The State of the Organs of the Female Reproductive System after a 5-Day “Dry” Immersion
Abstract
1. Introduction
2. Results
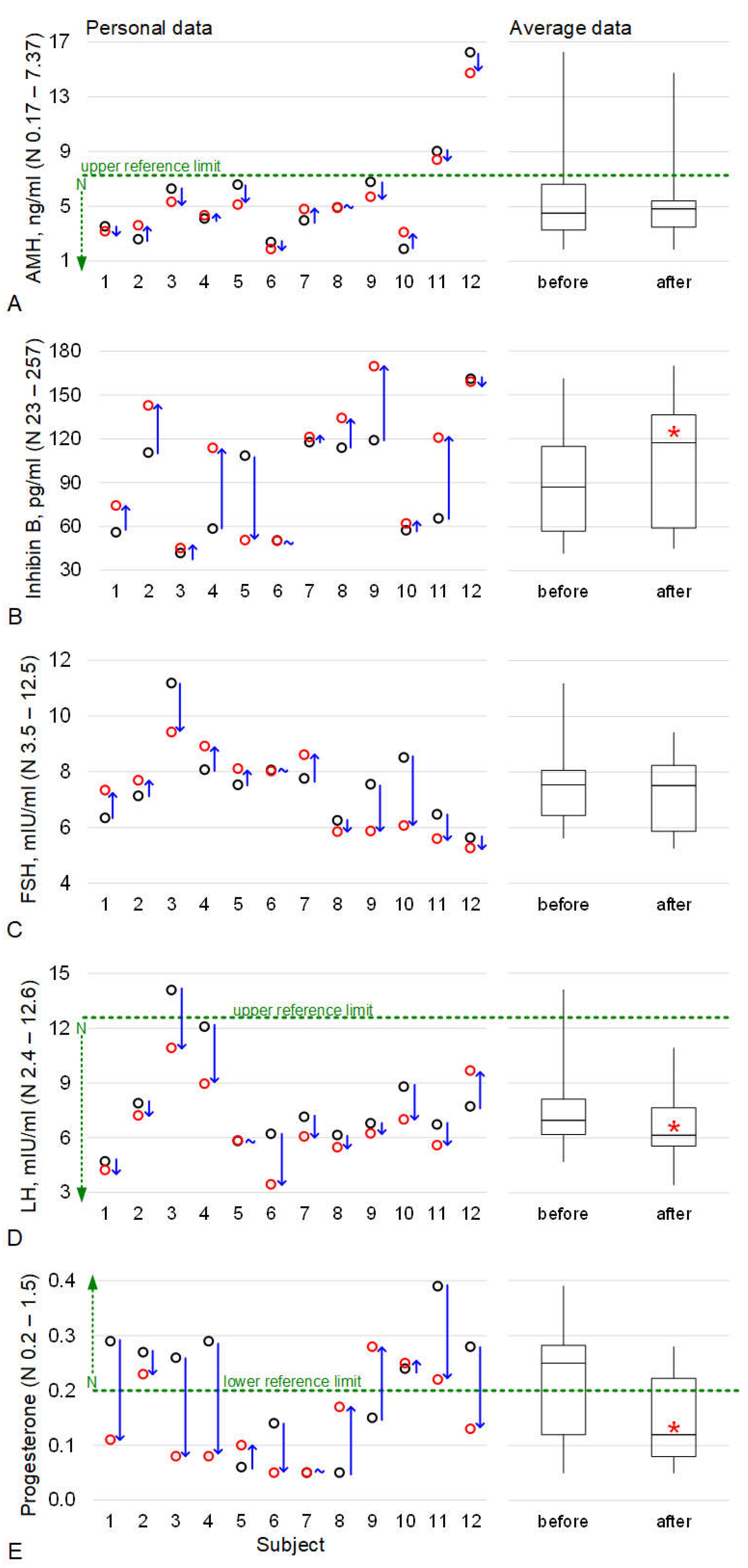
2.1. The Levels of Hormones Involved in the Functioning of the Reproductive Tissue
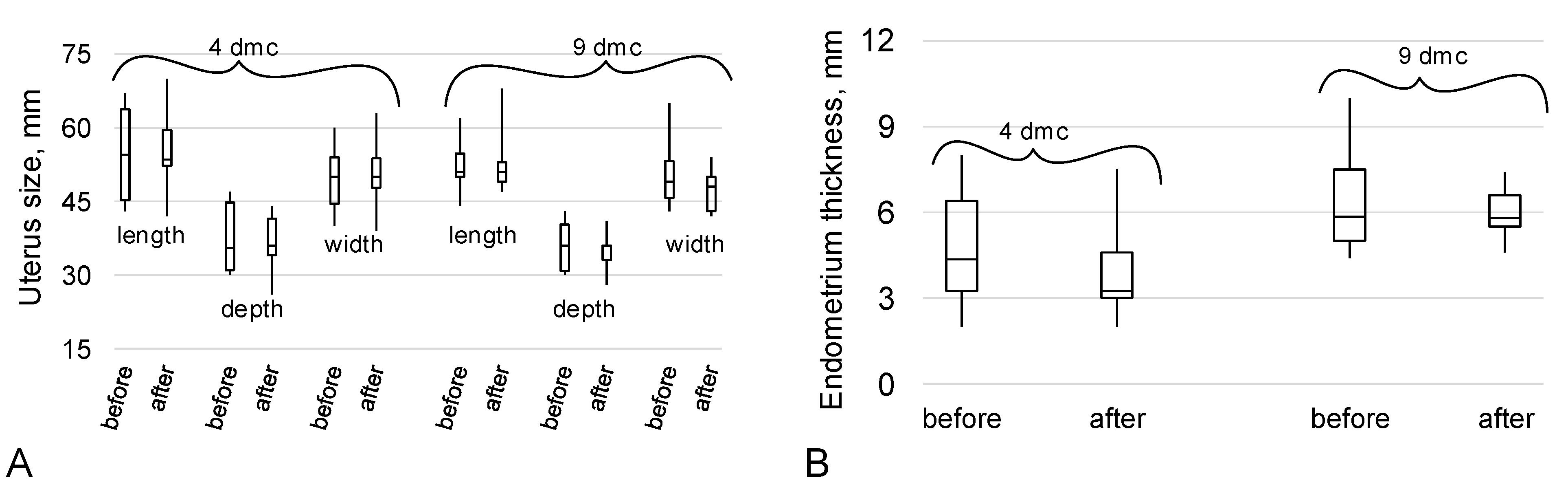
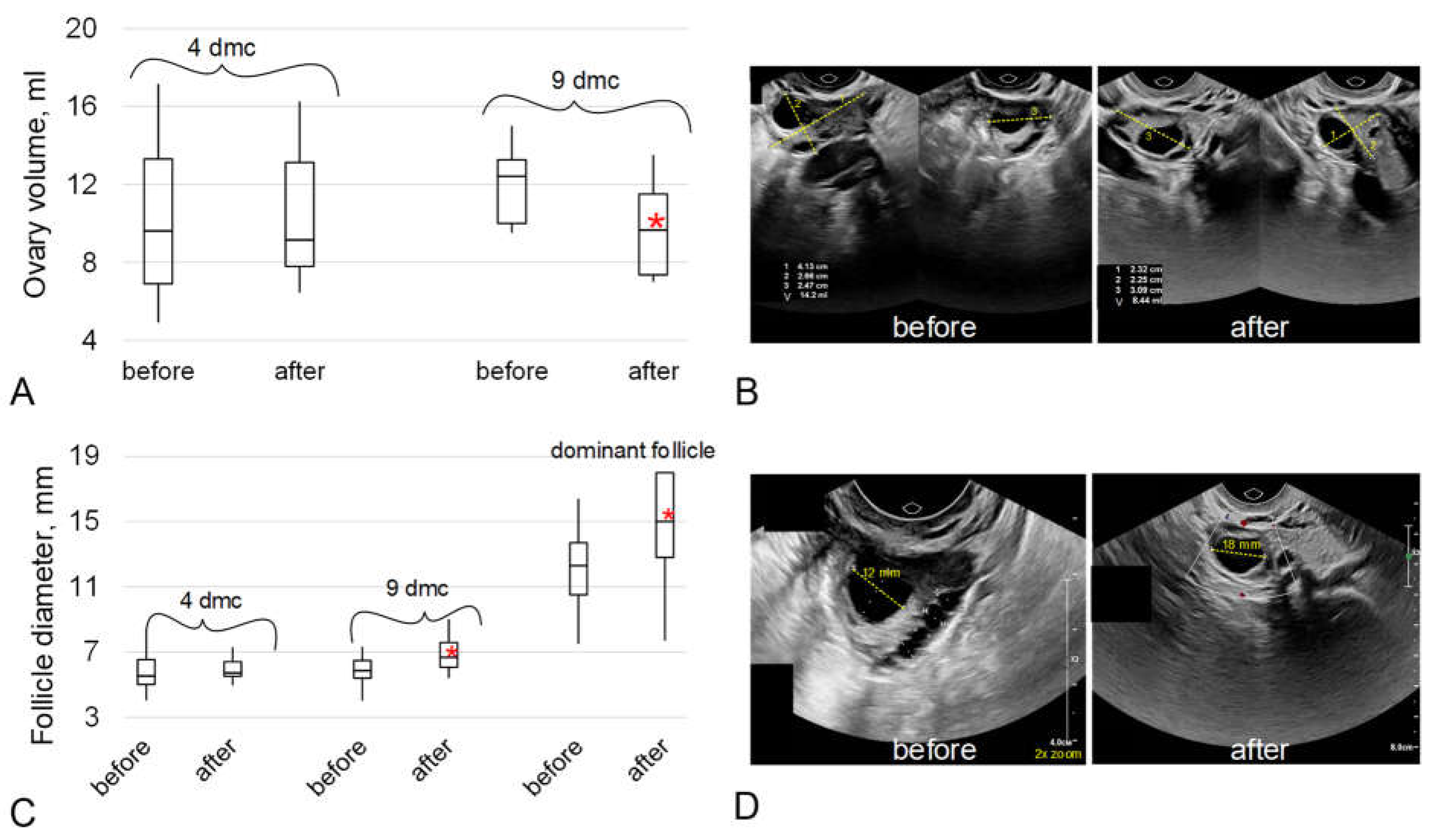
2.2. The Data of the Ultrasound Examination of the Organs of the Reproductive System
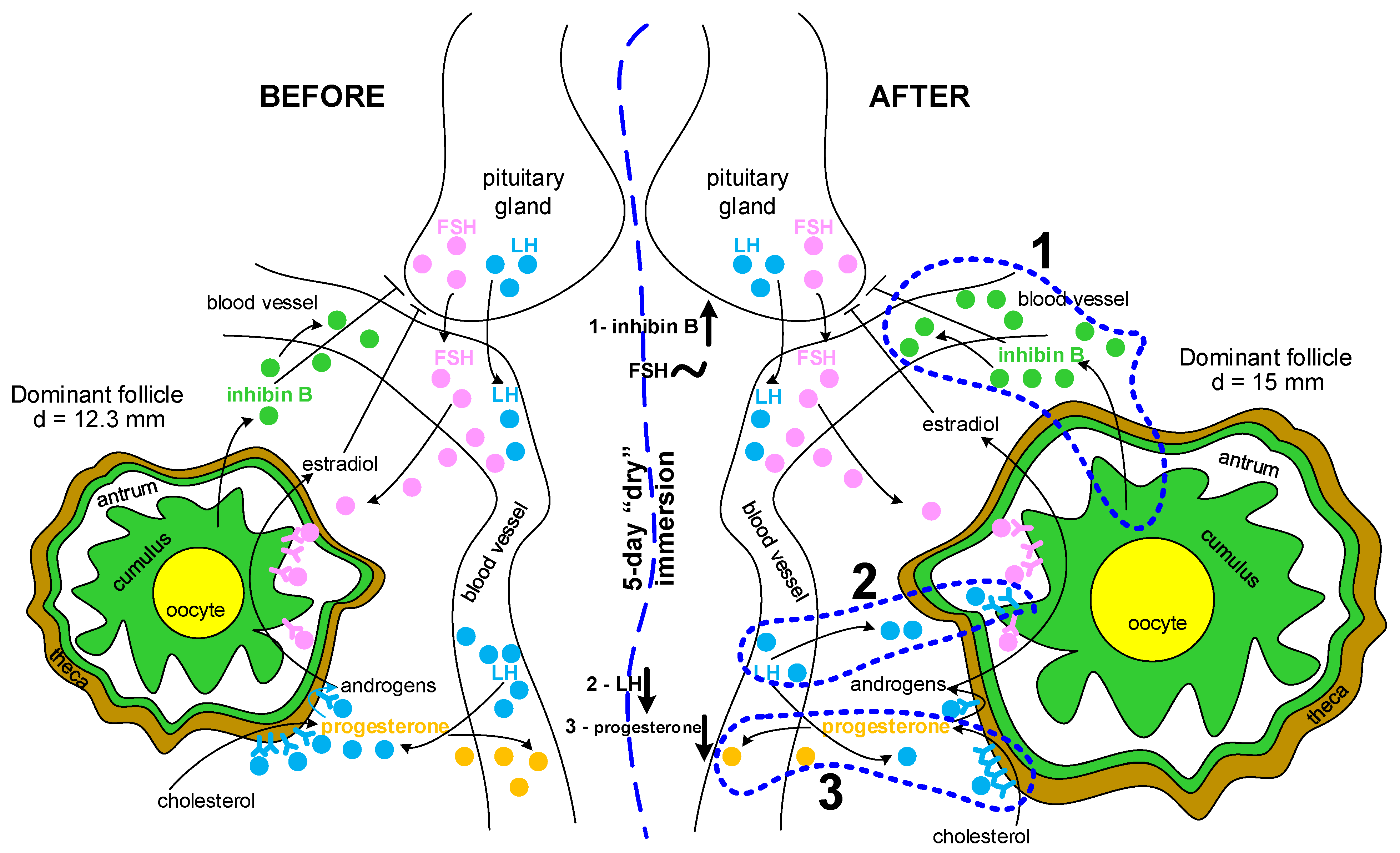
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Blood Sampling and Measurements of Hormones
4.3. Ultrasound Examination
4.4. Statistical Analysis
5. Conclusions
6. Limitations of the Study
- -
- the small number of subjects and high variability of the baseline parameters;
- -
- the phase of the menstrual cycle was determined by the date of the beginning of the last menstrual period but, due to the different reproductive histories, ages, etc., it could have shifted the longevity of the phases;
- -
- due to technical limitations during the organization of the experiment, the different days of the menstrual cycle at the beginning of exposure in the immersion bath could have caused the variability of some of the effects;
- -
- due to the same reasons mentioned above, our examinations were possible only on the fourth and ninth days of the menstrual cycle. The optimal dmc for estimating hormone levels is 2–3 dmc, for counting antral follicles it is 5–6 dmc, and for estimating the dominant follicle it is 11–12 dmc. In addition, it would be better to expand the range of detectable