Transcriptomic and Proteomic Analyses of Celery Cytoplasmic Male Sterile Line and Its Maintainer Line
Abstract
1. Introduction
2. Results
2.1. Morphology of the Sterile and Fertile Flowers
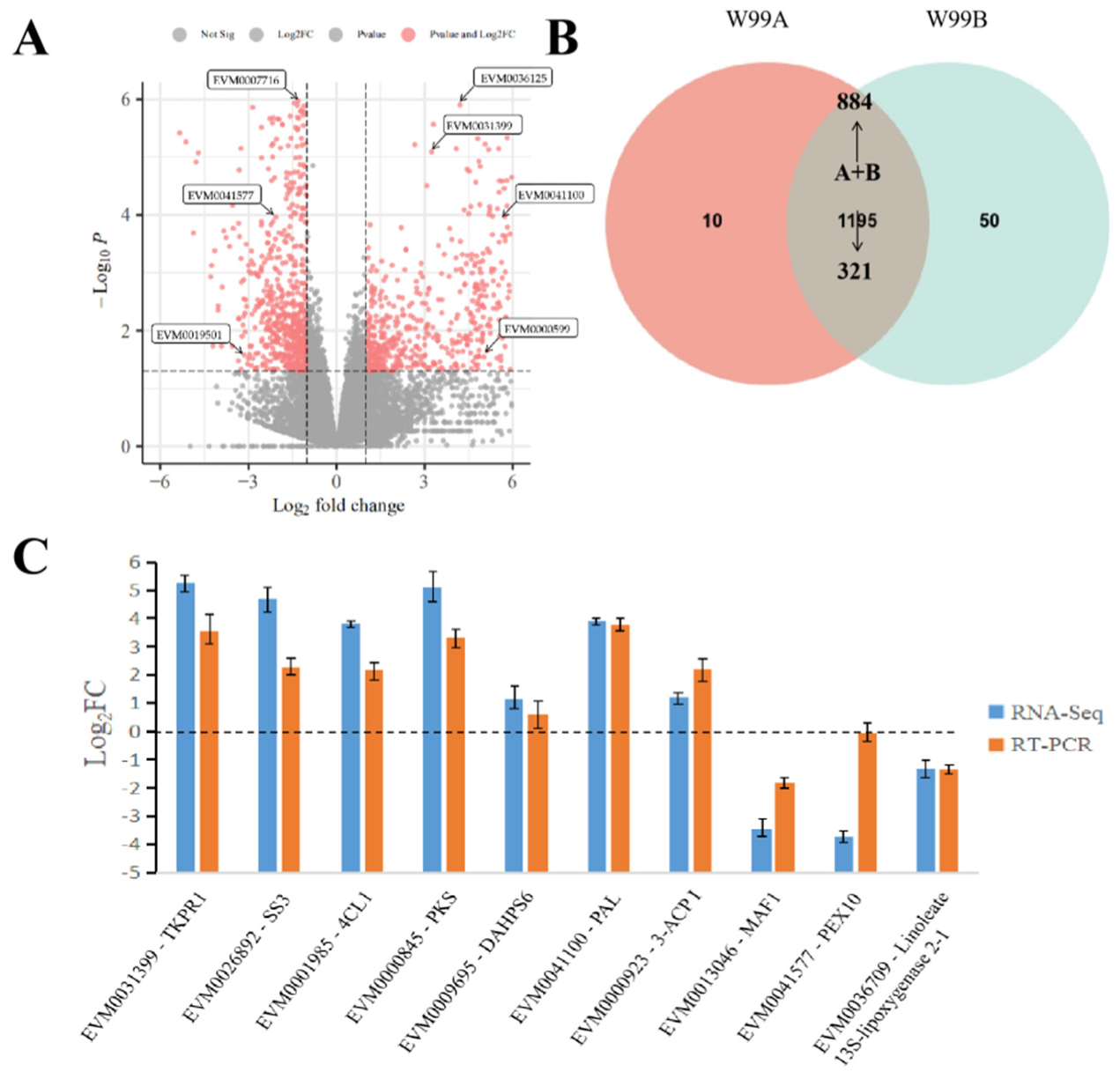
2.2. Overview of the RNA-Seq Data
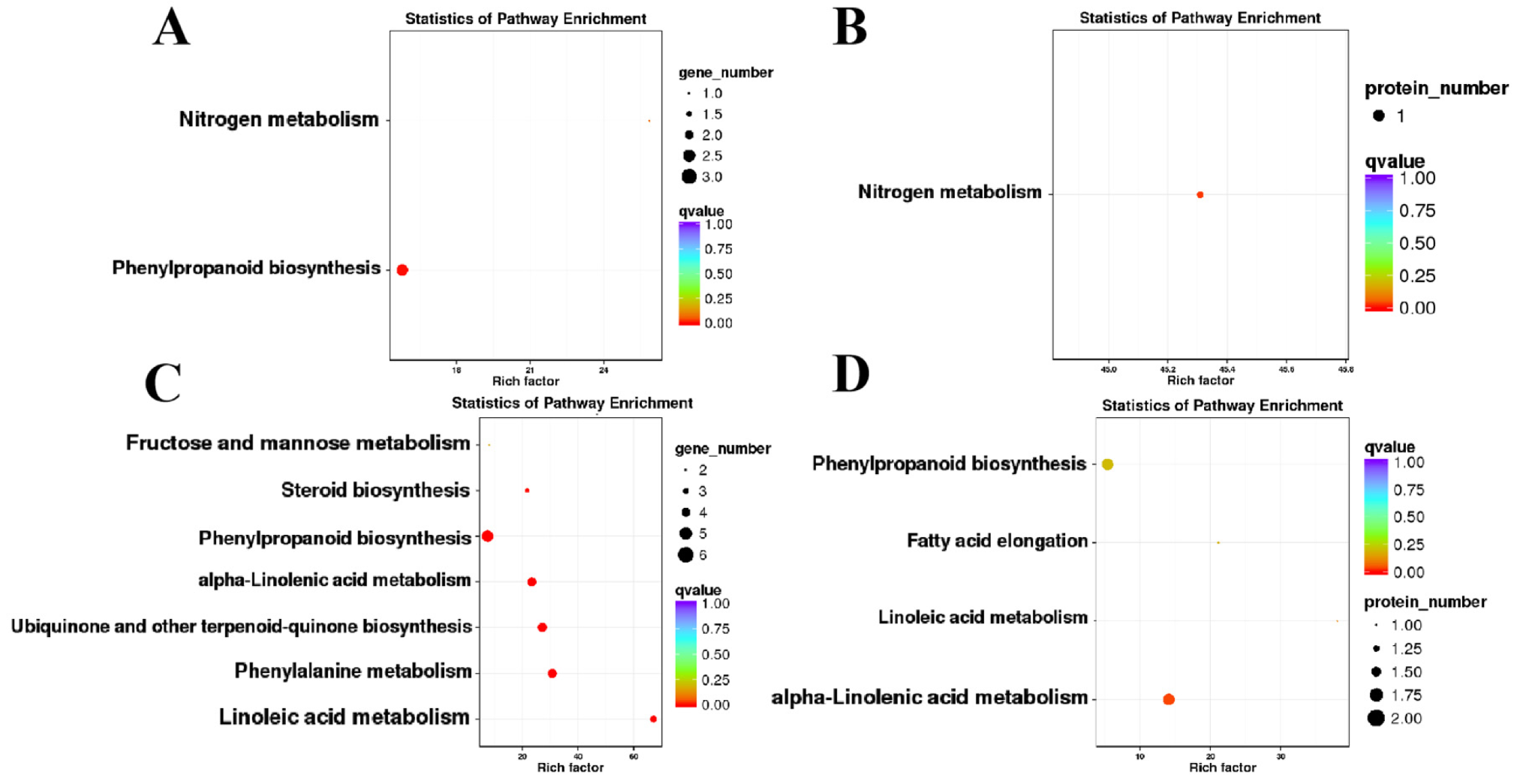
2.3. GO and KEGG Analyses
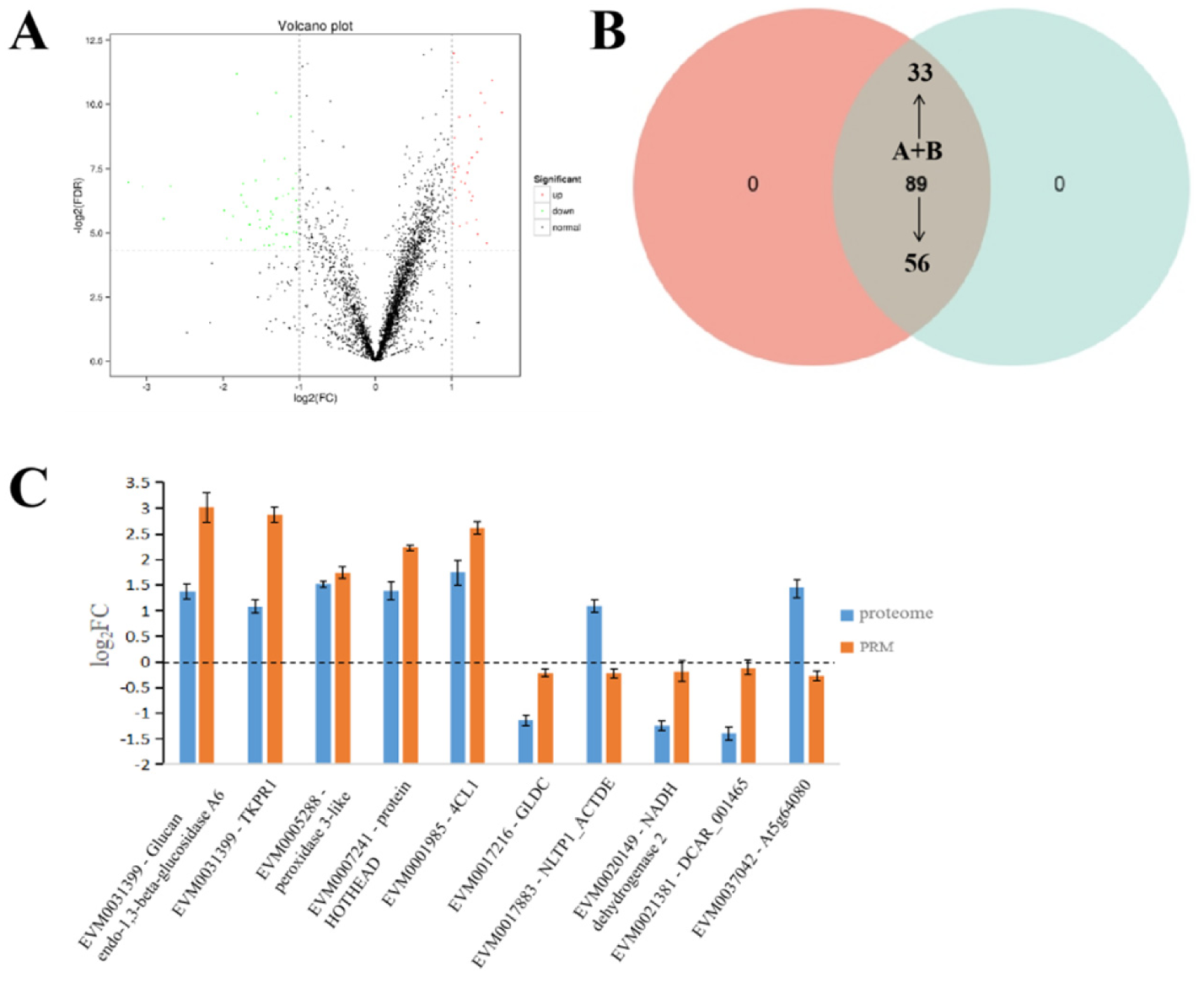
2.4. Differentially Expressed Proteins between W99A and W99B
2.5. Functional Classification of DEPs
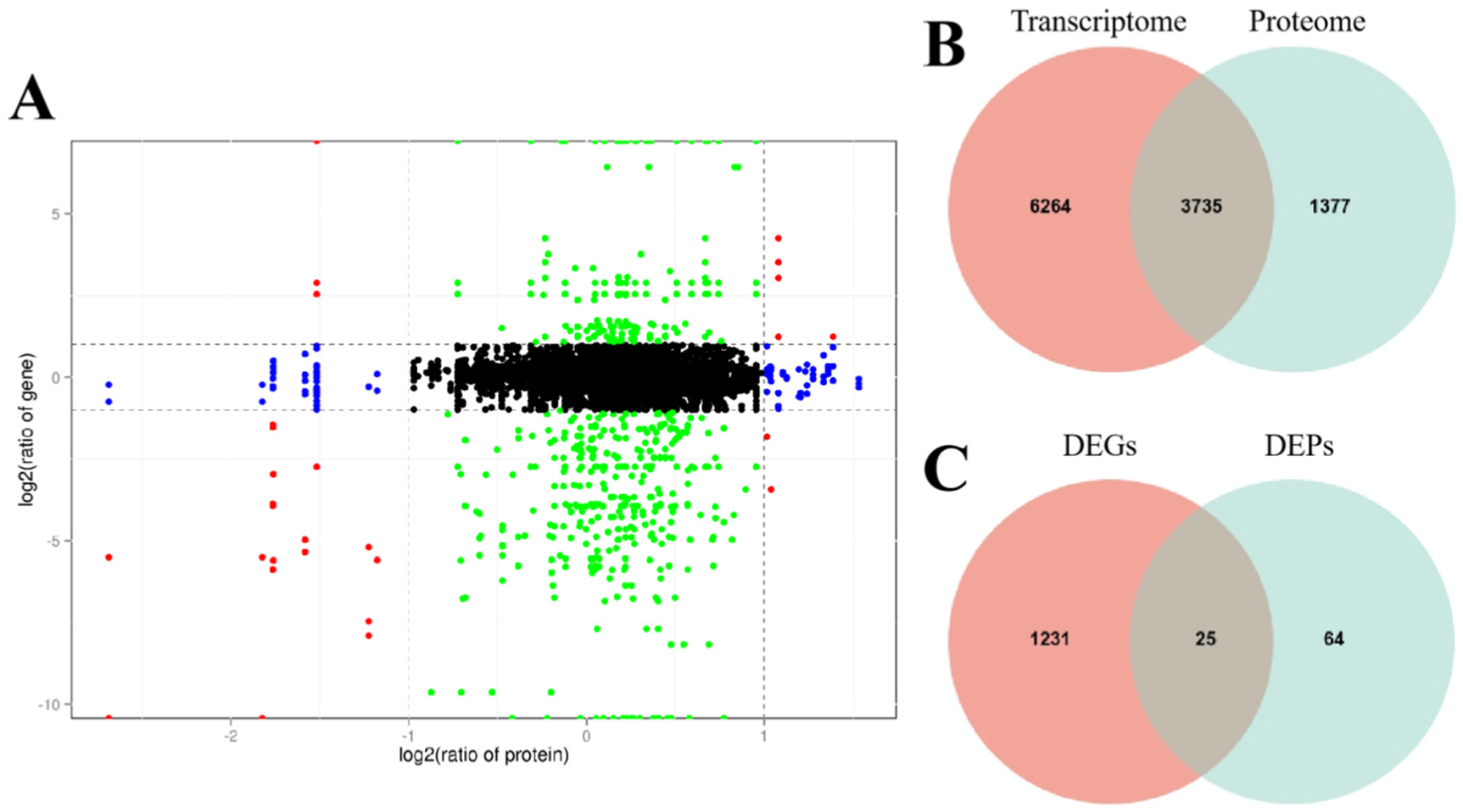
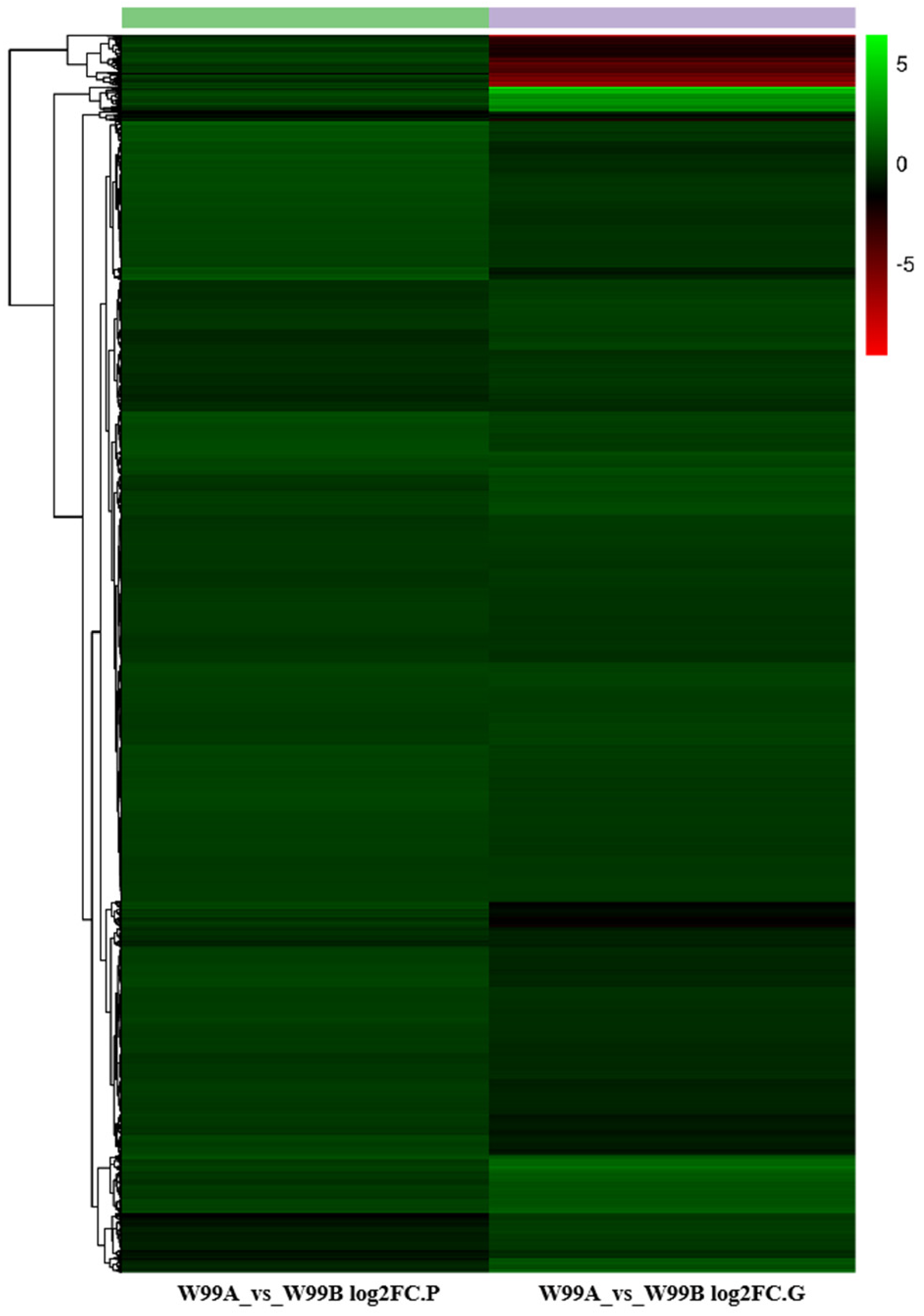
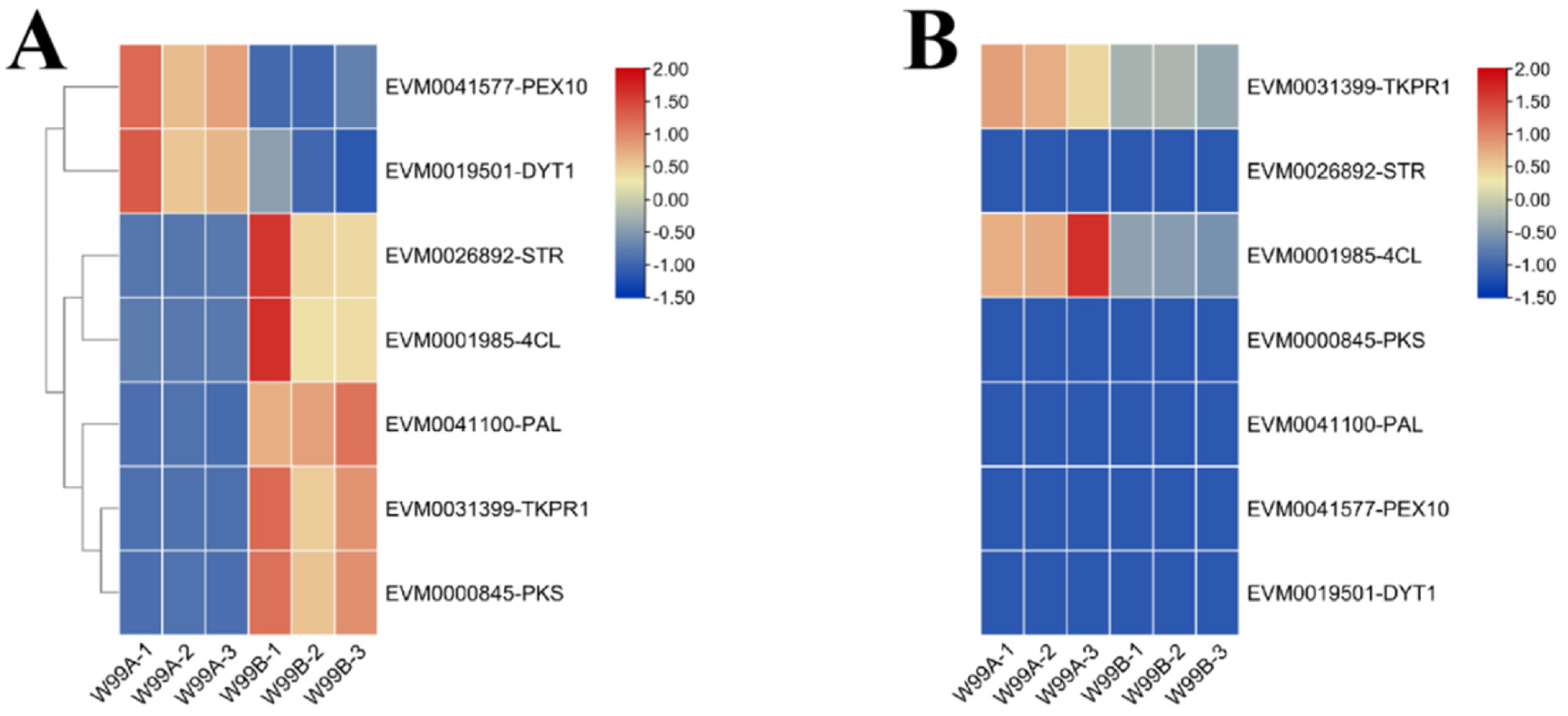
2.6. Transcriptome and Proteome Conjoint Analysis
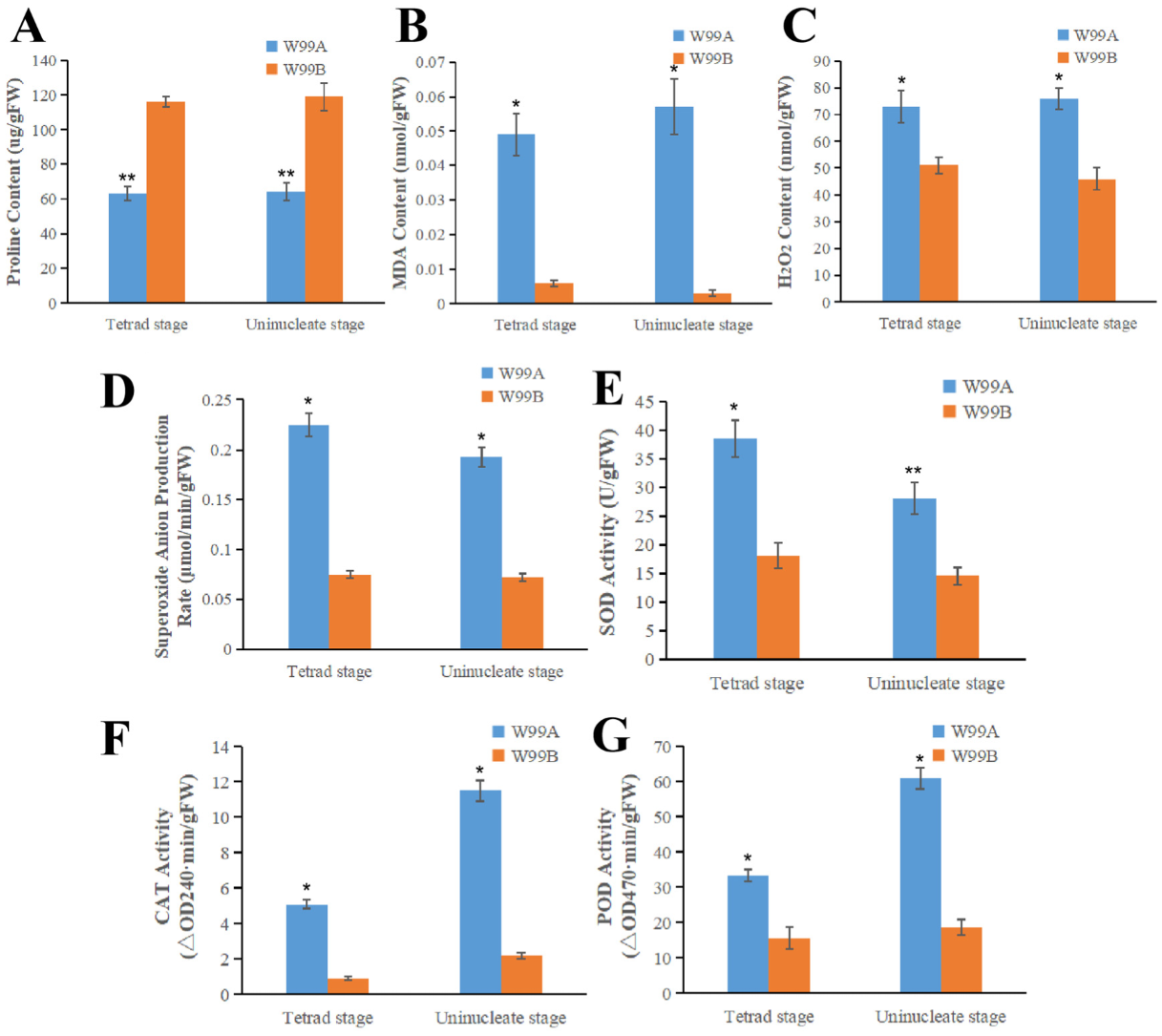
2.7. Determination of the Peroxides and Activities of Peroxides Eliminating Enzymes
2.8. Comparative Study of the Expression of Fertility-Related Genes between W99A and W99B
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Transcriptomic Analysis
4.3. Proteomic Analysis
4.4. Bioinformatics Analysis
4.5. Quantitative Real-Time PCR Validation
4.6. PRM Verification of DEPs
4.7. Biochemical Assay of Male Sterile Lines W99A and Maintainer Line W99B in Celery
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Q.; Wang, P.; Li, T.; Liu, J.; Zhang, Y.; Wang, Y.; Sun, L.; Shen, H. Apium graveolensComplete Mitochondrial Genome Sequence and Identification of a Candidate Gene Responsible for Cytoplasmic Male Sterility in Celery (L.). Int. J. Mol. Sci. 2021, 22, 8584. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.G. Male Sterility and Fertility Restoration in Crops. Annu. Rev. Plant Biol. 2013, 65, 579–606. [Google Scholar] [CrossRef]
- Afjani, J.A.; Najafabadi, M.S.; Mirfakhraei, R.G. Gene-Based Marker to Differentiate Among B, A, and R Lines in Hybrid Production of Rapeseed Ogura System. Iran. J. Biotechnol. 2019, 17, e1870. [Google Scholar]
- Liu, H.; Cui, P.; Zhan, K.; Lin, Q.; Zhuo, G.; Guo, X.; Ding, F.; Yang, W.; Liu, D.; Hu, S.; et al. Comparative analysis of mitochondrial genomes between a wheat K-type cytoplasmic male sterility (CMS) line and its maintainer line. BMC Genom. 2011, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Schnable, P.S.; Wise, R.P. The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci. 1998, 3, 175–180. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Beals, T.P.; Sanders, P.M. Anther Development: Basic Principles and Practical Applications. Plant cell 1993, 5, 1217–1229. [Google Scholar]
- McCormick, S. Control of Male Gametophyte Development. Plant Cell 2004, 16, S142. [Google Scholar] [CrossRef]
- Scott, R.; Hodge, R.; Paul, W.; Draper, J. The molecular biology of anther differentiation. Plant Sci. 1991, 80, 167–191. [Google Scholar] [CrossRef]
