 in Mammary Tumor Cells. J. Med. Food 2006, 9, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Staedler, D.; Idrizi, E.; Kenzaoui, B.H.; Juillerat-Jeanneret, L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. [Google Scholar] [CrossRef]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. Pharmacother. 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Zhou, Q.; Ye, M.; Lu, Y.; Zhang, H.; Chen, Q.; Huang, S.; Su, S. Curcumin Improves the Tumoricidal Effect of Mitomycin C by Suppressing ABCG2 Expression in Stem Cell-Like Breast Cancer Cells. PLoS ONE 2015, 10, e0136694. [Google Scholar] [CrossRef]
- Germain, E.; Chajès, V.; Cognault, S.; Lhcillery, C.; Bougnoux, P. Enhancement of doxorubicin cytotoxicity by polyun-saturated fatty acids in the human breast tumor cell line MDA-MB-231: Relationship to lipid peroxidation. Int. J. Cancer 1998, 75, 578–583. [Google Scholar] [CrossRef]
- Qian, J.; Xia, M.; Liu, W.; Li, L.; Yang, J.; Mei, Y.; Meng, Q.; Xie, Y. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemo-therapeutic agents. Eur. J. Pharmacol. 2019, 852, 231–243. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Agarwal, C.; Chan, D.C.; Agarwal, R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol. Rep. 2004, 11, 493–499. [Google Scholar] [CrossRef]
- Kalyani, C.; Narasu, M.L.; Devi, Y.P. Synergistic growth inhibitory effect of flavonol–kaempferol and conventional chemotherapeutic drugs on cancer cells. Int. J. Pharm. Pharm. Sci. 2017, 9, 123. [Google Scholar] [CrossRef]
- Nabekura, T.; Yamaki, T.; Ueno, K.; Kitagawa, S. Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals. Cancer Chemother. Pharmacol. 2008, 62, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, X.-N.; Chen, J.; Wang, W.-X.; Lu, Z.-F. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of Multidrug Resistance in Cancer by Multi-Functional Flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/flavonoids reverse breast cancer resistance pro-tein/ABCG2-mediated multidrug resistance. Cancer Res. 2004, 64, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Sen, G.S.; Mohanty, S.; Hossain, D.M.S.; Bhattacharyya, S.; Banerjee, S.; Chakraborty, J.; Saha, S.; Ray, P.; Bhattacharjee, P.; Mandal, D.; et al. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53-p300 in breast cancer. J. Biol. Chem. 2011, 286, 42232–42247. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Ji, C. Quercetin: A potential drug to reverse multidrug resistance. Life Sci. 2010, 87, 333–338. [Google Scholar] [CrossRef]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of Polyphenol Liposomes: A Challenge Ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yashodhara, B.M.; Umakanth, S.; Pappachan, J.M.; Bhat, S.K.; Kamath, R.; Choo, B.H. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Wu, T.; Lim, K. Omega-3 polyunsaturated fatty acids and cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Nifli, A.-P.; Notas, G.; Castanas, E. Polyphenols and cancer cell growth. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 159, pp. 79–113. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef]
- Wiggs, A.; Molina, S.; Sumner, S.J.; Rushing, B.R. A Review of Metabolic Targets of Anticancer Nutrients and Nutraceuticals in Pre-Clinical Models of Triple-Negative Breast Cancer. Nutrients 2022, 14, 1990. [Google Scholar] [CrossRef]
- Zaal, E.A.; Berkers, C.R. The influence of metabolism on drug response in cancer. Front. Oncol. 2018, 8, 500. [Google Scholar] [CrossRef]
- Morandi, A.; Indraccolo, S. Linking metabolic reprogramming to therapy resistance in cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2017, 1868, 1–6. [Google Scholar] [CrossRef]
- Zhao, Y.; Butler, E.B.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S. RefMet: A reference nomenclature for metabolomics. Nat. Methods 2020, 17, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Boroughs, L.K.; DeBerardinis, R.J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 2015, 17, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Hasan, M.R. Cancer Metabolism and Drug Resistance. Metabolites 2015, 5, 571–600. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Fan, M.; Liu, Z.; Li, X.; Wang, H. Serine, glycine and one-carbon metabolism in cancer (Review). Int. J. Oncol. 2021, 58, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef]
- Wajner, M.; Amaral, A.U. Mitochondrial dysfunction in fatty acid oxidation disorders: Insights from human and animal studies. Biosci. Rep. 2016, 36, e00281. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Neufer, P.D. Lipid-Induced Mitochondrial Stress and Insulin Action in Muscle. Cell Metab. 2012, 15, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Re-sistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wieckowski, M.; Wojtczak, L. Fatty acid-induced uncoupling of oxidative phosphorylation is partly due to opening of the mitochondrial permeability transition pore. FEBS Lett. 1998, 423, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, H.; Hoek, J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability tran-sition pore. Aging Cell 2017, 16, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Furuno, T.; Kanno, T.; Arita, K.; Asami, M.; Utsumi, T.; Doi, Y.; Inoue, M.; Utsumi, K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem. Pharmacol. 