Genomic Survey of Flavin Monooxygenases in Wild and Cultivated Rice Provides Insight into Evolution and Functional Diversities
Abstract
1. Introduction
2. Results
2.1. The Number of FMO Genes Varies in Wild and Cultivated Species of Rice
2.2. The Distribution of FMO Genes on Chromosomes Reveals the Presence of Gene Clusters
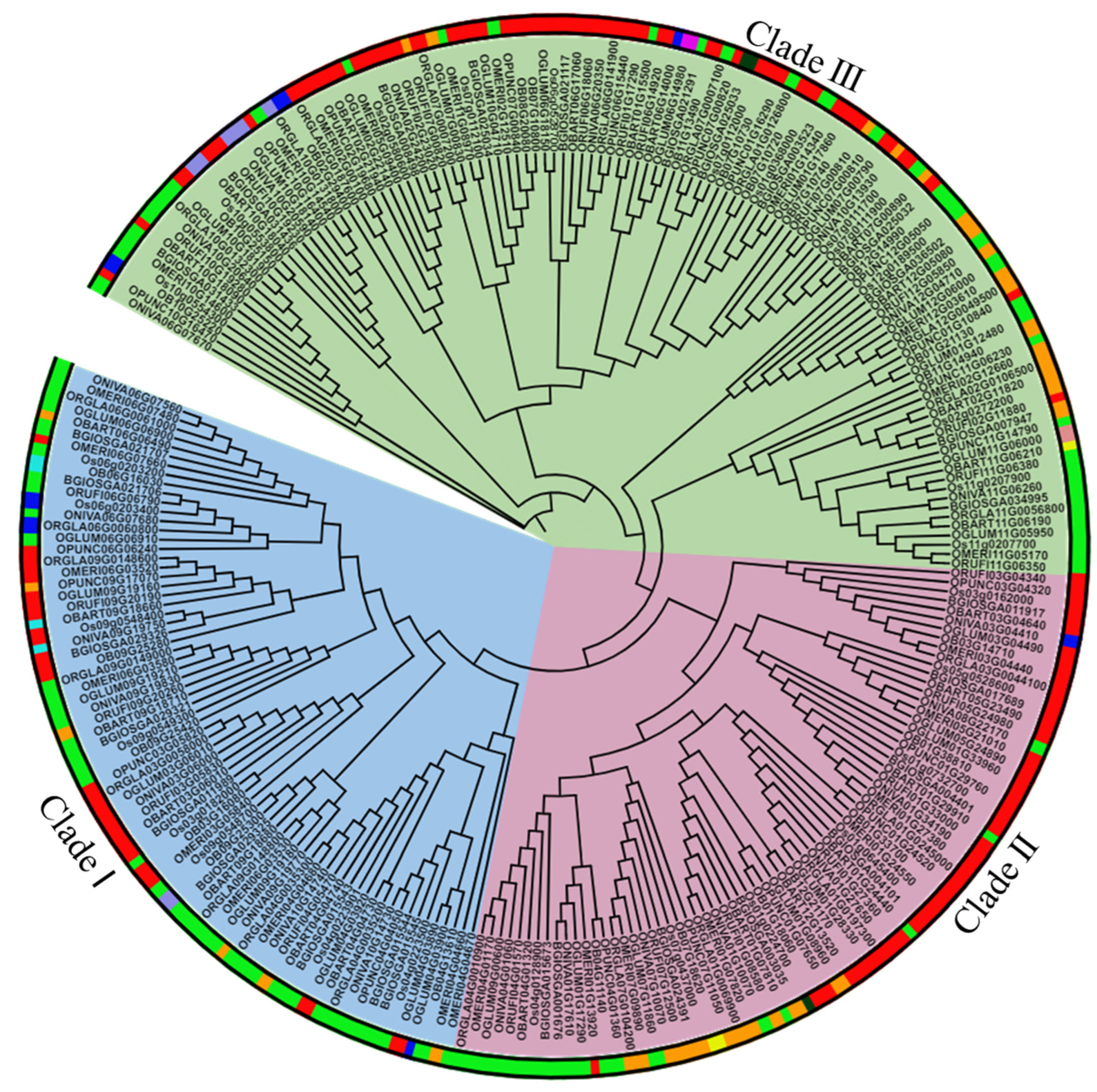
2.3. Phylogenetic Analysis Shows Conservation among FMO Family and the Majority of Them Belongs to the S-Oxygenating Clade in Different Oryza Species
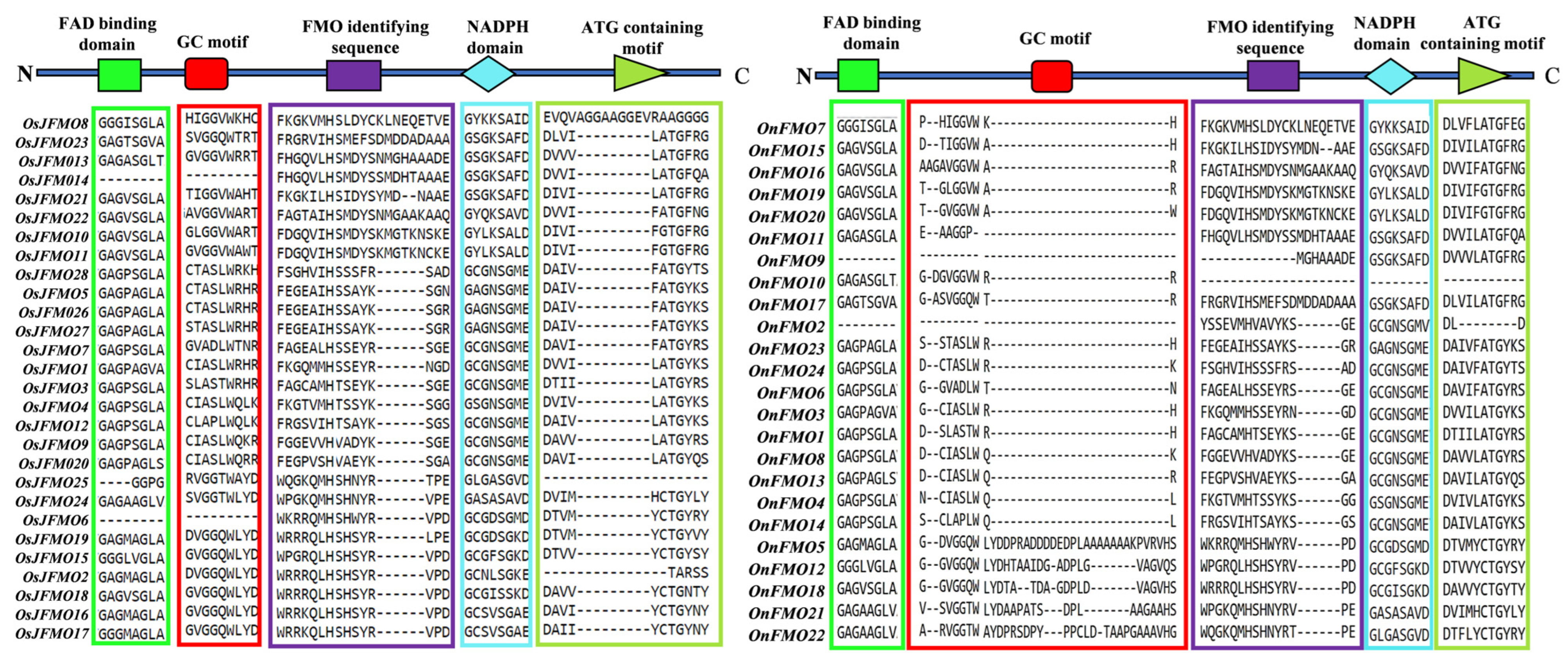
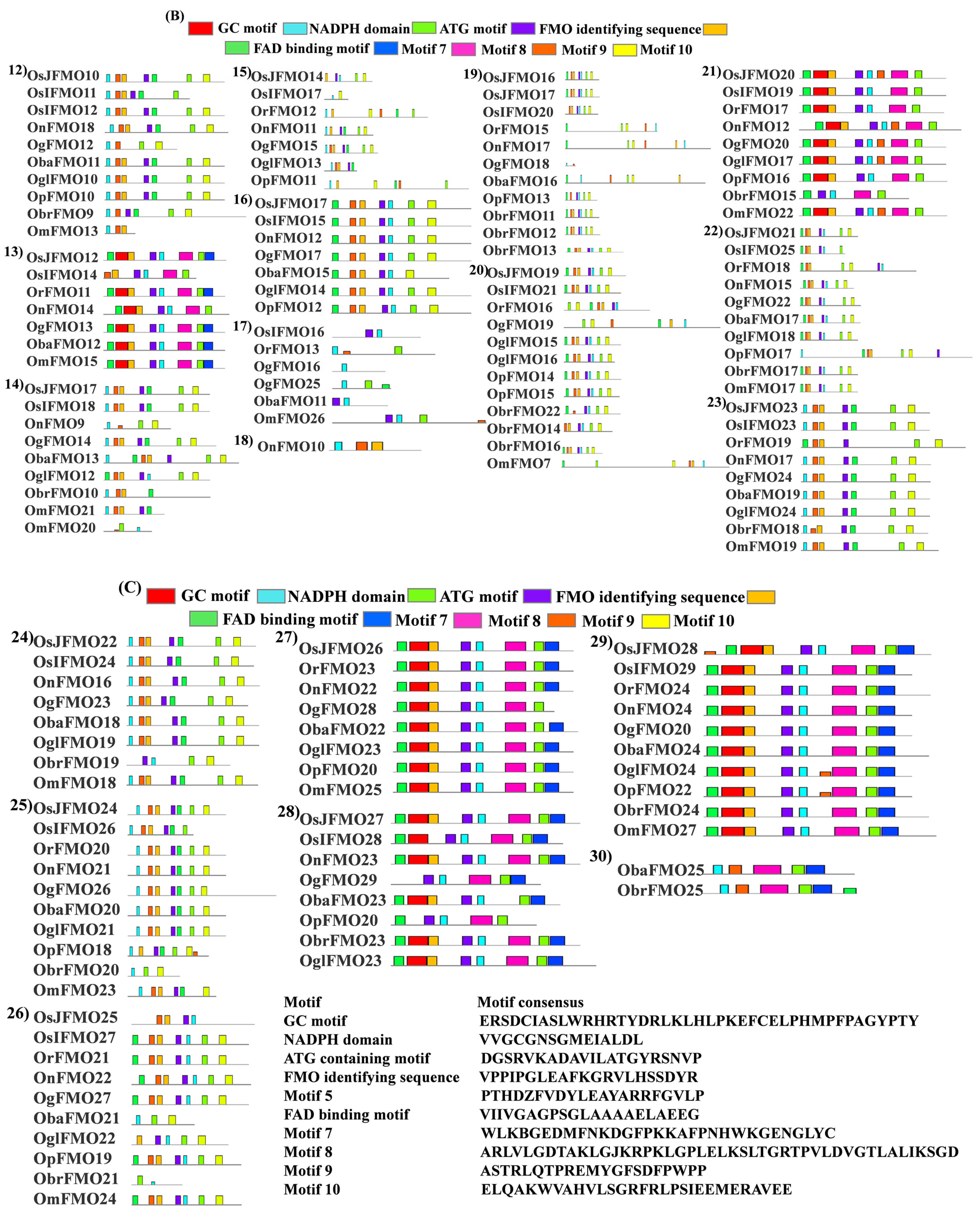
2.4. Protein Motif Analysis Reveals Five Conserved Motifs in FMO Members from Different Oryza Species
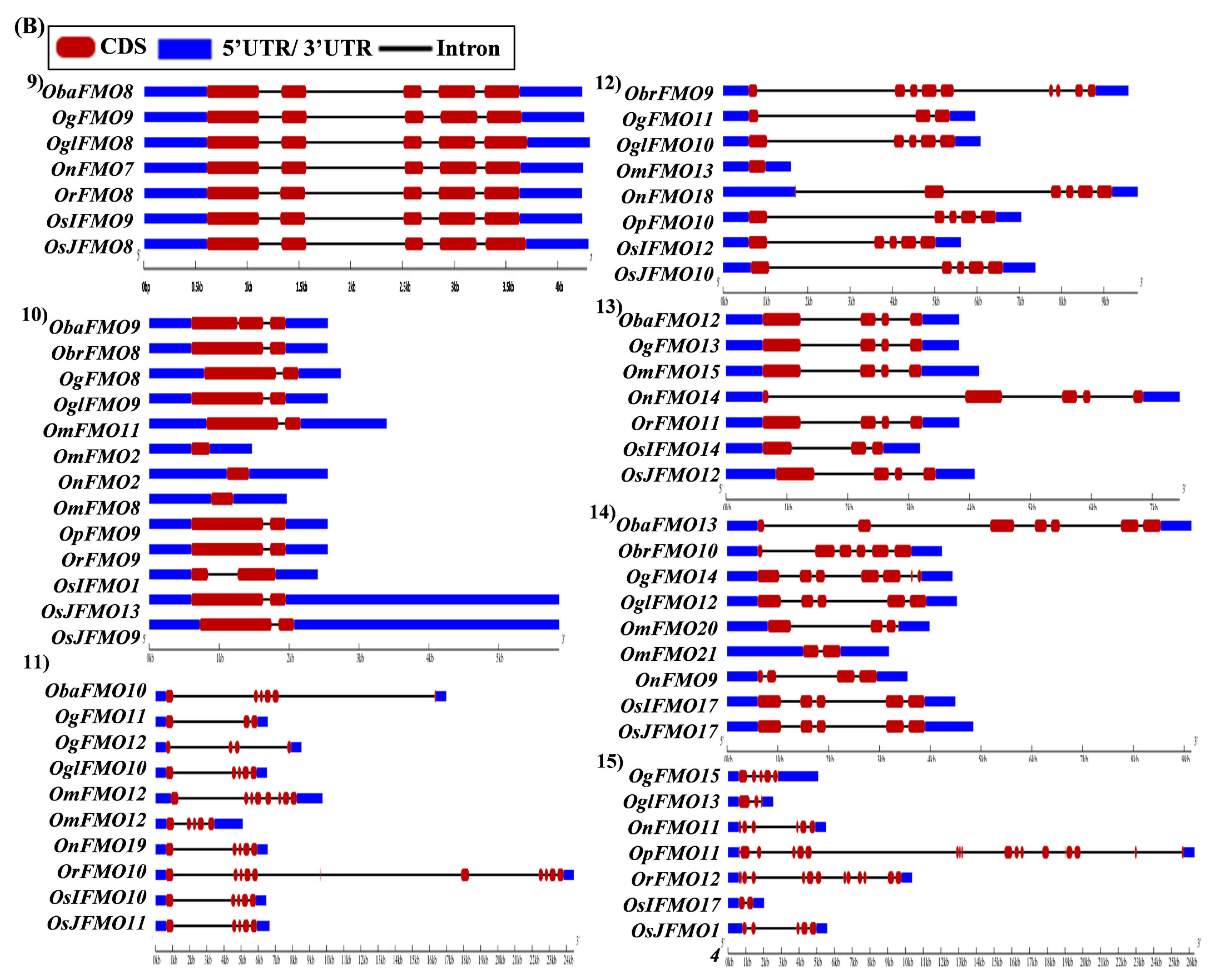
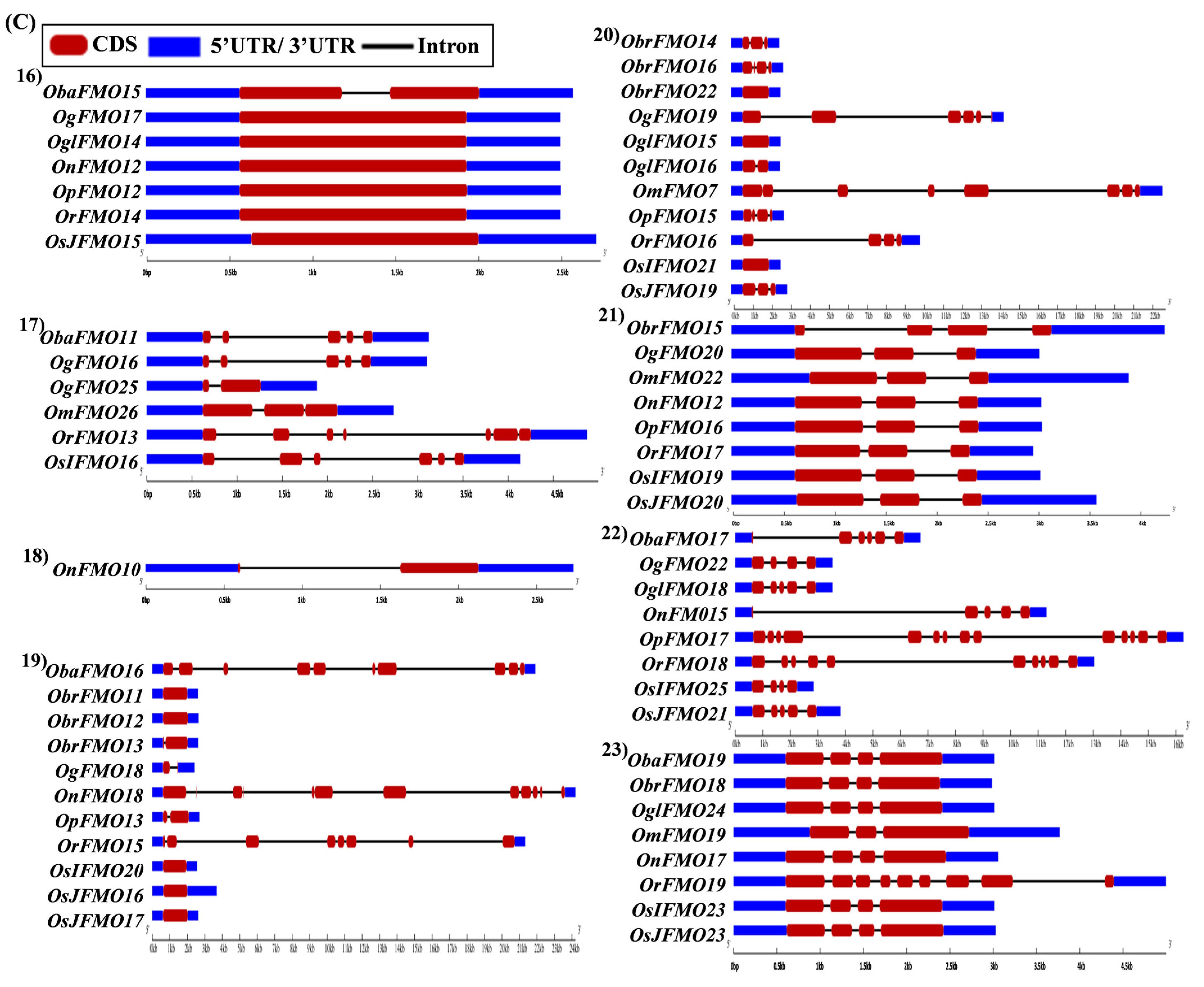
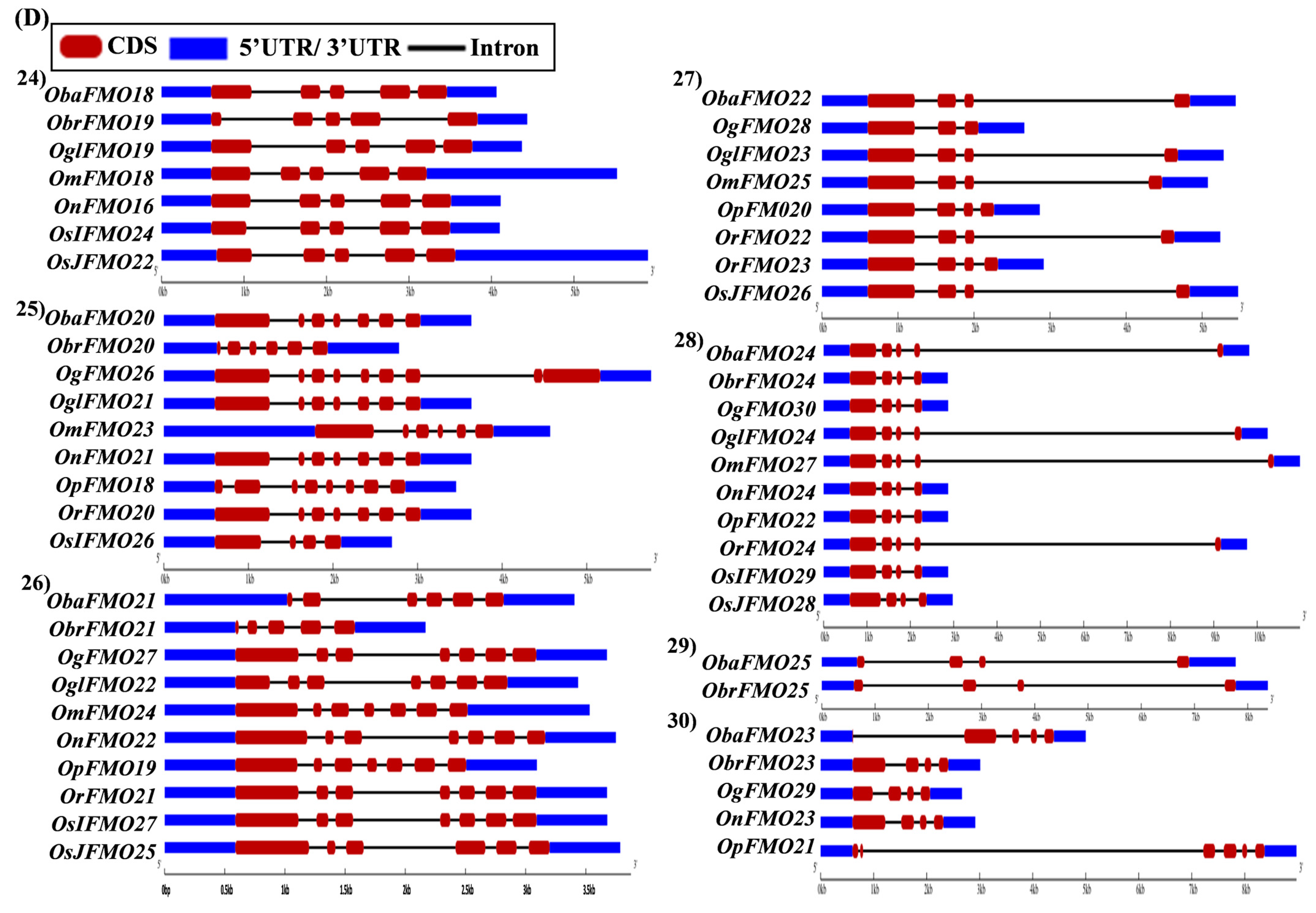
2.5. Analysis of Gene Architecture Depicts Conservation among Orthologs from Different Oryza Species
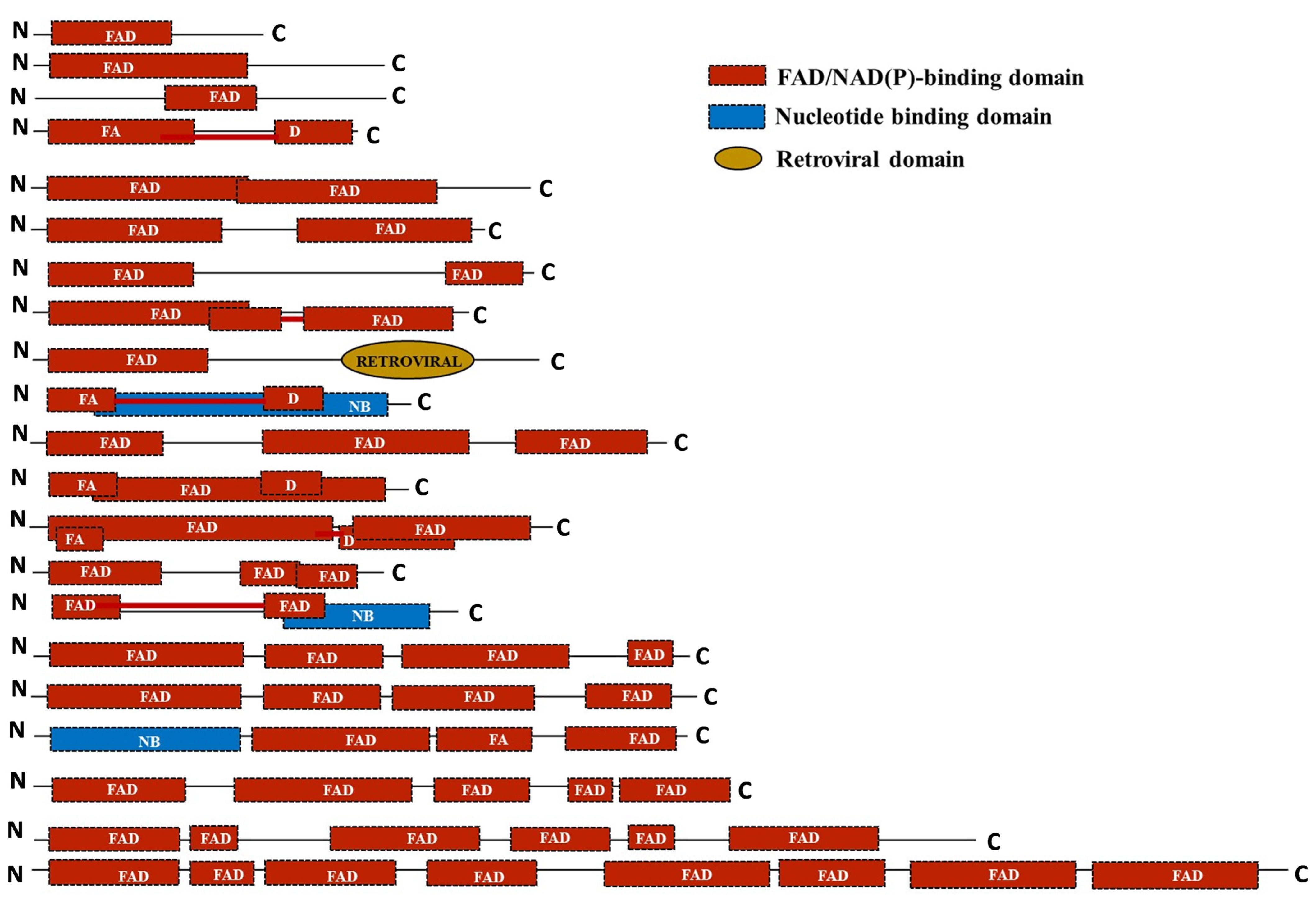
2.6. Domain Assessment Reveals Loss of Extra Copies of FAD Domain during the Course of Domestication
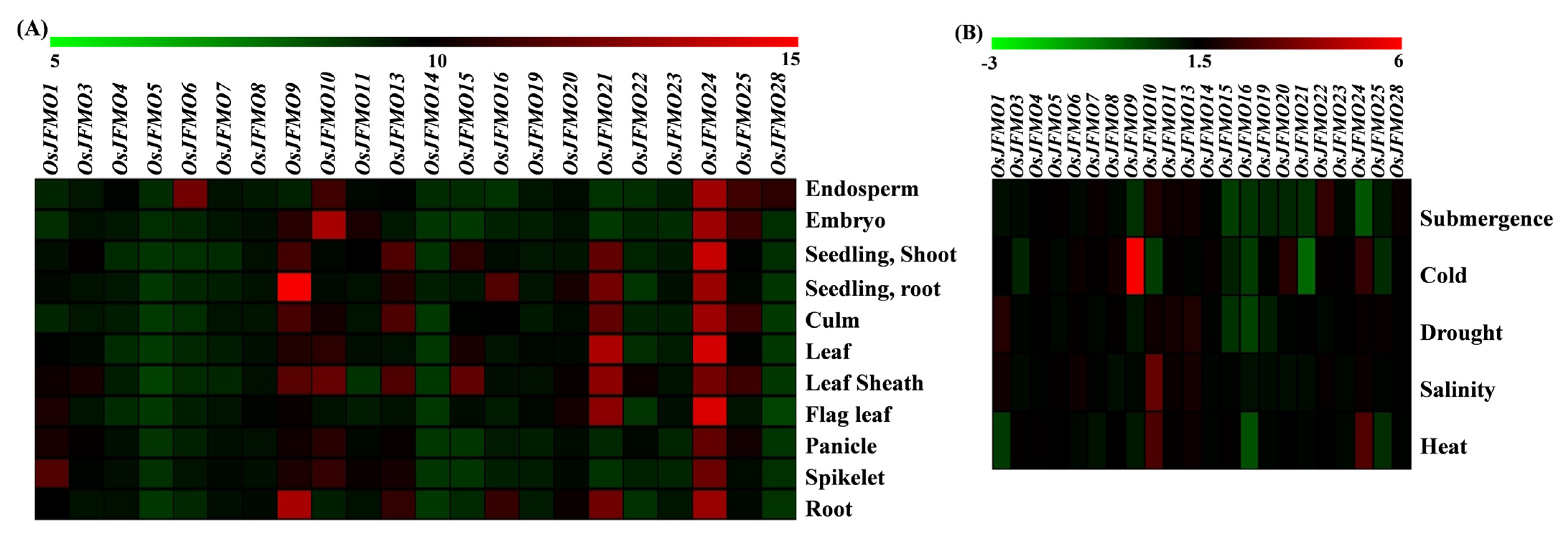
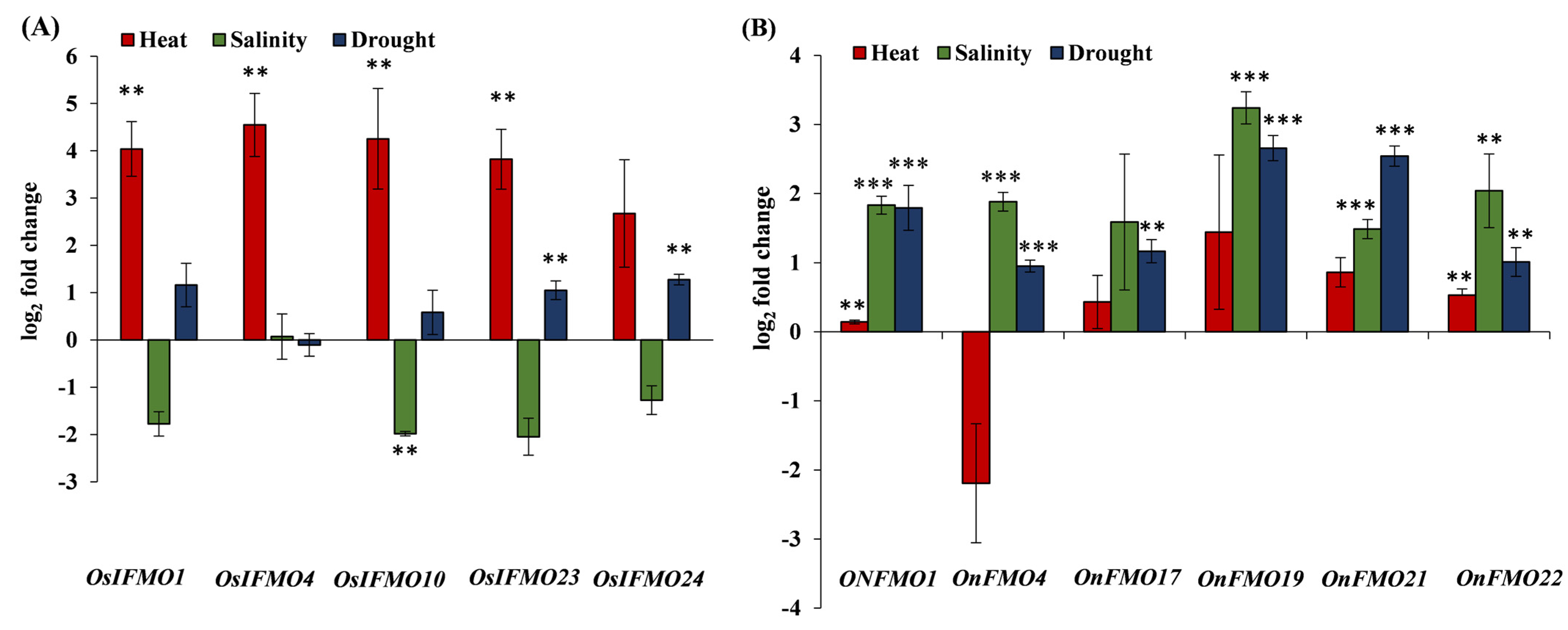
2.7. Developmental and Stress-Mediated Expression Profiling of FMO Genes Reveals Tissue Specificity and Perturbations in a Subset of Genes in Different Abiotic Stresses
3. Discussion
4. Materials and Methods
4.1. Identification of the FMO Family Members in Rice
4.2. Phylogenetic Analysis
4.3. Motif Analysis
4.4. Evaluation of Domain Architecture of the FMO Family Proteins
4.5. Evaluation of the Gene Structure
4.6. Developmental and Stress-Mediated Expression Profiling of the FMO Encoding Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pettit, F.H.; Ziegler, D.M. The catalytic demethylation of N, N-dimethylaniline-N-oxide by liver microsomes. Biochem. Biophys. Res. Commun. 1963, 13, 193–197. [Google Scholar] [CrossRef]
- Bickel, M.H. The pharmacology and biochemistry of N-oxides. Pharmacol. Rev. 1969, 21, 325–355. [Google Scholar] [PubMed]
- Jakoby, W.B.; Ziegler, D.M. The enzymes of detoxication. J. Biol. Chem. 1990, 265, 20715–20718. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.M.E.; Montersino, S.; Westphal, A.H.; Tischler, D.; Van-Berkel, W.J.H. Flavin dependent monooxygenases. Arch. Biochem. Biophys. 2014, 544, 2–17. [Google Scholar] [CrossRef]
- Mascotti, M.L.; Lapadula, W.J.; Ayub, M.J. The origin and evolution of Baeyer—Villiger Monooxygenases (BVMOs): An ancestral family of flavin monooxygenases. PLoS ONE 2015, 10, e0132689. [Google Scholar] [CrossRef]
- Nicoll, C.R.; Bailleul, G.; Fiorentini, F.; Mascotti, M.L.; Fraaije, M.W.; Mattevi, A. Ancestral-sequence reconstruction unveils the structural basis of function in mammalian FMOs. Nat. Struct. Mol. Biol. 2020, 1, 14–24. [Google Scholar] [CrossRef]
- Thodberg, S.; Jakobsen Neilson, E.H. The “Green” FMOs: Diversity, Functionality and Application of Plant Flavoproteins. Catalysts 2020, 10, 329. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wu, Y.F.; Wang, Z.P.; Qiu, J.G.; Li, X.L.; Cai, F.; Xiao, K.Q.; Sun, X.X.; Rosen, B.P.; et al. Oxidation of organoarsenicals and antimonite by a novel flavin monooxygenase widely present in soil bacteria. Environ. Microbiol. 2022, 24, 752–761. [Google Scholar] [CrossRef]
- Ziegler, D.M.; Pettit, F.H. Formation of an intermediate N oxide in the oxidative demethylation of N, N-dimethylaniline catalyzed by liver microsomes. Biochem. Biophys. Res. Commun. 1964, 15, 188–193. [Google Scholar] [CrossRef]
- Cashman, J.R.; Zhang, J. Human flavin-containing monooxygenases. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 65–100. [Google Scholar] [CrossRef]
- Mascotti, M.L.; Juri-Ayub, M.; Furnham, N.; Tornton, J.M.; Laskowski, R.A. Chopping and changing: The evolution of the flavin-dependent monooxygenases. J. Mol. Biol. 2016, 428, 3131–3146. [Google Scholar] [CrossRef] [PubMed]
- Rendić, S.P.; Crouch, R.D.; Guengerich, F.P. Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: Review and compilation of reactions. Arch. Toxicol. 2022, 96, 2145–2246. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, Q.; Wu, Y.; Chen, X.; Zhong, F. Properties and mechanisms of flavin-dependent monooxygenases and their applications in natural product synthesis. Int. J. Mol. Sci. 2022, 23, 2622. [Google Scholar] [CrossRef] [PubMed]
