Polymer Chemical Identity as a Key Factor in Microplastic–Insecticide Antagonistic Effects during Embryogenesis of Sea Urchin Arbacia lixula
Abstract
1. Introduction
2. Results
2.1. Adsorption Analysis
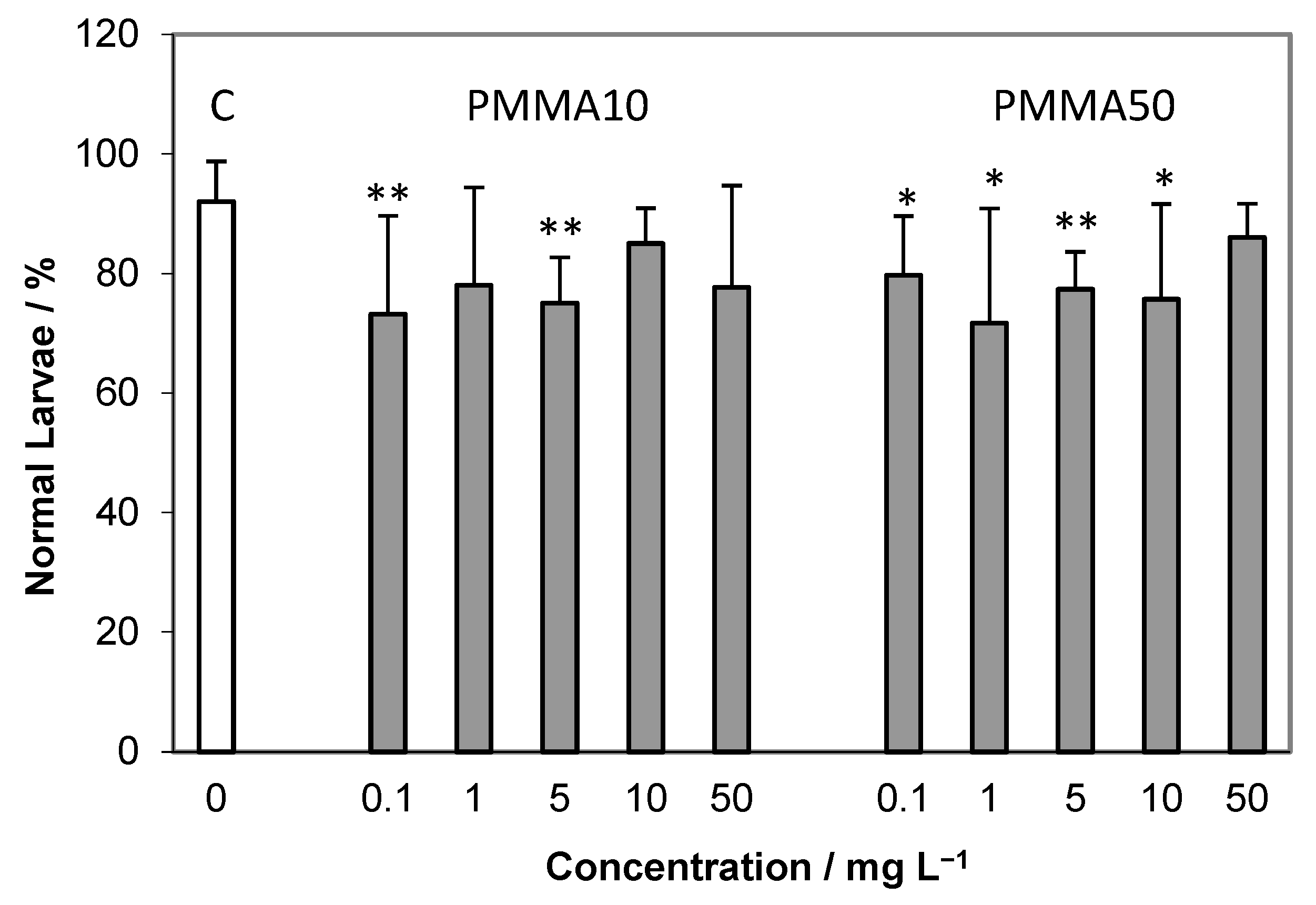
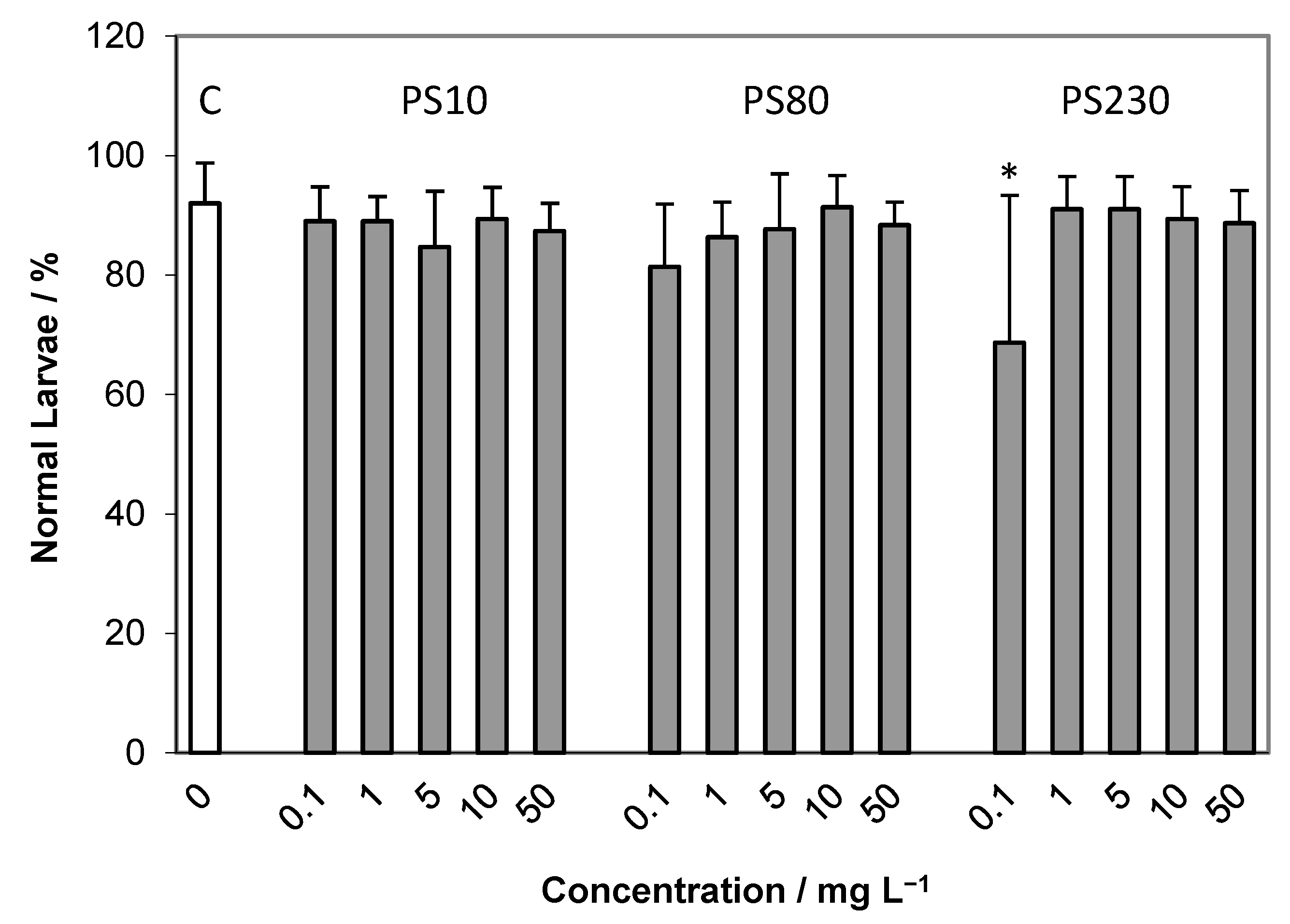
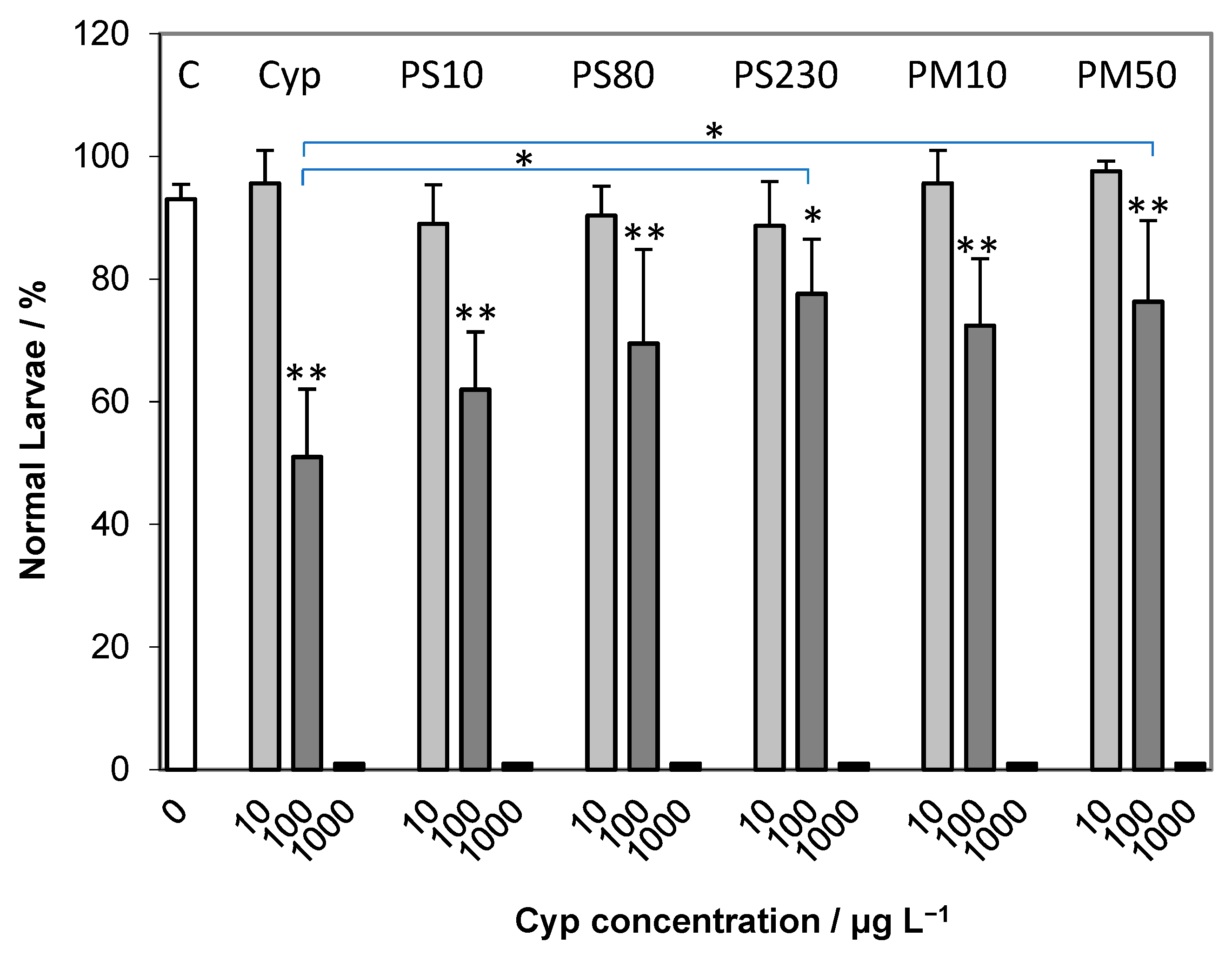
2.2. Embryotoxicity Assay
2.3. Spermiotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Adsorption of Cypermethrin on Plastic Microparticles
4.3. Sea Urchin Embryo Development Test
4.4. Spermiotoxicity Test and Offspring Quality
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, I.J.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific garbage patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the marine environment: Current trends in environmental pollution and mechanisms of toxicological profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Perono, G.; Tommasi, F.; Pagano, G.; Oral, R.; Burić, P.; Kovačić, I.; Toscanesi, M.; Trifuoggi, M.; Lyons, D.M. Resolving the effects of environmental micro- and nanoplastics exposure in biota: A knowledge gap analysis. Sci. Total Environ. 2021, 780, 146534. [Google Scholar] [CrossRef] [PubMed]
- Bacha, A.-U.-R.; Nabi, I.; Zaheer, M.; Jin, W.; Yang, L. Biodegradation of macro- and micro-plastics in environment: A review on mechanism, toxicity, and future perspectives. Sci. Total Environ. 2023, 858, 160108. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Yang, L.; Xiao, L.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 14373–14396. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Nordensvan, I.; Dave, G. Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile-butadiene-styrene, and epoxy to Daphnia magna. Environ. Sci. Pollut. Res. Int. 2012, 19, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, K.N.; Karapanagioti, H.N. Surface properties of beached plastic pellets. Mar. Environ. Res. 2012, 81, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.M.; Moreira, F.T.; Pereira, C.D.S.; Abessa, D.M.S.; Turra, A. Continuous exposure to microplastics does not cause physiological effects in the cultivated mussel Perna perna. Arch. Environ. Contam. Toxicol. 2018, 74, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Gandara e Silva, P.P.; Nobre, C.R.; Resaffe, P.; Pereira, C.D.S.; Gusmão, F. Leachate from microplastics impairs larval development in brown mussels. Water Res. 2016, 106, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Magni, S.; Gagné, F.; André, C.; Della Torre, C.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Colom, E.; Constenla, M.; Soler-Membrives, A.; Cartes, J.E.; Baeza, M.; Padrós, F.; Carrassón, M. Spatial occurrence and effects of microplastic ingestion on the deep-water shrimp Aristeus antennatus. Mar. Pollut. Bull. 2018, 133, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A.J.; Kunej, U.; Skalar, T. Screening study of four environmentally relevant microplastic pollutants: Uptake and effects on Daphnia magna and Artemia franciscana. Chemosphere 2018, 208, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; Tato, T. Microplastics do not increase toxicity of a hydrophobic organic chemical to marine plankton. Mar. Pollut. Bull. 2019, 138, 58–62. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Oral, R.; Pavičić-Hamer, D.; Burić, P.; Kovačić, I.; Siciliano, A.; Toscanesi, M.; Thomas, P.J.; Paduano, L.; et al. Microplastic-induced damage in early embryonal development of sea urchin Sphaerechinus granularis. Environ. Res. 2019, 179, 108815. [Google Scholar] [CrossRef]
