MitoSNARE Assembly and Disassembly Factors Regulate Basal Autophagy and Aging in C. elegans
Abstract
1. Introduction
2. Results
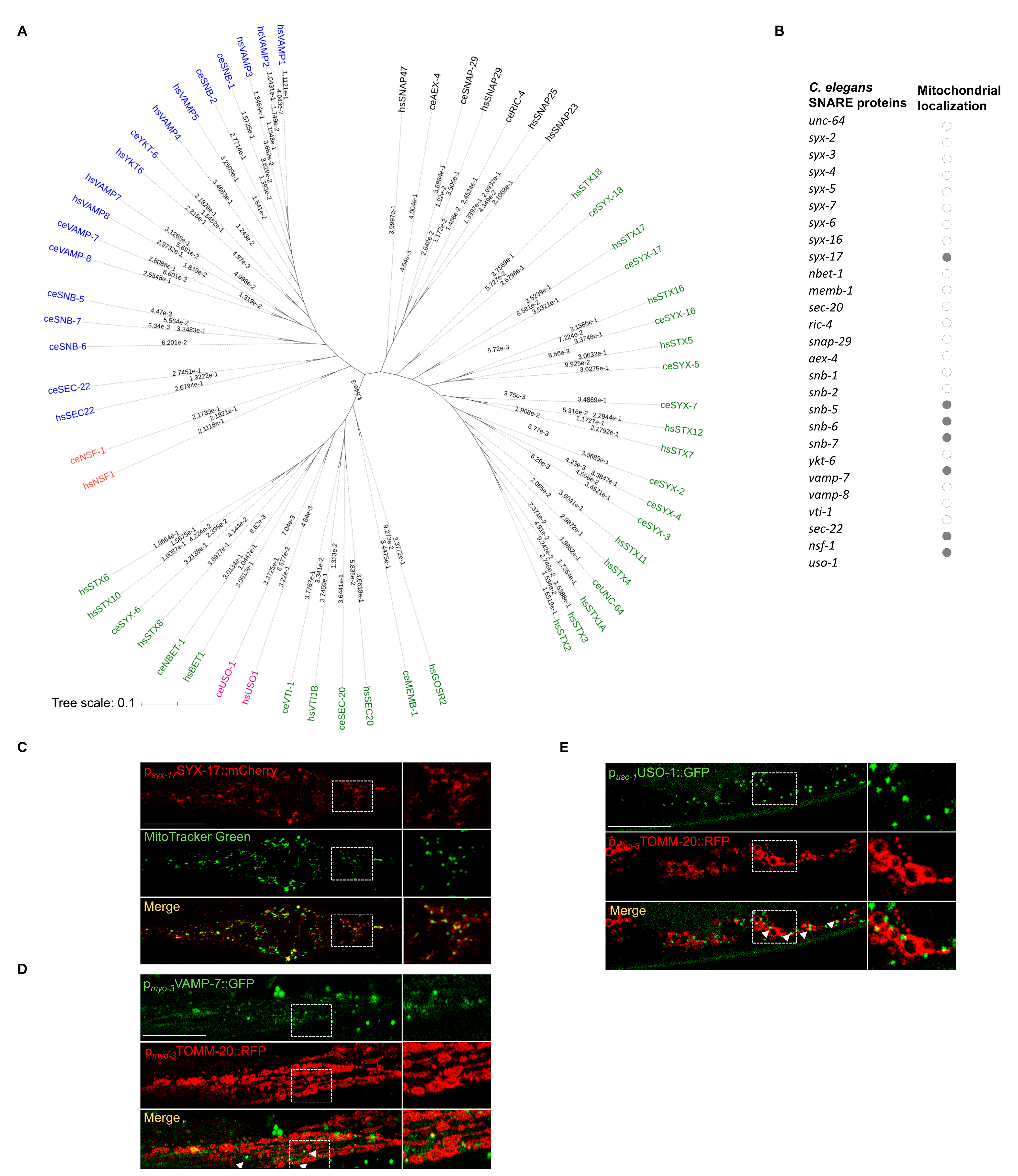
2.1. A Subset of SNARE Proteins Is Localized to Mitochondria
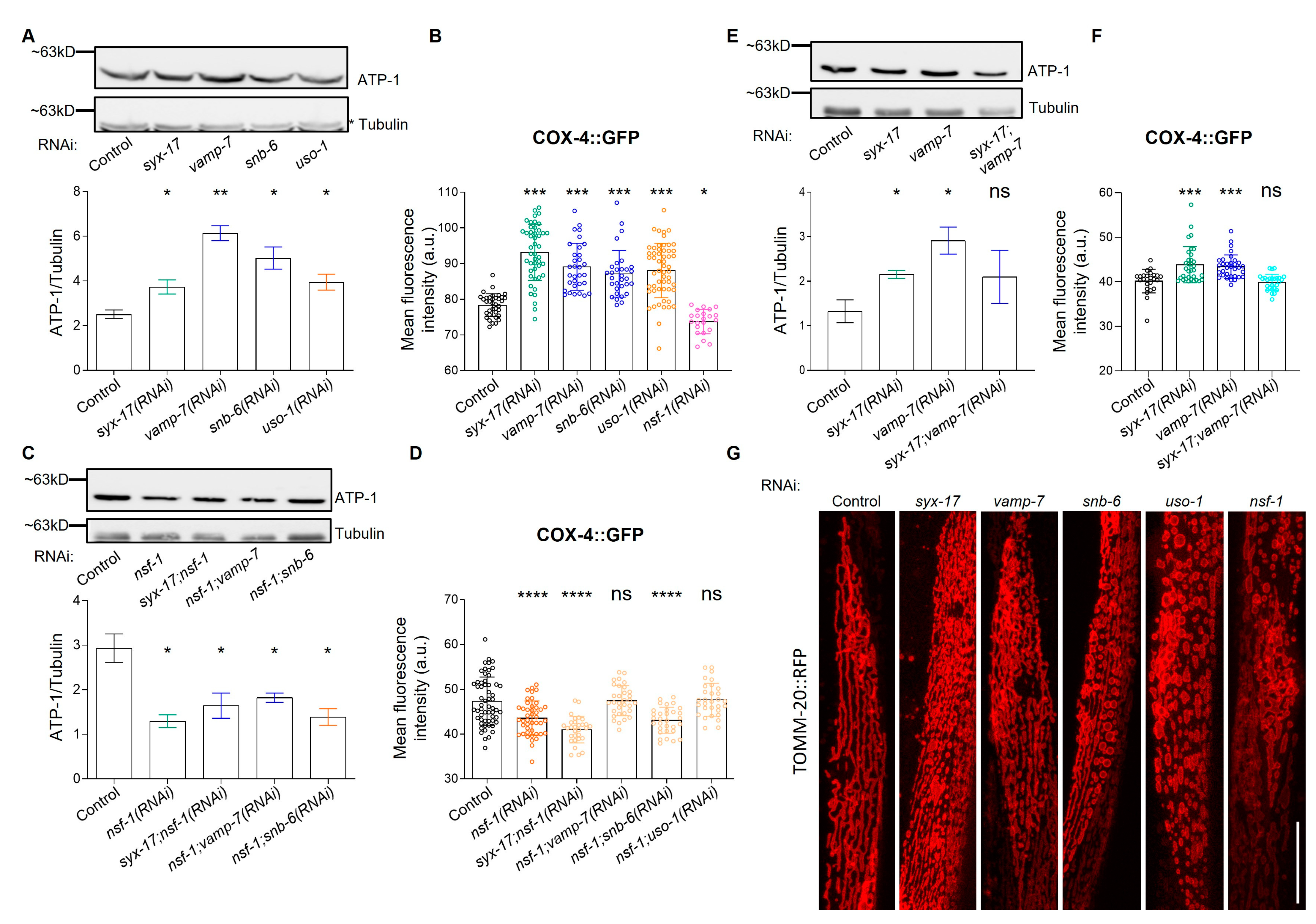
2.2. mitoSNARE Proteins Regulate Mitochondrial Morphology and Mass
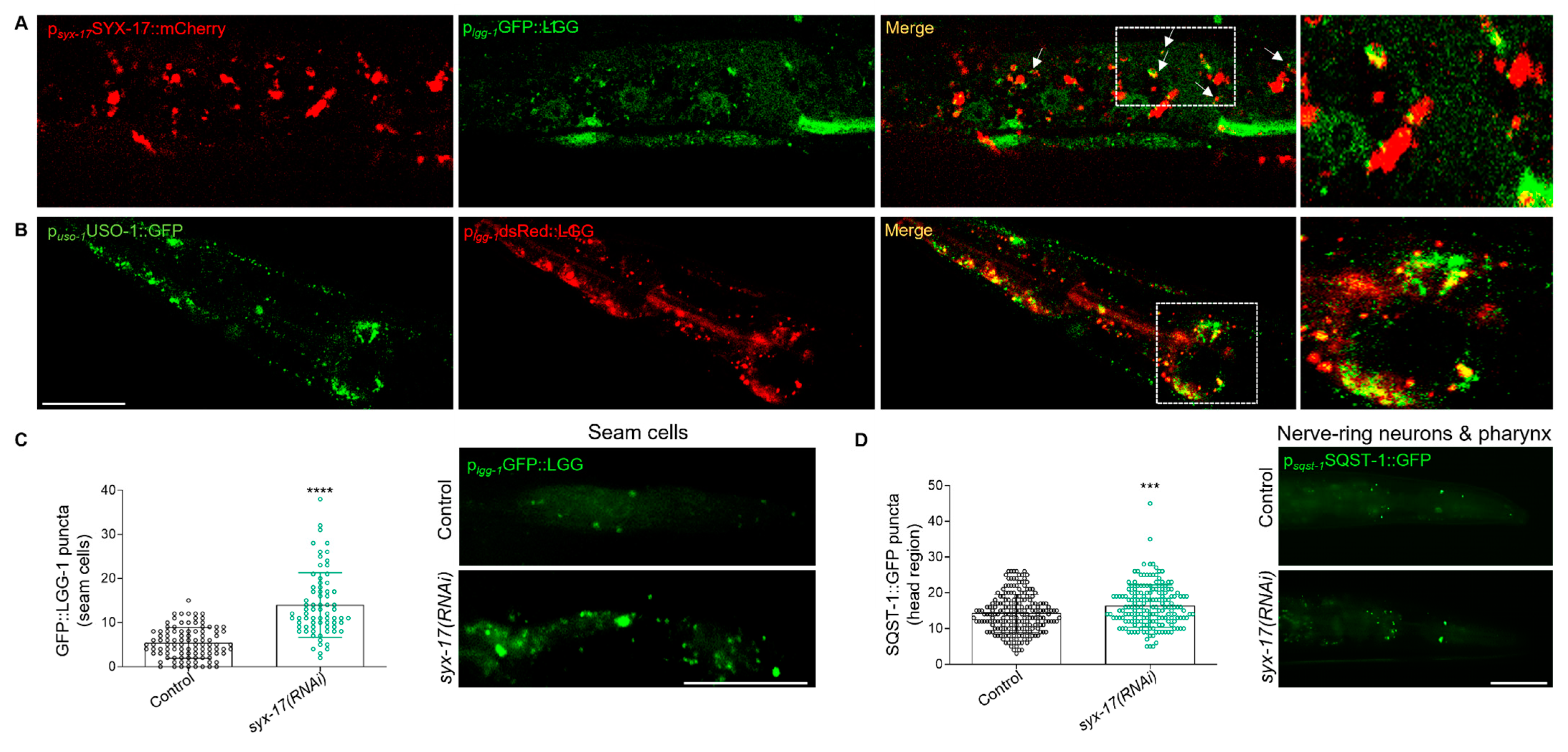
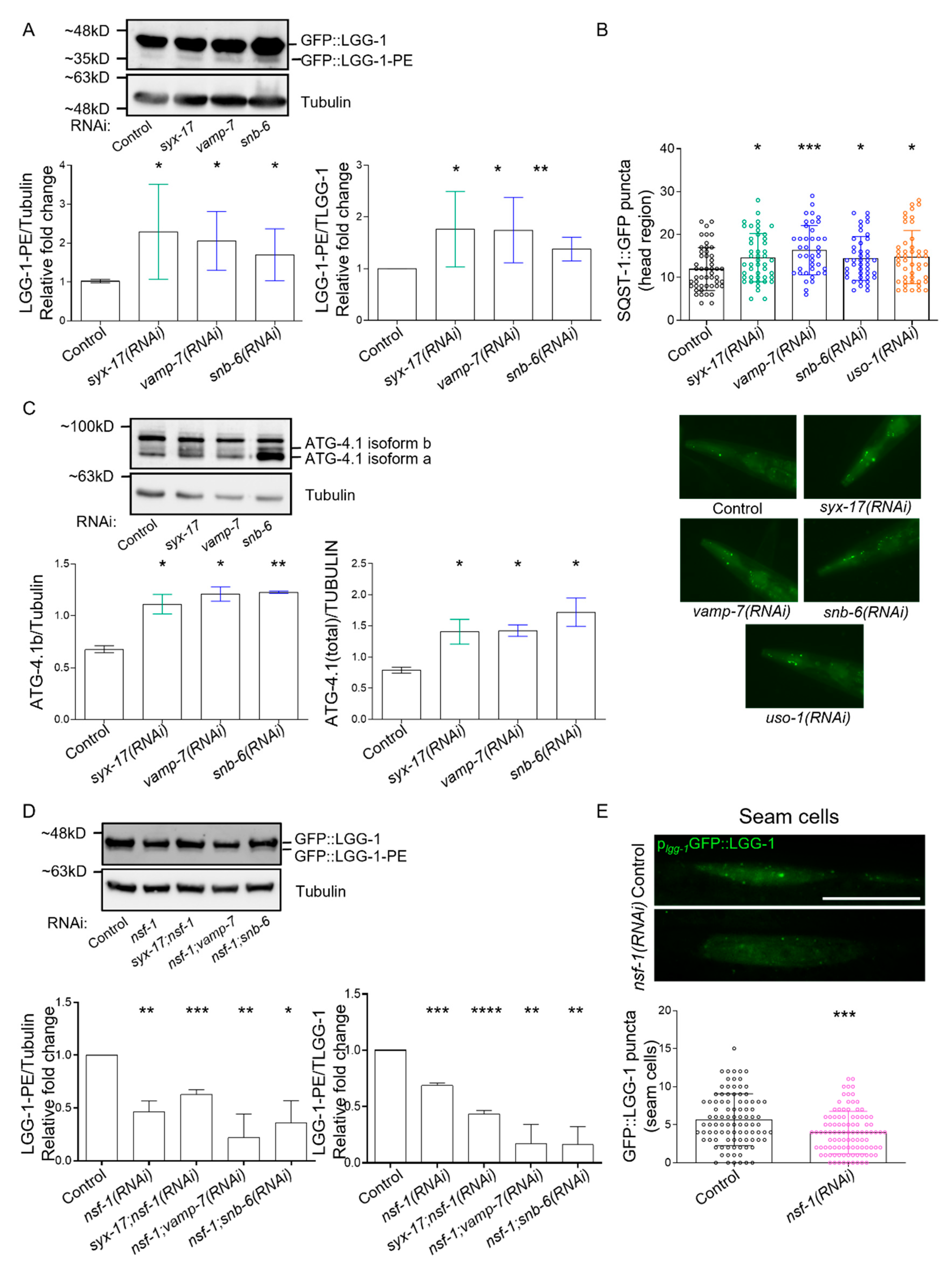
2.3. mitoSNARE Activity Is Required during Basal Autophagy
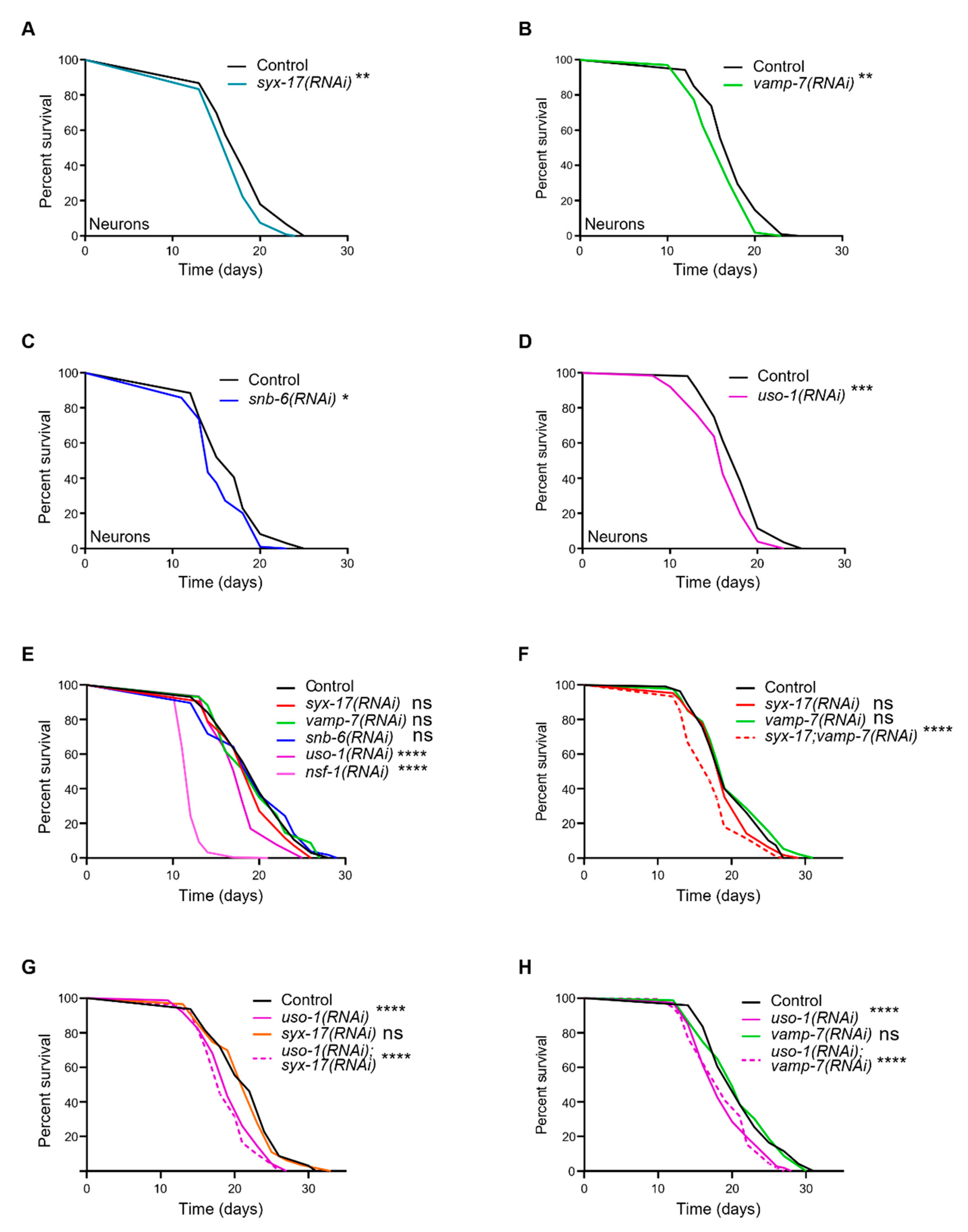
2.4. mitoSNAREs in Control of Aging in C. elegans
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Feeding RNAi
4.3. Molecular Cloning
4.4. Prediction of Mitochondrial Localization
4.5. Phylogenetic Analysis of SNARE Proteins
4.6. Immunoblotting
4.7. Lifespan Assays
4.8. Detection of Autophagy
4.9. Mitotracker Staining
4.10. Microparticle Bombardment
4.11. RNA Isolation and qPCR Analysis
4.12. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.Y.; Munson, M. SNARE complex assembly and disassembly. Curr. Biol. CB 2018, 28, R397–R401. [Google Scholar] [CrossRef] [PubMed]
- Sauvola, C.W.; Littleton, J.T. SNARE Regulatory Proteins in Synaptic Vesicle Fusion and Recycling. Front. Mol. Neurosci. 2021, 14, 733138. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C. The molecular machinery of neurotransmitter release (Nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12696–12717. [Google Scholar] [CrossRef] [PubMed]
- Sollner, T.; Whiteheart, S.W.; Brunner, M.; Erdjument-Bromage, H.; Geromanos, S.; Tempst, P.; Rothman, J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature 1993, 362, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Grabski, R.; Sztul, E.; Hay, J.C. p115-SNARE interactions: A dynamic cycle of p115 binding monomeric SNARE motifs and releasing assembled bundles. Traffic 2015, 16, 148–171. [Google Scholar] [CrossRef]