- Schlaich, N.L. Flavin-containing monooxygenases in plants: Looking beyond detox. Trends Plant Sci. 2007, 12, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.K.; Poulson, L.L.; Ziegler, D.M.; Robertus, J.D. Molecular cloning and kinetic characterization of a flavin-containing monooxygenase from Saccharomyces cerevisiae. Arch. Biochem. Biophys. 1996, 336, 268–274. [Google Scholar] [CrossRef]
- Lawton, M.P.; Cashman, J.R.; Cresteil, T.; Dolphin, C.T.; Elfarra, A.A.; Hines, R.N.; Hodgson, E.; Kimura, T.; Ozols, J.; Phillips, I.R. A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch. Biochem. Biophys. 1994, 308, 254–257. [Google Scholar] [CrossRef]
- Longin-Sauvageon, C.; Lattard, V.; Lilaz-Michel, C.; Buronfosse, T.; Benoit, E. Expression of two different FMOs in sheep liver. Drug Metab. Dispos. 1998, 26, 284–287. [Google Scholar]
- Veeravalli, S.; Phillips, I.R.; Freire, R.T.; Varshavi, D.; Everett, J.R.; Shephard, E.A. Flavin-containing monooxygenase 1 catalyzes the production of taurine from hypotaurine. Drug Metab. Dispos. 2020, 48, 378–385. [Google Scholar] [CrossRef]
- Uno, Y.; Shimizu, M.; Ogawa, Y.; Makiguchi, M.; Kawaguchi, H.; Yamato, O.; Ishizuka, M.; Yamazaki, H. Molecular and functional characterization of flavin-containing monooxygenases in pigs, dogs, and cats. Biochem. Pharmacol. 2022, 202, 115125. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Hansen, B.G.; Kliebenstein, D.J.; Halkier, B.A. Identification of a flavin-monooxygenase as the S-oxygenating enzyme in aliphatic glucosinolate biosynthesis in Arabidopsis. Plant J. 2007, 50, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.P.; Robson, P.R.H.; Smith, H.; Estelle, M.; Klee, H. Transgene-mediated auxin overproduction in Arabidopsis: Hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol. Biol. 1995, 27, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Marsch-Martinez, N.; Greco, R.; Arkel, G.V.; Herrera-Estrella, L.; Pereira, A. Activation tagging using the En-I maize transposon system in Arabidopsis. Plant Physiol. 2002, 129, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Mishina, T.E.; Zeier, J. The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol. 2006, 141, 1666–1675. [Google Scholar] [CrossRef]
- Bartsch, M.; Gobbato, E.; Bednarek, P.; Debey, S.; Schultze, J.L.; Bautor, J.; Parker, J.E. Salicylic acid-independent enhanced disease susceptibility1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the nudix hydrolase NUDT7. Plant Cell 2006, 18, 1038–1051. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 2007, 19, 2430–2439. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Woo, Y.M.; Park, H.J.; Su’udi, M.; Yangm, J.I.; Park, J.J.; Back, K.; Park, Y.M.; An, G. Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Mol. Biol. 2007, 65, 125–136. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef]
- Liu, H.; Ying, Y.Y.; Zhang, L.; Gao, Q.H.; Li, J.; Zhang, Z.; Fang, J.G.; Duan, K. Isolation and characterization of two YUCCA flavin monooxygenase genes from cultivated strawberry (Fragaria× ananassa Duch.). Plant Reprod. 2012, 31, 1425–1435. [Google Scholar] [CrossRef]
- Kim, J.I.; Baek, D.; Park, H.C.; Chun, H.J.; Oh, D.H.; Lee, M.K.; Cha, J.Y.; Kim, W.Y.; Kim, M.C.; Chung, W.S.; et al. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol. Plant. 2013, 6, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zaitoon, Y.M. Phylogenetic analysis of putative genes involved in the tryptophan-dependent pathway of auxin biosynthesis in rice. Appl. Biochem. Biotechnol. 2014, 172, 2480–2495. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yin, N.; Niu, Z.; Hui, W.; Song, J.; Huang, C.; Wang, H.; Kong, L.; Feng, D. Isolation and characterization of three TaYUC10 genes from wheat. Gene 2014, 546, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, Z.; Ji, C.Y.; Jeong, J.C.; Lee, H.S.; Li, H.; Li, H.; Xu, B.; Deng, X.; Kwak, S.S. Transgenic poplar expressing Arabidopsis YUCCA6 exhibits auxin-overproduction phenotypes and increased tolerance to abiotic stress. Plant Physiol. Biochem. 2015, 94, 19–27. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Zhang, X. Genome-wide analysis and expression patterns of the YUCCA genes in maize. J. Genet. Genom. 2015, 42, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Liu, H.H.; Wang, S.P.; Li, H.G. Genome-wide identification and expression analysis of the YUCCA gene family in soybean (Glycine max L.). J. Plant Growth Regul. 2017, 81, 265–275. [Google Scholar] [CrossRef]