2001, 62, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Berezhnov, A.V.; Fedotova, E.I.; Nenov, M.N.; Kasymov, V.A.; Pimenov, O.Y.; Dynnik, V.V. Dissecting Cellular Mechanisms of Long-Chain Acylcarnitines-Driven Cardiotoxicity: Disturbance of Calcium Homeostasis, Activation of Ca2+-Dependent Phospholipases, and Mitochondrial Energetics Collapse. Int. J. Mol. Sci. 2020, 21, 7461. [Google Scholar] [CrossRef]
- D'Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research Strategies in the Study of the Pro-Oxidant Nature of Polyphenol Nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxidative Med. Cell. Longev. 2015, 2016, 1–17. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Margreiter, R.; Amberger, A.; Saks, V.; Grimm, M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 1144–1152. [Google Scholar] [CrossRef]
- Dornfeld, K.; Bjork, J.; Folkert, G.; Skildum, A.; Wallace, K.B. Mitochondrial activities play a pivotal role in regulating cell cycle in response to doxorubicin. Cell Cycle 2021, 20, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Uygur, R.; Aktas, C.; Tulubas, F.; Alpsoy, S.; Topcu, B.; Ozen, O.A. Cardioprotective effects of fish omega-3 fatty acids on doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 2013, 33, 435–445. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; El-Afify, D.; Khedr, R.; Ibrahim, A.M. Omega 3 fatty acids can reduce early doxorubicin-induced cardio-toxicity in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2022, 69, 1–8. [Google Scholar] [CrossRef]
- Razavi-Azarkhiavi, K.; Iranshahy, M.; Sahebkar, A.; Shirani, K.; Karimi, G. The Protective Role of Phenolic Compounds Against Doxorubicin-induced Cardiotoxicity: A Comprehensive Review. Nutr. Cancer 2016, 68, 892–917. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Roscilli, G.; Marra, E.; Mori, F.; Di Napoli, A.; Mancini, R.; Serlupi-Crescenzi, O.; Virmani, A.; Aurisicchio, L.; Ciliberto, G. Carnitines slow down tumor development of colon cancer in the DMH-chemical carcinogenesis mouse model. J. Cell. Biochem. 2013, 114, 1665–1673. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Ekrami, E.M.; Aghdas, S.A.M.; Mihanfar, A.; Hallaj, S.; Yousefi, B.; Safa, A.; Majidinia, M. Targeting PI3K/Akt/mTOR signaling pathway by polyphenols: Implication for cancer therapy. Life Sci. 2020, 255, 117481. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Babichev, Y.; Kabaroff, L.; Datti, A.; Uehling, D.; Isaac, M.; Al-Awar, R.; Prakesch, M.; Sun, R.X.; Boutros, P.C.; Venier, R.; et al. PI3K/AKT/mTOR inhibition in combination with doxorubicin is an effective therapy for leiomyosarcoma. J. Transl. Med. 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.L.; Da Ros, M.; Pisano, C.; de Martino, M.; Genitori, L.; Sardi, I. Combined Treatment with Doxorubicin and Rapamycin Is Effective against In Vitro and In Vivo Models of Human Glioblastoma. J. Clin. Med. 2019, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, W.; Hao, H.; Wang, Q.; Xue, L. Rapamycin enhanced the antitumor effects of doxorubicin in myelogenous leukemia K562 cells by downregulating the mTOR/p70S6K pathway. Oncol. Lett. 2019, 18, 2694–2703. [Google Scholar] [CrossRef]
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: Evidence from a phase 1 trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2017, 3, 509–515. [Google Scholar] [CrossRef]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 2019, 8, 1584. [Google Scholar] [CrossRef]
- Peron, G.; Sut, S.; Ben, S.D.; Voinovich, D.; Dall'Acqua, S. Untargeted UPLC-MS metabolomics reveals multiple changes of urine composition in healthy adult volunteers after consumption of curcuma longa L. extract. Food Res. Int. 2019, 127, 108730. [Google Scholar] [CrossRef]
- Gimeno-Mallench, L.; Mas-Bargues, C.; Inglés, M.; Olaso, G.; Borras, C.; Gambini, J.; Vina, J. Resveratrol shifts energy metabolism to increase lipid oxidation in healthy old mice. Biomed. Pharmacother. 2019, 118, 109130. [Google Scholar] [CrossRef]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948. [Google Scholar] [CrossRef]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mi-tochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta.-Gen. Subj. 2016, 1860, 727–745. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M.; Nabavi, S.F. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016, 34, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Do, G.-M.; Kwon, E.-Y.; Ha, T.-Y.; Park, Y.B.; Kim, H.-J.; Jeon, S.-M.; Lee, M.-K.; Choi, M.-S. Tannic acid is more effective than clofibrate for the elevation of hepatic β-oxidation and the inhibition of 3-hydroxy-3-methyl-glutaryl-CoA reductase and aortic lesion formation in apo E-deficient mice. Br. J. Nutr. 2011, 106, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R. Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 2016, 29, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Li, H.; Jia, W.; Shou, Q.; Zhu, Y.; Mao, L.; Wang, W.; Wu, F.; Chen, X.; Wan, X. Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 2021, 9, 185. [Google Scholar] [CrossRef]
