Molecular Responses of Daphnids to Chronic Exposures to Pharmaceuticals
Abstract
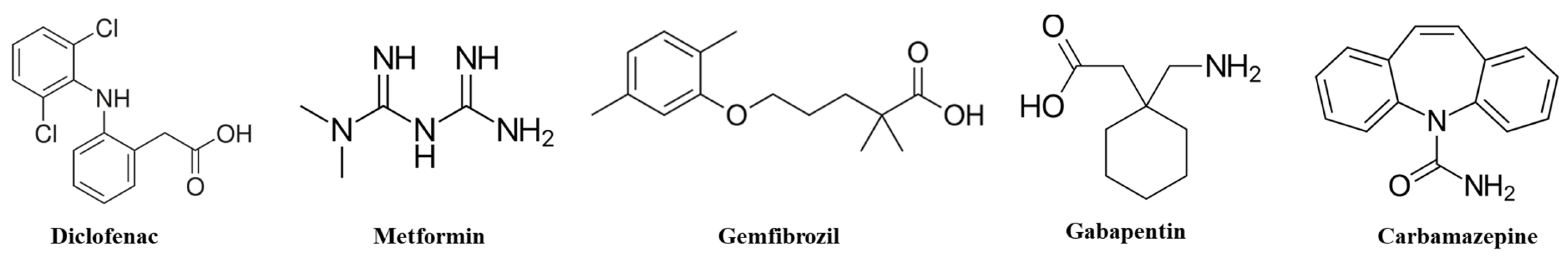
1. Introduction
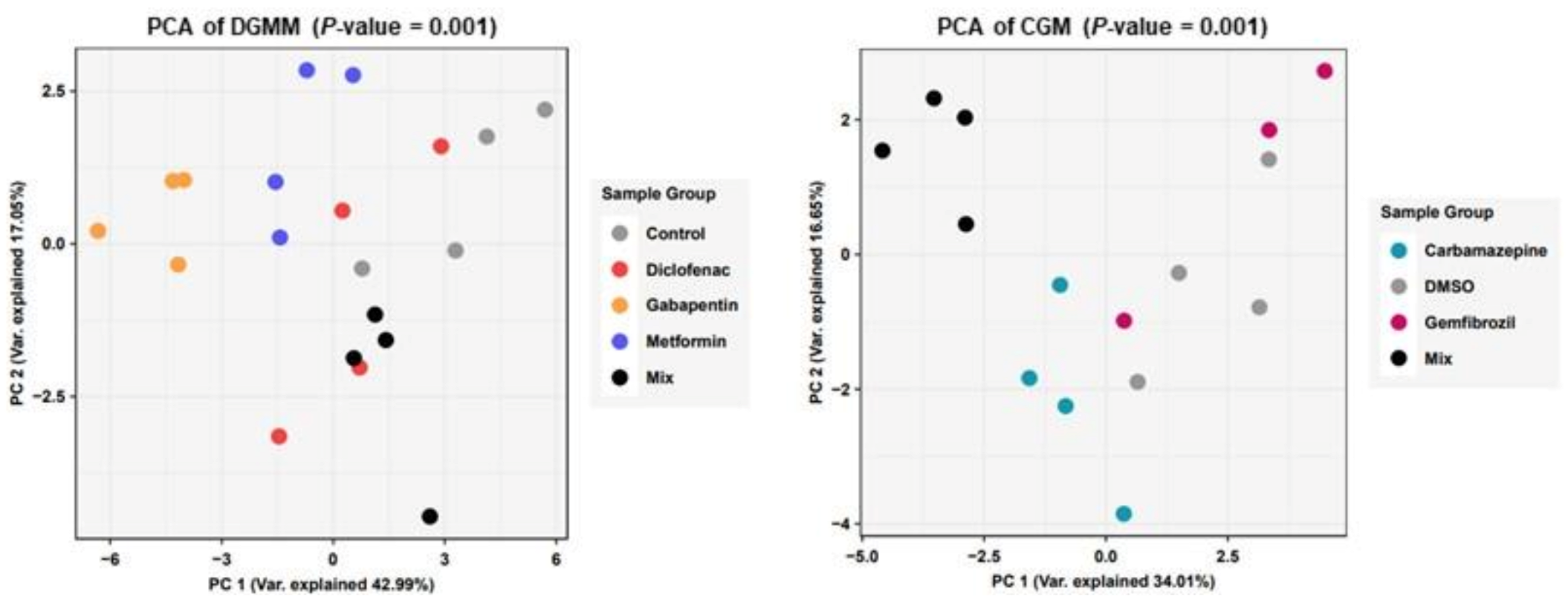
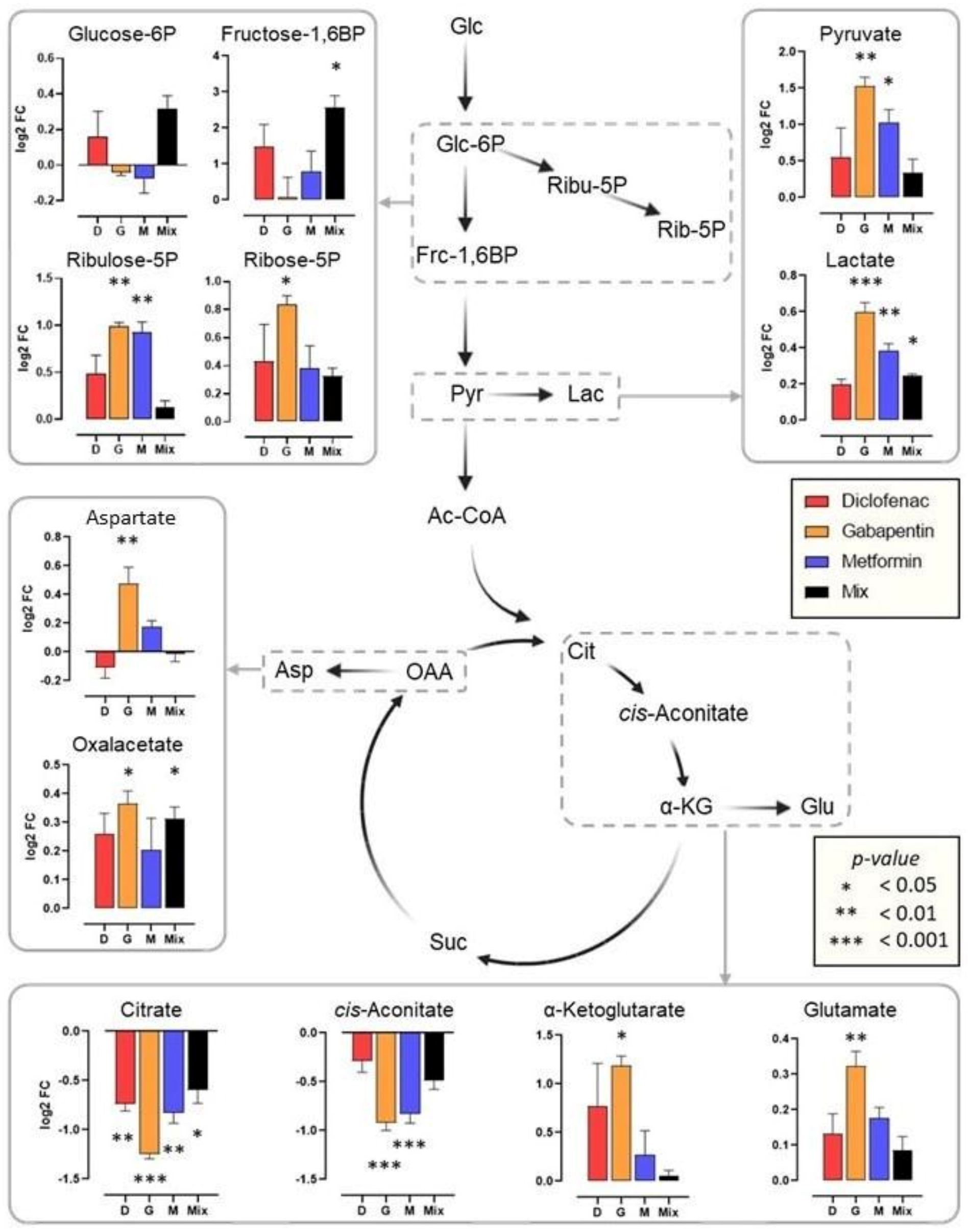
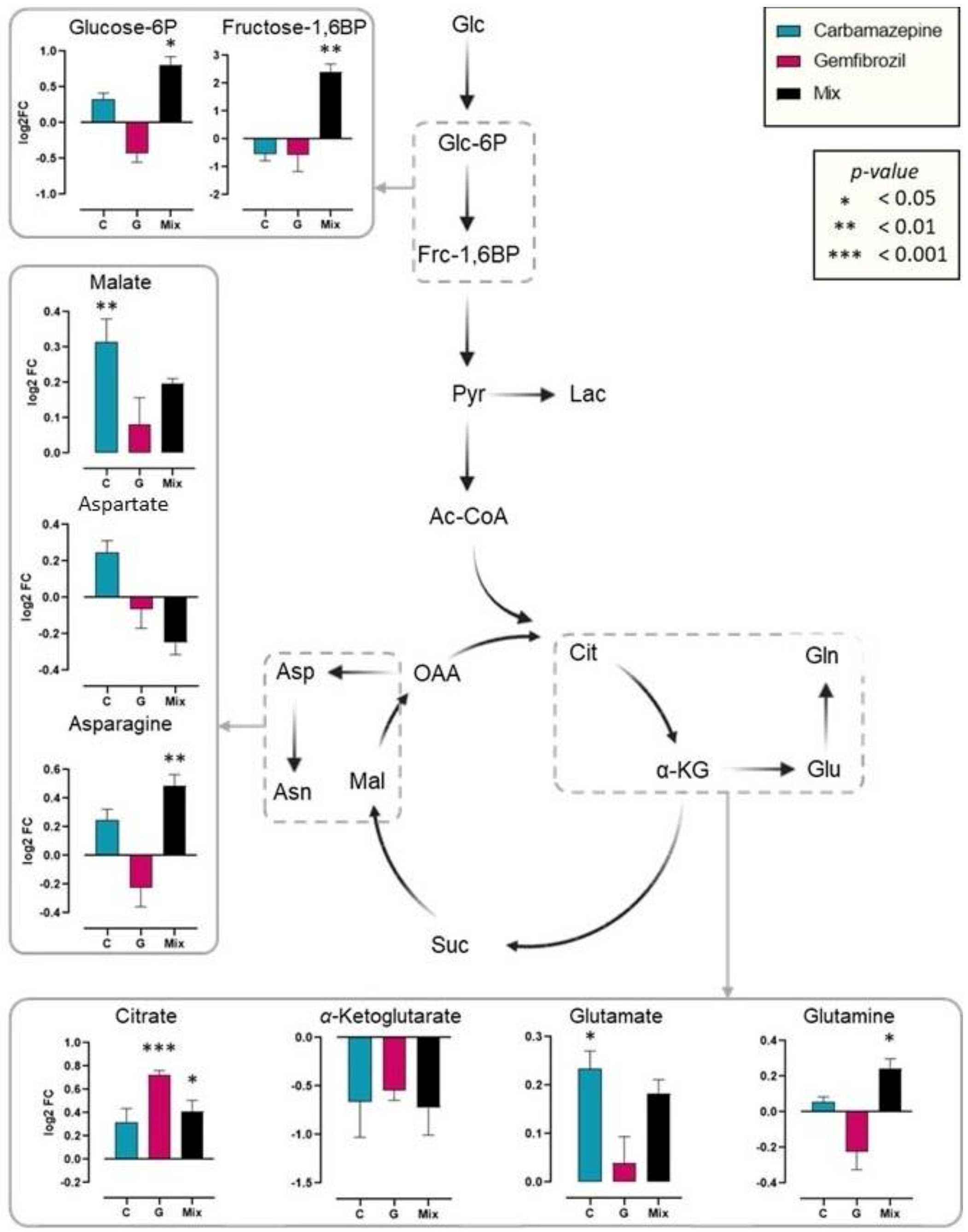
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Culturing Daphnids and Exposures to Pharmaceuticals
4.3. Sample Homogenization for Biochemical Assays
4.4. Sample Homogenization for Metabolomics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez Pena, O.I.; Lopez Zavala, M.A.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Paxeus, N.; Lo Giudice, R.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.J.; Wilson, C.J.; Brain, R.A.; Solomon, K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Brack, W.; Aissa, S.A.; Backhaus, T.; Dulio, V.; Escher, B.I.; Faust, M.; Hilscherova, K.; Hollender, J.; Hollert, H.; Müller, C.; et al. Effect-based methods are key. The European Collaborative Project SOLUTIONS recommends integrating effect-based methods for diagnosis and monitoring of water quality. Environ. Sci. Eur. 2019, 31, 10. [Google Scholar] [CrossRef]
- Könemann, S.; Kase, R.; Simon, E.; Swart, K.; Buchinger, S.; Schlüsener, M.; Hollert, H.; Escher, B.I.; Werner, I.; Aït-Aïssa, S.; et al. Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC Trends Anal. Chem. 2018, 102, 225–235. [Google Scholar] [CrossRef]
- Kruger, A.; Pieters, R.; Horn, S.; van Zijl, C.; Aneck-Hahn, N. The role of effect-based methods to address water quality monitoring in South Africa: A developing country’s struggle. Environ. Sci. Pollut. Res. Int. 2022, 29, 84049–84055. [Google Scholar] [CrossRef]
- Punt, A.; Bouwmeester, H.; Blaauboer, B.J.; Coecke, S.; Hakkert, B.; Hendriks, D.F.G.; Jennings, P.; Kramer, N.I.; Neuhoff, S.; Masereeuw, R.; et al. New approach methodologies (NAMs) for human-relevant biokinetics predictions. Meeting the paradigm shift in toxicology towards an animal-free chemical risk assessment. Altex 2020, 37, 607–622. [Google Scholar] [CrossRef]
- Westmoreland, C.; Bender, H.J.; Doe, J.E.; Jacobs, M.N.; Kass, G.E.N.; Madia, F.; Mahony, C.; Manou, I.; Maxwell, G.; Prieto, P.; et al. Use of New Approach Methodologies (NAMs) in regulatory decisions for chemical safety: Report from an EPAA Deep Dive Workshop. Regul. Toxicol. Pharmacol. RTP 2022, 135, 105261. [Google Scholar] [CrossRef]
