Polymorphisms in Lymphotoxin-Alpha as the “Missing Link” in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review
Abstract
1. Introduction
1.1. Pathogenesis of DED Is Inflammatory
1.2. Normal Immune Response Arc: Innate and Adaptive Immunity
1.3. Inflammation in DED Is Due to Dysregulation of Innate and Adaptive Immunity
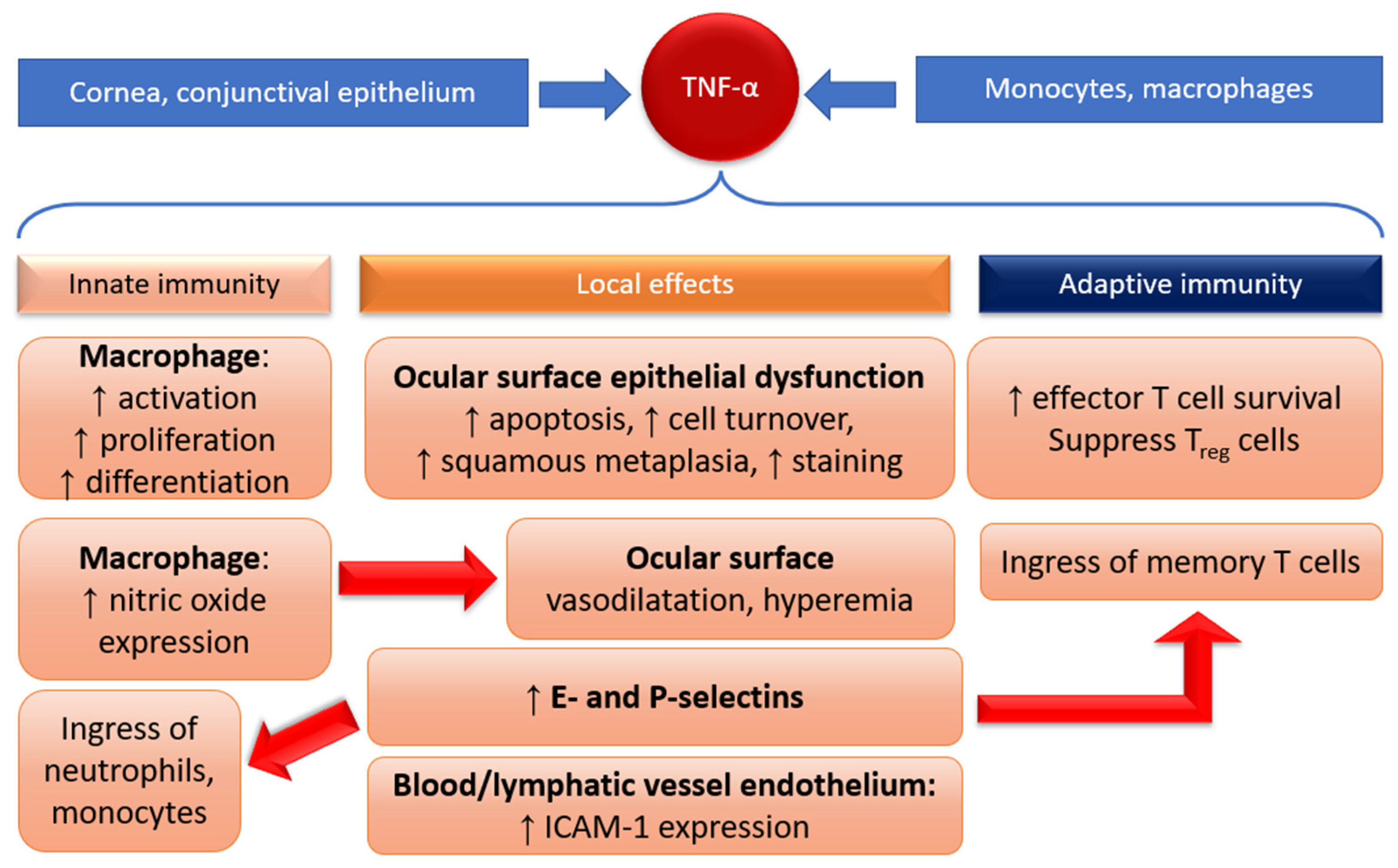
1.3.1. Innate Immunity
1.3.2. Adaptive Immunity
1.4. Inflammation Is Regulated by Genetics and Environment
2. Role of Omega-3 in Dampening Inflammation
2.1. Omega-3 Fatty Acids as a Treatment for DED
2.2. Effect of Omega-3 Fatty Acids on Innate and Adaptive Immunity
3. Roles of Inflammatory Cytokines TNF-α and LT-α in DED Inflammation
3.1. Tumor Necrosis Factor Alpha (TNF-α)
3.2. Lymphotoxin Alpha (LT-α)
4. Role of Genetics in Prognosticating Anti-Inflammatory Effects of Omega-3
4.1. Genetic Basis for Variation in Omega-3 Response
4.2. Genetic Basis for Variation in Production of TNF-α
4.3. Polymorphisms to TNF-α Related Genes Might Predict Omega-3 Response
4.4. Accounting for How Polymorphisms in LT-α Alter the Expression and Activity of Cytokines
4.5. Implications for Testing and Other Potential Testing Targets
4.6. Implications for Specific Patient Populations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.; Benítez-Del-Castillo, J.; Boboridis, K.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Daggy, B.P. The Role of Fish Oil in Inflammatory Eye Diseases. Biomed. Hub 2017, 2, 455818. [Google Scholar] [CrossRef] [PubMed]
- 2020–2021 BCSC Basic and Clinical Science CourseTM. Available online: https://www.aao.org/bcscsnippetdetail.aspx?id=e15abb31-d82f-4c41-abe1-8a4d9aece885 (accessed on 16 November 2022).
- Annunziato, F.; Cosmi, L.; Liotta, F.; Maggi, E.; Romagnani, S. Human T helper type 1 dichotomy: Origin, phenotype and biological activities. Immunology 2014, 144, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Wu, X.; Tian, J.; Wang, S. Insight into Non-Pathogenic Th17 Cells in Autoimmune Diseases. Front. Immunol. 2018, 9, 1112. [Google Scholar] [CrossRef]
- Soifer, M.; Azar, N.S.; Mousa, H.M.; Perez, V.L. Ocular Surface Inflammatory Disorders (OSID): A Collective of Systemic Etiologies Which Cause or Amplify Dry Eye Syndrome. Front. Med. 2022, 9, 1112. [Google Scholar] [CrossRef]
- Ong, H.S.; Dart, J.K. Managing ocular surface disease: A common-sense approach. Community Eye Health 2016, 29, 44–46. [Google Scholar] [PubMed]