- Hu, J.; Huang, W.; Huang, Q.; Qin, X.; Yu, C.; Wang, L.; Li, S.; Zhu, R.; Zhu, Y. Mitochondria and cytoplasmic male sterility in plants. Mitochondrion 2014, 19, 282–288. [Google Scholar] [CrossRef]
- Duroc, Y.; Hiard, S.; Vrielynck, N.; Ragu, S.; Budar, F. The Ogura sterility-inducing protein forms a large complex without interfering with the oxidative phosphorylation components in rapeseed mitochondria. Plant Mol. Biol. 2009, 70, 123. [Google Scholar] [CrossRef]
- Du, K.; Liu, Q.; Wu, X.; Jiang, J.; Wu, J.; Fang, Y.; Li, A.; Wang, Y. Morphological Structure and Transcriptome Comparison of the Cytoplasmic Male Sterility Line in Brassica napus (SaNa-1A) Derived from Somatic Hybridization and Its Maintainer Line SaNa-1B. Front. Plant Sci. 2016, 7, 1313. [Google Scholar] [CrossRef]
- An, H.; Yang, Z.; Yi, B.; Wen, J.; Shen, J.; Tu, J.; Ma, C.; Fu, T. Comparative transcript profiling of the fertile and sterile flower buds of pol CMS in B. napus. BMC Genom. 2014, 15, 258. [Google Scholar] [CrossRef]
- Suzuki, H.; Rodriguez-Uribe, L.; Xu, J.; Zhang, J. Transcriptome analysis of cytoplasmic male sterility and restoration in CMS-D8 cotton. Plant Cell Rep. 2013, 32, 1531–1542. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Zhang, M.; Zhang, J.; Mcd, S.J.; Xing, C.; Wu, J.; Jin, S. Transcriptome, cytological and biochemical analysis of cytoplasmic male sterility and maintainer line in CMS-D8 cotton. Plant Mol. Biol. 2018, 97, 537–551. [Google Scholar] [CrossRef]
- Sheoran, I.S.; Sawhney, V.K. Proteome analysis of the normal and Ogura (ogu) CMS anthers of Brassica napus to identify proteins associated with male sterility. Botany 2010, 88, 217–230. [Google Scholar] [CrossRef]
- Miaomiao, X.; Chao, S.; Hailong, L.; Shilin, H.; Lei, L.; Jungen, K.; Maoteng, L. Integrated analysis of transcriptome and proteome changes related to the Ogura cytoplasmic male sterility in cabbage. PLoS ONE 2018, 13, e0193462. [Google Scholar]
- Ning, L.; Wang, H.; Li, D.; Lin, Z.; Li, M. Transcriptomic and Proteomic Analysis of Shaan2A Cytoplasmic Male Sterility and Its Maintainer Line in Brassica napus. Front. Plant Sci. 2019, 10, 252. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, T.; Ai, Y.; Lu, Q.; Wang, Y.; Sun, L.; Shen, H. Complementary Transcriptomic and Proteomic Analysis Reveals a Complex Network Regulating Pollen Abortion in GMS (msc-1) Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2019, 20, 1789. [Google Scholar] [CrossRef]
- Saxena, S.; Sahu, S.; Kaila, T.; Nigam, D.; Chaduvla, P.K.; Rao, A.R.; Sanand, S.; Singh, N.K.; Gaikwad, K. Transcriptome profiling of differentially expressed genes in cytoplasmic male-sterile line and its fertility restorer line in pigeon pea (Cajanus cajan L.). BMC Plant Biology 2020, 20, 74. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Qu, Y.; Peng, R.; Huang, J. Transcriptomic and proteomic analyses of a new cytoplasmic male sterile line with a wild Gossypium bickii genetic background. BMC Genom. 2020, 21, 859. [Google Scholar] [CrossRef]
