Optimization of Primary Human Bronchial Epithelial 3D Cell Culture with Donor-Matched Fibroblasts and Comparison of Two Different Culture Media
Abstract
1. Introduction
2. Results
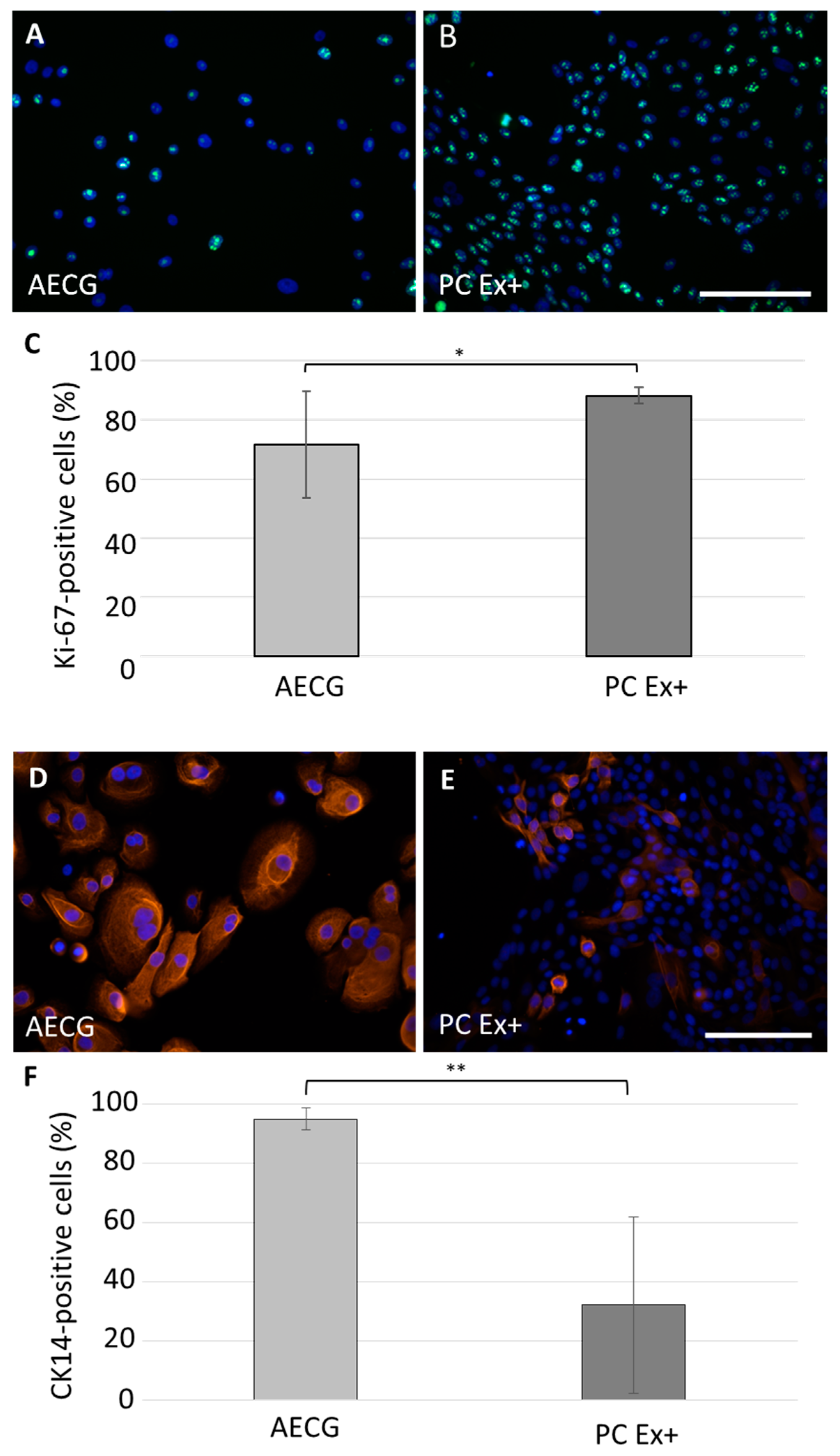
2.1. Optimized Cell Isolation and Expansion
2.2. Morphological Differentiation of 3D Models
2.3. Mucus Production
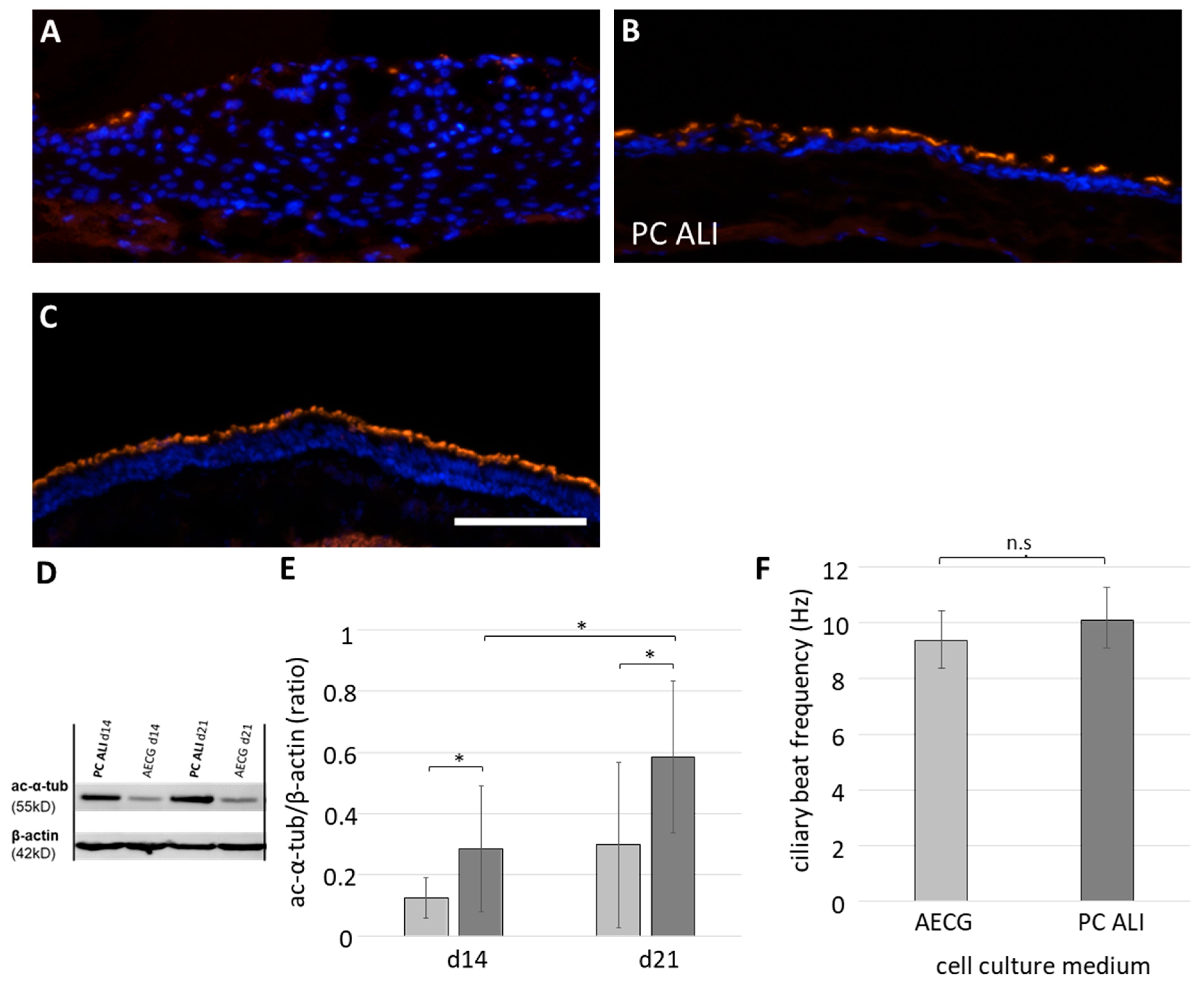