- Yang, Y.L.; Li, N.; Hui, W.R.; Yuan, B.J.; Fan, P.; Liu, J.X.; Wang, H.G.; Feng, D.S. Seed-specific expression of TaYUC10 significantly increases auxin and protein content in wheat seeds. Plant Reprod. 2020, 10, 301–314. [Google Scholar] [CrossRef]
- Li, J.; Hansen, B.G.; Ober, J.A.; Kliebenstein, D.J.; Halkier, B.A. Subclade of flavin-monooxygenases involved in aliphatic glucosinolate biosynthesis. Plant Physiol. 2008, 148, 1721–1733. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Onuma, M.; Mizuno, S.; Sugino, Y.; Nakabayashi, R.; Imai, S.; Tsuneyoshi, T.; Sumi, S.; Saito, K. Identification of a flavin-containing S-oxygenating monooxygenase involved in alliin biosynthesis in garlic. Plant J. 2015, 83, 941–951. [Google Scholar] [CrossRef]
- Kong, W.; Li, J.; Yu, Q.; Cang, W.; Xu, R.; Wang, Y.; Ji, W. Two novel flavin-containing monooxygenases involved in biosynthesis of aliphatic glucosinolates. Front. Plant Sci. 2016, 7, 1292. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Zeier, T.; Bernsdorff, F.; Reichel-Deland, V.; Kim, D.; Hohmann, M.; Scholten, N.; Schuck, S.; Bräutigam, A.; Hölzel, T.; et al. Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 2018, 173, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Saito, K. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. J. Exp. Bot. 2019, 70, 4123–4137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Sawada, Y.; Boerzhijin, S.; Kuwahara, A.; Sato, M.; Hirai, M.Y. Abscisic acid-mediated induction of FLAVIN-CONTAINING MONOOXYGENASE 2 leads to reduced accumulation of methylthioalkyl glucosinolates in Arabidopsis thaliana. Plant Sci. 2021, 303, 110764. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Kim, W.Y.; Kang, S.B.; Kim, J.I.; Baek, D.; Jung, I.J.; Kim, M.R.; Li, N.; Kim, H.J.; Nakajima, M.; et al. A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nat. Commun. 2015, 6, 8041. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Kim, M.R.; Jung, I.J.; Kang, S.B.; Park, H.J.; Kim, M.G.; Yun, D.J.; Kim, W.Y. The thiol reductase activity of YUCCA6 mediates delayed leaf senescence by regulating genes involved in auxin redistribution. Front. Plant Sci. 2016, 7, 626. [Google Scholar] [CrossRef]
- Bremer, G. Problems in breeding and cytology of sugar cane. Euphytica 1961, 10, 59–78. [Google Scholar] [CrossRef]
- Roach, B.T. “Nobilisation of sugarcane”. Proc. Int. Soc. Sugar Cane Technol. 1972, 14, 206–216. [Google Scholar]
- Santchurn, D.; Badaloo, M.G.H.; Zhou, M.; Labuschagne, M.T. Contribution of sugarcane crop wild relatives in the creation of improved varieties in Mauritius. Plant Genet. Resour. 2019, 17, 151–163. [Google Scholar] [CrossRef]
- Pradheep, K.; Ahlawat, S.P.; Nivedhitha, S.; Gupta, V. Crop wild relatives in India: Inventorization, prioritization and conservation. Indian J. Plant Genet. Res. 2022, 35, 124–130. [Google Scholar] [CrossRef]
- Brown, W.L. Global Crop Resources: Gene Banks and the World’s Food. Science 1987, 236, 617–618. [Google Scholar] [CrossRef] [PubMed]
- Fornasiero, A.; Wing, R.A.; Ronald, P. Rice domestication. Curr. Biol. 2022, 32, R20–R24. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Gutaker, R.M.; Chater, C.C.; Brinton, J.; Castillo-Lorenzo, E.; Breman, E.; Pironon, S. Scaling up neodomestication for climate-ready crops. Curr. Opin. Plant Biol. 2022, 66, 102169. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Agarwal, P.; Parida, S.K.; Raghuvanshi, S.; Kapoor, S.; Khurana, P.; Khurana, J.P.; Tyagi, A.K. Rice improvement through genome-based functional analysis and molecular breeding in India. Rice 2016, 9, 1. [Google Scholar] [CrossRef]
- Nair, K.P. Utilizing crop wild relatives to combat global warming. Adv. Agron. 2019, 153, 175–258. [Google Scholar]
- Coyne, C.J.; Kumar, S.; Wettberg, E.J.; Marques, E.; Berger, J.D.; Redden, R.J.; Noel Ellis, T.H.; Brus, J.; Zablatzká, L.; Smýkal, P. Potential and limits of exploitation of crop wild relatives for pea, lentil, and chickpea improvement. Legume Sci. 2020, 2, e36. [Google Scholar] [CrossRef]
- Das, S. Contributions of crop-wild relatives toward broadening the list of leafy vegetables. Int. J. Veg. Sci. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Wambugu, P.W.; Henry, R. Supporting in situ conservation of the genetic diversity of crop wild relatives using genomic technologies. Mol. Ecol. 2022, 31, 2207–2222. [Google Scholar] [CrossRef]