- Nie, H.; Cheng, C.; Hua, J. Mitochondrial proteomic analysis reveals that proteins relate to oxidoreductase activity play a central role in pollen fertility in cotton. J. Proteom. 2020, 225, 103861. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Wang, X.; Wang, J.; Yang, X.; Zhang, L.; Song, X. TaEXPB5 functions as a gene related to pollen development in thermo-sensitive male-sterility wheat with Aegilops kotschyi cytoplasm. Plant Sci. Int. J. Exp. Plant Biol. 2022, 323, 111377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Wang, R.; Jiang, Q.; Zhang, D.; Mu, R.; Xu, Y.; Nnaemeka, V.; Mei, J.; Zhao, Y.; Cai, F.; et al. Identification and functional analysis of a pollen fertility-associated gene GhGLP4 of Gossypium hirsutum L. TAG. Theor. Appl. Genetics. Theor. Und Angew. Genet. 2021, 134, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Mayandi, S.; Luke, M.; Glenn, F.; Punyasena, S.W.; Marcus, A.I. Capturing the Surface Texture and Shape of Pollen: A Comparison of Microscopy Techniques. PLoS ONE 2012, 7, e39129. [Google Scholar]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.; Zhang, D. Genetic and Biochemical Mechanisms of Pollen Wall Development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef]
- Cigan, A.M.; Singh, M.; Benn, G.; Feigenbutz, L.; Young, J. Targeted mutagenesis of a conserved anther-expressed P450 gene confers male sterility in monocots. Plant Biotechnol. J. 2017, 15, 379–389. [Google Scholar] [CrossRef]
- de Azevedo Souza, C.; Kim, S.; Koch, S.; Kienow, L.; Schneider, K.; McKim, S.; Haughn, G.; Kombrink, E.; Douglas, C. A novel fatty Acyl-CoA Synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 2009, 21, 507–525. [Google Scholar] [CrossRef]
- Kim, S.; Grienenberger, E.; Lallemand, B.; Colpitts, C.; Kim, S.; Souza, C.A.; Geoffroy, P.; Heintz, D.; Krahn, D.; Kaiser, M.; et al. LAP6/POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B encode hydroxyalkyl α-pyrone synthases required for pollen development and sporopollenin biosynthesis in Arabidopsis thaliana. Plant Cell 2010, 22, 4045–4066. [Google Scholar] [CrossRef]
- Lallemand, B.; Erhardt, M.; Heitz, T.; Legrand, M. Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells. Plant Physiol. 2013, 162, 616–625. [Google Scholar] [CrossRef]
- Grienenberger, E.; Kim, S.; Lallemand, B.; Geoffroy, P.; Heintz, D.; Souza, C.A.; Heitz, T.; Douglas, C.; Legrand, M. Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell 2010, 22, 4067–4083. [Google Scholar] [CrossRef]