2.4. Differentiation of Apical Cilia with Physiological Function
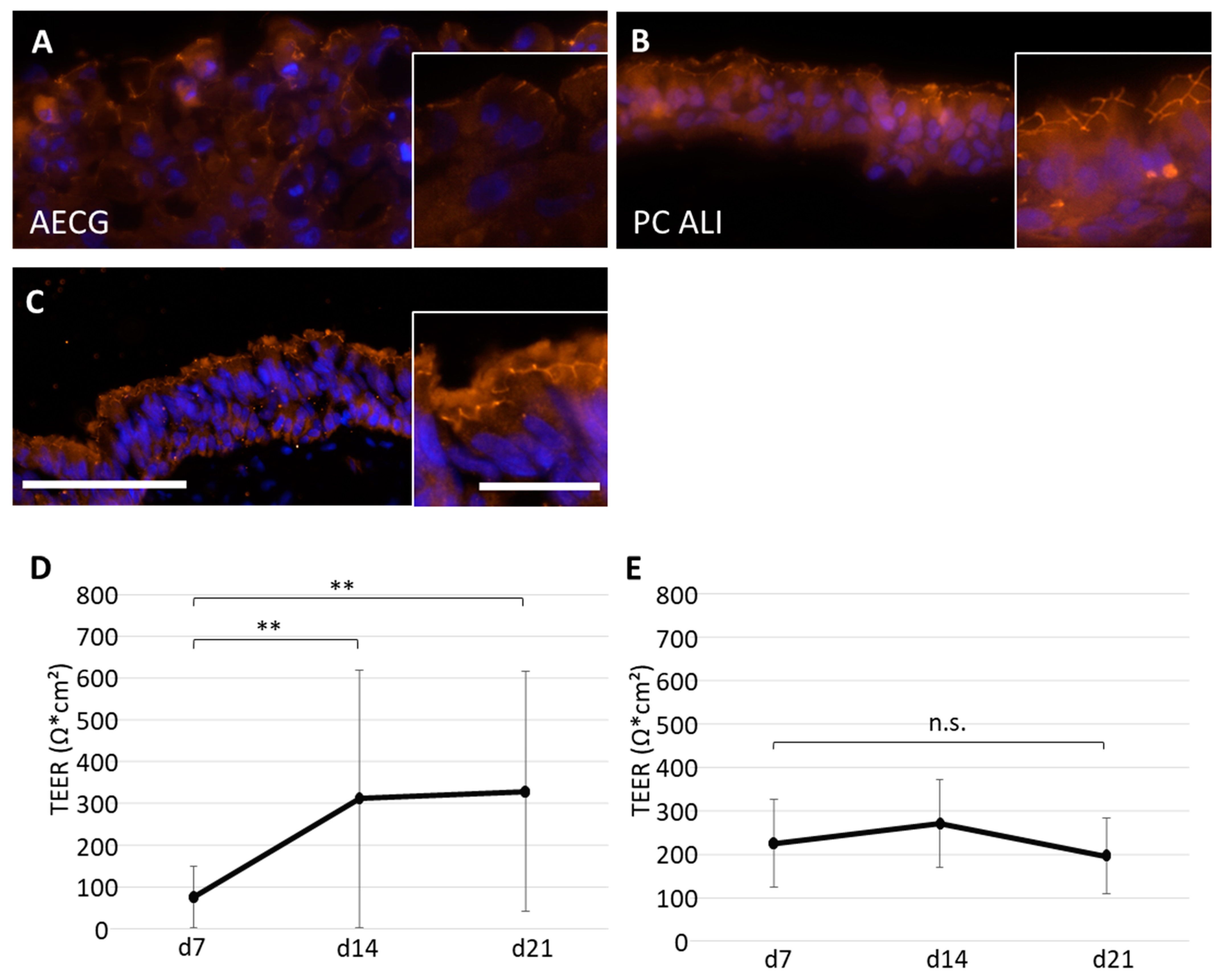
2.5. Evaluation of the Barrier Function
3. Discussion
4. Materials and Methods
4.1. Donor Tissue, Cell Isolation, and Cultivation
4.2. Generation and Cultivation of 3D Airway Models
4.3. Assessment of Epithelial Barrier Function
4.4. Assessment of Ciliary Beat Frequency
4.5. Histological and Immunofluorescence Staining
4.6. Western Blot Analysis
4.7. Mucin Score and Intraepithelial Mucus Cysts
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Respiratory Society. The Burden of Lung Disease. In European Lung White Book; European Respiratory Society: Lausanne, Switzerland, 2012; Available online: https://www.erswhitebook.org/chapters/the-burden-of-lung-disease/ (accessed on 28 August 2022).