- Baker, R.W.; Hughson, F.M. Chaperoning SNARE assembly and disassembly. Nat. Rev. Mol. Cell Biol. 2016, 17, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, S.; Wang, X.; Fan, F.; Zhou, Q.; Lu, S.; Cao, Y.; Wang, Q.W.; Dong, M.Q.; Yao, J.; et al. Mechanistic insights into the SNARE complex disassembly. Sci. Adv. 2019, 5, eaau8164. [Google Scholar] [CrossRef]
- Chen, F.; Chen, H.; Chen, Y.; Wei, W.; Sun, Y.; Zhang, L.; Cui, L.; Wang, Y. Dysfunction of the SNARE complex in neurological and psychiatric disorders. Pharmacol. Res. 2021, 165, 105469. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, Y.; Liu, J.; Wang, C.; Long, J. Neurodegenerative Disease Related Proteins Have Negative Effects on SNARE-Mediated Membrane Fusion in Pathological Confirmation. Front. Mol. Neurosci. 2017, 10, 66. [Google Scholar] [CrossRef]
- Sharma, S.; Lindau, M. Molecular mechanism of fusion pore formation driven by the neuronal SNARE complex. Proc. Natl. Acad. Sci. USA 2018, 115, 12751–12756. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Yan, H.; Farley, J.A.; Sonntag, W.E.; Freeman, W.M. Aging alters the expression of neurotransmission-regulating proteins in the hippocampal synaptoproteome. J. Neurochem. 2010, 113, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Stroupe, C. Autophagy: Cells SNARE selves. Curr. Biol. CB 2011, 21, R697–R699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Hou, C.; Lai, Y.; Long, J.; Liu, J.; Zhong, Q.; Diao, J. SNARE-mediated membrane fusion in autophagy. Semin. Cell Dev. Biol. 2016, 60, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Nair, U.; Jotwani, A.; Geng, J.; Gammoh, N.; Richerson, D.; Yen, W.L.; Griffith, J.; Nag, S.; Wang, K.; Moss, T.; et al. SNARE proteins are required for macroautophagy. Cell 2011, 146, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Itakura, E.; Kishi-Itakura, C.; Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 2012, 151, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Jiang, P.; Nakano, S.; Sakamaki, Y.; Yamamoto, H.; Mizushima, N. Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J. Cell Biol. 2018, 217, 2633–2645. [Google Scholar] [CrossRef]
- Babcock, D.T.; Shen, W.; Ganetzky, B. A Neuroprotective Function of NSF1 Sustains Autophagy and Lysosomal Trafficking in Drosophila. Genetics 2014, 199, 511–522. [Google Scholar] [CrossRef]
- Mazelova, J.; Ransom, N.; Astuto-Gribble, L.; Wilson, M.C.; Deretic, D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J. Cell Sci. 2009, 122, 2003–2013. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhuang, X.P.; Cai, D.L.; Cao, C.; Gu, Q.S.; Liu, X.N.; Zheng, B.B.; Guan, B.J.; Yu, L.; Li, J.K.; et al. Let-7a regulates EV secretion and mitochondrial oxidative phosphorylation by targeting SNAP23 in colorectal cancer. J. Exp. Clin. Cancer Res. CR 2021, 40, 31. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, S.; Khew-Goodall, Y.; Gamble, J.; Vadas, M.; Wattenberg, B.W. A splice-isoform of vesicle-associated membrane protein-1 (VAMP-1) contains a mitochondrial targeting signal. Mol. Biol. Cell 1998, 9, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Jagerstrom, S.; Polesie, S.; Wickstrom, Y.; Johansson, B.R.; Schroder, H.D.; Hojlund, K.; Bostrom, P. Lipid droplets interact with mitochondria using SNAP23. Cell Biol. Int. 2009, 33, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Hwang, J.; Zhou, C.; Veluthakal, R.; McCown, E.M.; Hamilton, A.; Oh, E.; Dai, W.; Fueger, P.T.; Jiang, L.; et al. Enrichment of the exocytosis protein STX4 in skeletal muscle remediates peripheral insulin resistance and alters mitochondrial dynamics via Drp1. Nat. Commun. 2022, 13, 424. [Google Scholar] [CrossRef]