- Grienenberger, E.; Quilichini, T. The Toughest Material in the Plant Kingdom: An Update on Sporopollenin. Front. Plant Sci. 2021, 12, 703864. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; So, J.; Du, Y.; Lo, C. Conserved metabolic steps for sporopollenin precursor formation in tobacco and rice. Physiol. Plant. 2013, 149, 13–24. [Google Scholar] [CrossRef]
- Colpitts, C.; Kim, S.; Posehn, S.; Jepson, C.; Kim, S.; Wiedemann, G.; Reski, R.; Wee, A.; Douglas, C.; Suh, D. PpASCL, a moss ortholog of anther-specific chalcone synthase-like enzymes, is a hydroxyalkylpyrone synthase involved in an evolutionarily conserved sporopollenin biosynthesis pathway. New Phytol. 2011, 192, 855–868. [Google Scholar] [CrossRef]
- Jepson, C.; Karppinen, K.; Daku, R.; Sterenberg, B.; Suh, D. Hypericum perforatum hydroxyalkylpyrone synthase involved in sporopollenin biosynthesis—Phylogeny, site-directed mutagenesis, and expression in nonanther tissues. FEBS J. 2014, 281, 3855–3868. [Google Scholar] [CrossRef]
- Qin, M.; Tian, T.; Xia, S.; Wang, Z.; Song, L.; Yi, B.; Wen, J.; Shen, J.; Ma, C.; Fu, T.; et al. Heterodimer Formation of BnPKSA or BnPKSB with BnACOS5 Constitutes a Multienzyme Complex in Tapetal Cells and is Involved in Male Reproductive Development in Brassica napus. Plant Cell Physiol. 2016, 57, 1643–1656. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, D.; Ke, F.; Zhu, M.; Xu, J.; Zhang, M. Seedless mutant ‘Wuzi Ougan’ (Citrus suavissima Hort. ex Tanaka ‘seedless’) and the wild type were compared by iTRAQ-based quantitative proteomics and integratedly analyzed with transcriptome to improve understanding of male sterility. BMC Genet. 2018, 19, 106. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Guo, Z.; Shi, Q.; Xiong, S.; Zhang, C.; Zhu, J.; Yang, Z. OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol. 2016, 16, 256. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, K.; Li, Y.; Zhou, L.; Xiong, S.; Han, Y.; Zhang, Y.; Yang, N.; Yang, Z.; Zhu, J. OsPKS1 is required for sexine layer formation, which shows functional conservation between rice and Arabidopsis. Plant Sci. Int. J. Exp. Plant Biol. 2018, 277, 145–154. [Google Scholar] [CrossRef]
- Zou, T.; Liu, M.; Xiao, Q.; Wang, T.; Chen, D.; Luo, T.; Yuan, G.; Li, Q.; Zhu, J.; Liang, Y.; et al. OsPKS2 is required for rice male fertility by participating in pollen wall formation. Plant Cell Rep. 2018, 37, 759–773. [Google Scholar] [CrossRef]
- Xu, D.; Qu, S.; Tucker, M.; Zhang, D.; Liang, W.; Shi, J. Ostkpr1 functions in anther cuticle development and pollen wall formation in rice. BMC Plant Biol. 2019, 19, 104. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Zhang, J.; Dong, S.; Liu, Q.; Wang, R. Prunus sibiricaTranscriptomic analysis reveals candidate genes for male sterility in. PeerJ 2021, 9, e12349. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.H.; Wen, J.F.; Huo, J.L.; Zhu, H.S.; Dai, X.Z.; Zhang, Z.Q.; Zhou, J.; Zhou, X.X. Relationship of metabolism of reactive oxygen species with cytoplasmic male sterility in pepper (Capsicum annuum L.). Sci. Hortic. Amst. 2012, 134, 232–236. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Yi, P.; Wan, C.; Zhu, C.Y. A Honglian CMS line of rice displays aberrant F0 of F0F1-ATPase. Plant Cell Rep. 2007, 26, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Edqvist, J.; Farbos, I.; Glimelius, K. Male-sterile tobacco displays abnormal mitochondrial atp1 transcript accumulation and reduced floral ATP/ADP ratio. Plant Mol. Biol. 2000, 42, 531. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.T.; Knorpp, C.; Glimelius, K. Modified sucrose, starch, and ATP levels in two alloplasmic male-sterile lines of B. napus. J. Exp. Bot. 2005, 56, 1245–1253. [Google Scholar] [CrossRef]
- Li, J.; Han, S.; Ding, X.; He, T.; Dai, J.; Yang, S.; Gai, J.; Tian, Z. Comparative Transcriptome Analysis between the Cytoplasmic Male Sterile Line NJCMS1A and Its Maintainer NJCMS1B in Soybean (Glycine max (L.) Merr.). PLoS ONE 2015, 10, e0126771. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Q.; Zhang, L.; Li, Z.; Wang, C.; Zhang, G. Comparative Proteomic Analysis of Developmental Changes in P-Type Cytoplasmic Male Sterile and Maintainer Anthers in Wheat. Int. J. Mol. Sci. 2021, 22, 2012. [Google Scholar] [CrossRef]
- Cui, X.; Wise, R.; Schnable, P. The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 1996, 272, 1334–1336. [Google Scholar] [CrossRef]
- Tsuji, H.; Tsutsumi, N.; Sasaki, T.; Hirai, A.; Nakazono, M. Organ-specific expressions and chromosomal locations of two mitochondrial aldehyde dehydrogenase genes from rice (Oryza sativa L.), ALDH2a and ALDH2b. Gene 2003, 305, 195–204. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, M.; Oliver, D.; Schnable, P. The roles of aldehyde dehydrogenases (ALDHs) in the PDH bypass of Arabidopsis. BMC Biochem. 2009, 10, 7. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, J.; Qin, C.; Hu, Z.; Yin, C.; Hu, K. Differential proteomic analysis of anthers between cytoplasmic male sterile and maintainer lines in Capsicum annuum L. Int. J. Mol. Sci. 2013, 14, 22982–22996. [Google Scholar] [CrossRef]
- Sun, L.; Sui, X.; Lucas, W.; Li, Y.; Feng, S.; Ma, S.; Fan, J.; Gao, L.; Zhang, Z. CsSUT1Down-regulation of the Sucrose Transporter Causes Male Sterility by Altering Carbohydrate Supply. Plant Physiol. 2019, 180, 986–997. [Google Scholar] [CrossRef]
- Mamun, E.; Alfred, S.; Cantrill, L.; Overall, R.; Sutton, B. Effects of chilling on male gametophyte development in rice. Cell Biol. Int. 2006, 30, 583–591. [Google Scholar] [CrossRef]
- Siddiqui, M.; Al-Khaishany, M.; Al-Qutami, M.; Al-Whaibi, M.; Grover, A.; Ali, H.; Al-Wahibi, M. Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi. J. Biol. Sci. 2015, 22, 656–663. [Google Scholar] [CrossRef]