- Thomas, P.J.; Oral, R.; Pagano, G.; Tez, S.; Toscanesi, M.; Ranieri, P.; Trifuoggi, M.; Lyons, D.M. Mild toxicity in Paracentrotus lividus early life stages may indicate species-specific sensitivity to polystyrene and polymethylmethacrylate microplastics. Mar. Environ. Res. 2020, 161, 105132. [Google Scholar] [CrossRef]
- Burić, P.; Jakšić, Ž.; Štajner, L.; Dutour Sikirić, M.; Jurašin, D.; Cascio, C.; Calzolai, L.; Lyons, D.M. Effect of silver nanoparticles on Mediterranean sea urchin embryonal development is species specific and depends on moment of first exposure. Mar. Environ. Res. 2015, 111, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Trifuoggi, M.; Pagano, G.; Guida, M.; Palumbo, A.; Siciliano, A.; Gravina, M.; Lyons, D.M.; Burić, P.; Levak, M.; Thomas, P.J.; et al. Comparative toxicity of seven rare earth elements in sea urchin early life stages. Environ. Sci. Pollut. Res. 2017, 24, 20803–20810. [Google Scholar] [CrossRef] [PubMed]
- Burić, P.; Čarapar, I.; Pavičić-Hamer, D.; Kovačić, I.; Jurković, L.; Dutour Sikirić, M.; Domazet Jurašin, D.; Mikac, N.; Bačić, N.; Lyons, D.M. Particle size modulates silver nanoparticle toxicity during embryogenesis of urchins Arbacia lixula and Paracentrotus Lividus. Int. J. Mol. Sci. 2023, 24, 745. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Trifuoggi, M.; Thomas, P.J.; Palumbo, A.; Romano, G.; Oral, R. Sea urchin bioassays in toxicity testing: I. Inorganics, organics, complex mixtures and natural products. Expert Opin. Environ. Biol. 2017, 6, 1. [Google Scholar] [CrossRef]