hormones involved in the functioning of the reproductive tissue, as well as the points of measurement. The lack of subject follow-up over future menstrual cycles and, as the result, the lack of data on the dynamics of the measured parameters is one of the most significant limitations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of sex and gender on adaptations to space: Reproductive health. J. Women’s Health 2014, 23, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Luderer, U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; E Wotring, V. Medically induced amenorrhea in female astronauts. NPJ Microgravity 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.T.; Baker, E.S. Gynecological and Reproductive Issues for Women in Space: A Review. Obstet. Gynecol. Surv. 2000, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.; Ronca, A.; Grindeland, R.; Wade, C. Models to Study Gravitational Biology of Mammalian Reproduction1. Biol. Reprod. 2002, 67, 1681–1687. [Google Scholar] [CrossRef]
- Sandler, H.; Winters, D. Physiological Responses of Women to Simulated Weightlessness. A Review of the Significant Findings of the First Female Bed Rest Study (NASA SP-340); NASA Scientific and Technical Information Office: Washington DC, USA, 1978. [Google Scholar]
- Rock, J.A.; Fortney, S.M. Medical and Surgical Considerations for Women in Spaceflight. Obstet. Gynecol. Surv. 1984, 39, 525–535. [Google Scholar] [CrossRef]
- Morgan, J.L.L.; Zwart, S.R.; Heer, M.; Ploutz-Snyder, R.; Ericson, K.; Smith, S.M. Bone metabolism and nutritional status during 30-day head-down-tilt bed rest. J. Appl. Physiol. 2012, 113, 1519–1529. [Google Scholar] [CrossRef]
- Koryak, Y.A. Influence of simulated microgravity on mechanical properties in the human triceps surae muscle in vivo. I: Effect of 120 days of bed-rest without physical training on human muscle musculo-tendinous stiffness and contractile properties in young women. Eur. J. Appl. Physiol. 2014, 114, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.A.; Macias, B.R.; Schneider, S.M.; Watenpaugh, D.E.; Lee, S.M.C.; Chang, D.G.; Hargens, A.R. WISE 2005: Aerobic and resistive countermeasures prevent paraspinal muscle deconditioning during 60-day bed rest in women. J. Appl. Physiol. 2016, 120, 1215–1222. [Google Scholar] [CrossRef]
- Frett, T.; Green, D.A.; Mulder, E.; Noppe, A.; Arz, M.; Pustowalow, W.; Petrat, G.; Tegtbur, U.; Jordan, J. Tolerability of daily intermittent or continuous short-arm centrifugation during 60-day 6o head down bed rest (AGBRESA study). PLOS ONE 2020, 15, e0239228. [Google Scholar] [CrossRef]
- Navasiolava, N.M.; Custaud, M.-A.; Tomilovskaya, E.S.; Larina, I.M.; Mano, T.; Gauquelin-Koch, G.; Gharib, C.; Kozlovskaya, I.B. Long-term dry immersion: Review and prospects. Eur. J. Appl. Physiol. 2011, 111, 1235–1260. [Google Scholar] [CrossRef] [PubMed]
- Watenpaugh, D.E. Analogs of microgravity: Head-down tilt and water immersion. J. Appl. Physiol. 2016, 120, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry Immersion as a Ground-Based Model of Microgravity Physiological Effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef]