- Guzmán, M.; Keitelman, I.; Sabbione, F.; Trevani, A.S.; Giordano, M.N.; Galletti, J.G. Desiccating stress-induced disruption of ocular surface immune tolerance drives dry eye disease. Clin. Exp. Immunol. 2016, 184, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, D.-Q.; Corrales, R.M.; Pflugfelder, S.C. Hyperosmolar Saline Is a Proinflammatory Stress on the Mouse Ocular Surface. Eye Contact Lens Sci. Clin. Pract. 2005, 31, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Pflugfelder, S.; Liu, Z.; Baudouin, C.; Kim, H.; Messmer, E.; Kruse, F.; Liang, L.; Liang, L.; Galeano, J.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271. [Google Scholar] [CrossRef]
- Jang, D.-I.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Enríquez-De-Salamanca, A.; Castellanos, E.; Stern, M.E.; Fernández, I.; Carreño, E.; García-Vázquez, C.; Herreras, J.M.; Calonge, M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol. Vis. 2010, 16, 862–873. [Google Scholar]
- Chauhan, S.K.; Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in Dry Eye Is Due to Resistance of Th17 to Treg Suppression. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D.; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef]
- Chennakesavalu, M.; Somala, S.R.R.; Dommaraju, S.R.; Peesapati, M.P.; Guo, K.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Corneal lymphangiogenesis as a potential target in dry eye disease—A systematic review. Surv. Ophthalmol. 2021, 66, 960–976. [Google Scholar] [CrossRef]
- Qi, M.; Liu, D.; Pan, L.; Lin, Y. Interleukin-10 gene -592C > A polymorphism and susceptibility to gastric cancer. Genet. Mol. Res. 2014, 13, 8954–8961. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’Shea, J.J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36. [Google Scholar] [CrossRef]
- Franke, A.; McGovern, D.P.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R.; et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 42, 1118–1125. [Google Scholar] [CrossRef]
- Hu, K.; Hou, S.; Jiang, Z.; Kijlstra, A.; Yang, P. JAK2 and STAT3 polymorphisms in a Han Chinese population with Behçet’s disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 538–541. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Ellinghaus, E.; Nair, R.P.; Stuart, P.E.; Esko, T.; Metspalu, A.; Debrus, S.; Raelson, J.V.; Tejasvi, T.; Belouchi, M.; et al. Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am. J. Hum. Genet. 2012, 90, 636–647. [Google Scholar] [CrossRef]