- Colbourne, J.; Viant, M.; Shaw, J. Phylotoxicology: Breaking the artificial divide between human- and eco-toxicology. Toxicol. Lett. 2015, 238, S12. [Google Scholar] [CrossRef]
- Groh, K.J.; Carvalho, R.N.; Chipman, J.K.; Denslow, N.D.; Halder, M.; Murphy, C.A.; Roelofs, D.; Rolaki, A.; Schirmer, K.; Watanabe, K.H. Development and application of the adverse outcome pathway framework for understanding and predicting chronic toxicity: I. Challenges and research needs in ecotoxicology. Chemosphere 2015, 120, 764–777. [Google Scholar] [CrossRef]
- Blenkharn, J.I. Healthcare Wastes☆. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2015; pp. 453–476. [Google Scholar]
- Bungau, S.; Tit, D.M.; Fodor, K.; Cioca, G.; Agop, M.; Iovan, C.; Cseppento, D.C.N.; Bumbu, A.; Bustea, C. Aspects Regarding the Pharmaceutical Waste Management in Romania. Sustainability 2018, 10, 2788. [Google Scholar] [CrossRef]
- Rogowska, J.; Zimmermann, A. Household Pharmaceutical Waste Disposal as a Global Problem-A Review. Int. J. Environ. Res. Public Health 2022, 19, 5798. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kummerer, K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes—Degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Insani, W.N.; Qonita, N.A.; Jannah, S.S.; Nuraliyah, N.M.; Supadmi, W.; Gatera, V.A.; Alfian, S.D.; Abdulah, R. Improper disposal practice of unused and expired pharmaceutical products in Indonesian households. Heliyon 2020, 6, e04551. [Google Scholar] [CrossRef]
- Seehusen, D.A.; Edwards, J. Patient practices and beliefs concerning disposal of medications. J. Am. Board Fam. Med. JABFM 2006, 19, 542–547. [Google Scholar] [CrossRef]
- Topp, E.; Monteiro, S.C.; Beck, A.; Coelho, B.B.; Boxall, A.B.; Duenk, P.W.; Kleywegt, S.; Lapen, D.R.; Payne, M.; Sabourin, L.; et al. Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Sci. Total Environ. 2008, 396, 52–59. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E. Applications of mass spectrometry to identifying pharmaceutical transformation products in water treatment. TrAC Trends Anal. Chem. 2008, 27, 807–820. [Google Scholar] [CrossRef]
- Shaw, J.R.; Pfrender, M.E.; Eads, B.D.; Klaper, R.; Callaghan, A.; Sibly, R.M.; Colson, I.; Jansen, B.; Gilbert, D.; Colbourne, J.K. Daphnia as an emerging model for toxicological genomics. In Advances in Experimental Biology; Hogstrand, C., Kille, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 2, pp. 165–328. [Google Scholar]