- Plehuna, A.; Green, D.A.; Amirova, L.E.; Tomilovskaya, E.S.; Rukavishnikov, I.V.; Kozlovskaya, I.B. Dry immersion induced acute low back pain and its relationship with trunk myofascial viscoelastic changes. Front. Physiol. 2022, 13, 1039924. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.; Navasiolava, N.; Gauquelin-Koch, G.; Gharib, C.; Custaud, M.-A.; Treffel, L. Spinal changes after 5-day dry immersion as shown by magnetic resonance imaging (DI-5-CUFFS). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R310–R318. [Google Scholar] [CrossRef]
- Guilhot, C.; Fovet, T.; Delobel, P.; Dargegen, M.; Jasmin, B.J.; Brioche, T.; Chopard, A.; Py, G. Severe Muscle Deconditioning Triggers Early Extracellular Matrix Remodeling and Resident Stem Cell Differentiation into Adipocytes in Healthy Men. Int. J. Mol. Sci. 2022, 23, 5489. [Google Scholar] [CrossRef]
- Linossier, M.-T.; Peurière, L.; Fernandez, P.; Normand, M.; Beck, A.; Bareille, M.-P.; Bonneau, C.; Gauquelin-Koch, G.; Vico, L. DI-5-Cuffs: Bone Remodelling and Associated Metabolism Markers in Humans After Five Days of Dry Immersion to Simulate Microgravity. Front. Physiol. 2022, 13, 801448. [Google Scholar] [CrossRef]
- Tomilovskaya, E.; Amirova, L.; Nosikova, I.; Rukavishnikov, I.; Chernogorov, R.; Lebedeva, S.; Saveko, A.; Ermakov, I.; Ponomarev, I.; Zelenskaya, I.; et al. The First Female Dry Immersion (NAIAD-2020): Design and Specifics of a 3-Day Study. Front. Physiol. 2021, 12, 661959. [Google Scholar] [CrossRef]