- Planté-Bordeneuve, T.; Pilette, C.; Froidure, A. The Epithelial-Immune Crosstalk in Pulmonary Fibrosis. Front. Immunol. 2021, 12, 631235. [Google Scholar] [CrossRef] [PubMed]
- Gon, Y.; Hashimoto, S. Role of Airway Epithelial Barrier Dysfunction in Pathogenesis of Asthma. Allergol. Int. 2018, 67, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ahookhosh, K.; Pourmehran, O.; Aminfar, H.; Mohammadpourfard, M.; Sarafraz, M.M.; Hamishehkar, H. Development of Human Respiratory Airway Models: A Review. Eur. J. Pharm. Sci. 2020, 145, 105233. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, J.; Clevers, H. Airway Organoids as Models of Human Disease. J. Intern. Med. 2021, 289, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Varner, V.D.; Nelson, C.M. Computational Models of Airway Branching Morphogenesis. Semin. Cell Dev. Biol. 2017, 67, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Bennet, T.J.; Randhawa, A.; Hua, J.; Cheung, K.C. Airway-On-A-Chip: Designs and Applications for Lung Repair and Disease. Cells 2021, 10, 1602. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.F.; Picher, M.; Blackburn, M.R. Animal Models of Airway Diseases. In Purinergic Regulation of Respiratory Diseases; Picher, M., Boucher, R.C., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2011; Volume 55, pp. 195–234. ISBN 978-94-007-1216-4. [Google Scholar]
- Van der Velden, J.; Snibson, K.J. Airway Disease: The Use of Large Animal Models for Drug Discovery. Pulm. Pharmacol. Ther. 2011, 24, 525–532. [Google Scholar] [CrossRef]
- Tanner, L.; Single, A.B. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef]
- Herminghaus, A.; Kozlov, A.V.; Szabó, A.; Hantos, Z.; Gylstorff, S.; Kuebart, A.; Aghapour, M.; Wissuwa, B.; Walles, T.; Walles, H.; et al. A Barrier to Defend—Models of Pulmonary Barrier to Study Acute Inflammatory Diseases. Front. Immunol. 2022, 13, 895100. [Google Scholar] [CrossRef]
- Sims, A.C.; Burkett, S.E.; Yount, B.; Pickles, R.J. SARS-CoV Replication and Pathogenesis in an In Vitro Model of the Human Conducting Airway Epithelium. Virus Res. 2008, 133, 33–44. [Google Scholar] [CrossRef]
- Ventura-López, C.; Cervantes-Luevano, K.; Aguirre-Sánchez, J.S.; Flores-Caballero, J.C.; Alvarez-Delgado, C.; Bernaldez-Sarabia, J.; Sánchez-Campos, N.; Lugo-Sánchez, L.A.; Rodríguez-Vázquez, I.C.; Sander-Padilla, J.G.; et al. Treatment with Metformin Glycinate Reduces SARS-CoV-2 Viral Load: An in Vitro Model and Randomized, Double-Blind, Phase IIb Clinical Trial. Biomed. Pharmacother. 2022, 152, 113223. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Ollé, L.; Sabaté-Brescó, M.; Guo, Y.; Muñoz-Cano, R.; Wagner, A.; Gastaminza, G.; Martín, M. SARS-CoV-2 Vaccine Excipients Polyethylene Glycol and Trometamol Do Not Induce Mast Cell Degranulation, in an in Vitro Model for Non-IgE-Mediated Hypersensitivity. Front. Allergy 2022, 3, 1046545. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Stabile, A.M.; Bastianelli, S.; Giustarini, D.; Pierucci, S.; Busti, C.; Vacca, C.; Gidari, A.; Francisci, D.; Castronari, R.; et al. SARS-CoV2 Infection Impairs the Metabolism and Redox Function of Cellular Glutathione. Redox Biol. 2021, 45, 102041. [Google Scholar] [CrossRef] [PubMed]
- Lodes, N.; Seidensticker, K.; Perniss, A.; Nietzer, S.; Oberwinkler, H.; May, T.; Walles, T.; Hebestreit, H.; Hackenberg, S.; Steinke, M. Investigation on Ciliary Functionality of Different Airway Epithelial Cell Lines in Three-Dimensional Cell Culture. Tissue Eng. Part A 2020, 26, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Wiese-Rischke, C.; Murkar, R.S.; Walles, H. Biological Models of the Lower Human Airways—Challenges and Special Requirements of Human 3D Barrier Models for Biomedical Research. Pharmaceutics 2021, 13, 2115. [Google Scholar] [CrossRef]