- Colbourne, J.K.; Pfrender, M.E.; Gilbert, D.; Thomas, W.K.; Tucker, A.; Oakley, T.H.; Tokishita, S.; Aerts, A.; Arnold, G.J.; Basu, M.K.; et al. The ecoresponsive genome of Daphnia pulex. Science 2011, 331, 555–561. [Google Scholar] [CrossRef]
- Ebert, D. Daphnia as a versatile model system in ecology and evolution. EvoDevo 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Bonnefille, B.; Gomez, E.; Alali, M.; Rosain, D.; Fenet, H.; Courant, F. Metabolomics assessment of the effects of diclofenac exposure on Mytilus galloprovincialis: Potential effects on osmoregulation and reproduction. Sci. Total Environ. 2018, 613–614, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, M.; Bebianno, M.J. Effects of non-steroidal anti-inflammatory drug (NSAID) diclofenac exposure in mussel Mytilus galloprovincialis. Aquat. Toxicol. 2014, 148, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Leverett, D.; Merrington, G.; Crane, M.; Ryan, J.; Wilson, I. Environmental quality standards for diclofenac derived under the European Water Framework Directive: 1. Aquatic organisms. Environ. Sci. Eur. 2021, 33, 133. [Google Scholar] [CrossRef]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- Oliveira, L.L.; Antunes, S.C.; Goncalves, F.; Rocha, O.; Nunes, B. Evaluation of ecotoxicological effects of drugs on Daphnia magna using different enzymatic biomarkers. Ecotoxicol. Environ. Saf. 2015, 119, 123–131. [Google Scholar] [CrossRef]
- Gomez-Olivan, L.M.; Galar-Martinez, M.; Islas-Flores, H.; Garcia-Medina, S.; SanJuan-Reyes, N. DNA damage and oxidative stress induced by acetylsalicylic acid in Daphnia magna. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2014, 164, 21–26. [Google Scholar] [CrossRef]
- Nkoom, M.; Lu, G.; Liu, J.; Dong, H.; Yang, H. Bioconcentration, behavioral, and biochemical effects of the non-steroidal anti-inflammatory drug diclofenac in Daphnia magna. Environ. Sci. Pollut. Res. Int. 2019, 26, 5704–5712. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Pan, B.; Wang, C.; Bao, S.; Nie, X. Toxic effects of diclofenac on life history parameters and the expression of detoxification-related genes in Daphnia magna. Aquat. Toxicol. 2017, 183, 104–113. [Google Scholar] [CrossRef]