- Remmers, E.F.; Plenge, R.M.; Lee, A.T.; Graham, R.R.; Hom, G.; Behrens, T.W.; de Bakker, P.I.; Le, J.M.; Lee, H.S.; Batliwalla, F.; et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 2007, 357, 977–986. [Google Scholar] [CrossRef]
- Duetsch, G.; Illig, T.; Loesgen, S.; Rohde, K.; Klopp, N.; Herbon, N.; Gohlke, H.; Altmueller, J.; Wjst, M. STAT6 as an asthma candidate gene: Polymorphism-screening, association and haplotype analysis in a Caucasian sib-pair study. Hum Mol Genet. 2002, 11, 613–621. [Google Scholar] [CrossRef]
- Grad, R. Cod and the consumptive: A brief history of cod-liver oil in the treatment of pulmonary tuberculosis. Pharm. Hist. 2004, 46, 106–120. [Google Scholar] [PubMed]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Yashodhara, B.M.; Umakanth, S.; Pappachan, J.M.; Bhat, S.K.; Kamath, R.; Choo, B.H. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Griffing, G.T. Mother was right about cod liver oil. Am. J. Med. 2008, 10, 8. [Google Scholar]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Sheppe, A.E.F.; Edelmann, M.J. Roles of Eicosanoids in Regulating Inflammation and Neutrophil Migration as an Innate Host Response to Bacterial Infections. Infect. Immun. 2021, 89, e00095-21. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, Y.; Valera, I.; Municio, C.; Hugo, E.; Padrón, F.; Blanco, L.; Rodríguez, M.; Fernández, N.; Crespo, M.S. Eicosanoids in the Innate Immune Response: TLR and Non-TLR Routes. Mediat. Inflamm. 2010, 2010, 201929. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.; Ng, S.M.; Lindsley, K.B.; Akpek, E.K. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Horwath-Winter, J.; Messmer, E.M.; Rolando, M.; Aragona, P.; Kinoshita, S. The role of systemic and topical fatty acids for dry eye treatment. Prog. Retin. Eye Res. 2017, 61, 23–34. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Hong, S.; Lu, Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: Targeting novel lipid mediator pathways in mitigation of acute kidney injury. Front. Immunol. 2013, 4, 13. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef]
- Liu, A.; Ji, J. Omega-3 essential fatty acids therapy for dry eye syndrome: A meta-analysis of randomized controlled studies. Med. Sci. Monit. 2014, 20, 1583–1589. [Google Scholar]
- O’Byrne, C.; O’Keeffe, M. Omega-3 fatty acids in the management of dry eye disease—An updated systematic review and meta-analysis. Acta Ophthalmol. 2022, 101, e118–e134. [Google Scholar] [CrossRef]
- Dry Eye Assessment and Management Study Research Group. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690.