- Fan, W.-H.; Wang, F.-C.; Jin, Z.; Zhu, L.; Zhang, J.-X. Curcumin Synergizes with Cisplatin to Inhibit Colon Cancer through Targeting the MicroRNA-137-Glutaminase Axis. Curr. Med Sci. 2021, 42, 108–117. [Google Scholar] [CrossRef]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.A.; Lalk, M. The Impact of Soy Isoflavones on MCF-7 and MDA-MB-231 Breast Cancer Cells Using a Global Metabolomic Approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef]
- Freeman, M.R.; Kim, J.; Lisanti, M.P.; Di Vizio, D. A metabolic perturbation by U0126 identifies a role for glutamine in resveratrol-induced cell death. Cancer Biol. Ther. 2011, 12, 966–977. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Wang, K.; Han, W.; Wang, X.; Gao, M.; Wang, Z.; Sun, Y.; Yan, H.; Zhang, H.; et al. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 2020, 881, 173185. [Google Scholar] [CrossRef]
- Rushing, B.R.; Tilley, S.; Molina, S.; Schroder, M.; Sumner, S. Commonalities in Metabolic Reprogramming between Tobacco Use and Oral Cancer. Int. J. Environ. Res. Public Health 2022, 19, 10261. [Google Scholar] [CrossRef]
- Rushing, B.R.; Schroder, M.; Sumner, S.C.J. Comparison of Lysis and Detachment Sample Preparation Methods for Cultured Triple-Negative Breast Cancer Cells Using UHPLC–HRMS-Based Metabolomics. Metabolites 2022, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Rushing, B.R.; Fogle, H.M.; Sharma, J.; You, M.; McCormac, J.P.; Molina, S.; Sumner, S.; Krupenko, N.I.; Krupenko, S.A. Exploratory Metabolomics Underscores the Folate Enzyme ALDH1L1 as a Regulator of Glycine and Methylation Reactions. Molecules 2022, 27, 8394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Rushing, B.R.; Hall, M.S.; Helke, K.L.; McRitchie, S.L.; Krupenko, N.I.; Sumner, S.J.; Krupenko, S.A. Sex-Specific Metabolic Effects of Dietary Folate Withdrawal in Wild-Type and Aldh1l1 Knockout Mice. Metabolites 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rushing, B.; Schroder, M.; Sumner, S.; Kay, C.D. Exploring the Contribution of (Poly)phenols to the Dietary Exposome Using High Resolution Mass Spectrometry Untargeted Metabolomics. Mol. Nutr. Food Res. 2022, 66, 2100922. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Rushing, B.R.; Harris, S.E.; McRitchie, S.L.; Dominguez, D.; Sumner, S.J.; Dohlman, H.G. Multi-Omics Analysis of Multiple Glucose-Sensing Receptor Systems in Yeast. Biomolecules 2022, 12, 175. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Rushing, B.R.; Harris, S.E.; McRitchie, S.L.; Jones, J.C.; Dominguez, D.; Sumner, S.J.; Dohlman, H.G. Multi-omics analysis of glucose-mediated signaling by a moonlighting Gβ protein Asc1/RACK1. PLoS Genet. 2021, 17, 1–30. [Google Scholar] [CrossRef]
