Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of the Participants
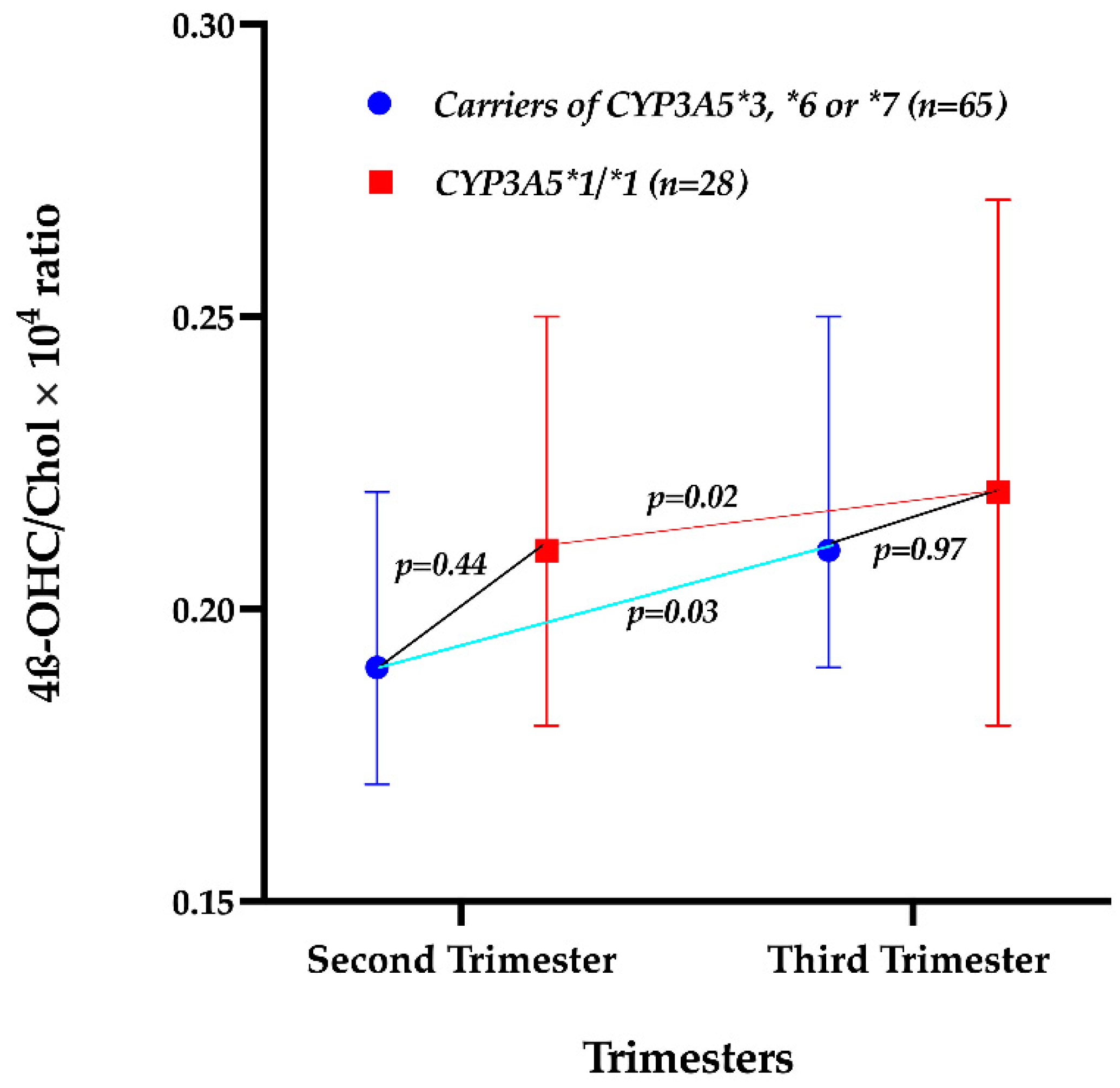
2.2. Change in the 4β-OHC/Chol Ratio during Pregnancy
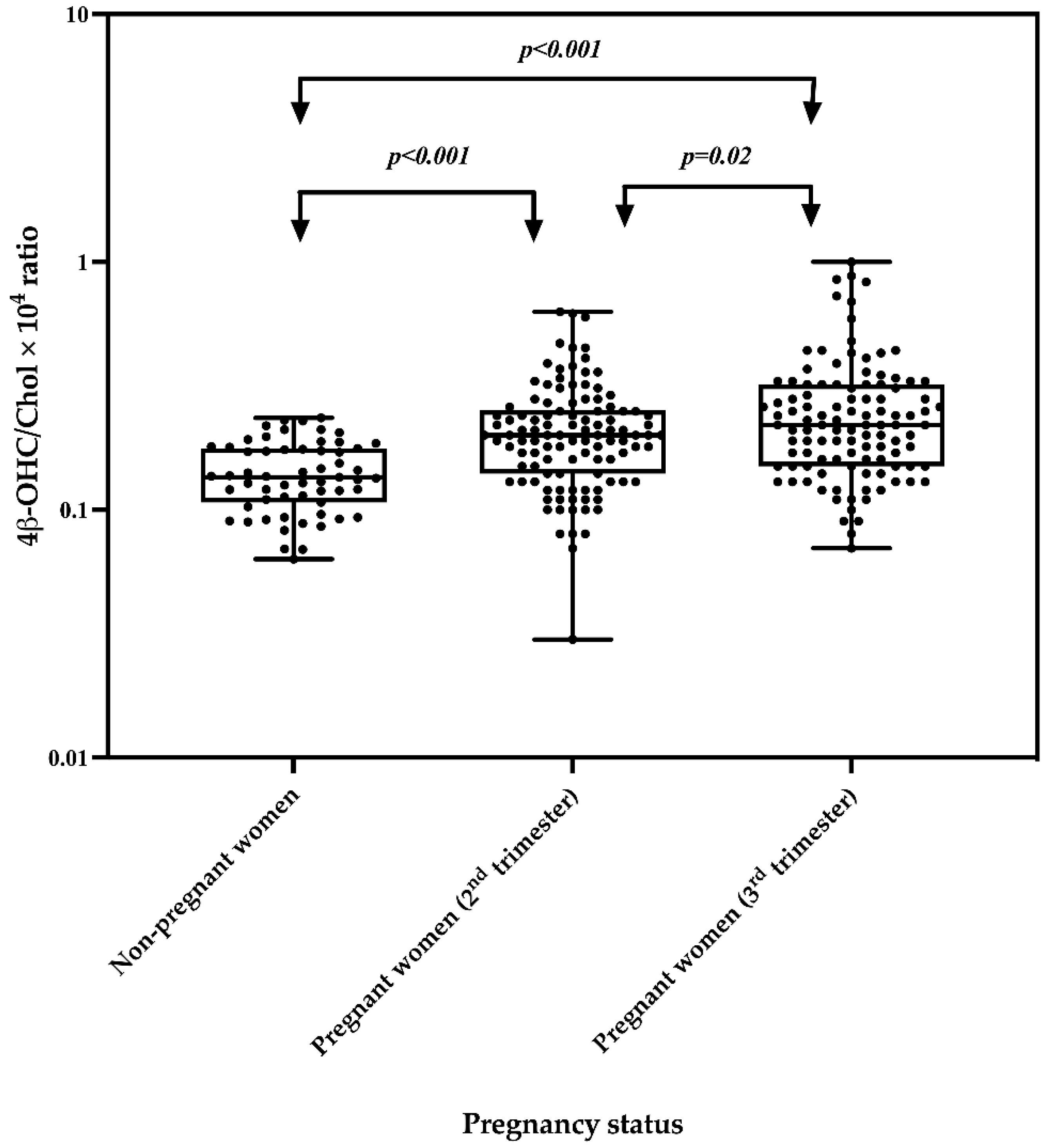
2.3. Effect of Pregnancy on the 4β-OHC/Chol Ratio
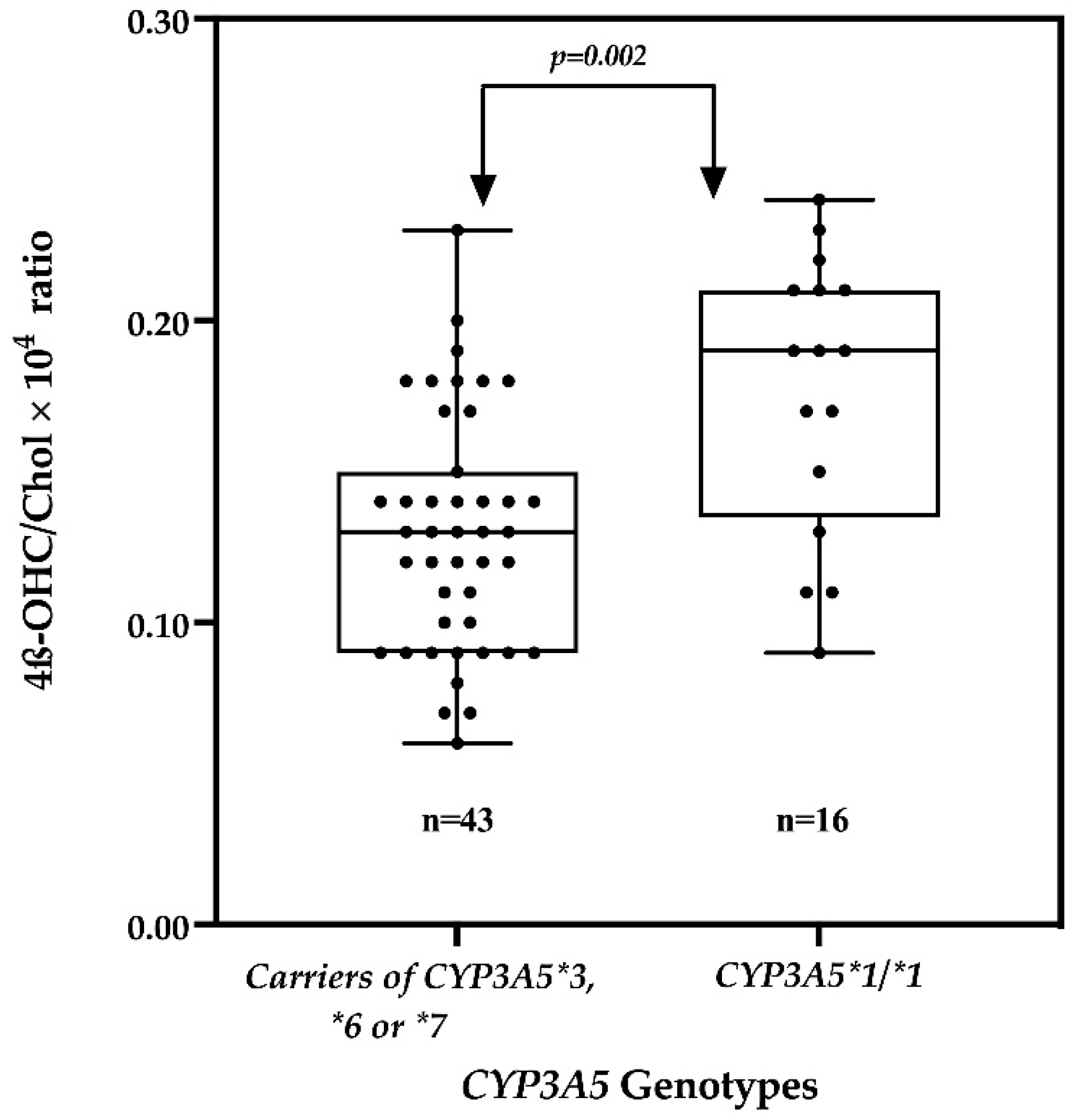
2.4. Effect of the CYP3A5 Genotype on the 4β-OHC/Chol Ratio
3. Discussion
4. Materials and Methods
4.1. Participant Recruitment, Sample Collection and Processing
4.2. Determination of Plasma 4β-OHC Levels
4.3. Determination of Plasma Cholestrol Levels
4.4. Genotyping
4.5. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eichelbaum, M.; Burk, O. CYP3A genetics in drug metabolism. Nat. Med. 2001, 7, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Masica, A.L.; Mayo, G.; Wilkinson, G.R. In vivo comparisons of constitutive cytochrome P450 3A activity assessed by alprazolam, triazolam, and midazolam. Clin. Pharmacol. Ther. 2004, 76, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Galteau, M.M.; Shamsa, F. Urinary 6beta-hydroxycortisol: A validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals. Eur. J. Clin. Pharmacol. 2003, 59, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.S.; Venkataramanan, R.; Glover, D.D.; Caritis, S.N. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am. J. Obstet. Gynecol. 2005, 192, 633–639. [Google Scholar] [CrossRef]
- Keller, G.A.; Gago, M.L.F.; Diez, R.A.; Di Girolamo, G. In vivo Phenotyping Methods: Cytochrome P450 Probes with Emphasis on the Cocktail Approach. Curr. Pharm. Des. 2017, 23, 2035–2049. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Nylén, H.; Elander, P.; Bertilsson, L. 4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br. J. Clin. Pharmacol. 2011, 71, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Wójcikowski, J.; Daniel, W.A. The role of the nervous system in the regulation of liver cytochrome p450. Curr. Drug. Metab. 2011, 12, 124–138. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Grepper, S.; Unadkat, J.D. Induction of hepatic CYP3A enzymes by pregnancy-related hormones: Studies in human hepatocytes and hepatic cell lines. Drug. Metab. Dispos. 2013, 41, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Ngaimisi, E.; Minzi, O.; Mugusi, S.; Sasi, P.; Riedel, K.D.; Suda, A.; Ueda, N.; Bakari, M.; Janabi, M.; Mugusi, F.; et al. Pharmacokinetic and pharmacogenomic modelling of the CYP3A activity marker 4β-hydroxycholesterol during efavirenz treatment and efavirenz/rifampicin co-treatment. J. Antimicrob. Chemother. 2014, 69, 3311–3319. [Google Scholar] [CrossRef] [Green Version]
- Abduljalil, K.; Pansari, A.; Jamei, M. Prediction of maternal pharmacokinetics using physiologically based pharmacokinetic models: Assessing the impact of the longitudinal changes in the activity of CYP1A2, CYP2D6 and CYP3A4 enzymes during pregnancy. J. Pharmacokinet. Pharmacodyn. 2020, 47, 361–383. [Google Scholar] [CrossRef]
- Bukkems, V.E.; Colbers, A.; Marzolini, C.; Molto, J.; Burger, D.M. Drug-Drug Interactions with Antiretroviral Drugs in Pregnant Women Living with HIV: Are They Different from Non-Pregnant Individuals? Clin. Pharmacokinet. 2020, 59, 1217–1236. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Kim, B.; Rhee, S.J.; Lee, Y.; Park, J.S.; Lee, S.M.; Kim, S.M.; Lee, S.; Yu, K.S.; Jang, I.J.; et al. Assessment of induced CYP3A activity in pregnant women using 4β-hydroxycholesterol: Cholesterol ratio as an appropriate metabolic marker. Drug. Metab. Pharmacokinet. 2018, 33, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Nylén, H.; Sergel, S.; Forsberg, L.; Lindemalm, S.; Bertilsson, L.; Wide, K.; Diczfalusy, U. Cytochrome P450 3A activity in mothers and their neonates as determined by plasma 4β-hydroxycholesterol. Eur. J. Clin. Pharmacol. 2011, 67, 715–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug. Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef]