- Hussain, M.; Shtein, R.M.; Pistilli, M.; Maguire, M.G.; Oydanich, M.; Asbell, P.A. The Dry Eye Assessment and Management (DREAM) extension study—A randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease. Ocul. Surf. 2020, 18, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clària, J. Resolvin D1 and Its Precursor Docosahexaenoic Acid Promote Resolution of Adipose Tissue Inflammation by Eliciting Macrophage Polarization toward an M2-Like Phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS–) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; de la Rosa, A.J.C. Efficacy of Fish Oil on Serum of TNFα, IL-1β, and IL-6 Oxidative Stress Markers in Multiple Sclerosis Treated with Interferon Beta-1b. Oxidative Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, A.M.; Saedisomeolia, A.; Djalali, M.; Djazayery, A.; Pooya, S.; Sojoudi, F. Efficacy of omega-3 fatty acid supplementation on serum levels of tumour necrosis factor-alpha, C-reactive protein and interleukin-2 in type 2 diabetes mellitus patients. Singap. Med. J. 2012, 53, 615–619. [Google Scholar]
- Natto, Z.S.; Yaghmoor, W.; Alshaeri, H.K.; Van Dyke, T.E. Omega-3 Fatty Acids Effects on Inflammatory Biomarkers and Lipid Profiles among Diabetic and Cardiovascular Disease Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 18867. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of Marine-Derived n-3 Polyunsaturated Fatty Acids on C-Reactive Protein, Interleukin 6 and Tumor Necrosis Factor α: A Meta-Analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.; Mantzioris, E.; Gibson, R.; Cleland, L.G.; James, M.J. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G. Inflammatory Cytokines in Nonpathological States. Physiology 2000, 15, 298–303. [Google Scholar] [CrossRef]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P. Analysis of Inflammatory Cytokines in the Tears of Dry Eye Patients. Cornea 2009, 28, 1023–1027. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef]
- Wesemann, D.R.; Benveniste, E.N. STAT-1 alpha and IFN-gamma as modulators of TNF-αlpha signaling in macrophages: Regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J. Immunol. 2003, 171, 5313–5319. [Google Scholar] [CrossRef]
- Yarilina, A.; Park-Min, K.-H.; Antoniv, T.T.; Hu, X.; Ivashkiv, L.B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon–response genes. Nat. Immunol. 2008, 9, 378–387. [Google Scholar] [CrossRef]
- Takada, Y.; Aggarwal, B.B. Evidence that genetic deletion of the TNF receptor p60 or p80 in macrophages modulates RANKL-induced signaling. Blood 2004, 104, 4113–4121. [Google Scholar] [CrossRef]
- Yoon, K.-C.; Jeong, I.-Y.; Park, Y.-G.; Yang, S.-Y. Interleukin-6 and Tumor Necrosis Factor-α Levels in Tears of Patients with Dry Eye Syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef]