- Tou, J.C.L.; Grindeland, R.E.; Wade, C.E. Effects of diet and exposure to hindlimb suspension on estrous cycling in Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E425–E433. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, D.; Wu, Y.; Lin, W.; Chen, Z.; Meng, L.; Liu, J.; Zhou, Y. Simulated Microgravity Using a Rotary Culture System Compromises the In Vitro Development of Mouse Preantral Follicles. PLOS ONE 2016, 11, e0151062. [Google Scholar] [CrossRef]
- Gougeon, A. Qualitative changes in medium and large antral follicles in the human ovary during the menstrual cycle. Ann. de Biol. Anim. Biochim. Biophys. 1979, 19, 1461–1468. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Updat. 2012, 18, 73–91. [Google Scholar] [CrossRef]
- Baker, T.G. A quantitative and cytological study of germ cells in human ovaries. Proc. R. Soc. London. Ser. B Boil. Sci. 1963, 158, 417–433. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The Mammalian Ovary from Genesis to Revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef]
- de Castro, F.C.; Cruz, M.H.C.; Leal, C.L.V. Role of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 in Ovarian Function and Their Importance in Mammalian Female Fertility—A Review. Asian-Australasian J. Anim. Sci. 2016, 29, 1065–1074. [Google Scholar] [CrossRef]