- Rushing, B.R.; McRitchie, S.; Arbeeva, L.; Nelson, A.E.; Azcarate-Peril, M.A.; Li, Y.-Y.; Qian, Y.; Pathmasiri, W.; Sumner, S.C.; Loeser, R.F. Fecal metabolomics reveals products of dysregulated proteolysis and altered microbial metabolism in obesity-related osteoarthritis. Osteoarthr. Cartil. 2021, 30, 81–91. [Google Scholar] [CrossRef]
- Välikangas, T.; Suomi, T.; Elo, L.L. A systematic evaluation of normalization methods in quantitative label-free proteomics. Brief. Bioinform. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Bender, R.; Lange, S. Adjusting for multiple testing—When and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]

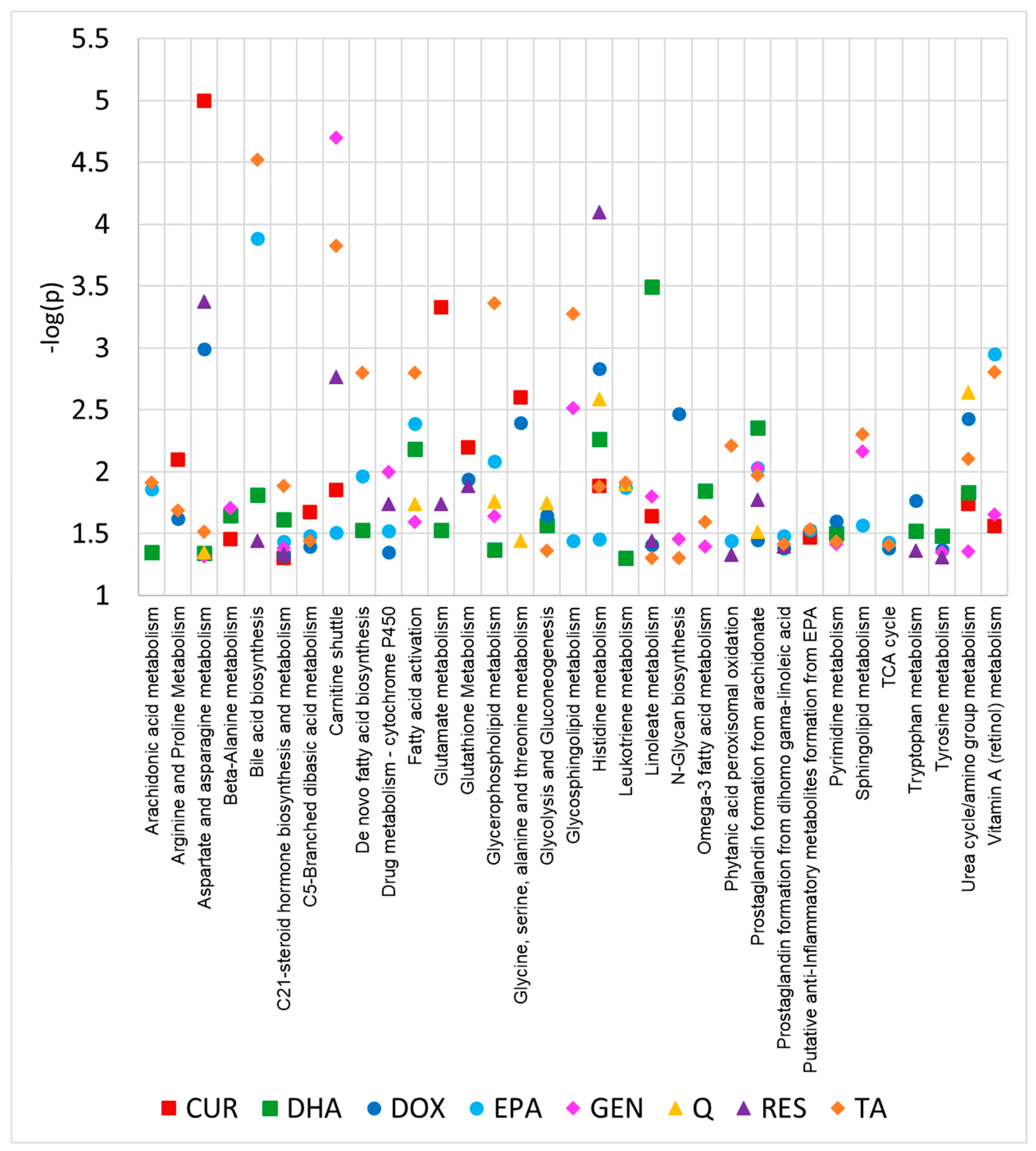
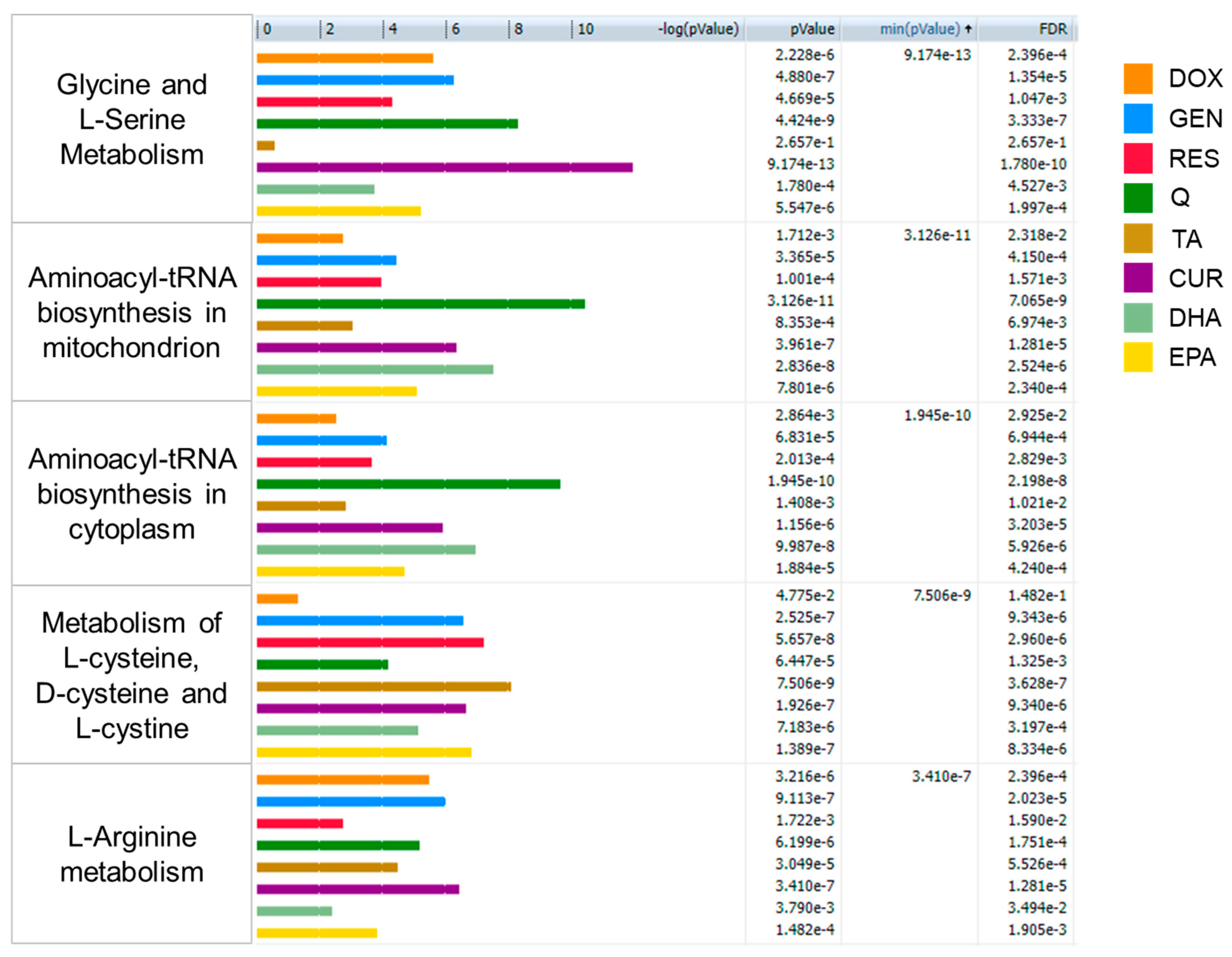
| Doxorubicin | EPA | DHA | Quercetin | Genistein | Resveratrol | Tannic Acid | Curcumin | |
|---|---|---|---|---|---|---|---|---|
| C21-steroid hormone biosynthesis and metabolism | 4.7 × 10−2 | 3.6 × 10−2 | 2.4 × 10−2 | 4.1 × 10−2 | 4.6 × 10−2 | 1.3 × 10−2 | 4.9 × 10−2 | |
| Histidine metabolism | 1.4 × 10−3 | 2.4 × 10−2 | 1.6 × 10−2 | 5.5 × 10−3 | 6.0 × 10−5 | 1.3 × 10−2 | 1.5 × 10−2 | |
| Aspartate and asparagine metabolism | 1.0 × 10−3 | 4.5 × 10−2 | 4.8 × 10−2 | 5.3 × 10−4 | 3.0 × 10−2 | <1.0 × 10−5 | ||
| Linoleate metabolism | 3.9 × 10−2 | 3.2 × 10−4 | 1.6 × 10−2 | 3.6 × 10−2 | 5.0 × 10−2 | 2.3 × 10−2 | ||
| Prostaglandin formation from arachidonate | 3.5 × 10−2 | 9.2 × 10−3 | 4.4 × 10−3 | 9.2 × 10−3 | 1.7 × 10−2 | 1.1 × 10−2 | ||
| Urea cycle/amino group metabolism | 6.9 × 10−3 | 1.5 × 10−2 | 3.5 × 10−2 | 4.4 × 10−2 | 7.9 × 10−3 | 1.8 × 10−2 | ||
| Carnitine shuttle | 3.1 × 10−2 | 2.0 × 10−5 | 1.7 × 10−3 | 1.5 × 10−4 | 1.4 × 10−2 | |||
| Drug metabolism—cytochrome P450 | 4.4 × 10−2 | 3.0 × 10−2 | 9.9 × 10−3 | 1.8 × 10−2 | 3.0 × 10−2 | |||
| Glycerophospholipid metabolism | 8.2 × 10−3 | 4.2 × 10−2 | 4.5 × 10−2 | 2.3 × 10−2 | 4.3 × 10−4 | |||
| Pyrimidine metabolism | 2.5 × 10−2 | 3.7 × 10−2 | 3.1 × 10−2 | 3.8 × 10−2 | 3.7 × 10−2 | |||
| Bile acid biosynthesis | 1.3 × 10−4 | 1.5 × 10−2 | 2.3 × 10−2 | 3.0 × 10−5 | ||||
| C5-Branched dibasic acid metabolism | 3.4 × 10−2 | 3.2 × 10−2 | 3.5 × 10−2 | 2.1 × 10−2 | ||||