- Vallance, P.; Chan, N. Endothelial function and nitric oxide: Clinical relevance. Heart 2001, 85, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Larson, D.F.; Hunter, K.; Gorman, M.; Yang, B. Comparison of tumor necrosis factor-α effect on the expression of iNOS in macrophage and cardiac myocytes. Perfusion 2001, 16, 67–74. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.-Q.; Pflugfelder, S.C. Hyperosmolarity-Induced Apoptosis in Human Corneal Epithelial Cells Is Mediated by Cytochrome c and MAPK Pathways. Cornea 2007, 26, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Byun, Y.J.; Choi, W.; Jeong, E.; Kim, J.S.; Noh, H.; Kim, E.S.; Song, Y.J.; Park, S.K.; Lee, H.K. Neutralization of Ocular Surface TNF-α Reduces Ocular Surface and Lacrimal Gland Inflammation Induced by In Vivo Dry Eye. Investig. Opthalmol. Vis. Sci. 2013, 54, 7557–7566. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Noh, H.; Yeo, A.; Jang, H.; Ahn, H.K.; Song, Y.J.; Lee, H.K. The Effect of TNF-α Blocker HL036337 and Its Best Concentration to Inhibit Dry Eye Inflammation. Korean J. Ophthalmol. 2016, 30, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Shettle, L.; McLaurin, E.; Martel, J.; Seaman, J.W.; Weissgerber, G. Topical Anti-TNFα Agent Licaminlimab (OCS-02) Relieves Persistent Ocular Discomfort in Severe Dry Eye Disease: A Randomized Phase II Study. Clin. Ophthalmol. 2022, 16, 2167–2177. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, S.; Cong, L.; Zhang, T.; Cheng, J.; Yang, N.; Qu, X.; Li, D.; Zhou, X.; Wang, H.; et al. TNF-α inhibitor tanfanercept (HBM9036) improves signs and symptoms of dry eye in a phase 2 trial in the controlled adverse environment in China. Int. Ophthalmol. 2022, 42, 2459–2472. [Google Scholar] [CrossRef]
- Rogler, G.; Herfarth, H.; Hibi, T.; Nielsen, O.H. Anti-Tumor Necrosis Factor Therapy in Inflammatory Bowel Disease; Karger Medical and Scientific Publishers: Basel, Switzerland, 2015. [Google Scholar]
- Clark, J.; Vagenas, P.; Panesar, M.; Cope, A.P. What does tumour necrosis factor excess do to the immune system long term? Ann. Rheum. Dis. 2005, 64, iv70–iv76. [Google Scholar] [CrossRef]
- Calmon-Hamaty, F.; Combe, B.; Hahne, M.; Morel, J. Lymphotoxin α revisited: General features and implications in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, 232–235. [Google Scholar] [CrossRef]
- Chiang, E.Y.; Kolumam, G.; Yu, X.; Francesco, M.; Ivelja, S.; Peng, I.; Gribling, P.; Shu, J.; Lee, W.P.; Refino, C.J.; et al. Targeted depletion of lymphotoxin-α–expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 2009, 15, 766–773. [Google Scholar] [CrossRef]
- Etemadi, N.; Holien, J.K.; Chau, D.; Dewson, G.; Murphy, J.M.; Alexander, W.S.; Parker, M.W.; Silke, J.; Nachbur, U. Lymphotoxin α induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J. 2013, 280, 5283–5297. [Google Scholar] [CrossRef] [PubMed]
- Cuff, C.; Schwartz, J.; Bergman, C.M.; Russell, K.S.; Bender, J.R.; Ruddle, N.H. Lymphotoxin alpha3 induces chemokines and adhesion molecules: Insight into the role of LT alpha in inflammation and lymphoid organ development. J. Immunol. 1998, 161, 6853–6860. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Campos-Neto, A.; Hanson, M.S.; Ruddle, N.H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 1996, 183, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β (lymphotoxin α) can activate the inflammatory environment in human chondrocytes. Arthritis Res. Ther. 2013, 15, R202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Takahashi, N.; Matsui, N.; Tetsuka, T.; Okamoto, T. The NF-κB Activation in Lymphotoxin β Receptor Signaling Depends on the Phosphorylation of p65 at Serine 536 *. J. Biol. Chem. 2003, 278, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Bangalore, S.; Schmieder, R.E. Wilder’s principle: Pre-treatment value determines post-treatment response. Eur. Heart J. 2014, 36, 576–579. [Google Scholar] [CrossRef]
