Interaction of Amphipathic Peptide from Influenza Virus M1 Protein with Mitochondrial Cytochrome Oxidase
Abstract
1. Introduction
2. Results
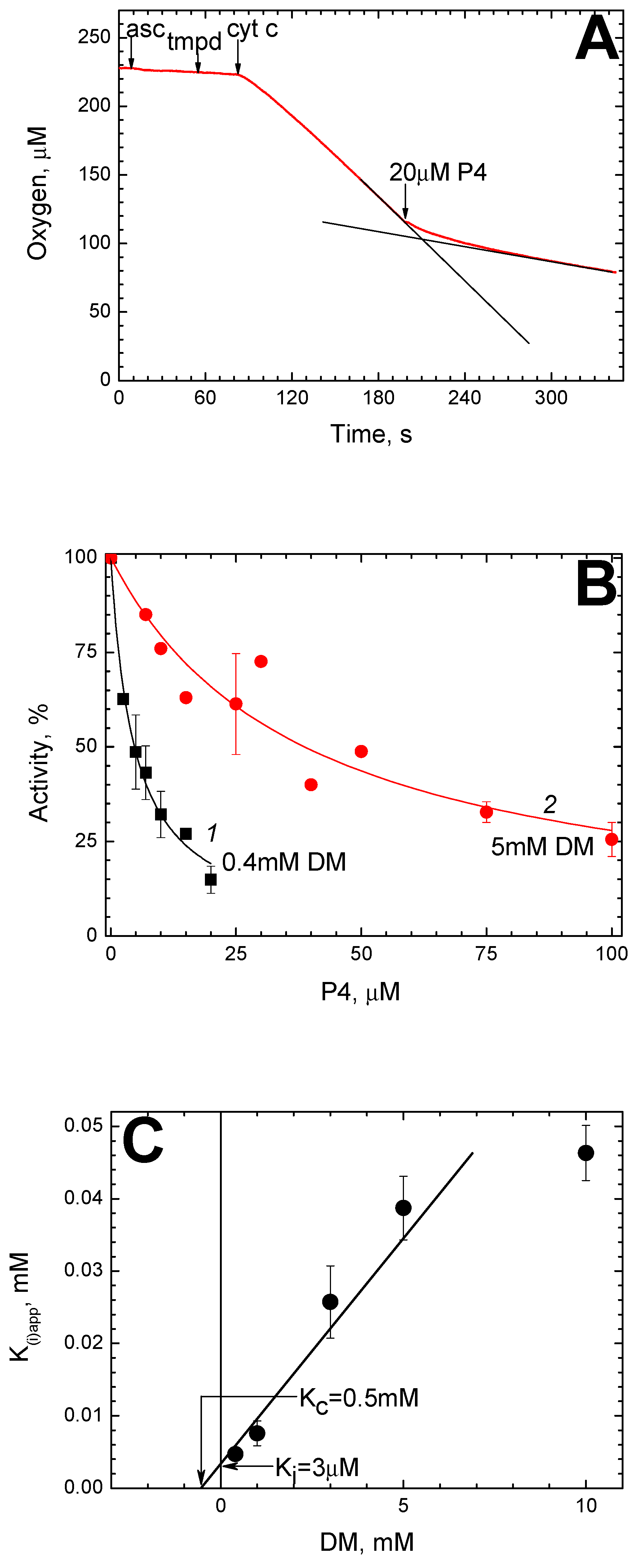
2.1. P4 Inhibits CсO Activity in Solution
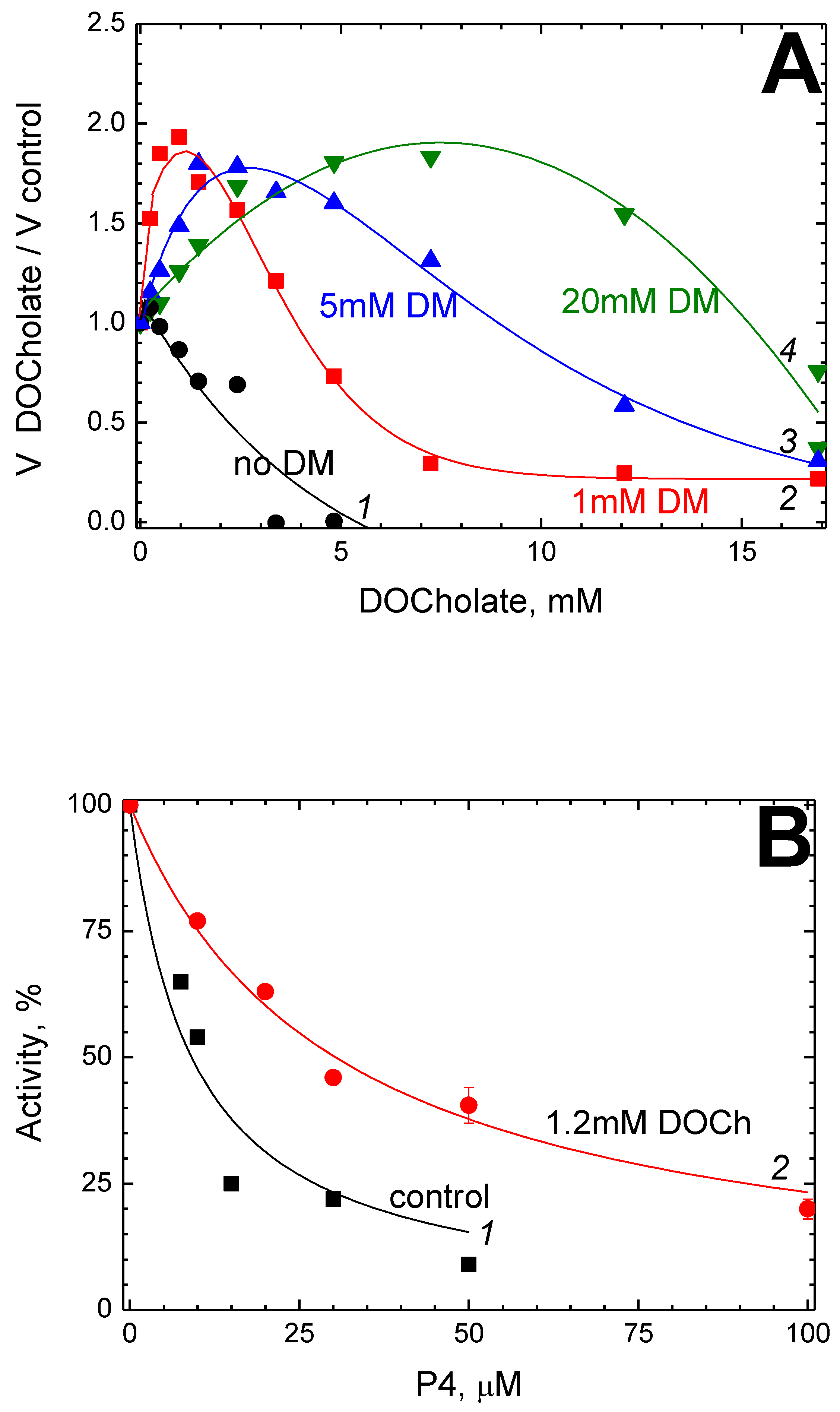
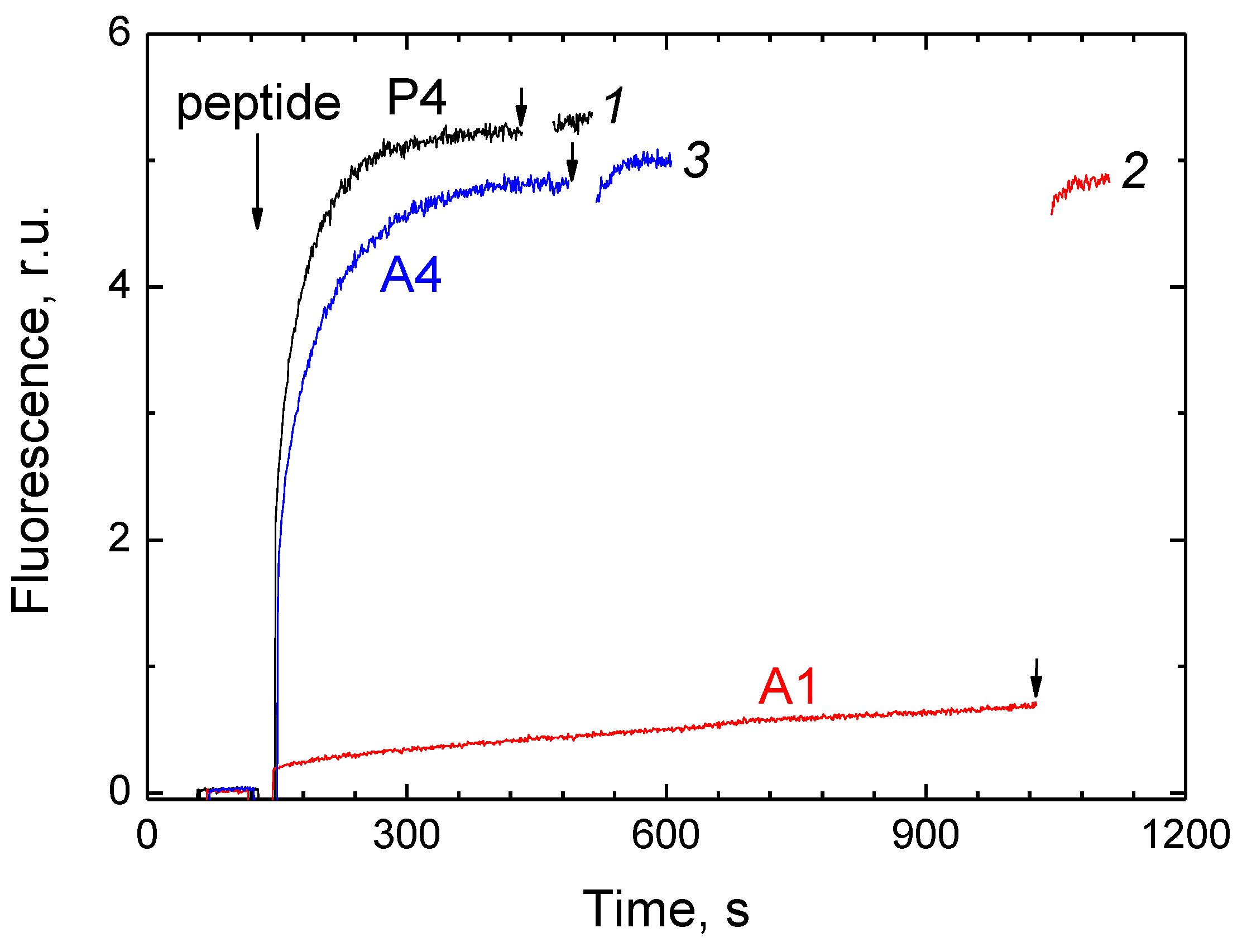
2.2. P4 Competes for Binding to CcO with Dodecyl Maltoside
2.3. P4 Competes for Binding to CcO with Deoxycholate
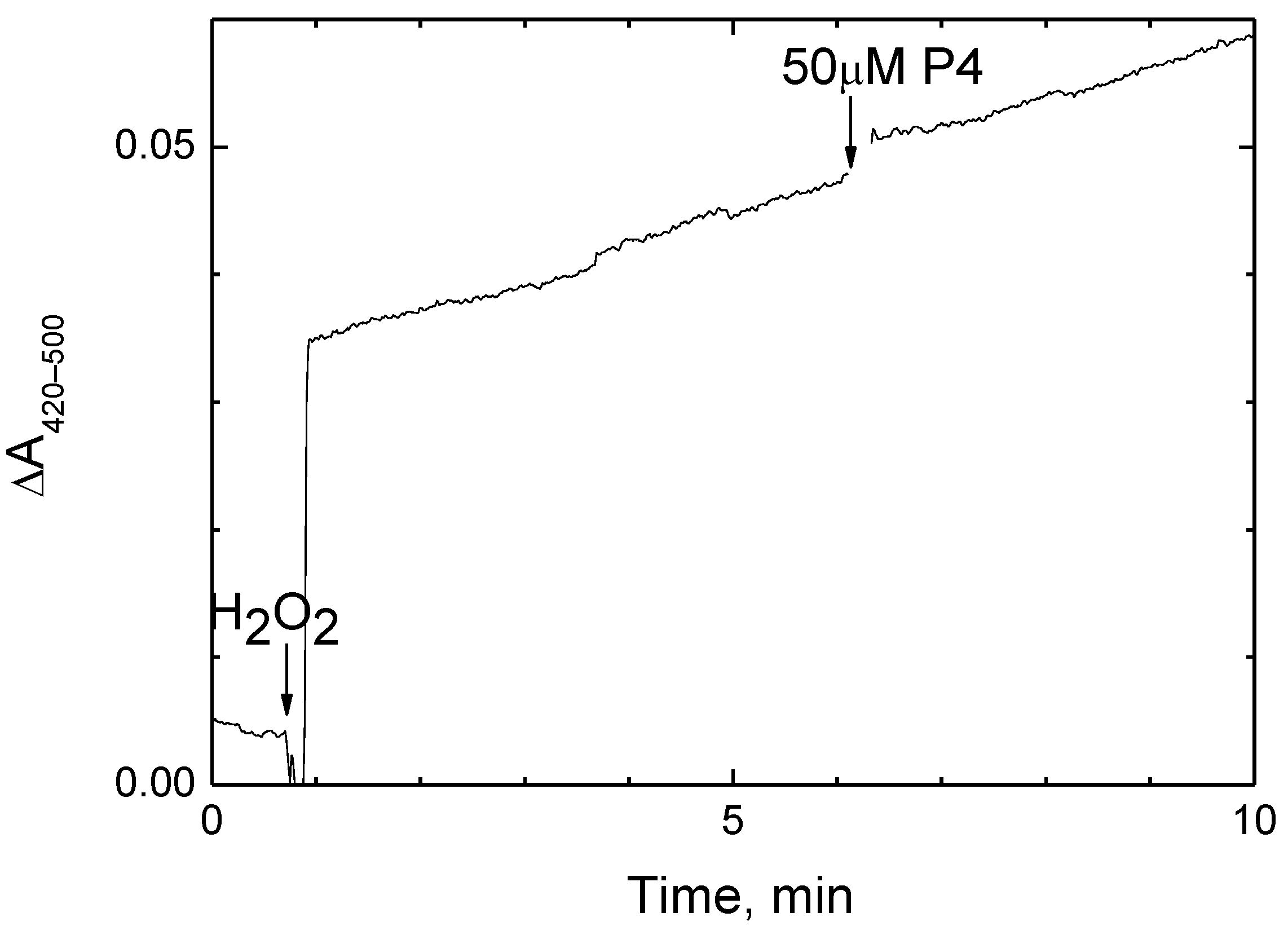
2.4. P4 Does Not Inhibit the Peroxidase Activity of CcO
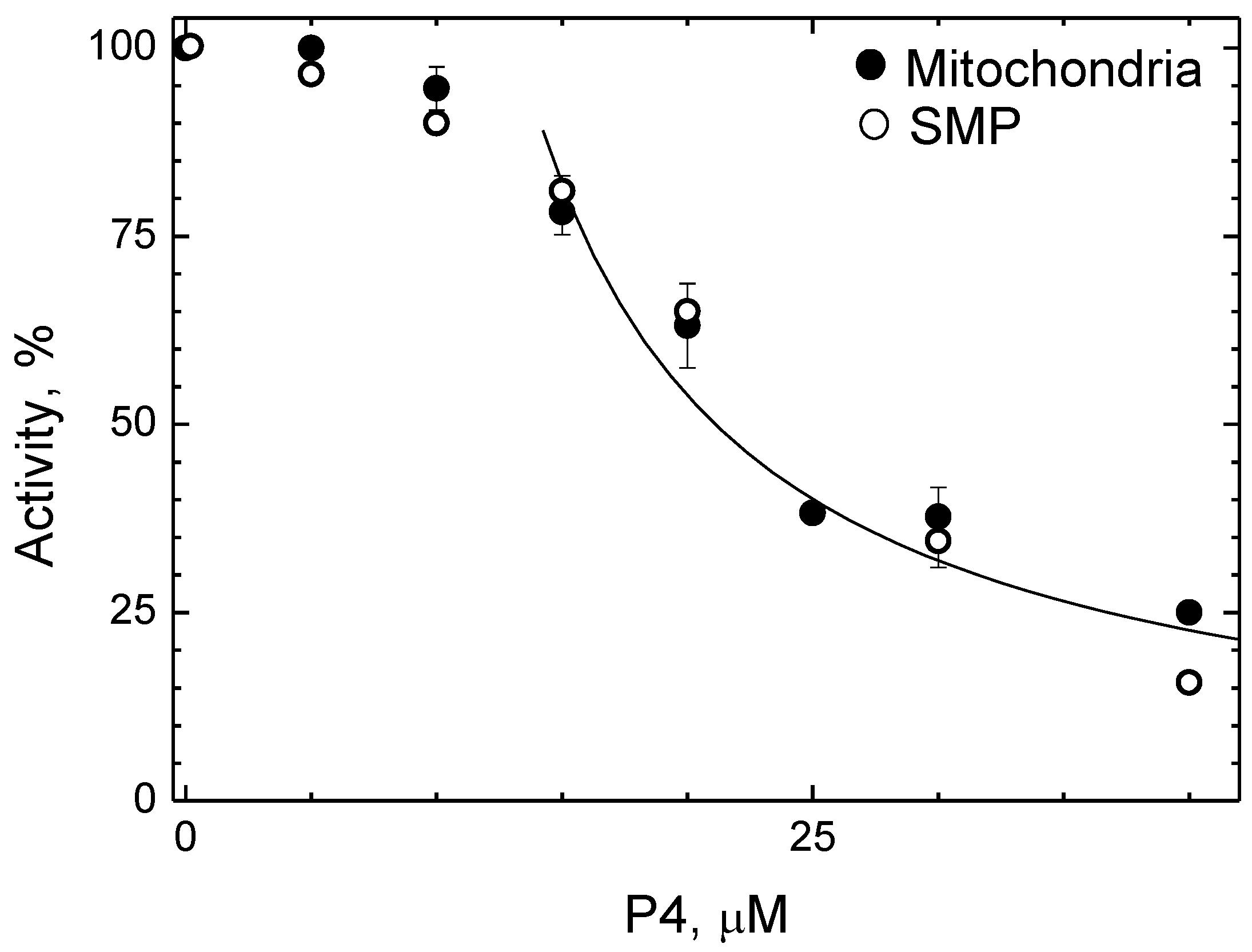
2.5. P4 Inhibits CcO Activity in Mitochondria and Submitochondrial Particles but Not in Proteoliposomes
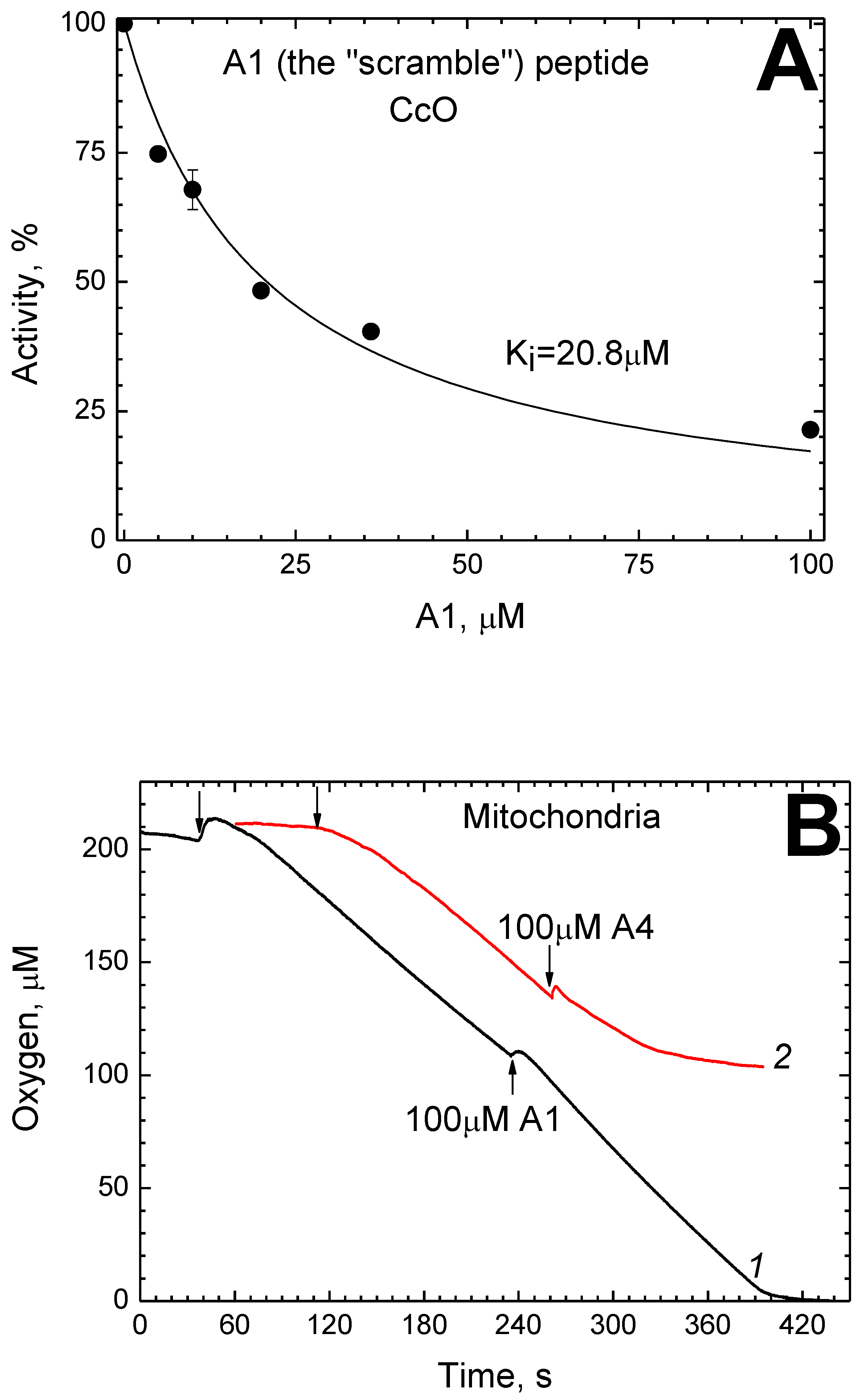
2.6. Scramble Analogue of P4 Inhibits CcO in Solution, but Not in Mitochondria
2.7. Efficacy of Various P4 Analogs as CcO Inhibitors
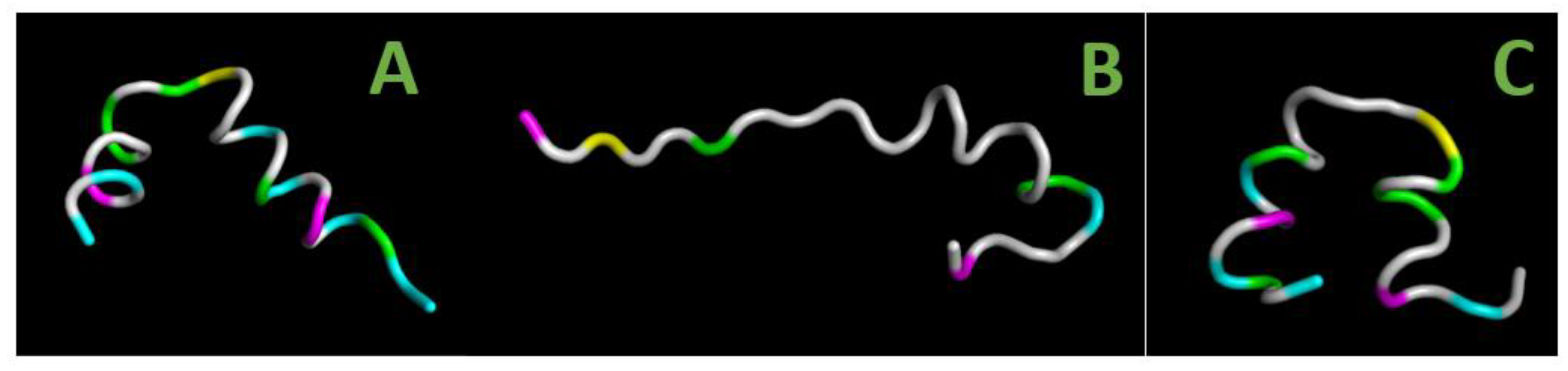
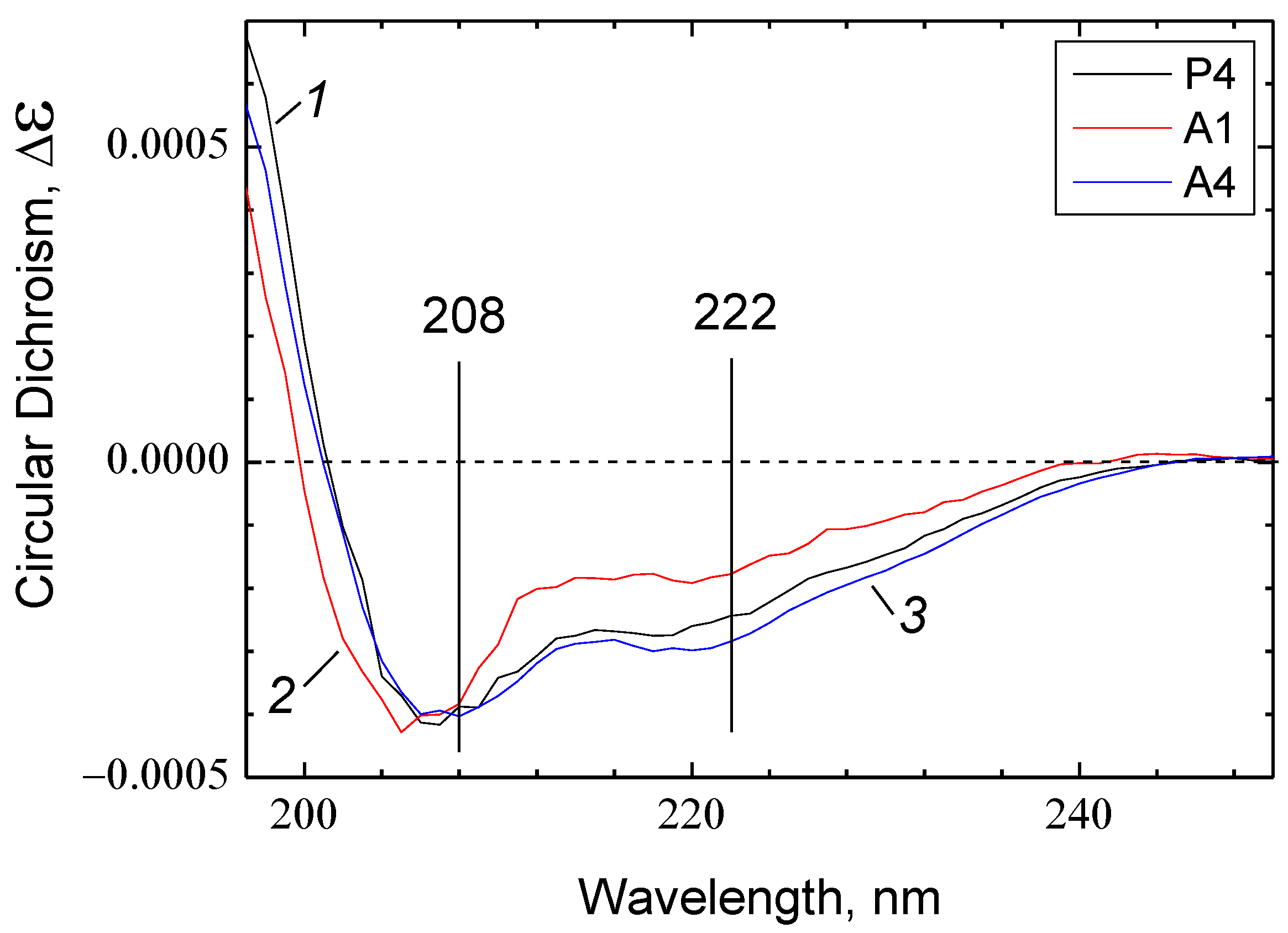
2.8. Secondary Structure of the Inhibitory Peptides
3. Discussion
3.1. The Site of Interaction between P4 and CcO Is BABS
3.2. Influence of Primary Sequence on the Efficiency of Inhibition
3.3. Possible Mechanism of Inhibition
3.4. Possible Role of the Secondary Structure
3.5. Perspectives
3.6. Conclusions
4. Materials and Methods
Chemicals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindgren, M.; Hällbrink, M.; Prochiantz, A.; Langel, Ü. Cell-penetrating peptides. Trends Pharm. Sci. 2000, 21, 99–103. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef]
- Gautam, A.; Singh, H.; Tyagi, A.; Chaudhary, K.; Kumar, R.; Kapoor, P.; Raghava, G.P.S. CPP site: A curated database of cell penetrating peptides. Database 2012, 2012. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodefi ciency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Elliott, G.; O’Hare, P. Intercellular trafficking and protein delivery by a herpes virus structural protein. Cell 1997, 88, 223–233. [Google Scholar] [CrossRef]
- Yu, W.; Zhan, Y.; Xue, B.; Dong, Y.; Wang, Y.; Jiang, P.; Wang, A.; Sun, Y.; Yang, Y. Highly efficient cellular uptake of a cell-penetrating peptide (CPP) derived from the capsid protein of porcine circovirus type 2. J. Biol. Chem. 2018, 293, 15221–15232. [Google Scholar] [CrossRef]
- Zhang, P.; Monteiro da Silva, G.; Deatherage, C.; Burd, C.; Di Maio, D. Cell-Penetrating Peptide Mediates Intracellular Membrane Passage of Human Papillomavirus L2 Protein to Trigger Retrograde Trafficking. Cell 2018, 174, 1465–1476. [Google Scholar] [CrossRef]