- Fu, Q.; Scheidegger, A.; Laczko, E.; Hollender, J. Metabolomic Profiling and Toxicokinetics Modeling to Assess the Effects of the Pharmaceutical Diclofenac in the Aquatic Invertebrate Hyalella azteca. Environ. Sci. Technol. 2021, 55, 7920–7929. [Google Scholar] [CrossRef]
- Copolovici, L.; Timis, D.; Taschina, M.; Copolovici, D.; Cioca, G.; Bungau, S. Diclofenac Influence on photosynthetic parameters and volatile organic compounds emision from Phaseolus vulgaris L. Plants. Rev. Chim. 2017, 68, 2076–2078. [Google Scholar] [CrossRef]
- Fu, Q.; Fedrizzi, D.; Kosfeld, V.; Schlechtriem, C.; Ganz, V.; Derrer, S.; Rentsch, D.; Hollender, J. Biotransformation Changes Bioaccumulation and Toxicity of Diclofenac in Aquatic Organisms. Environ. Sci. Technol. 2020, 54, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W. Metabolomic Approaches to Investigate the Effect of Metformin: An Overview. Int. J. Mol. Sci. 2021, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Briones, R.M.; Sarmah, A.K.; Padhye, L.P. A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Knoll, S.; Huhn, C.; Kohler, H.R.; Tisler, S.; Zwiener, C.; Triebskorn, R. Effects of guanylurea, the transformation product of the antidiabetic drug metformin, on the health of brown trout (Salmo trutta f. fario). PeerJ 2019, 7, e7289. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xu, S.; Lu, Y.; Wong, K.F.; Sun, L.; Hasan, K.M.M.; Ma, A.C.H.; Tse, G.; Manno, S.H.C.; Tian, L.; et al. Metformin accelerates zebrafish heart regeneration by inducing autophagy. NPJ Regen. Med. 2021, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Jonas-Closs, R.A.; Yampolsky, L.Y.; Kirschner, M.W.; Peshkin, L. Intelligent high-throughput intervention testing platform in Daphnia. Aging Cell 2022, 21, e13571. [Google Scholar] [CrossRef]
- Lee, J.W.; Shin, Y.J.; Kim, H.; Kim, H.; Kim, J.; Min, S.A.; Kim, P.; Yu, S.D.; Park, K. Metformin-induced endocrine disruption and oxidative stress of Oryzias latipes on two-generational condition. J. Hazard. Mater. 2019, 367, 171–181. [Google Scholar] [CrossRef]
- Niemuth, N.J.; Jordan, R.; Crago, J.; Blanksma, C.; Johnson, R.; Klaper, R.D. Metformin exposure at environmentally relevant concentrations causes potential endocrine disruption in adult male fish. Environ. Toxicol. Chem. 2015, 34, 291–296. [Google Scholar] [CrossRef]