- Chen, L.; Ding, X.; Zhang, H.; He, T.; Li, Y.; Wang, T.; Li, X.; Jin, L.; Song, Q.; Yang, S.; et al. Comparative analysis of circular RNAs between soybean cytoplasmic male-sterile line NJCMS1A and its maintainer NJCMS1B by high-throughput sequencing. BMC Genom. 2018, 19, 663. [Google Scholar] [CrossRef]
- Luo, D.; Hong, X.; Liu, Z.; Guo, J.; Liu, Y.G. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573. [Google Scholar] [CrossRef]
- Pei, H.; Xie, H.; Wang, X.; Yan, X.; Wang, B.; Feng, H.; Zhao, Y.; Gao, J.; Gao, J. Capsicum annuumProteomic analysis of differential anther development from sterile/fertile lines in L. PeerJ 2022, 10, e13168. [Google Scholar] [CrossRef]
- Hu, J.; Chen, X.; Zhang, H.; Ding, Y. Genome-wide analysis of DNA methylation in photoperiod- and thermo-sensitive male sterile rice Peiai 64S. BMC Genom. 2015, 16, 102. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, X.; Shao, Z.; Wei, Z.; Wang, Y. Rice UDP-Glucose Pyrophosphorylase1 Is Essential for Pollen Callose Deposition and Its Cosuppression Results in a New Type of Thermosensitive Genic Male Sterility. Plant Cell 2007, 19, 847–861. [Google Scholar] [CrossRef]
- Huang, P.; Ju, H.W.; Min, J.H.; Zhang, X.; Kim, S.H.; Yang, K.Y.; Kim, C.S. Overexpression of L-type lectin-like protein kinase 1 confers pathogen resistance and regulates salinity response in Arabidopsis thaliana. Plant Sci. 2013, 203–204, 98–106. [Google Scholar] [CrossRef]
- Rai, R.K.; Stoskopf, N.C. Amino acid comparisons in male sterile wheat derived from Triticum timopheevi zhuk. cytoplasm and its fertile counterpart. Theor. Appl. Genet. 1974, 44, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zheng, X.; Zhou, C.; Kanwar, M.; Zhou, J. Functions of Redox Signaling in Pollen Development and Stress Response. Antioxidants 2022, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, B.; Zhou, X.; Wang, G. The Rice Dynamin-Related Protein OsDRP1E Negatively Regulates Programmed Cell Death by Controlling the Release of Cytochrome c from Mitochondria. PLoS Path. 2017, 13, e1006157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, X.; Li, L.; Jiao, Z.; Huang, Q. Metabolism of Reactive Oxygen Species in Cytoplasmic Male Sterility of Rice by Marking Upmost Pulvinus Interval. Appl. Biochem. Biotechnol. 2015, 175, 1263–1269. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Wei, C.; Sun, J.; Wang, Q. Using Transcriptome Analysis to Screen for Key Genes and Pathways Related to Cytoplasmic Male Sterility in Cotton (Gossypium hirsutum L.). Int. J. Mol. Sci. 2019, 20, 5120. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, A. Reactive Oxygen Species Accumulation Strongly Allied with Genetic Male Sterility Convertible to Cytoplasmic Male Sterility in Kenaf. Int. J. Mol. Sci. 2021, 22, 1107. [Google Scholar]