- Yang, R.; Wang, P.; Chen, Z.; Zhang, L.; Ye, N.; You, Z. Genome-wide identification and analysis of CPP transcription factor family in tea plants. Acta Bot. Boreali-Occident. Sin. 2019, 39, 1024–1032. [Google Scholar]
- Ullah, U.; Buttar, Z.A.; Shalmani, A.; Muhammad, I.; Ud-Din, A.; Ali, H. Genome-wide identification and expression analysis of CPP-like gene family in Triticum aestivum L. under different hormone and stress conditions. Open Life Sci. 2022, 17, 544–562. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engstrom, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Malmsten, M.; Schmidtchen, A. Antimicrobial peptides: A key component of innate immunity. Crit. Rev. Biotechnol. 2011, 32, 143–171. [Google Scholar] [CrossRef]
- Ye, J.; Liu, E.; Yu, Z.; Pei, X.; Chen, S.; Zhang, P.; Shin, M.C.; Gong, J.; He, H.; Yang, V.C. CPP-assisted intracellular drug delivery, what is next? Int. J. Mol. Sci. 2016, 17, 1892. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Langel, Ü. Recent CPP-based applications in medicine. Expert Opin. Drug Deliv. 2019, 16, 1183–1191. [Google Scholar] [CrossRef]
- Taylor, R.E.; Zahid, M. Cell penetrating peptides, novel vectors for gene therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef]
- Takakura, H.; Sato, H.; Nakajima, K.; Suzuki, M.; Ogawa, M. In Vitro and In Vivo Cell Uptake of a Cell-Penetrating Peptide Conjugated with Fluorescent Dyes Having Different Chemical Properties. Cancers 2021, 13, 2245. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapere, J.J.; Lindemann, P.L.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef]
- Song, Y.; Kenworthy, A.K.; Sanders, C.R. Cholesterol as a co-solvent and a ligand for membrane proteins. Prot. Sci. 2014, 23, 1–22. [Google Scholar] [CrossRef]
- Epand, R.M. Proteins and cholesterol-rich domains. Biochim. Biophys. Acta 2008, 1778, 1576–1582. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Dou, X.; Li, Y.; Han, J.; Zarlenga, D.S.; Zhu, W.; Ren, X.; Dong, N.; Li, X.; Li, G. Cholesterol of lipid rafts is a key determinant for entry and post-entry control of porcine rotavirus infection. BMC Vet. Res. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Sviridov, D.; Mukhamedova, N.; Makarov, A.A.; Adzhubei, A.; Bukrinsky, M. Comorbidities of HIV infection: Role of Nef-induced impairment of cholesterol metabolism and lipid raft functionality. AIDS 2020, 34, 1–13. [Google Scholar] [CrossRef]