- Yan, M.; Qi, H.; Xia, T.; Zhao, X.; Wang, W.; Wang, Z.; Lu, C.; Ning, Z.; Chen, H.; Li, T.; et al. Metabolomics profiling of metformin-mediated metabolic reprogramming bypassing AMPKalpha. Metab. Clin. Exp. 2019, 91, 18–29. [Google Scholar] [CrossRef]
- Li, X.; Zhou, S.; Qian, Y.; Xu, Z.; Yu, Y.; Xu, Y.; He, Y.; Zhang, Y. The assessment of the eco-toxicological effect of gabapentin on early development of zebrafish and its antioxidant system. RSC Adv. 2018, 8, 22777–22784. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, X.; Jia, D.; Zhang, W.; Zhang, T.; Yu, Y.; Xu, Y.; Zhang, Y. A transcriptomics-based analysis of the toxicity mechanisms of gabapentin to zebrafish embryos at realistic environmental concentrations. Environ. Pollut. 2019, 251, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Fenz, R.; Blaschke, A.P.; Clara, M.; Kroiss, H.; Mascher, D.; Zessner, M. Monitoring of carbamazepine concentrations in wastewater and groundwater to quantify sewer leakage. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2005, 52, 205–213. [Google Scholar] [CrossRef]
- Tian, Y.; Xia, X.; Wang, J.; Zhu, L.; Wang, J.; Zhang, F.; Ahmad, Z. Chronic Toxicological Effects of Carbamazepine on Daphnia magna Straus: Effects on Reproduction Traits, Body Length, and Intrinsic Growth. Bull. Environ. Contam. Toxicol. 2019, 103, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Oropesa, A.L.; Floro, A.M.; Palma, P. Assessment of the effects of the carbamazepine on the endogenous endocrine system of Daphnia magna. Environ. Sci. Pollut. Res. Int. 2016, 23, 17311–17321. [Google Scholar] [CrossRef] [PubMed]
- Lurling, M.; Sargant, E.; Roessink, I. Life-history consequences for Daphnia pulex exposed to pharmaceutical carbamazepine. Environ. Toxicol. 2006, 21, 172–180. [Google Scholar] [CrossRef]
- Dumas, T.; Courant, F.; Almunia, C.; Boccard, J.; Rosain, D.; Duporte, G.; Armengaud, J.; Fenet, H.; Gomez, E. An integrated metabolomics and proteogenomics approach reveals molecular alterations following carbamazepine exposure in the male mussel Mytilus galloprovincialis. Chemosphere 2022, 286, 131793. [Google Scholar] [CrossRef]