- Zscheppang, K.; Berg, J.; Hedtrich, S.; Verheyen, L.; Wagner, D.E.; Suttorp, N.; Hippenstiel, S.; Hocke, A.C. Human Pulmonary 3D Models for Translational Research. Biotechnol. J. 2018, 13, 1700341. [Google Scholar] [CrossRef] [PubMed]
- Bovard, D.; Giralt, A.; Trivedi, K.; Neau, L.; Kanellos, P.; Iskandar, A.; Kondylis, A.; Luettich, K.; Frentzel, S.; Hoeng, J.; et al. Comparison of the Basic Morphology and Function of 3D Lung Epithelial Cultures Derived from Several Donors. Curr. Res. Toxicol. 2020, 1, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Dong, J.; Yuan, X.; Bo, H.; Li, S.; Wang, C.; Duan, Z.; Zheng, L. Anti-H7N9 Avian Influenza A Virus Activity of Interferon in Pseudostratified Human Airway Epithelium Cell Cultures. Virol. J. 2019, 16, 44. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for In Vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef]
- Myerburg, M.M.; Latoche, J.D.; McKenna, E.E.; Stabile, L.P.; Siegfried, J.S.; Feghali-Bostwick, C.A.; Pilewski, J.M. Hepatocyte Growth Factor and Other Fibroblast Secretions Modulate the Phenotype of Human Bronchial Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1352–L1360. [Google Scholar] [CrossRef]
- Steinke, M.; Gross, R.; Walles, H.; Gangnus, R.; Schütze, K.; Walles, T. An Engineered 3D Human Airway Mucosa Model Based on an SIS Scaffold. Biomaterials 2014, 35, 7355–7362. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Ishimori, K.; Ito, S. A 3D Epithelial–Mesenchymal Co-Culture Model of Human Bronchial Tissue Recapitulates Multiple Features of Airway Tissue Remodeling by TGF-Β1 Treatment. Respir. Res. 2017, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Harrington, H.; Cato, P.; Salazar, F.; Wilkinson, M.; Knox, A.; Haycock, J.W.; Rose, F.; Aylott, J.W.; Ghaemmaghami, A.M. Immunocompetent 3D Model of Human Upper Airway for Disease Modeling and In Vitro Drug Evaluation. Mol. Pharm. 2014, 11, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, S.; Dieken, H.; Krischenowski, O.; Förster, C.; Branscheid, D.; Aufderheide, M. Evaluation of E-Cigarette Liquid Vapor and Mainstream Cigarette Smoke after Direct Exposure of Primary Human Bronchial Epithelial Cells. Int. J. Environ. Res. Public Health 2015, 12, 3915–3925. [Google Scholar] [CrossRef]
- Chilvers, M.A.; O’Callaghan, C. Analysis of Ciliary Beat Pattern and Beat Frequency Using Digital High Speed Imaging: Comparison with the Photomultiplier and Photodiode Methods. Thorax 2000, 55, 314–317. [Google Scholar] [CrossRef]
- Nettesheim, P.; Koo, J.S.; Gray, T. Regulation of Differentiation of the Tracheobronchial Epithelium. J. Aerosol. Med. 2000, 13, 207–218. [Google Scholar] [CrossRef]
- Ng-Blichfeldt, J.-P.; Schrik, A.; Kortekaas, R.K.; Noordhoek, J.A.; Heijink, I.H.; Hiemstra, P.S.; Stolk, J.; Königshoff, M.; Gosens, R. Retinoic Acid Signaling Balances Adult Distal Lung Epithelial Progenitor Cell Growth and Differentiation. EBioMedicine 2018, 36, 461–474. [Google Scholar] [CrossRef]
- Smirnova, N.F.; Schamberger, A.C.; Nayakanti, S.; Hatz, R.; Behr, J.; Eickelberg, O. Detection and Quantification of Epithelial Progenitor Cell Populations in Human Healthy and IPF Lungs. Respir. Res. 2016, 17, 83. [Google Scholar] [CrossRef]