- Carballeira, C.; Ramos-Gómez, J.; Martín-Díaz, L.; DelValls, T.A. Identification of specific malformations of sea urchin larvae for toxicity assessment: Application to marine pisciculture effluents. Mar. Environ. Res. 2012, 77, 12–22. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; León, V.M.; Calles, S.; Gomáriz-Olcina, M.; Vethaak, A.D. The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res. 2017, 130, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, C.; Morgana, S.; Bramini, M.; Rotini, A.; Manfra, L.; Migliore, L.; Piazza, V.; Garaventa, F.; Faimali, M. Ecotoxicological effects of polystyrene microbeads in a battery of marine organisms belonging to different trophic levels. Mar. Environ. Res. 2018, 141, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Messinetti, S.; Mercurio, S.; Parolini, M.; Sugni, M.; Pennati, R. Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. Pollut. 2018, 237, 1080–1087. [Google Scholar] [CrossRef]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Oliviero, M.; Tato, T.; Schiavo, S.; Fernández, V.; Manzo, S.; Beiras, R. Leachates of micronized plastic toys provoke embryotoxic effects upon sea urchin Paracentrotus lividus. Environ. Pollut. 2019, 247, 706–715. [Google Scholar] [CrossRef]
- Kaposi, K.L.; Mos, B.; Kelaher, B.P.; Dworjanyn, S.A. Ingestion of microplastic has limited impact on a marine larva. Environ. Sci. Technol. 2014, 48, 1638–1645. [Google Scholar] [CrossRef]