- Renzi, J.P.; Coyne, C.J.; Berger, J.; Wettberg, E.; Nelson, M.; Ureta, S.; Hernández, F.; Smýkal, P.; Brus, J. How Could the Use of Crop Wild Relatives in Breeding Increase the Adaptation of Crops to Marginal Environments? Front. Plant Sci. 2022, 13, 886162. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.; Qi, Y.; Peres, L.E.; Fernie, A.R.; Zsögön, A. Pathways to de novo domestication of crop wild relatives. Plant Physiol. 2022, 188, 1746–1756. [Google Scholar] [CrossRef]
- Stein, J.C.; Yu, Y.; Copetti, D.; Zwickl, D.J.; Zhang, L.; Zhang, C.; Chougule, K.; Gao, D.; Iwata, A.; Goicoechea, J.L.; et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover, and innovation across the genus Oryza. Nat. Genet. 2018, 50, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.; Subba, A.; Bala, M.; Singh, A.K.; Pareek, A.; Singla-Pareek, S.L. Genetic conservation of CBS domain containing protein family in Oryza Species and their association with abiotic stress responses. Int. J. Mol. Sci. 2022, 23, 1687. [Google Scholar] [CrossRef] [PubMed]
- Gauchan, D.; Timsina, K.P.; Gairhe, S.; Timsina, J.; Joshi, K.D. Cereal demand and production projections for 2050: Opportunities for achieving food self-sufficiency in Nepal. In Agriculture, Natural Resources and Food Security; Springer: Cham, Switzerland, 2022; pp. 19–35. [Google Scholar]
- Samal, P.; Babu, S.C.; Mondal, B.; Mishra, S.N. The global rice agriculture towards 2050: An inter-continental perspective. Outlook Agric. 2022, 51, 164–172. [Google Scholar] [CrossRef]
- Satori, D.; Tovar, C.; Faruk, A.; Hammond Hunt, E.; Muller, G.; Cockel, C.; Kühn, N.; Leitch, I.J.; Lulekal, E.; Pereira, L.; et al. Prioritising crop wild relatives to enhance agricultural resilience in sub-Saharan Africa under climate change. Plants People Planet 2022, 4, 269–282. [Google Scholar] [CrossRef]
- Kashyap, A.; Garg, P.; Tanwar, K.; Sharma, J.; Gupta, N.C.; Ha, P.T.T.; Bhattacharya, R.C.; Mason, A.S.; Rao, M. Strategies for utilization of crop wild relatives in plant breeding programs. Theor. Appl. Genet. 2022, 135, 4151–4167. [Google Scholar] [CrossRef]
- Kushwaha, H.R.; Singh, A.K.; Sopory, S.K.; Singla-Pareek, S.L.; Pareek, A. Genome-wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) and Oryza sativa (L.) reveals their developmental and stress regulation. BMC Genom. 2009, 10, 200. [Google Scholar] [CrossRef]
- Mustafiz, A.; Singh, A.K.; Pareek, A.; Sopory, S.K.; Singla-Pareek, S.L. Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct. Integr. Genom. 2011, 11, 293–305. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, R.; Tripathi, A.K.; Gupta, B.K.; Pareek, A.; Singla-Pareek, S.L. Genome-wide investigation and expression analysis of Sodium/Calcium exchanger gene family in rice and Arabidopsis. Rice 2015, 8, 54. [Google Scholar] [CrossRef]
- Kushwaha, H.R.; Joshi, R.; Pareek, A.; Singla-Pareek, S.L. MATH-domain family shows response toward abiotic stress in Arabidopsis and Rice. Front. Plant Sci. 2016, 7, 923. [Google Scholar] [CrossRef] [PubMed]
- Bhowal, B.; Bhattacharjee, A.; Goswami, K.; Sanan-Mishra, N.; Singla-Pareek, S.L.; Kaur, C.; Sopory, S. Serotonin and melatonin biosynthesis in plants: Genome-wide Identification of the genes and their expression reveal a conserved role in stress and development. Int. J. Mol. Sci. 2021, 22, 11034. [Google Scholar] [CrossRef] [PubMed]
- Tello-Ruiz, M.K.; Jaiswal, P.; Ware, D. Gramene: A Resource for comparative analysis of plants genomes and pathways. Methods Mol. Biol. 2022, 2443, 101–131. [Google Scholar] [PubMed]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 1–4. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Eswaramoorthy, S.; Bonanno, J.B.; Burley, S.K.; Swaminathan, S. Mechanism of action of a flavin containing monooxygenase. Proc. Natl. Acad. Sci. USA 2006, 103, 9832–9837. [Google Scholar] [CrossRef]
- Stehr, M.; Diekmann, H.; Smau, L.; Seth, O.; Ghisla, S.; Singh, M.; Macheroux, P. A hydrophobic sequence motif common to N-hydroxylating enzymes. Trends Biochem. Sci. 1998, 23, 56–57. [Google Scholar] [CrossRef]
- Bo, H.; Jinpu, J.; An-Yuan, G.; He, Z.; Jingchu, L.; Ge, G. GSDS 2.0: An upgraded gene features visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar]
- Gough, J.; Karplus, K.; Hughey, R.; Chothia, C. Assignment of homology to genome sequences using a library of Hidden Markov Models that represent all proteins of known structure. J. Mol. Biol. 2001, 313, 903–919. [Google Scholar] [CrossRef]