| Fatty acid activation | 4.0 × 10−3 | 1.2 × 10−2 | 2.6 × 10−2 | 1.6 × 10−3 | ||||
| Leukotriene metabolism | 1.3 × 10−2 | 5.0 × 10−2 | 3.0 × 10−2 | 1.2 × 10−2 | ||||
| N-Glycan biosynthesis | 3.3 × 10−3 | 5.0 × 10−2 | 3.5 × 10−2 | 5.0 × 10−2 | ||||
| Purine metabolism | 3.3 × 10−2 | 4.6 × 10−2 | 3.3 × 10−2 | 4.3 × 10−2 | ||||
| Vitamin A (retinol) metabolism | 1.1 × 10−3 | 2.2 × 10−2 | 1.6 × 10−3 | 2.8 × 10−2 | ||||
| Arachidonic acid metabolism | 1.3 × 10−2 | 4.4 × 10−2 | 1.2 × 10−2 | |||||
| De novo fatty acid biosynthesis | 1.1 × 10−2 | 2.9 × 10−2 | 1.6 × 10−3 | |||||
| Glutathione metabolism | 1.6 × 10−2 | 8.7 × 10−3 | 9.0 × 10−3 | |||||
| Glycolysis and gluconeogenesis | 2.2 × 10−2 | 4.7 × 10−3 | 4.3 × 10−2 | |||||
| Glycosphingolipid metabolism | 3.6 × 10−2 | 3.0 × 10−3 | 5.3 × 10−4 | |||||
| Prostaglandin formation from dihomo gama-linoleic acid | 3.9 × 10−2 | 4.0 × 10−2 | 3.6 × 10−2 | |||||
| Putative anti-inflammatory metabolites formation from EPA | 2.8 × 10−2 | 2.9 × 10−2 | 3.3 × 10−2 | |||||
| Sphingolipid metabolism | 2.8 × 10−2 | 6.9 × 10−3 | 5.0 × 10−3 | |||||
| Tryptophan metabolism | 4.3 × 10−2 | 3.0 × 10−2 | 4.3 × 10−2 | |||||
| Tyrosine metabolism | 4.3 × 10−2 | 3.3 × 10−2 | 4.5 × 10−2 | |||||
| Alanine and aspartate metabolism | 4.7 × 10−2 | 6.5 × 10−3 | ||||||
| Arginine and proline metabolism | 2.0 × 10−2 | 7.7 × 10−3 | ||||||
| Beta-Alanine metabolism | 1.9 × 10−2 | 5.0 × 10−2 | ||||||
| Fatty acid metabolism | 9.6 × 10−3 | 8.6 × 10−3 | ||||||
| Fatty acid oxidation, peroxisome | 2.4 × 10−2 | 9.4 × 10−3 | ||||||
| Glutamate metabolism | 1.6 × 10−2 | 5.4 × 10−4 | ||||||
| Glycine, serine, alanine and threonine metabolism | 4.0 × 10−3 | 2.4 × 10−3 | ||||||
| Methionine and cysteine metabolism | 2.2 × 10−2 | 4.3 × 10−2 | ||||||
| Nitrogen metabolism | 2.8 × 10−2 | 4.7 × 10−2 | ||||||
| Omega-3 fatty acid metabolism | 2.9 × 10−2 | 2.6 × 10−2 | ||||||
| Pyruvate metabolism | 4.6 × 10−3 | 5.0 × 10−2 | ||||||
| Saturated fatty acid beta-oxidation | 1.5 × 10−2 | 4.4 × 10−2 | ||||||
| Sialic acid metabolism | 3.2 × 10−2 | 2.6 × 10−2 | ||||||
| Squalene and cholesterol biosynthesis | 3.6 × 10−2 | 3.6 × 10−2 | ||||||
| TCA cycle | 3.7 × 10−2 | 3.7 × 10−2 | ||||||
| Vitamin E metabolism | 4.3 × 10−2 | 2.1 × 10−2 | ||||||
| 3-oxo-10R-octadecatrienoate beta-oxidation | 3.6 × 10−2 | |||||||
| Aminosugars metabolism | 4.3 × 10−2 | |||||||
| Blood group biosynthesis | 2.3 × 10−2 | |||||||
| Carbon fixation | 8.3 × 10−3 | |||||||
| Di-unsaturated fatty acid beta-oxidation | 4.8 × 10−2 | |||||||