- Khurana, G.K.; Vishwakarma, P.; Puri, N.; Lynn, A.M. Phylogenetic Analysis of the vesicular fusion SNARE machinery revealing its functional divergence across Eukaryotes. Bioinformation 2018, 14, 361–368. [Google Scholar] [CrossRef]
- Kloepper, T.H.; Kienle, C.N.; Fasshauer, D. An elaborate classification of SNARE proteins sheds light on the conservation of the eukaryotic endomembrane system. Mol. Biol. Cell 2007, 18, 3463–3471. [Google Scholar] [CrossRef]
- Kloepper, T.H.; Kienle, C.N.; Fasshauer, D. SNAREing the basis of multicellularity: Consequences of protein family expansion during evolution. Mol. Biol. Evol. 2008, 25, 2055–2068. [Google Scholar] [CrossRef]
- Sato, K.; Norris, A.; Sato, M.; Grant, B.D.C. elegans as a model for membrane traffic. WormBook Online Rev. C. Elegans Biol. 2014, 1–47. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Feldmann, A.; Winterstein, C.; White, R.; Trotter, J.; Kramer-Albers, E.M. Comprehensive analysis of expression, subcellular localization, and cognate pairing of SNARE proteins in oligodendrocytes. J. Neurosci. Res. 2009, 87, 1760–1772. [Google Scholar] [CrossRef]
- McLelland, G.L.; Lee, S.A.; McBride, H.M.; Fon, E.A. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J. Cell Biol. 2016, 214, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Anderson, A.J.; Stojanovski, D. Mitochondrial protein import dysfunction: Mitochondrial disease, neurodegenerative disease and cancer. FEBS Lett. 2021, 595, 1107–1131. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Peikert, C.D.; Lubbert, P.; Suppanz, I.; Klemm, C.; Alka, O.; Steiert, C.; Naumenko, N.; Schendzielorz, A.; Melchionda, L.; et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metab. 2021, 33, 2464–2483.e2418. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef]