- Ouweneel, A.B.; Thomas, M.G.; Sorci-Thomas, M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles and cell membrane. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Bharti, D.; Levental, I. Membrane Heterogeneity Beyond the Plasma Membrane. Front Cell Dev. Biol. 2020, 8, 580814. [Google Scholar] [CrossRef]
- Ciarlo, L.; Manganelli, V.; Garofalo, T.; Matarrese, P.; Tinari, A.; Misasi, R.; Malorni, W.; Sorice, M. Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ. 2010, 17, 1047–1058. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Mitochondrial targeting involving cholesterol-rich lipid rafts in the mechanism of action of the antitumor ether lipid and alkylphospholipid analog edelfosine. Pharmaceutics 2021, 13, 763. [Google Scholar] [CrossRef]
- Tsfasman, T.; Kost, V.; Markushin, S.; Lotte, V.; Koptiaeva, I.; Bogacheva, E.; Baratova, L.; Radyukhin, V. Amphipathic alpha-helices and putative cholesterol binding domains of the influenza virus matrix M1 protein are crucial for virion structure organisation. Virus Res. 2015, 210, 114–118. [Google Scholar] [CrossRef]
- Radyukhin, V.A.; Dadinova, L.A.; Orlov, I.A.; Baratova, L.A. Amphipathic secondary structure elements and putative cholesterol recognizing amino acid consensus (CRAC) motifs as governing factors of highly specific matrix protein interactions with raft-type membranes in enveloped viruses. J. Biomol. Struct. Dyn. 2018, 36, 1351–1359. [Google Scholar] [CrossRef]
- Dunina-Barkovskaya, A.Y.; Vishnyakova, K.S.; Baratova, L.A.; Radyukhin, V.A. Modulation of cholesterol-dependent activity of macrophages IC-21 by a peptide containing two CRAC-motifs from protein M1 of influenza virus. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2019, 13, 268–276. [Google Scholar] [CrossRef]
- Dunina-Barkovskaya, A.Y.; Vishnyakova, K.S. Modulation of the cholesterol-dependent activity of macrophages IC-21 by CRAC peptides with substituted motif-forming amino acids. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2020, 14, 331–343. [Google Scholar] [CrossRef]
- Koksharova, O.; Safronova, N.; Dunina-Barkovskaya, A. New Antimicrobial Peptide with Two CRAC Motifs: Activity against Escherichia coli and Bacillus subtilis. Microorganisms 2022, 10, 1538. [Google Scholar] [CrossRef]