- Hom, M.M.; Asbell, P.; Barry, B. Omegas and Dry Eye: More Knowledge, More Questions. Optom. Vis. Sci. 2015, 92, 948–956. [Google Scholar] [CrossRef]
- Wierzejska, R.E. Dietary Supplements—For Whom? The Current State of Knowledge about the Health Effects of Selected Supplement Use. Int. J. Environ. Res. Public Health 2021, 18, 8897. [Google Scholar] [CrossRef]
- Takeuchi, T.; Miyasaka, N.; Tatsuki, Y.; Yano, T.; Yoshinari, T.; Abe, T.; Koike, T. Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 1208–1215. [Google Scholar] [CrossRef]
- Zhang, R.-F.; Liu, S.; Wang, Y.-W.; Li, J. Potential molecular biomarkers used to predict the response to biological therapies in ulcerative colitis. Chin. Med. J. 2021, 134, 1058–1060. [Google Scholar] [CrossRef]
- Khan, S.; Mandal, R.K.; Jawed, A.; Dar, S.A.; Wahid, M.; Panda, A.K.; Areeshi, M.Y.; Khan, E.A.; Haque, S. TNF-α -308 G > A (rs1800629) Polymorphism is Associated with Celiac Disease: A Meta-analysis of 11 Case-Control Studies. Sci. Rep. 2016, 6, 32677. [Google Scholar] [CrossRef] [PubMed]
- El-Tahan, R.R.; Ghoneim, A.M.; El-Mashad, N. TNF-α gene polymorphisms and expression. Springerplus 2016, 5, 1508. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.L.; Davis, M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front. Immunol. 2013, 4, 478. [Google Scholar] [CrossRef] [PubMed]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional Control of the TNF Gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.-P.; Ottenhoff, T.H.M.; Verweij, C.L. Is there a future for TNF promoter polymorphisms? Genes Immun. 2004, 5, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Asotra, K.; Matata, B.M.; Mastana, S.S. Tumor necrosis factor alpha-308 gene locus promoter polymorphism: An analysis of association with health and disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2009, 1792, 163–172. [Google Scholar] [CrossRef]
- Mekinian, A.; Tamouza, R.; Pavy, S.; Gestermann, N.; Ittah, M.; Mariette, X.; Miceli-Richard, C. Functional study of TNF-α promoter polymorphisms: Literature review and meta-analysis. Eur. Cytokine Netw. 2011, 22, 88–102. [Google Scholar] [CrossRef]
- Pociot, F.; Briant, L.; Jongeneel, C.V.; Mölvig, J.; Worsaae, H.; Abbal, M.; Thomsen, M.; Nerup, J.; Cambon-Thomsen, A. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-αlpha and TNF-beta by human mononuclear cells: A possible link to insulin-dependent diabetes mellitus. Eur. J. Immunol. 1993, 23, 224–231. [Google Scholar] [CrossRef]
- Aderka, D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996, 7, 231–240. [Google Scholar] [CrossRef]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef]
- Sainz, J.; Salas-Alvarado, I.; López-Fernández, E.; Olmedo, C.; Comino, A.; García, F.; Blanco, A.; Gómez-Lopera, S.; Oyonarte, S.; Bueno, P.; et al. TNFR1 mRNA Expression Level and TNFR1 Gene Polymorphisms are Predictive Markers for Susceptibility to Develop Invasive Pulmonary Aspergillosis. Int. J. Immunopathol. Pharmacol. 2010, 23, 423–436. [Google Scholar] [CrossRef]