- Mutagonda, R.F.; Minzi, O.M.S.; Massawe, S.N.; Asghar, M.; Färnert, A.; Kamuhabwa, A.A.R.; Aklillu, E. Pregnancy and CYP3A5 Genotype Affect Day 7 Plasma Lumefantrine Concentrations. Drug Metab. Dispos. 2019, 47, 1415–1424. [Google Scholar] [CrossRef] [Green Version]
- Mirghani, R.A.; Sayi, J.; Aklillu, E.; Allqvist, A.; Jande, M.; Wennerholm, A.; Eriksen, J.; Herben, V.M.; Jones, B.C.; Gustafsson, L.L.; et al. CYP3A5 genotype has significant effect on quinine 3-hydroxylation in Tanzanians, who have lower total CYP3A activity than a Swedish population. Pharm. Genom. 2006, 16, 637–645. [Google Scholar] [CrossRef]
- Matthaei, J.; Bonat, W.H.; Kerb, R.; Tzvetkov, M.V.; Strube, J.; Brunke, S.; Sachse-Seeboth, C.; Sehrt, D.; Hofmann, U.; von Bornemann Hjelmborg, J.; et al. Inherited and Acquired Determinants of Hepatic CYP3A Activity in Humans. Front. Genet. 2020, 11, 944. [Google Scholar] [CrossRef]
- Gebeyehu, E.; Engidawork, E.; Bijnsdorp, A.; Aminy, A.; Diczfalusy, U.; Aklillu, E. Sex and CYP3A5 genotype influence total CYP3A activity: High CYP3A activity and a unique distribution of CYP3A5 variant alleles in Ethiopians. Pharmacogenomics 2011, 11, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Hole, K.; Gjestad, C.; Heitmann, K.M.; Haslemo, T.; Molden, E.; Bremer, S. Impact of genetic and nongenetic factors on interindividual variability in 4β-hydroxycholesterol concentration. Eur. J. Clin. Pharmacol. 2017, 73, 317–324. [Google Scholar] [CrossRef]
- Aweeka, F.T.; Hu, C.; Huang, L.; Best, B.M.; Stek, A.; Lizak, P.; Burchett, S.K.; Read, J.S.; Watts, H.; Mirochnick, M.; et al. Alteration in cytochrome P450 3A4 activity as measured by a urine cortisol assay in HIV-1-infected pregnant women and relationship to antiretroviral pharmacokinetics. HIV Med. 2015, 16, 176–183. [Google Scholar] [CrossRef]