- Rich, P.R. Mitochondrial cytochrome c oxidase: Catalysis, coupling and controversies. Biochem. Soc. Trans. 2017, 45, 813–829. [Google Scholar] [CrossRef]
- Wikström, M.; Sharma, V. Proton pumping by cytochrome c oxidase—A 40 year anniversary. Biochim. Biophys. Acta (Bioenerg.) 2018, 1859, 692–698. [Google Scholar] [CrossRef]
- Kadenbach, B.; Hüttemann, M. The subunit composition and function of mammalian cytochrome c oxidase. Mitochondrion 2015, 24, 64–76. [Google Scholar] [CrossRef]
- Helling, S.; Hüttemann, M.; Ramzan, R.; Kim, S.H.; Lee, I.; Müller, T.; Langenfeld, E.; Meyer, H.E.; Kadenbach, B.; Vogt, S.; et al. Multiple phosphorylations of cytochrome c oxidase and their functions. Proteomics 2012, 12, 950–959. [Google Scholar] [CrossRef]
- Kadenbach, B. Regulation of Mammalian 13-Subunit Cytochrome c Oxidase and Binding of other Proteins: Role of NDUFA4. Trends Endocrinol. Metab. 2017, 28, 761–770. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Vygodina, T.V.; Mukhaleva, E.; Azarkina, N.V.; Konstantinov, A.A. Cytochrome c oxidase inhibition by calcium at physiological ionic composition of the medium: Implications for physiological significance of the effect. Biochim. Biophys. Acta 2017, 1858, 982–990. [Google Scholar] [CrossRef]
- Qin, L.; Mills, D.A.; Buhrow, L.; Hiser, C.; Ferguson-Miller, S. A Conserved steroid binding site in cytochrome c oxidase. Biochemistry 2008, 47, 9931–9933. [Google Scholar] [CrossRef]
- Hiser, C.; Buhrow, L.; Liu, J.; Kuhn, L.; Ferguson-Miller, S. A conserved amphipathic ligand binding region influences K-path-dependent activity of cytochrome c oxidase. Biochemistry 2013, 52, 1385–1396. [Google Scholar] [CrossRef]
- Buhrow, L.; Hiser, C.; van Voorst, J.R.; Ferguson-Miller, S.; Kuhn, L.A. Computational prediction and in vitro analysis of potential physiological ligands of the bile acid binding site in cytochrome c oxidase. Biochemistry 2013, 52, 6995–7006. [Google Scholar] [CrossRef]
- Oleynikov, I.P.; Azarkina, N.V.; Vygodina, T.V.; Konstantinov, A.A. Interaction of cytochrome c oxidase with steroid hormones. Cells 2020, 9, 2211. [Google Scholar] [CrossRef]
- Oleynikov, I.P.; Azarkina, N.V.; Vygodina, T.V.; Konstantinov, A.A. Mechanism of inhibition of cytochrome c oxidase by Triton X-100. Biochem. (Mosc.) 2021, 86, 44–58. [Google Scholar] [CrossRef]