- Barreto, A.; Luis, L.G.; Paiga, P.; Santos, L.; Delerue-Matos, C.; Soares, A.; Hylland, K.; Loureiro, S.; Oliveira, M. A multibiomarker approach highlights effects induced by the human pharmaceutical gemfibrozil to gilthead seabream Sparus aurata. Aquat. Toxicol. 2018, 200, 266–274. [Google Scholar] [CrossRef]
- Steinkey, D.; Lari, E.; Woodman, S.G.; Luong, K.H.; Wong, C.S.; Pyle, G.G. Effects of gemfibrozil on the growth, reproduction, and energy stores of Daphnia magna in the presence of varying food concentrations. Chemosphere 2018, 192, 75–80. [Google Scholar] [CrossRef]
- Salesa, B.; Ferrando, M.D.; Villarroel, M.J.; Sancho, E. Effect of the lipid regulator Gemfibrozil in the Cladocera Daphnia magna at different temperatures. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 228–234. [Google Scholar] [CrossRef]
- Quinn, B.; Schmidt, W.; O’Rourke, K.; Hernan, R. Effects of the pharmaceuticals gemfibrozil and diclofenac on biomarker expression in the zebra mussel (Dreissena polymorpha) and their comparison with standardised toxicity tests. Chemosphere 2011, 84, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Krausz, K.W.; Fang, Z.Z.; Brocker, C.; Qu, A.; Gonzalez, F.J. Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARalpha and its relevance to hepatotoxicity. Arch. Toxicol. 2014, 88, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Ploessl, F.; Bracher, F.; Laforsch, C. Single and combined toxicity of pharmaceuticals at environmentally relevant concentrations in Daphnia magna—A multigenerational study. Chemosphere 2010, 79, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Jeong, T.-Y.; Simpson, M.J. Daphnia magna metabolic profiling as a promising water quality parameter for the biological early warning system. Water Res. 2019, 166, 115033. [Google Scholar] [CrossRef]
- Taylor, N.S.; Gavin, A.; Viant, M.R. Metabolomics Discovers Early-Response Metabolic Biomarkers that Can Predict Chronic Reproductive Fitness in Individual Daphnia magna. Metabolites 2018, 8, 42. [Google Scholar] [CrossRef]
- Michalaki, A.; McGivern, A.R.; Poschet, G.; Buttner, M.; Altenburger, R.; Grintzalis, K. The Effects of Single and Combined Stressors on Daphnids-Enzyme Markers of Physiology and Metabolomics Validate the Impact of Pollution. Toxics 2022, 10, 604. [Google Scholar] [CrossRef]
- Worthington, K.; Worthington, V. Worthington Enzyme Manual. Available online: https://www.worthington-biochem.com/index/manual.html (accessed on 9 September 2022).