- Li, H.X.; Getzinger, G.J.; Ferguson, P.L.; Orihuela, B.; Zhu, M.; Rittschof, D. Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle Amphibalanus amphitrite. Environ. Sci. Technol. 2016, 50, 924–931. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Q.; Hu, Z.; Yan, L.; Ieong, U.-I.; Xu, Y. Adsorption of chlorophenols on polyethylene terephthalate microplastics from aqueous environments: Kinetics, mechanisms and influencing factors. Environ. Pollut. 2020, 265, 114926. [Google Scholar] [CrossRef]
- Levak, M.; Burić, P.; Dutour Sikirić, M.; Domazet Jurašin, D.; Mikac, N.; Bačić, N.; Drexel, R.; Meier, F.; Jakšić, Ž.; Lyons, D.M. Effect of protein corona on silver nanoparticle stabilization and ion release kinetics in artificial seawater. Environ. Sci. Technol. 2017, 51, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Kleber, M. Molecular-level interactions in soils and sediments: The role of aromatic π-systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Reichel, J.; Graßmann, J.; Knoop, O.; Letzel, T.; Drewes, J.E. A novel analytical approach to assessing sorption of trace organic compounds into micro- and nanoplastic particles. Biomolecules 2022, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Reichel, J.; Graßmann, J.; Letzel, T.; Drewes, J.E. Systematic development of a simultaneous determination of plastic particle identity and adsorbed organic compounds by thermodesorption-pyrolysis GC/MS (TD-Pyr-GC/MS). Molecules 2020, 25, 4985. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 445–464. [Google Scholar]
- Beiras, R.; Bellas, J.; Cachot, J.; Cormier, B.; Cousin, X.; Engwall, M.; Gambardella, C.; Garaventa, F.; Keiter, S.; Le Bihanic, F.; et al. Ingestion and contact with polyethylene microplastics does not cause acute toxicity on marine zooplankton. J. Hazard. Mater. 2018, 360, 452–460. [Google Scholar] [CrossRef]