- Oleynikov, I.P.; Sudakov, R.V.; Azarkina, N.V.; Vygodina, T.V. Direct Interaction of Mitochondrial Cytochrome c Oxidase with Thyroid Hormones: Evidence for Two Binding Sites. Cells 2022, 11, 908. [Google Scholar] [CrossRef]
- Konstantinov, A.A. Cytochrome c oxidase: Intermediates of the catalytic cycle and their energy-coupled interconversion. FEBS Lett. 2012, 586, 630–639. [Google Scholar] [CrossRef]
- Konstantinov, A.A.; Vygodina, T.; Capitanio, N.; Papa, S. Ferrocyanide-peroxidase activity of cytochrome c oxidase. Biochim. Biophys. Acta 1998, 1363, 11–23. [Google Scholar] [CrossRef]
- Musatov, A.P.; Berka, V.; Ksenzenko, M.Y.; Vygodina, T.V.; Konstantinov, A.A. Effect of polycations on the interaction of cytochrome oxidase with artificial electron donors and cyanide. Biol. Membr. 1991, 8, 229–234. [Google Scholar]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; DiNola, A.R.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Perevoshchikova, I.V.; Davydova, L.I.; Agapov, I.A.; Bogush, V.G. Interaction of recombinant analogs of spider silk proteins 1F9 and 2E12 with phospholipid membranes. Biochim. Biophys. Acta (Biomembr.) 2010, 1798, 1172–1178. [Google Scholar] [CrossRef]
- Chertkova, R.V.; Firsov, A.M.; Brazhe, N.A.; Nikelshparg, E.I.; Bochkova, Z.V.; Bryantseva, T.V.; Semenova, M.A.; Baizhumanov, A.A.; Kotova, E.A.; Kirpichnikov, M.P.; et al. Multiple Mutations in the Non-Ordered Red Ω-Loop Enhance the Membrane-Permeabilizing and Peroxidase-like Activity of Cytochrome c. Biomolecules 2022, 12, 665. [Google Scholar] [CrossRef]
- Vygodina, T.V.; Pecoraro, C.; Mitchell, D.; Gennis, R.; Konstantinov, A.A. Mechanism of inhibition of electron transfer by amino acid replacement K362M in a proton channel of Rhodobacter sphaeroides cytochrome c oxidase. Biochemistry 1998, 37, 3053–3061. [Google Scholar] [CrossRef]
- Konstantinov, A. Cytochrome c oxidase as a proton-pumping peroxidase: Reaction cycle and electrogenic mechanism. J. Bioenerg. Biomembr. 1998, 30, 121–130. [Google Scholar] [CrossRef]
- Qin, L.; Mills, D.A.; Hiser, C.; Murphree, A.; Garavito, R.M.; Ferguson-Miller, S.; Hosler, J. Crystallographic location and mutational analysis of Zn and Cd inhibitory sites and role of lipidic carboxylates in rescuing proton path mutants in cytochrome c oxidase. Biochemistry 2007, 46, 6239–6248. [Google Scholar] [CrossRef]