- Thumuluri, V.; Almagro Armenteros, J.J.; Johansen, A.R.; Nielsen, H.; Winther, O. DeepLoc 2.0: Multi-label subcellular localization prediction using protein language models. Nucleic Acids Res. 2022, 50, W228–W234. [Google Scholar] [CrossRef]
- Backes, S.; Hess, S.; Boos, F.; Woellhaf, M.W.; Godel, S.; Jung, M.; Muhlhaus, T.; Herrmann, J.M. Tom70 enhances mitochondrial preprotein import efficiency by binding to internal targeting sequences. J. Cell Biol. 2018, 217, 1369–1382. [Google Scholar] [CrossRef]
- Claros, M.G.; Vincens, P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996, 241, 779–786. [Google Scholar] [CrossRef]
- Ventura, A.; Maccarana, M.; Raker, V.A.; Pelicci, P.G. A cryptic targeting signal induces isoform-specific localization of p46Shc to mitochondria. J. Biol. Chem. 2004, 279, 2299–2306. [Google Scholar] [CrossRef]
- Kato, S.; Arasaki, K.; Tokutomi, N.; Imai, Y.; Inoshita, T.; Hattori, N.; Sasaki, T.; Sato, M.; Wakana, Y.; Inoue, H.; et al. Syntaxin 17, an ancient SNARE paralog, plays different and conserved roles in different organisms. J. Cell Sci. 2021, 134, jcs258669. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef]
- Grabski, R.; Balklava, Z.; Wyrozumska, P.; Szul, T.; Brandon, E.; Alvarez, C.; Holloway, Z.G.; Sztul, E. Identification of a functional domain within the p115 tethering factor that is required for Golgi ribbon assembly and membrane trafficking. J. Cell Sci. 2012, 125, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Sohda, M.; Misumi, Y.; Yoshimura, S.; Nakamura, N.; Fusano, T.; Sakisaka, S.; Ogata, S.; Fujimoto, J.; Kiyokawa, N.; Ikehara, Y. Depletion of vesicle-tethering factor p115 causes mini-stacked Golgi fragments with delayed protein transport. Biochem. Biophys. Res. Commun. 