- Li, H.; Anderson, S.K. Association of TNFRSF1B Promoter Polymorphisms with Human Disease: Further Studies Examining T-Regulatory Cells Are Required. Front. Immunol. 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
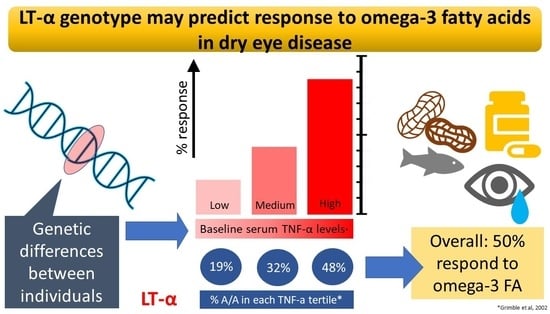
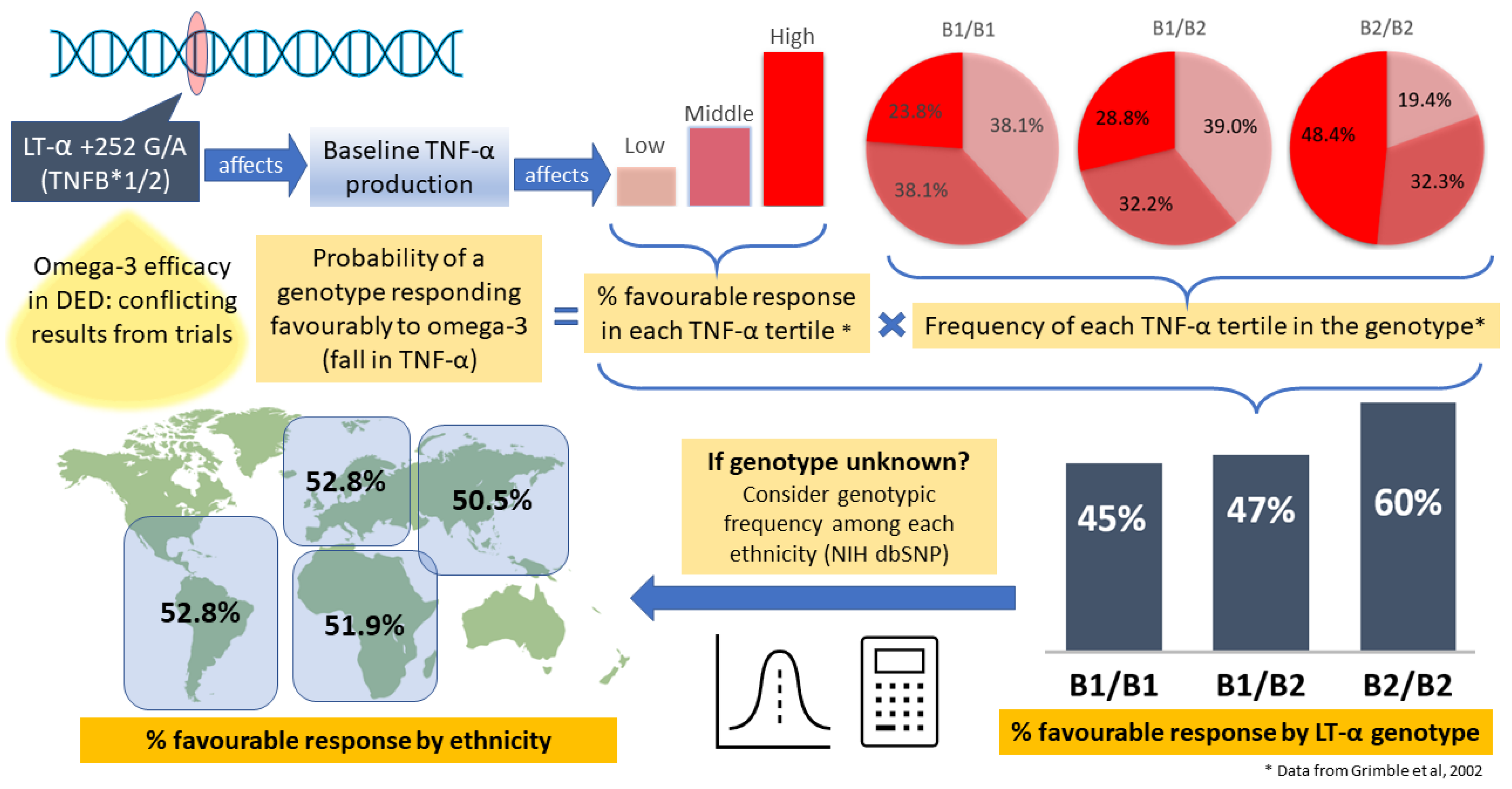
- Grimble, R.F.; Howell, W.M.; O’Reilly, G.; Turner, S.J.; Markovic, O.; Hirrell, S.; East, J.M.; Calder, P.C. The ability of fish oil to suppress tumor necrosis factor α production by peripheral blood mononuclear cells in healthy men is associated with polymorphisms in genes that influence tumor necrosis factor α production. Am. J. Clin. Nutr. 2002, 76, 454–459. [Google Scholar] [CrossRef]
- Bilolikar, H.; Nam, A.R.; Rosenthal, M.; Davies, J.; Henderson, D.C.; Balfour-Lynn, I.M. Tumour necrosis factor gene polymorphisms and childhood wheezing. Eur. Respir. J. 2005, 26, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Abramovs, N.; Brass, A.; Tassabehji, M. Hardy-Weinberg Equilibrium in the Large Scale Genomic Sequencing Era. Front. Genet. 2020, 11, 210. [Google Scholar] [CrossRef]
- Vejbaesya, S.; Luangtrakool, P.; Luangtrakool, K.; Kalayanarooj, S.; Vaughn, D.W.; Endy, T.P.; Mammen, M.P.; Green, S.; Libraty, D.H.; Ennis, F.A.; et al. TNF and LTA Gene, Allele, and Extended HLA Haplotype Associations with Severe Dengue Virus Infection in Ethnic Thais. J. Infect. Dis. 2009, 199, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.G.; Diakite, M.; Auburn, S.; Campino, S.; Fry, A.E.; Green, A.; Richardson, A.; Small, K.; Teo, Y.Y.; Wilson, J.; et al. Tumor Necrosis Factor and Lymphotoxin-α Polymorphisms and Severe Malaria in African Populations. J. Infect. Dis. 2009, 199, 569–575. [Google Scholar] [CrossRef]
- Tsytsykova, A.V.; Rajsbaum, R.; Falvo, J.V.; Ligeiro, F.; Neely, S.R.; Goldfeld, A.E. Activation-dependent intrachromosomal interactions formed by the TNF gene promoter and two distal enhancers. Proc. Natl. Acad. Sci. USA 2007, 104, 16850–16855. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, H.; Liang, L.; Zhong, Y.; Liang, Y.; Yu, Y.; Huang, S.; Lu, X. Evaluation of Tear Protein Markers in Dry Eye Disease with Different Lymphotoxin-Alpha Expression Levels. Am. J. Ophthalmol. 2020, 217, 198–211. [Google Scholar] [CrossRef]
- Ambaw, Y.A.; Chao, C.; Ji, S.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Prabhawasat, P.; Kojima, T.; Grueterich, M.; Espana, E.; Goto, E. The challenge of dry eye diagnosis. Clin. Ophthalmol. 2008, 2, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Messer, G.; Spengler, U.; Jung, M.C.; Honold, G.; Blömer, K.; Pape, G.R.; Riethmüller, G.; Weiss, E.H. Polymorphic structure of the tumor necrosis factor (TNF) locus: An NcoI polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J. Exp. Med. 1991, 173, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Whichelow, C.E.; Hitman, G.A.; Raafat, I.; Bottazzo, G.F.; Sachs, J.A. The effect of tnf*b gene polymorphism on tnf-? and -? secretion levels in patients with insulin-dependent diabetes mellitus and healthy controls. Eur. J. Immunogenet. 1996, 23, 425–435. [Google Scholar] [CrossRef]