- Fetter, J.; Sharpe, M.; Qian, J.; Mills, D.; Ferguson-Miller, S.; Nicholls, P. Fatty acids stimulate activity and restore respiratory control in a proton channel mutant of cytochrome c oxidase. FEBS Lett. 1996, 393, 155–160. [Google Scholar] [CrossRef]
- Smirnova, I.A.; Adelroth, P.; Gennis, R.B.; Brzezinski, P. Aspartate-132 in cytochrome c oxidase from Rhodobacter sphaeroides is involved in a two-step proton transfer during oxo-ferryl formation. Biochemistry 1999, 38, 6826–6833. [Google Scholar] [CrossRef]
- Checover, S.; Nachliel, E.; Dencher, N.A.; Gutman, M. Mechanism of proton entry into the cytoplasmic section of the proton-conducting channel of bacteriorhodopsin. Biochemistry 1997, 36, 13919–13928. [Google Scholar] [CrossRef]
- Napiwotzki, J.; Shinzawa-Itoh, K.; Yoshikawa, S.; Kadenbach, B. ATP and ADP bind to cytochrome c oxidase and regulate its activity. Biol. Chem. 1997, 378, 1013–1021. [Google Scholar] [CrossRef]
- Tsukihara, T.; Aoyama, H.; Yamashita, E.; Tomizaki, T.; Yamaguchi, H.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yoshikawa, S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 1996, 272, 1136–1144. [Google Scholar] [CrossRef]
- Ramzan, R.; Kadenbach, B.; Vogt, S. Multiple mechanisms regulate eukaryotic cytochrome c oxidase. Cells 2021, 10, 514. [Google Scholar] [CrossRef]
- Arnold, S.; Kadenbach, B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur. J. Biochem. 1997, 249, 350–354. [Google Scholar] [CrossRef]
- Musatov, A.; Robinson, N.C. Cholate-induced dimerization of detergent- or phospholipid-solubilized bovine cytochrome c oxidase. Biochemistry 2002, 41, 4371–4376. [Google Scholar] [CrossRef]
- Meidleman, S.L. Phospholipids Handbook; Cevc, G., Ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 23–38. [Google Scholar]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef]
- Elustondo, P.; Martin, L.A.; Karten, B. Mitochondrial cholesterol import. Biochim. Biophys. Acta (Mol. Cell Biol. Lipids) 2017, 1862, 90–101. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Berg, K.B.; Foster, L.J. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 2009, 50, 988–998. [Google Scholar] [CrossRef]
- Garner, A.E.; Smith, D.A.; Hooper, N.M. Visualization of detergent solubilization of membranes: Implications for the isolation of rafts. Biophys. J. 2008, 94, 1326–1340. [Google Scholar] [CrossRef]