2005, 338, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Tabara, L.C.; Tilokani, L.; Paupe, V.; Anand, H.; Pogson, J.H.; Zunino, R.; McBride, H.M.; Prudent, J. Golgi-derived PI(4)P-containing vesicles drive late steps of mitochondrial division. Science 2020, 367, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Teng, J.; Chen, J. New insights regarding SNARE proteins in autophagosome-lysosome fusion. Autophagy 2021, 17, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- McLelland, G.L.; Soubannier, V.; Chen, C.X.; McBride, H.M.; Fon, E.A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014, 33, 282–295. [Google Scholar] [CrossRef]
- Sugo, M.; Kimura, H.; Arasaki, K.; Amemiya, T.; Hirota, N.; Dohmae, N.; Imai, Y.; Inoshita, T.; Shiba-Fukushima, K.; Hattori, N.; et al. Syntaxin 17 regulates the localization and function of PGAM5 in mitochondrial division and mitophagy. EMBO J. 2018, 37, e98899. [Google Scholar] [CrossRef]
- Arasaki, K.; Shimizu, H.; Mogari, H.; Nishida, N.; Hirota, N.; Furuno, A.; Kudo, Y.; Baba, M.; Baba, N.; Cheng, J.; et al. A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev. Cell 2015, 32, 304–317. [Google Scholar] [CrossRef]
- Raiders, S.A.; Eastwood, M.D.; Bacher, M.; Priess, J.R. Binucleate germ cells in Caenorhabditis elegans are removed by physiological apoptosis. PLoS Genet. 2018, 14, e1007417. [Google Scholar] [CrossRef]
- Sahani, M.H.; Itakura, E.; Mizushima, N. Expression of the autophagy substrate SQSTM1/p62 is restored during prolonged starvation depending on transcriptional upregulation and autophagy-derived amino acids. Autophagy 2014, 10, 431–441. [Google Scholar] [CrossRef]
- Chapin, H.C.; Okada, M.; Merz, A.J.; Miller, D.L. Tissue-specific autophagy responses to aging and stress in C. elegans. Aging 2015, 7, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Henis-Korenblit, S.; Meléndez, A. Methods to Determine the Role of Autophagy Proteins in C. elegans Aging. Methods Mol. Biol. 2019, 1880, 561–586. [Google Scholar] [CrossRef]