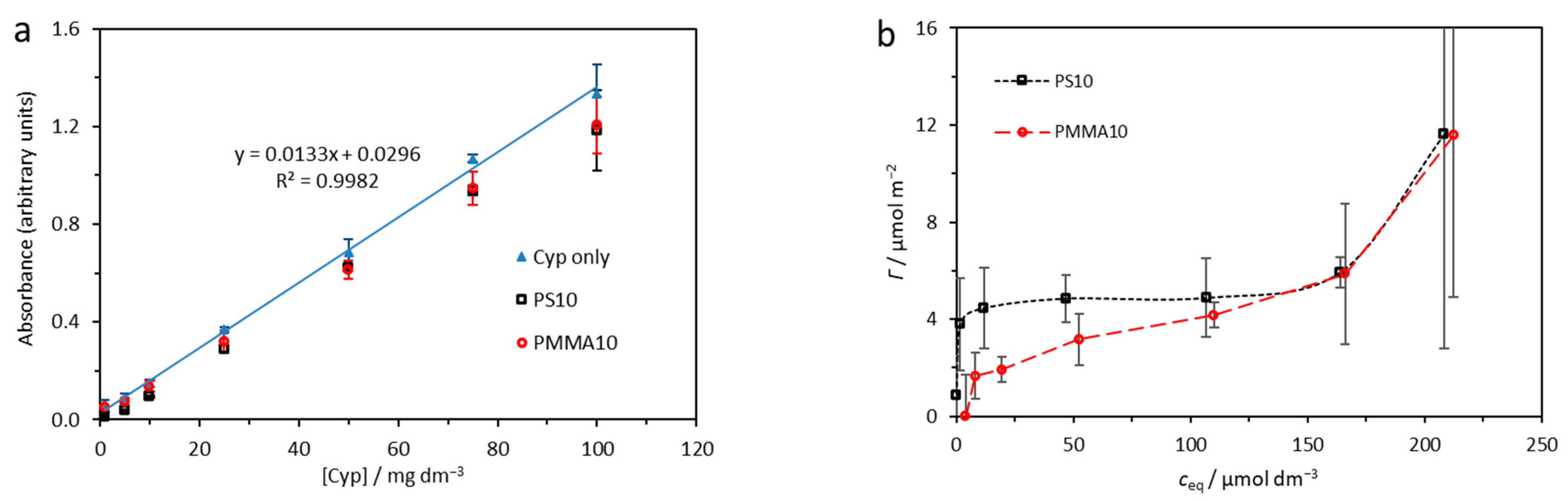


| Γmax/μmol m−2 | Kad/μmol−1 dm3 | α | β | |
|---|---|---|---|---|
| PS10 | 5.0 ± 0.3 | 1.53 ± 0.98 | 3.8 ± 0.1 | 16.5 ± 2.2 |
| PMMA10 | 4.0 ± 0.9 | 0.06 ± 0.04 | 0.4 ± 0.2 | 2.0 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burić, P.; Kovačić, I.; Jurković, L.; Tez, S.; Oral, R.; Landeka, N.; Lyons, D.M. Polymer Chemical Identity as a Key Factor in Microplastic–Insecticide Antagonistic Effects during Embryogenesis of Sea Urchin Arbacia lixula. Int. J. Mol. Sci. 2023, 24, 4136. https://doi.org/10.3390/ijms24044136
Burić P, Kovačić I, Jurković L, Tez S, Oral R, Landeka N, Lyons DM. Polymer Chemical Identity as a Key Factor in Microplastic–Insecticide Antagonistic Effects during Embryogenesis of Sea Urchin Arbacia lixula. International Journal of Molecular Sciences. 2023; 24(4):4136. https://doi.org/10.3390/ijms24044136
Chicago/Turabian StyleBurić, Petra, Ines Kovačić, Lara Jurković, Serkan Tez, Rahime Oral, Nediljko Landeka, and Daniel M. Lyons. 2023. "Polymer Chemical Identity as a Key Factor in Microplastic–Insecticide Antagonistic Effects during Embryogenesis of Sea Urchin Arbacia lixula" International Journal of Molecular Sciences 24, no. 4: 4136. https://doi.org/10.3390/ijms24044136
APA StyleBurić, P., Kovačić, I., Jurković, L., Tez, S., Oral, R., Landeka, N., & Lyons, D. M. (2023). Polymer Chemical Identity as a Key Factor in Microplastic–Insecticide Antagonistic Effects during Embryogenesis of Sea Urchin Arbacia lixula. International Journal of Molecular Sciences, 24(4), 4136. https://doi.org/10.3390/ijms24044136