- Schiffer, M.; Chang, C.H.; Stevens, F.J. The function of tryptophan residues in membrane proteins. Protein Eng. Des. Sel. 1992, 5, 213–214. [Google Scholar] [CrossRef]
- Landolt-Marticorena, C.; Williams, K.A.; Deber, C.M.; Reithmeier, R.A. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J. Mol. Biol. 1993, 229, 602–608. [Google Scholar] [CrossRef]
- Killian, J.A.; Salemink, I.; De Planque, M.R.; Lindblom, G.; Koeppe, R.E.; Greathouse, D.V. Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane α-helical peptides: Importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry 1996, 35, 1037–1045. [Google Scholar] [CrossRef]
- Gleason, N.J.; Vostrikov, V.V.; Greathouse, D.V.; Grant, C.V.; Opella, S.J.; Koeppe, R.E. Tyrosine replacing tryptophan as an anchor in GWALP peptides. Biochemistry 2012, 51, 2044–2053. [Google Scholar] [CrossRef]
- Sharma, V.; Wikström, M. The role of the K-channel and the active-site tyrosine in the catalytic mechanism of cytochrome c oxidase. Biochim. Biophys. Acta (Bioenerg.) 2016, 1857, 1111–1115. [Google Scholar] [CrossRef]
- Stegmaier, V.; Gorriz, R.F.; Imhof, P. Protonation Dynamics in the K-Channel of Cytochrome c Oxidase Estimated from Molecular Dynamics Simulations. Processes 2021, 9, 265. [Google Scholar] [CrossRef]
- Supekar, S.; Kaila, V.R. Dewetting transitions coupled to K-channel activation in cytochrome c oxidase. Chem. Sci. 2018, 9, 6703–6710. [Google Scholar] [CrossRef]
- Di Trani, J.M.; Moe, A.; Riepl, D.; Saura, P.; Kaila, V.R.; Brzezinski, P.; Rubinstein, J.L. Structural basis of mammalian complex IV inhibition by steroids. Proc. Natl. Acad. Sci. USA 2022, 119, e2205228119. [Google Scholar] [CrossRef]
- Lee, M.T.; Hung, W.C.; Chen, F.Y.; Huang, H.W. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 5087–5092. [Google Scholar] [CrossRef]
- Shinzawa-Itoh, K.; Sugimura, T.; Misaki, T.; Tadehara, Y.; Yamamoto, S.; Hanada, M.; Yano, N.; Nakagawa, T.; Uene, S.; Yamada, T.; et al. Monomeric structure of an active form of bovine cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2019, 116, 19945–19951. [Google Scholar] [CrossRef]
- Fowler, L.; Richardson, S.; Hatefi, Y. A rapid method for the preparation of highly purified cytochrome oxidase. Biochim. Biophys. Acta 1962, 64, 170–173. [Google Scholar] [CrossRef]
- Vygodina, T.V.; Kirichenko, A.; Konstantinov, A.A. Cation binding site of cytochrome c oxidase: Progress report. Biochim. Biophys. Acta 2014, 1837, 1188–1195. [Google Scholar] [CrossRef]