- Ruysseveldt, E.; Martens, K.; Steelant, B. Airway Basal Cells, Protectors of Epithelial Walls in Health and Respiratory Diseases. Front. Allergy 2021, 2, 787128. [Google Scholar] [CrossRef]
- Feriani, L.; Juenet, M.; Fowler, C.J.; Bruot, N.; Chioccioli, M.; Holland, S.M.; Bryant, C.E.; Cicuta, P. Assessing the Collective Dynamics of Motile Cilia in Cultures of Human Airway Cells by Multiscale DDM. Biophys. J. 2017, 113, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.E.X.; Tarsi, J.; Ramkumar, T.; Horiuchi, T.K.; Cochran, R.; DeMartino, S.; Schechtman, K.B.; Hussain, I.; Holtzman, M.J.; Castro, M. Epithelial Cell Proliferation Contributes to Airway Remodeling in Severe Asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Tsartsali, L.; Hislop, A.A.; McKay, K.; James, A.L.; Elliot, J.; Zhu, J.; Rosenthal, M.; Payne, D.N.; Jeffery, P.K.; Bush, A.; et al. Development of the Bronchial Epithelial Reticular Basement Membrane: Relationship to Epithelial Height and Age. Thorax 2011, 66, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Wadsworth, S.J.; Yang, S.J.; Dorscheid, D.R. Structural and Functional Variations in Human Bronchial Epithelial Cells Cultured in Air-Liquid Interface Using Different Growth Media. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1063–L1073. [Google Scholar] [CrossRef] [PubMed]
- Yamaya, M.; Finkbeiner, W.E.; Chun, S.Y.; Widdicombe, J.H. Differentiated Structure and Function of Cultures from Human Tracheal Epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 1992, 262, L713–L724. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Rojanasakul, Y.; Wang, L.; Bhat, M.; Glover, D.D.; Malanga, C.J.; Ma, J.K.H. The Transport Barrier of Epithelia: A Comparative Study on Membrane Permeability and Charge Selectivity in the Rabbit. Pharm. Res. 1992, 9, 1029–1034. [Google Scholar] [CrossRef]
- Hoppensack, A.; Kazanecki, C.C.; Colter, D.; Gosiewska, A.; Schanz, J.; Walles, H.; Schenke-Layland, K. A Human In Vitro Model That Mimics the Renal Proximal Tubule. Tissue Eng. Part C Methods 2014, 20, 599–609. [Google Scholar] [CrossRef]
- Schweinlin, M.; Rossi, A.; Lodes, N.; Lotz, C.; Hackenberg, S.; Steinke, M.; Walles, H.; Groeber, F. Human Barrier Models for the in Vitro Assessment of Drug Delivery. Drug Deliv. Transl. Res. 2017, 7, 217–227. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, J.; Walles, T.; Wiese-Rischke, C. Optimization of Primary Human Bronchial Epithelial 3D Cell Culture with Donor-Matched Fibroblasts and Comparison of Two Different Culture Media. Int. J. Mol. Sci. 2023, 24, 4113. https://doi.org/10.3390/ijms24044113
Maurer J, Walles T, Wiese-Rischke C. Optimization of Primary Human Bronchial Epithelial 3D Cell Culture with Donor-Matched Fibroblasts and Comparison of Two Different Culture Media. International Journal of Molecular Sciences. 2023; 24(4):4113. https://doi.org/10.3390/ijms24044113
Chicago/Turabian StyleMaurer, Julian, Thorsten Walles, and Cornelia Wiese-Rischke. 2023. "Optimization of Primary Human Bronchial Epithelial 3D Cell Culture with Donor-Matched Fibroblasts and Comparison of Two Different Culture Media" International Journal of Molecular Sciences 24, no. 4: 4113. https://doi.org/10.3390/ijms24044113
APA StyleMaurer, J., Walles, T., & Wiese-Rischke, C. (2023). Optimization of Primary Human Bronchial Epithelial 3D Cell Culture with Donor-Matched Fibroblasts and Comparison of Two Different Culture Media. International Journal of Molecular Sciences, 24(4), 4113. https://doi.org/10.3390/ijms24044113