- Vree, T.B.; Reekers-Ketting, J.J.; Fragen, R.J.; Arts, T.H. Placental transfer of midazolam and its metabolite 1-hydroxymethylmidazolam in the pregnant ewe. Anesth. Analg. 1984, 63, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Kasichayanula, S.; Boulton, D.W.; Luo, W.L.; Rodrigues, A.D.; Yang, Z.; Goodenough, A.; Lee, M.; Jemal, M.; LaCreta, F. Validation of 4β-hydroxycholesterol and evaluation of other endogenous biomarkers for the assessment of CYP3A activity in healthy subjects. Br. J. Clin. Pharmacol. 2014, 78, 1122–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.; Martin, I.; McLeod, J.; Nolan, G.; van Horn, R.; Vourvahis, M.; Lin, Y.S. Perspective: 4β-hydroxycholesterol as an emerging endogenous biomarker of hepatic CYP3A. Drug Metab. Rev. 2017, 49, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Maisa Feghali, R.V.; Steve, C. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol. 2015, 39, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Diczfalusy, U.; Miura, J.; Roh, H.K.; Mirghani, R.A.; Sayi, J.; Larsson, H.; Bodin, K.G.; Allqvist, A.; Jande, M.; Kim, J.W.; et al. 4Beta-hydroxycholesterol is a new endogenous CYP3A marker: Relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharm. Genom. 2008, 18, 201–208. [Google Scholar] [CrossRef]
- Gaohua, L.; Abduljalil, K.; Jamei, M.; Johnson, T.N.; Rostami-Hodjegan, A. A pregnancy physiologically based pharmacokinetic (p-PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br. J. Clin. Pharmacol. 2012, 74, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Hebert, M.F.; Easterling, T.R.; Kirby, B.; Carr, D.B.; Buchanan, M.L.; Rutherford, T.; Thummel, K.E.; Fishbein, D.P.; Unadkat, J.D. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: A University of Washington specialized center of research study. Clin. Pharmacol. Ther. 2008, 84, 248–253. [Google Scholar] [CrossRef]
- Shin, K.H.; Choi, M.H.; Lim, K.S.; Yu, K.S.; Jang, I.J.; Cho, J.Y. Evaluation of endogenous metabolic markers of hepatic CYP3A activity using metabolic profiling and midazolam clearance. Clin. Pharmacol. Ther. 2013, 94, 601–609. [Google Scholar] [CrossRef]