| Dimethyl-branched-chain fatty acid mitochondrial beta-oxidation | 3.1 × 10−2 | |||||||
| Fructose and mannose metabolism | 7.0 × 10−3 | |||||||
| Galactose metabolism | 2.4 × 10−2 | |||||||
| Glycosphingolipid biosynthesis—ganglioseries | 2.6 × 10−2 | |||||||
| Glycosphingolipid biosynthesis—lactoseries | 2.3 × 10−2 | |||||||
| Glycosphingolipid biosynthesis—neolactoseries | 2.3 × 10−2 | |||||||
| Hexose phosphorylation | 1.7 × 10−2 | |||||||
| Limonene and pinene degradation | 3.4 × 10−2 | |||||||
| Lysine metabolism | 3.0 × 10−2 | |||||||
| Omega-6 fatty acid metabolism | 2.1 × 10−2 | |||||||
| Pentose and glucuronate interconversions | 4.9 × 10−2 | |||||||
| Pentose phosphate pathway | 2.7 × 10−2 | |||||||
| Phytanic acid peroxisomal oxidation | 1.1 × 10−2 | |||||||
| Polyunsaturated fatty acid biosynthesis | 3.8 × 10−2 | |||||||
| Selenoamino acid metabolism | 2.1 × 10−2 | |||||||
| Valine, leucine and isoleucine degradation | 5.6 × 10−3 | |||||||
| Vitamin B3 (nicotinate and nicotinamide) metabolism | 2.4 × 10−2 | |||||||
| Vitamin B9 (folate) metabolism | 3.6 × 10−2 | |||||||
| Vitamin D3 (cholecalciferol) metabolism | 3.6 × 10−2 | |||||||
| Vitamin K metabolism | 2.1 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rushing, B.R.; Wiggs, A.; Molina, S.; Schroder, M.; Sumner, S. Metabolomics Analysis Reveals Novel Targets of Chemosensitizing Polyphenols and Omega-3 Polyunsaturated Fatty Acids in Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 4406. https://doi.org/10.3390/ijms24054406
Rushing BR, Wiggs A, Molina S, Schroder M, Sumner S. Metabolomics Analysis Reveals Novel Targets of Chemosensitizing Polyphenols and Omega-3 Polyunsaturated Fatty Acids in Triple Negative Breast Cancer Cells. International Journal of Molecular Sciences. 2023; 24(5):4406. https://doi.org/10.3390/ijms24054406
Chicago/Turabian StyleRushing, Blake R., Alleigh Wiggs, Sabrina Molina, Madison Schroder, and Susan Sumner. 2023. "Metabolomics Analysis Reveals Novel Targets of Chemosensitizing Polyphenols and Omega-3 Polyunsaturated Fatty Acids in Triple Negative Breast Cancer Cells" International Journal of Molecular Sciences 24, no. 5: 4406. https://doi.org/10.3390/ijms24054406
APA StyleRushing, B. R., Wiggs, A., Molina, S., Schroder, M., & Sumner, S. (2023). Metabolomics Analysis Reveals Novel Targets of Chemosensitizing Polyphenols and Omega-3 Polyunsaturated Fatty Acids in Triple Negative Breast Cancer Cells. International Journal of Molecular Sciences, 24(5), 4406. https://doi.org/10.3390/ijms24054406