- Tang, S.S.; Lin, C.C.; Chang, G.G. Metal-catalyzed oxidation and cleavage of octopus glutathione transferase by the Cu(II)-ascorbate system. Free Radic. Biol. Med. 1996, 21, 955–964. [Google Scholar] [CrossRef]
- Warholm, M.; Guthenberg, C.; Mannervik, B.; Pacifici, G.M.; Rane, A. Glutathione S-transferases in human fetal liver. Acta Chem. Scandinavica. Ser. B Org. Chem. Biochem. 1981, 35, 225–227. [Google Scholar] [CrossRef]
- Grintzalis, K.; Georgiou, C.D.; Schneider, Y.J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal. Biochem. 2015, 480, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, M.; Li, X.; Abdallah, M.A.; Stubbings, W.; Yan, N.; Barnard, M.; Guo, L.H.; Colbourne, J.K.; Orsini, L. Daphnia as a Sentinel Species for Environmental Health Protection: A Perspective on Biomonitoring and Bioremediation of Chemical Pollution. Environ. Sci. Technol. 2022, 56, 14237–14248. [Google Scholar] [CrossRef] [PubMed]
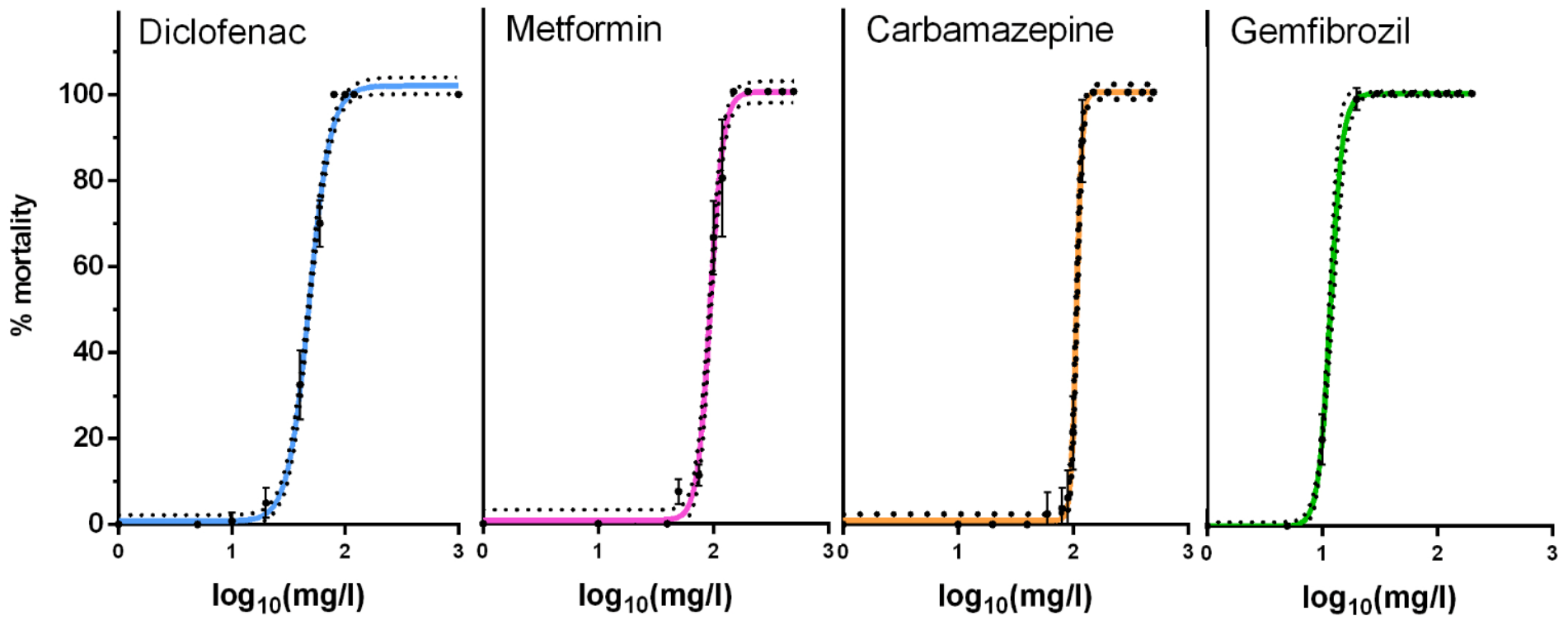
| Chemical | Hill Slope | EC50 | EC10 | EC5 | EC1 |
|---|---|---|---|---|---|
| Diclofenac | 4.6 | 101.3 | 62.8 | 53.4 | 37.3 |
| Metformin | 7.76 | 99.04 | 74.6 | 67.8 | 54.8 |
| Carbamazepine | 18.21 | 107.5 | 95.3 | 91.5 | 83.5 |
| Gemfibrozil | 8.3 | 100 | 76.7 | 70.1 | 57.5 |
| Enzyme | Control | Diclofenac | Metformin | Gabapentin | Mixture |
|---|---|---|---|---|---|
| ALP | 13.8 ± 1.42 | 11.7 ± 0.82 (−15.2%) * | 11.3 ± 0.71 (−18.1%) * | 13.4 ± 1.47 | 11.8 ± 1.66 |
| ACP | 4.4 ± 0.34 | 3.2 ± 0.01 (−27.3%) * | 4.1 ± 0.41 | 3.8 ± 0.24 (−13.6%) * | 3.6 ± 0.28 (−18.2%) * |
| βGAL | 3.2 ± 0.36 | 2.7 ± 0.32 | 2.5 ± 0.29 (−21.9%) * | 2.8 ± 0.14 | 3.1 ± 0.26 |
| LIP | 12.2 ± 0.44 | 12 ± 1.43 | 14.1 ± 2.4 | 14.8 ± 0.38 (+21.3%) * | 12 ± 1.45 |
| PEP | 137 ± 14.4 | 170 ± 11.3 (+24.1%) * | 148 ± 10.3 | 143 ± 15.6 | 154 ± 13.5 |
| LDH | 39.4 ± 8.36 | 31.2 ± 6 | 50.1 ± 4.23 | 46.2 ± 6.71 | 71.9 ± 11.2 (+82.5%) * |