- Liu, Z.; Shi, X.; Li, S.; Gan, H.; Zhang, L.; Song, X. Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat. Int. J. Mol. Sci. 2018, 19, 1708. [Google Scholar] [CrossRef]
- Xavier, S.K.; Haneefa, S.M.; Anand, D.R.; Polo, P.R.; Setty, M.M. Antioxidant and Nephroprotective Activities of the Extract and Fractions of Homonoia riparia Lour. Pharm. Mag. 2017, 13, 25. [Google Scholar]
- Sabela, M.I.; Mpanza, T.; Kanchi, S.; Sharma, D.; Bisetty, K. Electrochemical sensing platform amplified with a nanobiocomposite of L-phenylalanine ammonia-lyase enzyme for the detection of capsaicin. Biosens. Bioelectron. 2016, 83, 45–53. [Google Scholar] [CrossRef]
- Yao, J.; Li, R.; Cheng, Y.; Li, Z. A combined transcriptomic and proteomic analysis of chrysanthemum provides new insights into petal senescence. Planta 2021, 255, 22. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, K.; Liang, P.; Yang, L.; Zhang, L.; Gao, X. Myzus persicaeCombined Transcriptomic and Proteomic Analysis of the Green Peach Aphid, Infected with Cucumber Mosaic Virus. Insects 2021, 12, 372. [Google Scholar] [CrossRef]
- Hussain, T.; Asrar, H.; Zhang, W.; Gul, B.; Liu, X. Panicum antidotaleCombined Transcriptome and Proteome Analysis to Elucidate Salt Tolerance Strategies of the Halophyte Retz. Front. Plant. Sci. 2021, 12, 760589. [Google Scholar] [CrossRef]
- Shaw, L.; Sneddon, S.; Zeef, L.; Kimber, S.; Brison, D. Global gene expression profiling of individual human oocytes and embryos demonstrates heterogeneity in early development. PLoS ONE 2013, 8, e64192. [Google Scholar] [CrossRef]
- Morse, D.L.; Gillies, R.J. Molecular imaging and targeted therapies. Biochem. Pharmacol. 2010, 80, 731–738. [Google Scholar] [CrossRef]
- Schwanhusser, B.; Busse, D.; Li, N.; Dittmar, G.; Selbach, M. Corrigendum: Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Jay, A.; Reitz, D.; Namekawa, S.; Heyer, W. Cancer testis antigens and genomic instability: More than immunology. DNA Repair 2021, 108, 103214. [Google Scholar] [CrossRef]
- Silvester, E.; McWilliam, K.; Matthews, K. The Cytological Events and Molecular Control of Life Cycle Development of Trypanosoma brucei in the Mammalian Bloodstream. Pathogens 2017, 6, 29. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; Chen, C.; Fan, D.; Wu, X.; Zhao, L.; Shao, B.; Sun, Z.; Ji, Z. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: Involvement of miR-30c-5p/TCF7 axis. Molecular Cancer 2021, 20, 93. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Thran, M.; Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. CMLS 2019, 76, 301–328. [Google Scholar] [CrossRef]
- Méchin, V.; Damerval, C.; Zivy, M. Total protein extraction with TCA-acetone. Methods Mol. Biol. 2007, 355, 1–8. [Google Scholar] [CrossRef]