- Rimon-Dahari, N.; Yerushalmi-Heinemann, L.; Alyagor, L.; Dekel, N. Ovarian Folliculogenesis. Results Probl. Cell Differ. 2016, 58, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Orisaka, M.; Wang, H.; Orisaka, S.; Thompson, W.; Zhu, C.; Kotsuji, F.; Tsang, B.K. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: The delicate balance between life and death. Front. Biosci. 2007, 12, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Messinis, I.E. From Menarche to Regular Menstruation: Endocrinological Background. Ann. N. Y. Acad. Sci. 2006, 1092, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, A.R.; Adams, G.P.; A Pierson, R. A new model for ovarian follicular development during the human menstrual cycle. Fertil. Steril. 2003, 80, 116–122. [Google Scholar] [CrossRef]
- Andersen, C.Y. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Mol. Hum. Reprod. 2017, 23, 16–24. [Google Scholar] [CrossRef]
- Gougeon, A. Dynamics of follicular growth in the human: A model from preliminary results. Hum. Reprod. 1986, 1, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gershon, E.; Dekel, N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef] [PubMed]
- Elvin, J.A.; Yan, C.; Matzuk, M.M. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E 2 /EP2 receptor pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 10288–10293. [Google Scholar] [CrossRef] [PubMed]
- Vitt, U.; Hayashi, M.; Klein, C.; Hsueh, A. Growth Differentiation Factor-9 Stimulates Proliferation but Suppresses the Follicle-Stimulating Hormone-Induced Differentiation of Cultured Granulosa Cells from Small Antral and Preovulatory Rat Follicles1. Biol. Reprod. 2000, 62, 370–377. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef]