- Guo, Y.L. Gene family evolution in green plants with emphasis on the origination and evolution of Arabidopsis thaliana genes. Plant J. 2013, 73, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Birchler, J.A.; Yang, H. The multiple fates of gene duplications: Deletion, hypofunctionalization, subfunctionalization, neofunctionalization, dosage balance constraints, and neutral variation. Plant Cell 2022, 34, 2466–2474. [Google Scholar] [CrossRef]
- Hines, R.N.; Hopp, K.A.; Franco, J.; Saeian, K.; Begun, F.P. Alternative processing of the human FMO6 gene renders transcripts incapable of encoding a functional flavin-containing monooxygenase. Mol. Pharmacol. 2002, 62, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, E.S.; Ayala, F.J. Pseudogenes: Are they “junk” or functional DNA? Annu. Rev. Genet. 2003, 37, 123–151. [Google Scholar] [CrossRef] [PubMed]
- Lees-Murdock, D.J.; McLoughlin, G.A.; McDaid, J.R.; Quinn, L.M.; O’Doherty, A.; Hiripi, L.; Hack, C.J.; Walsh, C.P. Identification of 11 pseudogenes in the DNA methyltransferase gene family in rodents and humans and implications for the functional loci. Genomics 2004, 84, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Dhar, Y.V.; Srivastava, D.; Kidwai, M.; Chauhan, P.S.; Bag, S.K.; Asif, M.H.; Chakrabarty, D. Genome-wide analysis of rice dehydrin gene family: Its evolutionary conservedness and expression pattern in response to PEG induced dehydration stress. PLoS ONE 2017, 12, e0176399. [Google Scholar] [CrossRef]
- Chowrasia, S.; Panda, A.K.; Rawal, H.C.; Kaur, H.; Mondal, T.K. Identification of jumonjiC domain-containing gene family among the Oryza species and their expression analysis in FL478, a salt tolerant rice genotype. Plant Physiol. Biochem. 2018, 1, 43–53. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, T.; Wang, H.; Feng, D. Genome-wide identification and expression analysis of the TaYUCCA gene family in wheat. Mol. Biol. Rep. 2021, 48, 1269–1279. [Google Scholar] [CrossRef]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.; Li, W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 122–1234. [Google Scholar] [CrossRef]
- Freeling, M. Bias in plant gene content following different sorts of duplication: Tandem, whole-genome, segmental, or by transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Ann. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, L.; Wang, D.; Zhang, D.; Cai, W. Genome-wide Identification and Bioinformatics Analysis of NRL Gene Family in Rice. Mol. Plant Breed. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Guan, J.; Zhang, J.; Gong, D.; Zhang, Z.; Yu, Y.; Luo, G.; Somta, P.; Hu, Z.; Wang, S.; Yuan, X.; et al. Genomic analyses of rice bean landraces reveal adaptation and yield related loci to accelerate breeding. Nat. Commun. 2022, 13, 5707. [Google Scholar] [CrossRef]
- Barrett, L.W.; Fletcher, S.; Wilton, S.D. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell. Mol. Life Sci. 2012, 69, 3613–3634. [Google Scholar] [CrossRef]
- Ren, X.Y.; Vorst, O.; Fiers, M.W.; Stiekema, W.J.; Nap, J.P. In plants, highly expressed genes are the least compact. Trends Genet. 2006, 22, 528–532. [Google Scholar] [CrossRef]
- Chung, B.Y.; Simons, C.; Firth, A.E.; Brown, C.M.; Hellens, R.P. Effect of 5’UTR introns on gene expression in Arabidopsis thaliana. BMC Genom. 2006, 7, 120. [Google Scholar] [CrossRef]
- Bicknell, A.A.; Cenik, C.; Chua, H.N.; Roth, F.P.; Moore, M.J. Introns in UTRs: Why we should stop ignoring them. BioEssays 2012, 34, 1025–1034. [Google Scholar] [CrossRef]
- Kertész, S.; Kerényi, Z.; Mérai, Z.; Bartos, I.; Pálfy, T.; Barta, E.; Silhavy, D. Both introns and long 3’-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 2006, 34, 6147–6157. [Google Scholar] [CrossRef]
- Soukup, S.W. Evolution by gene duplication. S. Ohno. Springer-Verlag, New York. 160 pp. Teratology 1970, 9, 250–251. [Google Scholar] [CrossRef]
- Hidenori, T.; Tohru, K. Evolution of multigene families by gene duplication: A haploid model. Genetics 1998, 149, 2147–2158. [Google Scholar]
- Kolkman, J.A.; Stemmer, W.P. Directed evolution of proteins by exon shuffling. Nat. Biotechnol. 2001, 194, 23–28. [Google Scholar] [CrossRef]
- França, G.S.; Cancherini, D.V.; De Souza, S.J. Evolutionary history of exon shuffling. Genetica 2012, 140, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Kim, K.M.; Caetano-Anollés, G. Global patterns of protein domain gain and loss in superkingdoms. PLoS Comput. Biol. 2014, 30, e1003452. [Google Scholar] [CrossRef] [PubMed]