| GST | 39.4 ± 2.57 | 34.5 ± 11 | 50.9 ± 2.12 (+29.2%) * | 52 ± 4.62 (+31.2%) * | 55.6 ± 1.72 (+41.1%) * |
| Enzyme | Control | DMSO | Carbamazpine | Gemfibrozil | Mixture |
|---|---|---|---|---|---|
| ALP | 3.7 ± 0.33 | 4.5 ± 0.42 $ | 3.9 ± 0.31 | 5 ± 0.18 | 3.9 ± 0.28 |
| ACP | 2.9 ± 0.13 | 3.2 ± 0.19 $ | 2.7 ± 0.2 (−15.6%) * | 2.9 ± 0.02 (−9.4%) * | 3 ± 0.11 |
| βGAL | 1.6 ± 0.23 | 1.8 ± 0.19 | 1.9 ± 0.2 | 2.5 ± 0.22 (+38.9%) * | 2 ± 0.12 |
| LIP | 71.5 ± 6.71 | 86.2 ± 4.7 $ | 84.4 ± 6.35 | 82.4 ± 3.56 | 92.2 ± 6.15 |
| PEP | 319 ± 55.4 | 554 ± 15.5 $ | 300 ± 78.1 (−45.8%) * | 394 ± 33.2 (−28.9%) * | 379 ± 16.6 (−31.6%) * |
| LDH | 78.3 ± 13.3 | 148 ± 23.4 $ | 154 ± 32.5 | 126 ± 14.9 | 113 ± 10.9 (−23.6%) * |
| GST | 56.3 ± 10.8 | 79.6 ± 6.07 $ | 45.4 ± 6.88 (−43%) * | 71.4 ± 0.74 (−10.3%) * | 67.2 ± 7.83 (−15.6%) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Rourke, K.; Engelmann, B.; Altenburger, R.; Rolle-Kampczyk, U.; Grintzalis, K. Molecular Responses of Daphnids to Chronic Exposures to Pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 4100. https://doi.org/10.3390/ijms24044100
O’Rourke K, Engelmann B, Altenburger R, Rolle-Kampczyk U, Grintzalis K. Molecular Responses of Daphnids to Chronic Exposures to Pharmaceuticals. International Journal of Molecular Sciences. 2023; 24(4):4100. https://doi.org/10.3390/ijms24044100
Chicago/Turabian StyleO’Rourke, Katie, Beatrice Engelmann, Rolf Altenburger, Ulrike Rolle-Kampczyk, and Konstantinos Grintzalis. 2023. "Molecular Responses of Daphnids to Chronic Exposures to Pharmaceuticals" International Journal of Molecular Sciences 24, no. 4: 4100. https://doi.org/10.3390/ijms24044100
APA StyleO’Rourke, K., Engelmann, B., Altenburger, R., Rolle-Kampczyk, U., & Grintzalis, K. (2023). Molecular Responses of Daphnids to Chronic Exposures to Pharmaceuticals. International Journal of Molecular Sciences, 24(4), 4100. https://doi.org/10.3390/ijms24044100