- Nosikova, I.; Riabova, A.; Amirova, L.; Kitov, V.; Tomilovskaya, E. NAIAD-2020: Characteristics of Motor Evoked Potentials After 3-Day Exposure to Dry Immersion in Women. Front. Hum. Neurosci. 2021, 15, 753259. [Google Scholar] [CrossRef]
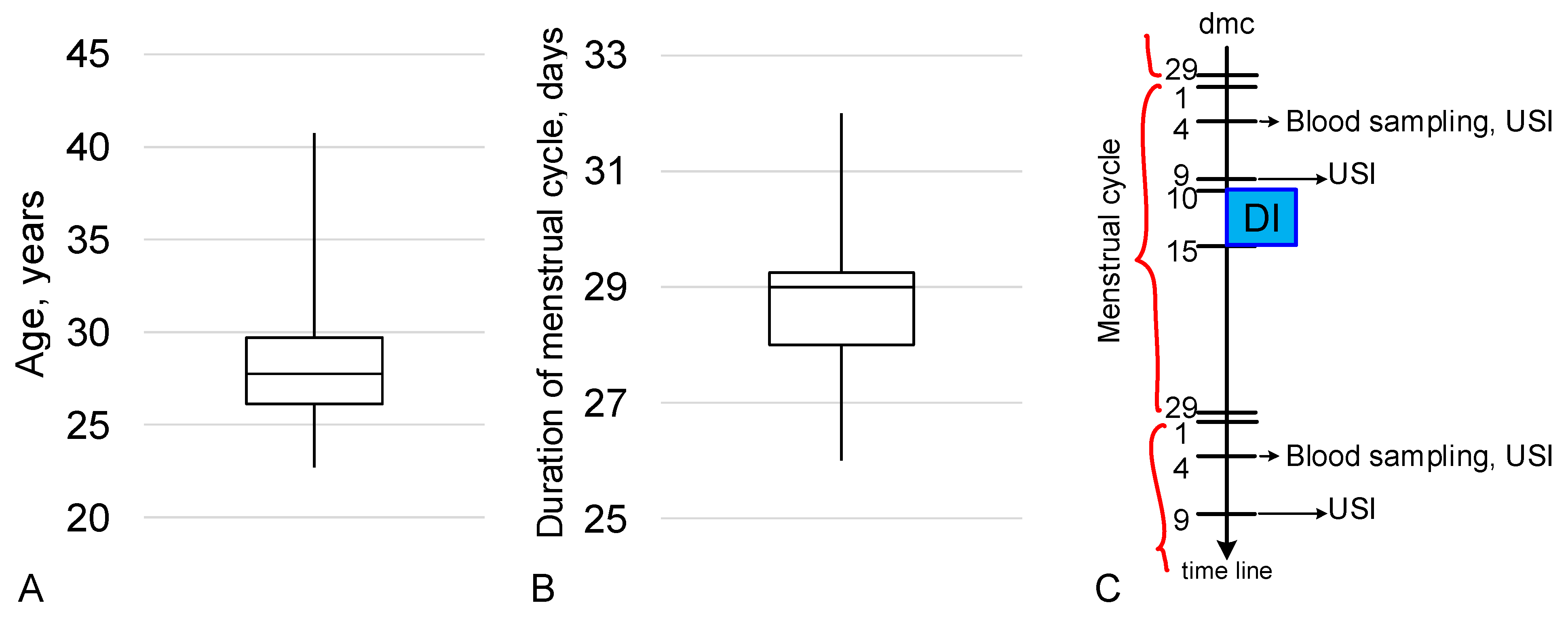
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbacheva, E.Y.; Toniyan, K.A.; Biriukova, Y.A.; Lukicheva, N.A.; Orlov, O.I.; Boyarintsev, V.V.; Ogneva, I.V. The State of the Organs of the Female Reproductive System after a 5-Day “Dry” Immersion. Int. J. Mol. Sci. 2023, 24, 4160. https://doi.org/10.3390/ijms24044160
Gorbacheva EY, Toniyan KA, Biriukova YA, Lukicheva NA, Orlov OI, Boyarintsev VV, Ogneva IV. The State of the Organs of the Female Reproductive System after a 5-Day “Dry” Immersion. International Journal of Molecular Sciences. 2023; 24(4):4160. https://doi.org/10.3390/ijms24044160
Chicago/Turabian StyleGorbacheva, Elena Yu., Konstantin A. Toniyan, Yulia A. Biriukova, Nadezhda A. Lukicheva, Oleg I. Orlov, Valery V. Boyarintsev, and Irina V. Ogneva. 2023. "The State of the Organs of the Female Reproductive System after a 5-Day “Dry” Immersion" International Journal of Molecular Sciences 24, no. 4: 4160. https://doi.org/10.3390/ijms24044160
APA StyleGorbacheva, E. Y., Toniyan, K. A., Biriukova, Y. A., Lukicheva, N. A., Orlov, O. I., Boyarintsev, V. V., & Ogneva, I. V. (2023). The State of the Organs of the Female Reproductive System after a 5-Day “Dry” Immersion. International Journal of Molecular Sciences, 24(4), 4160. https://doi.org/10.3390/ijms24044160