- Lee, S.Y.; Han, S.J.; Nam, S.M.; Yoon, S.; Ahn, J.M.; Kim, T.-I.; Kim, E.K.; Seo, K.Y. Analysis of Tear Cytokines and Clinical Correlations in Sjögren Syndrome Dry Eye Patients and Non–Sjögren Syndrome Dry Eye Patients. Am. J. Ophthalmol. 2013, 156, 247–253.e1. [Google Scholar] [CrossRef]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.; Piccoli, D.S.; Hamilton, J. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef]
- Bogdan, C.; Paik, J.; Vodovotz, Y.; Nathan, C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J. Biol. Chem. 1992, 267, 23301–23308. [Google Scholar] [CrossRef]
- Renz, H.; Gong, J.H.; Schmidt, A.; Nain, M.; Gemsa, D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J. Immunol. 1988, 141, 2388–2393. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, Z.; Wang, L.; Xu, X.; Wei, Z.; Zheng, P.; Cao, K.; Zhang, Z.; Chen, K.; Liang, Q. Dry Eye Disease in Patients with Schizophrenia: A Case-Control Study. Front. Med. 2022, 9, 23. [Google Scholar] [CrossRef]
| TNF-α Production | ||||
|---|---|---|---|---|
| Before Supplementation (ng/L) | After Supplementation (ng/L) | |||
| All Subjects (n = 111) | 4821 ± 4177 | 4643 ± 3388 | Subjects with Reduction of TNF-α | |
| Tertile of inherent (baseline) TNF-α production | Lowest | 1458 ± 600 | 3809 ± 2571 | 22% |
| Middle | 3728 ± 936 | 4796 ± 3270 | 43% | |
| Highest | 9277 ± 4338 | 5323 ± 3941 | 86% | |
| LT-α Genotype | ||||
|---|---|---|---|---|
| B1/B1 | B1/B2 | B2/B2 | ||
| All Subjects (n) | 21 | 59 | 31 | |
| Tertile of inherent (baseline) TNF-α production | Lowest | 8 0.381 | 23 0.390 | 6 0.194 |
| Middle | 8 0.381 | 19 0.322 | 10 0.323 | |
| Highest | 5 0.238 | 17 0.288 | 15 0.484 | |
| Caucasian | Prob in Tertile × Chance of Responding | Probability of Response to Omega-3 |
|---|---|---|
| B1/B1 | 0.238 × 0.86 + 0.381 × 0.43 + 0.381 × 0.22 | =0.452 |
| B1/B2 | 0.288 × 0.86 + 0.322 × 0.43 + 0.39 × 0.22 | =0.472 |
| B2/B2 | 0.484 × 0.86 + 0.323 × 0.43 + 0.194 × 0.22 | =0.598 |
| Proportion B1/B1 | P(B1/B1) | Proportion B1/B2 | P(B1/B2) | Proportion B2/B2 | P(B2/B2) | P(unknown) | |
|---|---|---|---|---|---|---|---|
| European | 0.102 | 0.452 | 0.435 | 0.472 | 0.463 | 0.598 | 0.528 |
| African | 0.142 | 0.452 | 0.470 | 0.472 | 0.389 | 0.598 | 0.519 |
| Asian | 0.204 | 0.452 | 0.495 | 0.472 | 0.300 | 0.598 | 0.505 |
| Latin America | 0.102 | 0.452 | 0.434 | 0.472 | 0.464 | 0.598 | 0.528 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paik, B.; Tong, L. Polymorphisms in Lymphotoxin-Alpha as the “Missing Link” in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4236. https://doi.org/10.3390/ijms24044236
Paik B, Tong L. Polymorphisms in Lymphotoxin-Alpha as the “Missing Link” in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(4):4236. https://doi.org/10.3390/ijms24044236
Chicago/Turabian StylePaik, Benjamin, and Louis Tong. 2023. "Polymorphisms in Lymphotoxin-Alpha as the “Missing Link” in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review" International Journal of Molecular Sciences 24, no. 4: 4236. https://doi.org/10.3390/ijms24044236
APA StylePaik, B., & Tong, L. (2023). Polymorphisms in Lymphotoxin-Alpha as the “Missing Link” in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review. International Journal of Molecular Sciences, 24(4), 4236. https://doi.org/10.3390/ijms24044236