- Finel, M.; Wikström, M. Studies on the role of the oligomeric state and subunit III of cytochrome oxidase in proton translocation. Biochim. Biophys. Acta 1986, 851, 99–108. [Google Scholar] [CrossRef]
- Johnson, D.; Lardy, H. Isolation of liver or kidney mitochondria. Meth. Enzymol. 1967, 10, 94–96. [Google Scholar]
- Vinogradov, A.D.; King, T.E. The Keilin-Hartree heart muscle preparation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1979; Volume 55, pp. 118–127. [Google Scholar]

| Name | Sequence a | Description b | Inhibition of CcO | ||
|---|---|---|---|---|---|
| Solubilized, Ki(app) c | Proteo- Liposomes | Mitochondrial Membrane | |||
| P4 | Ac-RTKLWEMLVELGNMDKAVKLWRKLKR-NH2 | α-helices 3 + 6 | 7.6 μM | - | + d |
| A1 | Ac-WVGMALENRKLKKDRLKVLKMLRWT-NH2 | “scramble” | 20.8 μM | - | - |
| A2 | Ac-STKLSEMLSELGNMDKASKLSRKLSR-NH2 | α-helices 3 + 6, R/K,V,W→S | >200 μM | - | - |
| A3 | Ac-RTKLSEMLVELGNMDKAVKLSRKLKR-NH2 | α-helices 3 + 6, W→S | 197 μM | - | - |
| A4 | Ac-STKLWEMLVELGNMDKAVKLWRKLSR-NH2 | α-helices 3 + 6, R/K→S | 22.3 μM | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleynikov, I.P.; Sudakov, R.V.; Radyukhin, V.A.; Arutyunyan, A.M.; Azarkina, N.V.; Vygodina, T.V. Interaction of Amphipathic Peptide from Influenza Virus M1 Protein with Mitochondrial Cytochrome Oxidase. Int. J. Mol. Sci. 2023, 24, 4119. https://doi.org/10.3390/ijms24044119
Oleynikov IP, Sudakov RV, Radyukhin VA, Arutyunyan AM, Azarkina NV, Vygodina TV. Interaction of Amphipathic Peptide from Influenza Virus M1 Protein with Mitochondrial Cytochrome Oxidase. International Journal of Molecular Sciences. 2023; 24(4):4119. https://doi.org/10.3390/ijms24044119
Chicago/Turabian StyleOleynikov, Ilya P., Roman V. Sudakov, Victor A. Radyukhin, Alexander M. Arutyunyan, Natalia V. Azarkina, and Tatiana V. Vygodina. 2023. "Interaction of Amphipathic Peptide from Influenza Virus M1 Protein with Mitochondrial Cytochrome Oxidase" International Journal of Molecular Sciences 24, no. 4: 4119. https://doi.org/10.3390/ijms24044119
APA StyleOleynikov, I. P., Sudakov, R. V., Radyukhin, V. A., Arutyunyan, A. M., Azarkina, N. V., & Vygodina, T. V. (2023). Interaction of Amphipathic Peptide from Influenza Virus M1 Protein with Mitochondrial Cytochrome Oxidase. International Journal of Molecular Sciences, 24(4), 4119. https://doi.org/10.3390/ijms24044119