- Tegude, H.; Schnabel, A.; Zanger, U.M.; Klein, K.; Eichelbaum, M.; Burk, O. Molecular Mechanism of Basal CYP3A4 Regulation by Hepatocyte Nuclear Factor 4α: Evidence for Direct Regulation in the Intestine. Drug Metab. Dispos. 2007, 35, 946–954. [Google Scholar] [CrossRef] [Green Version]
- Mukonzo, J.K.; Waako, P.; Ogwal-Okeng, J.; Gustafsson, L.L.; Aklillu, E. Genetic Variations in ABCB1 and CYP3A5 as well as Sex Influence Quinine Disposition Among Ugandans. Ther. Drug Monit. 2010, 32, 346–352. [Google Scholar] [CrossRef]
- Kagawa, Y.; Yamamoto, Y.; Ueno, A.; Maeda, T.; Obi, T. Impact of CYP2D6, CYP3A5, and ABCB1 Polymorphisms on Plasma Concentrations of Donepezil and Its Metabolite in Patients With Alzheimer Disease. Ther. Drug Monit. 2021, 43, 429–435. [Google Scholar] [CrossRef]
- Aklillu, E.; Zumla, A.; Habtewold, A.; Amogne, W.; Makonnen, E.; Yimer, G.; Burhenne, J.; Diczfalusy, U. Early or deferred initiation of efavirenz during rifampicin-based TB therapy has no significant effect on CYP3A induction in TB-HIV infected patients. Br J Pharmacol. 2021, 178, 3294–3308. [Google Scholar] [CrossRef]
- Mlugu, E.M.; Minzi, O.; Kamuhabwa, A.A.R.; Aklillu, E. Prevalence and Correlates of Asymptomatic Malaria and Anemia on First Antenatal Care Visit among Pregnant Women in Southeast, Tanzania. Int. J. Environ. Res. Public. Health. 2020, 17, 3123. [Google Scholar] [CrossRef]
| Variables | Pregnant Women (n = 110) | Non-Pregnant Women (n = 59) | |
|---|---|---|---|
| Median age (IQR) (years) | 27 (18 to 33) | 35 (24 to 41) | |
| Median weight (IQR) (kg) | 55 (50 to 62) | 65 (54 to 75) | |
| Median gestational age (IQR) (weeks) | 22 (19 to 24) | - | |
| Gravidity n (%) | Primigravida | 29 (26.4) | - |
| Secundigravida | 27 (24.5) | - | |
| Multigravida | 54 (49.1) | - | |
| Variables | N | Median | IQR | Change in Median Value (%) | p-Value | |
|---|---|---|---|---|---|---|
| 4β-OHC (ng/mL) | Second trimester | 110 | 30.25 | 24.62–40.60 | 9.43 | 0.003 |
| Third trimester | 110 | 34.80 | 29.30–46.50 | |||