- Yafremava, L.S.; Wielgos, M.; Thomas, S.; Nasir, A.; Wang, M.; Mittenthal, J.E.; Caetano-Anollés, G. A general framework of persistence strategies for biological systems helps explain domains of life. Front. Genet. 2013, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Rairdan, G.J.; Collier, S.M.; Sacco, M.A.; Baldwin, T.T.; Boettrich, T.; Moffett, P. The coiled-coil and nucleotide binding domains of the potato Rx disease resistance protein function in pathogen recognition and signaling. Plant Cell 2008, 20, 739–751. [Google Scholar] [CrossRef]
- Dalio, R.J.; Paschoal, D.; Arena, G.D.; Magalhaes, D.M.; Oliveira, T.S.; Merfa, M.V.; Maximo, H.J.; Machado, M.A. Hypersensitive response: From NLR pathogen recognition to cell death response. Ann. Appl. Biol. 2021, 178, 268–280. [Google Scholar] [CrossRef]
- Förderer, A.; Yu, D.; Li, E.; Chai, J. Resistosomes at the interface of pathogens and plants. Curr. Opin. Plant Biol. 2022, 1, 102212. [Google Scholar] [CrossRef]
- Parker, J.E.; Hessler, G.; Cui, H. A new biochemistry connecting pathogen detection to induced defense in plants. New Phytol. 2022, 234, 819–826. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Tang, B.; Wu, T.; Chen, G.; Xie, Q.; Hu, Z. Silencing of SlMYB55 affects plant flowering and enhances tolerance to drought and salt stress in tomato. Plant Sci. 2022, 316, 111166. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, P.; Zhang, X.; Xie, Q.; Chen, G.; Zhou, S.; Hu, Z. Silencing of SlMYB50 affects tolerance to drought and salt stress in tomato. Plant Physiol. Biochem. 2022, 193, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]



| (A) Chromosome | O. brachyantha | O. punctata | O. meridionalis | O. glumaepatula | O. glaberrima |
| 1 | ObrFMO1 (OB01G18060) | OpFMO1 (OPUNC01G07650) | OmFMO1(OMERI01G07820) | OgFMO1 (OGLUM01G08960) | OglFMO1 (ORGLA01G0069900) |
| ObrFMO2 (OB01G21130) | OpFMO2 (OPUNC01G10840) | OmFMO2 (OMERI01G13920) | OgFMO2 (OGLUM01G12480) | OglFMO2 (ORGLA01G0126800) | |
| ObrFMO3 (OB01G33700) | OpFMO3 (OPUNC01G16290) | OmFMO3 (OMERI01G14340) | OgFMO3 (OGLUM01G17290) | OglFMO3 (ORGLA01G0197300) | |
| ObrFMO4 (OB01G38810) | OpFMO4 (OPUNC01G24520) | OmFMO4 (OMERI01G24550) | OgFMO4 (OGLUM01G17860) | OglFMO4 (ORGLA01G0245000) | |
| OpFMO5 (OPUNC01G29760) | OmFMO5 (OMERI01G27380) | OgFMO5 (OGLUM01G28330) | |||
| OgFMO6 (OGLUM01G33960) | |||||
| 2 | ObrFMO5 (OB02G29400) | OpFMO6 (OPUNC02G19680) | OmFMO6 (OMERI02G00100) | OgFMO7 (OGLUM02G22140) | OglFMO5 (ORGLA02G0106500) |
| OmFMO7 (OMERI02G12510) | OglFMO6 (ORGLA02G0187600) | ||||
| OmFMO8 (OMERI02G12660) | |||||
| 3 | ObrFMO6 (OB03G14710) | OpFMO7 (OPUNC03G04320) | OmFMO9 (OMERI03G04440) | OgFMO8 (OGLUM03G04490) | OglFMO7 (ORGLA03G0044100) |
| ObrFMO7 (OB03G16080) | OpFMO8 (OPUNC03G05820) | OmFMO10 (OMERI03G05840) | OgFMO9 (OGLUM03G06010) | OglFMO8 (ORGLA03G0058000) | |
| 4 | ObrFMO8 (OB04G11140) | OpFMO9 (OPUNC04G01360) | OmFMO11 (OMERI04G01170) | OgFMO10 (OGLUM04G03890) | OglFMO9 (ORGLA04G0010900 |
| ObrFMO9 (OB04G13610) | OpFMO10 (OPUNC04G04050) | OmFMO12 (OMERI04G04660) | OgFMO11 (OGLUM04G03900) | OglFMO10 (ORGLA04G0035200) | |
| OmFMO13 (OMERI04G04670) | OgFMO12 (OGLUM04G03920) | OglFMO11 (ORGLA04G0035300) | |||
| OmFMO14 (OMERI04G04680) | |||||
| 5 | OmFMO15 (OMERI05G21010) | OgFMO13 (OGLUM05G24890) | |||
| OmFMO16 (OMERI05G09400) | |||||
| 6 | ObrFMO10 (OB06G16030) | OpFMO11 (OPUNC06G06240) | OmFMO17 (OMERI06G03520) | OgFMO14 (OGLUM06G06900) | OglFMO12 (ORGLA06G0061000) |
| OpFMO12 (OPUNC06G15440) | OmFMO18 (OMERI06G03530) | OgFMO15 (OGLUM06G06910) | OglFMO13 (ORGLA06G0060800) | ||
| OmFMO19 (OMERI06G03580) | OgFMO16 (OGLUM06G14980) | OglFMO14 (ORGLA06G0141900) | |||
| OmFMO20 (OMERI06G07480) | OgFMO17 (OGLUM06G18150) | ||||
| OmFMO21 (OMERI06G07660) | |||||
| 7 | ObrFMO11 (OB07G10720) | OpFMO13 (OPUNC07G00810) | OmFMO22 (OMERI07G09890) | OgFMO18 (OGLUM07G00790) | OglFMO15 (ORGLA07G0007100) |
| ObrFMO12 (OB07G10730) | OpFMO14 (OPUNC07G00820) | OgFMO19 (OGLUM07G00830) | OglFMO16 (ORGLA07G0007200) | ||
| ObrFMO13 (OB07G10740) | OpFMO15 (OPUNC07G00840) | OgFMO20 (OGLUM07G11860) | OglFMO17 (ORGLA07G0104200) | ||
| ObrFMO14 (OB07G10800) | OpFMO16 (OPUNC07G11050) | ||||
| ObrFMO15 (OB07G18620) | |||||
| 8 | ObrFMO16 (OB08G20080) | ||||
| 9 | ObrFMO17 (OB09G25280) | OpFMO17 (OPUNC09G17070) | OgFMO21 (OGLUM09G00600) | OglFMO18 (ORGLA09G0148600) | |