- Sun, L.; Xiong, H.; Chen, L.; Dai, X.; Yan, X.; Wu, Y.; Yang, M.; Shan, M.; Li, T.; Yao, J.; et al. Deacetylation of ATG4B promotes autophagy initiation under starvation. Sci. Adv. 2022, 8, eabo0412. [Google Scholar] [CrossRef]
- Margiotta, A. Role of SNAREs in Neurodegenerative Diseases. Cells 2021, 10, 991. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Jones, A.A.; Sawada, K.; Barr, A.M.; Bayer, T.A.; Falkai, P.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A.; Honer, W.G. Frontotemporal dysregulation of the SNARE protein interactome is associated with faster cognitive decline in old age. Neurobiol. Dis. 2018, 114, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A. Neurotransmitter changes in the aging brain. Dan. Med. Bull. 1985, 32 (Suppl. S1), 40–43. [Google Scholar] [PubMed]
- Verhage, M.; Sorensen, J.B. SNAREopathies: Diversity in Mechanisms and Symptoms. Neuron 2020, 107, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.; Ravikumar, B.; Renna, M.; Puri, C.; Rubinsztein, D.C. Autophagosome precursor maturation requires homotypic fusion. Cell 2011, 146, 303–317. [Google Scholar] [CrossRef]
- Moreau, K.; Renna, M.; Rubinsztein, D.C. Connections between SNAREs and autophagy. Trends Biochem. Sci. 2013, 38, 57–63. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Codogno, P.; Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 2021, 22, 733–750. [Google Scholar] [CrossRef]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef]
- Kumar, S.; Gu, Y.; Abudu, Y.P.; Bruun, J.A.; Jain, A.; Farzam, F.; Mudd, M.; Anonsen, J.H.; Rusten, T.E.; Kasof, G.; et al. Phosphorylation of Syntaxin 17 by TBK1 Controls Autophagy Initiation. Dev. Cell 2019, 49, 130–144.e136. [Google Scholar] [CrossRef]