- Zhi, N.; Bowen, L.; Xiao, Z.; Ling, W.; Dan, L.; Jialei, G.; Xuan, H.; Duojiang, G.; Shiqiang, G.; Shibin, G. Combined Transcriptome and Proteome Analysis of Maize (Zea mays L.) Reveals A Complementary Profile in Response to Phosphate Deficiency. Curr. Issues Mol. Biol. 2021, 43, 1142–1155. [Google Scholar]
- Wang, C.; Zhao, B.; He, L.; Zhou, S.; Liu, Y.; Zhao, W.; Guo, S.; Wang, R.; Bai, Q.; Li, Y. The WOX family transcriptional regulator SlLAM1 controls compound leaf and floral organ development in Solanum lycopersicum. J. Exp. Bot. 2020, 72, 1822–1835. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Sun, L.; Qiao, H.; Li, Z.; Li, M.; Cui, X.; Li, W.; Liu, S.; Wang, H.; Yang, W.; et al. Loci underlying leaf agronomic traits identified by re-sequencing celery accessions based on an assembled genome. iScience 2022, 25, 104565. [Google Scholar] [CrossRef] [PubMed]
- Attard, T.J.; Carter, M.D.; Mengxuan, F.; Johnson, R.C.; Reid, G.E. Structural Characterization and Absolute Quantification of Microcystin Peptides Using Collision-Induced and Ultraviolet Photo-Dissociation Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 1812–1825. [Google Scholar] [CrossRef]
- Lau, E.; Han, Y.; Williams, D.; Thomas, C.; Shrestha, R.; Wu, J.; Lam, M. Splice-Junction-Based Mapping of Alternative Isoforms in the Human Proteome. Cell Rep. 2019, 29, 3751–3765. [Google Scholar] [CrossRef]
- Guo, J.; Liu, C.; Wang, P.; Cheng, Q.; Sun, L.; Yang, W.; Shen, H. Aborted MicrosporesThe ()-Like Gene Is Required for Anther and Microspore Development in Pepper (L.). Int. J. Mol. Sci. 2018, 19, 1341. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cheng, Q.; Zhai, Z.; Cui, X.; Li, M.; Ye, R.; Sun, L.; Shen, H. Transcriptomic and Proteomic Analyses of Celery Cytoplasmic Male Sterile Line and Its Maintainer Line. Int. J. Mol. Sci. 2023, 24, 4194. https://doi.org/10.3390/ijms24044194
Wang H, Cheng Q, Zhai Z, Cui X, Li M, Ye R, Sun L, Shen H. Transcriptomic and Proteomic Analyses of Celery Cytoplasmic Male Sterile Line and Its Maintainer Line. International Journal of Molecular Sciences. 2023; 24(4):4194. https://doi.org/10.3390/ijms24044194
Chicago/Turabian StyleWang, Haoran, Qing Cheng, Ziqi Zhai, Xiangyun Cui, Mingxuan Li, Ruiquan Ye, Liang Sun, and Huolin Shen. 2023. "Transcriptomic and Proteomic Analyses of Celery Cytoplasmic Male Sterile Line and Its Maintainer Line" International Journal of Molecular Sciences 24, no. 4: 4194. https://doi.org/10.3390/ijms24044194
APA StyleWang, H., Cheng, Q., Zhai, Z., Cui, X., Li, M., Ye, R., Sun, L., & Shen, H. (2023). Transcriptomic and Proteomic Analyses of Celery Cytoplasmic Male Sterile Line and Its Maintainer Line. International Journal of Molecular Sciences, 24(4), 4194. https://doi.org/10.3390/ijms24044194