| Cholesterol (mmol/L) | Second trimester | 110 | 4.04 | 3.30–5.32 | 2.84 | 0.82 |
| Third trimester | 110 | 4.25 | 3.39–5.10 | |||
| 4β-OHC/Chol × 104 molar ratios | Second trimester | 110 | 0.20 | 0.14–0.25 | 7.31 | 0.02 |
| Third trimester | 110 | 0.22 | 0.15–0.32 | |||
| Variable | N | Pregnant Women in Second Trimester | N | Non-Pregnant Women | p-Value | Pregnant Women in Third Trimester | p-Value |
|---|---|---|---|---|---|---|---|
| Median 4β-OHC (IQR) (ng/mL) | 110 | 30.25 (24.62 to 40.60) | 59 | 23.19 (17.94 to 29.49) | <0.001 | 34.80 (29.30 to 46.50) | <0.001 |
| Median Chol (IQR) (mmol/L) | 110 | 4.04 (3.30 to 5.32) | 59 | 4.24 (3.74 to 4.81) | 0.54 | 4.25 (3.39 to 5.10) | 0.96 |
| Median 4β-OHC/Chol × 104 molar ratios (IQR) | 110 | 0.20 (0.14 to 0.25) | 59 | 0.14 (0.11 to 0.18) | <0.001 | 0.22 (0.15 to 0.32) | <0.001 |
| Geometric mean 4β-OHC/Chol × 104 molar ratios | 110 | 0.20 | 59 | 0.14 | <0.001 | 0.22 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlugu, E.M.; Minzi, O.M.; Kamuhabwa, A.A.R.; Diczfalusy, U.; Aklillu, E. Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio. Int. J. Mol. Sci. 2022, 23, 15168. https://doi.org/10.3390/ijms232315168
Mlugu EM, Minzi OM, Kamuhabwa AAR, Diczfalusy U, Aklillu E. Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio. International Journal of Molecular Sciences. 2022; 23(23):15168. https://doi.org/10.3390/ijms232315168
Chicago/Turabian StyleMlugu, Eulambius M., Omary M. Minzi, Appolinary A. R. Kamuhabwa, Ulf Diczfalusy, and Eleni Aklillu. 2022. "Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio" International Journal of Molecular Sciences 23, no. 23: 15168. https://doi.org/10.3390/ijms232315168
APA StyleMlugu, E. M., Minzi, O. M., Kamuhabwa, A. A. R., Diczfalusy, U., & Aklillu, E. (2022). Pregnancy Increases CYP3A Enzymes Activity as Measured by the 4β-Hydroxycholesterol/Cholesterol Ratio. International Journal of Molecular Sciences, 23(23), 15168. https://doi.org/10.3390/ijms232315168