- Ailion, M.; Inoue, T.; Weaver, C.I.; Holdcraft, R.W.; Thomas, J.H. Neurosecretory control of aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1999, 96, 7394–7397. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Miller, R.A.; Thurmond, D.C. Syntaxin 4 Overexpression Ameliorates Effects of Aging and High-Fat Diet on Glucose Control and Extends Lifespan. Cell Metab. 2015, 22, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Martinez-Campos, M.; Zipperlen, P.; Fraser, A.G.; Ahringer, J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001, 2, research0002.1. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Berezikov, E. Biolistic transformation of Caenorhabditis elegans. Methods Mol. Biol. 2013, 940, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011, 300, 723–742. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkikas, I.; Daskalaki, I.; Kounakis, K.; Tavernarakis, N.; Lionaki, E. MitoSNARE Assembly and Disassembly Factors Regulate Basal Autophagy and Aging in C. elegans. Int. J. Mol. Sci. 2023, 24, 4230. https://doi.org/10.3390/ijms24044230
Gkikas I, Daskalaki I, Kounakis K, Tavernarakis N, Lionaki E. MitoSNARE Assembly and Disassembly Factors Regulate Basal Autophagy and Aging in C. elegans. International Journal of Molecular Sciences. 2023; 24(4):4230. https://doi.org/10.3390/ijms24044230
Chicago/Turabian StyleGkikas, Ilias, Ioanna Daskalaki, Konstantinos Kounakis, Nektarios Tavernarakis, and Eirini Lionaki. 2023. "MitoSNARE Assembly and Disassembly Factors Regulate Basal Autophagy and Aging in C. elegans" International Journal of Molecular Sciences 24, no. 4: 4230. https://doi.org/10.3390/ijms24044230
APA StyleGkikas, I., Daskalaki, I., Kounakis, K., Tavernarakis, N., & Lionaki, E. (2023). MitoSNARE Assembly and Disassembly Factors Regulate Basal Autophagy and Aging in C. elegans. International Journal of Molecular Sciences, 24(4), 4230. https://doi.org/10.3390/ijms24044230