| ObrFMO18 (OB09G25420) | OgFMO22 (OGLUM09G19160) | OglFMO19 (ORGLA09G0148800) | |||
| ObrFMO19 (OB09G25300) | OgFMO23 (OGLUM09G19180) | OglFMO20 (ORGLA09G0149000) | |||
| OgFMO24 (OGLUM09G19210) | |||||
| 10 | ObrFMO20 (OB10G25150) | OpFMO18 (OPUNC10G16400) | OmFMO23 (OMERI10G14540) | OgFMO25 (OGLUM10G04710) | OglFMO21 (ORGLA10G0131800) |
| ObrFMO21 (OB10G25220) | OpFMO19 (OPUNC10G16470) | OmFMO24 (OMERI10G14600) | OgFMO26 (OGLUM10G18140) | OglFMO22 (ORGLA10G0132300) | |
| OgFMO27 (OGLUM10G18190) | |||||
| 11 | ObrFMO22 (OB11G13490) | OpFMO20 (OPUNC11G06230) | OmFMO25 (OMERI11G05170) | OgFMO28 (OGLUM11G05950) | OglFMO23 (ORGLA11G0056800) |
| ObrFMO23 (OB11G14940) | OpFMO21 (OPUNC11G14790) | OmFMO26 (OMERI11G08970) | OgFMO29 (OGLUM11G06000) | ||
| 12 | ObrFMO24 (OB12G14990) | OpFMO22 (OPUNC12G05050) | OmFMO27 (OMERI12G03610) | OgFMO30 (OGLUM12G06000) | OglFMO24 (ORGLA12G0049500) |
| ObrFMO25 (OB12G21170) | |||||
| (B) Chromosome | O. barthii | O. nivara | O. indica | O. rufipogon | O. japonica |
| 1 | ObaFMO1 (OBART01G07810) | OnFMO1 (ONIVA01G10070) | OsIFMO1 (BGIOSGA001676) | OrFMO1 (ORUFI01G08580) | OsJFMO1 (Os01g0224700) |
| ObaFMO2 (OBART01G15500) | OnFMO2 (ONIVA01G17610) | OsIFMO2 (BGIOSGA003035) | OrFMO2 (ORUFI01G17290) | OsJFMO2 (Os01g0368000) | |
| ObaFMO3 (OBART01G24440) | OnFMO3 (ONIVA01G27650) | OsIFMO3 (BGIOSGA003523) | OrFMO3 (ORUFI01G27360) | OsJFMO3 (Os01g0645400) | |
| ObaFMO4 (OBART01G29910) | OnFMO4 (ONIVA01G34190) | OsIFMO4 (BGIOSGA004101) | OrFMO4 (ORUFI01G33000) | OsJFMO4 (Os01g0732700) | |
| OsIFMO5 (BGIOSGA004401) | |||||
| OsIFMO6 (BGIOSGA007947) | |||||
| 2 | ObaFMO5 (OBART02G11820) | OnFMO5 (ONIVA02G24200) | OsIFMO7 (BGIOSGA008498) | OrFMO5 (ORUFI02G11880) | OsJFMO5 (Os02g0272200) |
| ObaFMO6 (OBART02G21940) | OrFMO6 (ORUFI02G23020) | OsJFMO6 (Os02g0580600) | |||
| 3 | ObaFMO7 (OBART03G04640) | OnFMO6 (ONIVA03G04410) | OsIFMO8 (BGIOSGA011917) | OrFMO7 (ORUFI03G04340) | OsJFMO7 (Os03g0162000) |
| ObaFMO8 (OBART03G06010) | OnFMO7 (ONIVA03G06000) | OsIFMO9 (BGIOSGA011989) | OrFMO8 (ORUFI03G05830) | OsJFMO8 (Os03g0182000) | |
| 4 | ObaFMO9 (OBART04G01320) | OnFMO8 (ONIVA04G01060) | OsIFMO10 (BGIOSGA015543) | OrFMO9 (ORUFI04G01570) | OsJFMO9 (Os04g0128900) |
| ObaFMO10 (OBART04G04730) | OsIFMO11 (BGIOSGA015544) | OrFMO10 (ORUFI04G05470) | OsJFMO10 (Os04g0223500) | ||
| ObaFMO11 (OBART04G04740) | OsIFMO12 (BGIOSGA015545) | OsJFMO11 (Os04g0223901) | |||
| OsIFMO13 (BGIOSGA015673) | |||||
| 5 | ObaFMO12 (OBART05G23490) | OsIFMO14 (BGIOSGA017689) | OrFMO11 (ORUFI05G24980) | OsJFMO12 (Os05g0528600) | |
| 6 | ObaFMO13 (OBART06G06490) | OnFMO9 (ONIVA06G07560) | OsIFMO15 (BGIOSGA021117) | OrFMO12 (ORUFI06G06790) | OsJFMO13 (Os06g0203200) |
| ObaFMO14 (OBART06G14000) | OnFMO10 (ONIVA06G07670) | OsIFMO16 (BGIOSGA021291) | OrFMO13 (ORUFI06G14920) | OsJFMO14 (Os06g0203400) | |
| ObaFMO15 (OBART06G17060) | OnFMO11 (ONIVA06G07680) | OsIFMO17 (BGIOSGA021706) | OrFMO14 (ORUFI06G18060) | OsJFMO15 (Os06g0528700) | |
| OnFMO12 (ONIVA06G20350) | OsIFMO18 (BGIOSGA021707) | ||||
| 7 | ObaFMO16 (OBART07G00890) | OnFMO13 (ONIVA07G10070) | OsIFMO19 (BGIOSGA024391) | OrFMO15 (ORUFI07G00810) | OsJFMO16 (Os07g0111700) |
| OsIFMO20 (BGIOSGA025032) | OrFMO16 (ORUFI07G00820) | OsJFMO17 (Os07g0111900) | |||
| OsIFMO21 (BGIOSGA025033) | OrFMO17 (ORUFI07G12500) | OsJFMO18 (Os07g0112000) | |||
| OsIFMO22 (BGIOSGA025034) | OsJFMO19 (Os07g0112100) | ||||
| OsJFMO20 (Os07g0437000) | |||||
| 8 | OnFMO14 (ONIVA08G22170) | ||||
| 9 | ObaFMO17 (OBART09G18660) | OnFMO15 (ONIVA09G19750) | OsIFMO23 (BGIOSGA029321) | OrFMO18 (ORUFI09G20190) | OsJFMO21 (Os09g0548400) |
| ObaFMO18 (OBART09G18680) | OnFMO16 (ONIVA09G19770) | OsIFMO24 (BGIOSGA029325) | OrFMO19 (ORUFI09G20260) | OsJFMO22 (Os09g0548700) | |
| ObaFMO19 (OBART09G18710) | OnFMO17 (ONIVA09G19830) | OsIFMO25 (BGIOSGA029326) | OsJFMO23 (Os09g0549300) | ||
| OnFMO18 (ONIVA10G13930) | |||||
| 10 | ObaFMO20 (OBART10G18040) | OnFMO19 (ONIVA10G14730) | OsIFMO26 (BGIOSGA031436) | OrFMO20 (ORUFI10G19230) | OsJFMO24 (Os10g0553800) |
| ObaFMO21 (OBART10G18090) | OnFMO20 (ONIVA10G14740) | OsIFMO27 (BGIOSGA031433) | OrFMO21 (ORUFI10G19280) | OsJFMO25 (Os10g0554300) | |
| OnFMO21 (ONIVA10G20590) | |||||
| OnFMO22 (ONIVA10G20640) | |||||
| 11 | ObaFMO22 (OBART11G06190) | OnFMO23 (ONIVA11G06260) | OsIFMO28 (BGIOSGA034995) | OrFMO22 (ORUFI11G06350) | OsJFMO26 (Os11g0207700) |
| ObaFMO23 (OBART11G06210) | OrFMO23 (ORUFI11G06380) | OsJFMO27 (Os11g0207900) | |||
| 12 | ObaFMO24 (OBART12G05080) | OnFMO24 (ONIVA12G04710) | OsIFMO29 (BGIOSGA036502) | OrFMO24 (ORUFI12G05850) | OsJFMO28 (Os12g0189500) |
| ObaFMO25 (OBART12G13520) |
| Chromosome | O. brachyantha | O. punctata | O. meridionalis | O. glumaepatula | O. glaberrima | O. barthii | O. nivara | O. indica | O. rufipogon | O. japonica |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ObrFMO1 | OpFMO1 | OmFMO1 | OgFMO1 | OglFMO1 | ObaFMO1 | OnFMO1 | OsIFMO2 | OrFMO1 | OsJFMO1 |
| ObrFMO2 | OpFMO2 | OgFMO2 | ||||||||
| OpFMO3 | OmFMO3 | OgFMO4 | OglFMO2 | ObaFMO2 | OsIFMO3 | OrFMO2 | OsJFMO2 | |||
| ObrFMO3 | OpFMO4 | OmFMO4 | OgFMO5 | OglFMO3 | ObaFMO3 | OnFMO3 | OsIFMO4 | OrFMO3 | OsJFMO3 | |
| ObrFMO4 | OpFMO5 | OmFMO5 | OgFMO6 | OglFMO4 | ObaFMO4 | OnFMO4 | OsIFMO5 | OrFMO4 | OsJFMO4 | |
| 2 | OmFMO8 | OglFMO5 | ObaFMO5 | OsIFMO6 | OrFMO5 | OsJFMO5 | ||||
| ObrFMO5 | OpFMO6 | OmFMO6, OmFMO16 * | OgFMO7 | OglFMO6 | ObaFMO6 | OnFMO5 | OsIFMO7 | OrFMO6 | OsJFMO6 | |
| 3 | ObrFMO6 | OpFMO7 | OmFMO9 | OgFMO8 | OglFMO7 | ObaFMO7 | OnFMO6 | OsIFMO8 | OrFMO7 | OsJFMO7 |
| ObrFMO7 | OpFMO8 | OmFMO10 | OgFMO9 | OglFMO8 | ObaFMO8 | OnFMO7 | OsIFMO9 | OrFMO8 | OsJFMO8 | |
| 4 | ObrFMO8 | OpFMO9 | OmFMO11, OmFMO2 * | OgFMO21 *, OgFMO3 | OglFMO9 | ObaFMO9 | OnFMO8, OnFMO2 * | OsIFMO1, OsIFMO13 | OrFMO9 | OsJFMO9 |
| ObrFMO9 | OpFMO10 | OmFMO13 | OgFMO11 | OglFMO10 | ObaFMO10 | OnFMO19 * | OsIFMO11, OsIFMO12 | OsJFMO10 | ||
| OmFMO12 OmFMO14 | OgFMO10, OgFMO12 | OglFMO11 | ObaFMO11 | OnFMO20 * | OsIFMO10 | OrFMO10 | OsJFMO11 | |||
| OmFMO15 | OgFMO13 | ObaFMO12 | OnFMO14 * | OsIFMO14 | OrFMO11 | OsJFMO12 | ||||
| 5 | ObrFMO10 | OmFMO20, OmFMO21 | OgFMO14 | OglFMO12 | ObaFMO13 | OnFMO9 | OsIFMO18 | OsJFMO13 | ||
| 6 | OnFMO10 | |||||||||
| OpFMO11 | OgFMO15 | OglFMO13 | OnFMO11 | OsIFMO17 | OrFMO12 | OsJFMO14 | ||||
| OpFMO12 | OgFMO17 | OglFMO14 | ObaFMO15 | OnFMO12 | OsIFMO15 | OrFMO14 | OsJFMO15 | |||
| OmFMO26 * | OgFMO25, OgFMO16 * | ObaFMO14 | OsIFMO16, OsIFMO22 | OrFMO13 | OsJFMO18 * | |||||
| 7 | ObrFMO11, ObrFMO12, ObrFMO13 | OpFMO13 | OgFMO18 | ObaFMO16 | OnFMO18 * | OsIFMO20 | OrFMO15 | OsJFMO16, OsJFMO17 | ||
| ObrFMO14, ObrFMO22, ObrFMO16 * | OpFMO14, OpFMO15 | OmFMO7 * | OgFMO19 | OglFMO15, OglFMO16 | OsIFMO21 | OrFMO16 | OsJFMO19 | |||
| ObrFMO15 | OpFMO16 | OmFMO22 | OgFMO20 | OglFMO17 | OnFMO13 | OsIFMO19 | OrFMO17 | OsJFMO20 | ||
| 9 | ObrFMO17 | OpFMO17 | OmFMO17 * | OgFMO22 | OglFMO18 | ObaFMO17 | OnFMO15 | OsIFMO25 | OrFMO18 | OsJFMO21 |
| ObrFMO19 | OmFMO18 * | OgFMO23 | OglFMO19 | ObaFMO18 | OnFMO16 | OsIFMO24 | - | OsJFMO22 | ||
| ObrFMO18 | OmFMO19 * | OgFMO24 | OglFMO20 | ObaFMO19 | OnFMO17 | OsIFMO23 | OrFMO19 | OsJFMO23 | ||
| 10 | ObrFMO20 | OpFMO18 | OmFMO23 | OgFMO26 | OglFMO21 | ObaFMO20 | OnFMO21 | OsIFMO26 | OrFMO20 | OsJFMO24 |
| ObrFMO21 | OpFMO19 | OmFMO24 | OgFMO27 | OglFMO22 | ObaFMO21 | OnFMO22 | OsIFMO27 | OrFMO21 | OsJFMO25 | |
| 11 | OpFMO20 | OmFMO25 | OgFMO28 | OglFMO23 | ObaFMO22 | OrFMO22, OrFMO23 | OsJFMO26 | |||
| ObrFMO23 | OpFMO21 | OgFMO29 | ObaFMO23 | OnFMO23 | OsIFMO28 | OsJFMO27 | ||||
| 12 | ObrFMO24 | OpFMO22 | OmFMO27 | OgFMO30 | OglFMO24 | ObaFMO24 | OnFMO24 | OsIFMO29 | OrFMO24 | OsJFMO28 |
| ObrFMO25 | ObaFMO25 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaba, Y.; Bhowal, B.; Pareek, A.; Singla-Pareek, S.L. Genomic Survey of Flavin Monooxygenases in Wild and Cultivated Rice Provides Insight into Evolution and Functional Diversities. Int. J. Mol. Sci. 2023, 24, 4190. https://doi.org/10.3390/ijms24044190
Gaba Y, Bhowal B, Pareek A, Singla-Pareek SL. Genomic Survey of Flavin Monooxygenases in Wild and Cultivated Rice Provides Insight into Evolution and Functional Diversities. International Journal of Molecular Sciences. 2023; 24(4):4190. https://doi.org/10.3390/ijms24044190
Chicago/Turabian StyleGaba, Yashika, Bidisha Bhowal, Ashwani Pareek, and Sneh Lata Singla-Pareek. 2023. "Genomic Survey of Flavin Monooxygenases in Wild and Cultivated Rice Provides Insight into Evolution and Functional Diversities" International Journal of Molecular Sciences 24, no. 4: 4190. https://doi.org/10.3390/ijms24044190
APA StyleGaba, Y., Bhowal, B., Pareek, A., & Singla-Pareek, S. L. (2023). Genomic Survey of Flavin Monooxygenases in Wild and Cultivated Rice Provides Insight into Evolution and Functional Diversities. International Journal of Molecular Sciences, 24(4), 4190. https://doi.org/10.3390/ijms24044190







