Integrative Roles of Dopamine Pathway and Calcium Channels Reveal a Link between Schizophrenia and Opioid Use Disorder
Abstract
1. Introduction
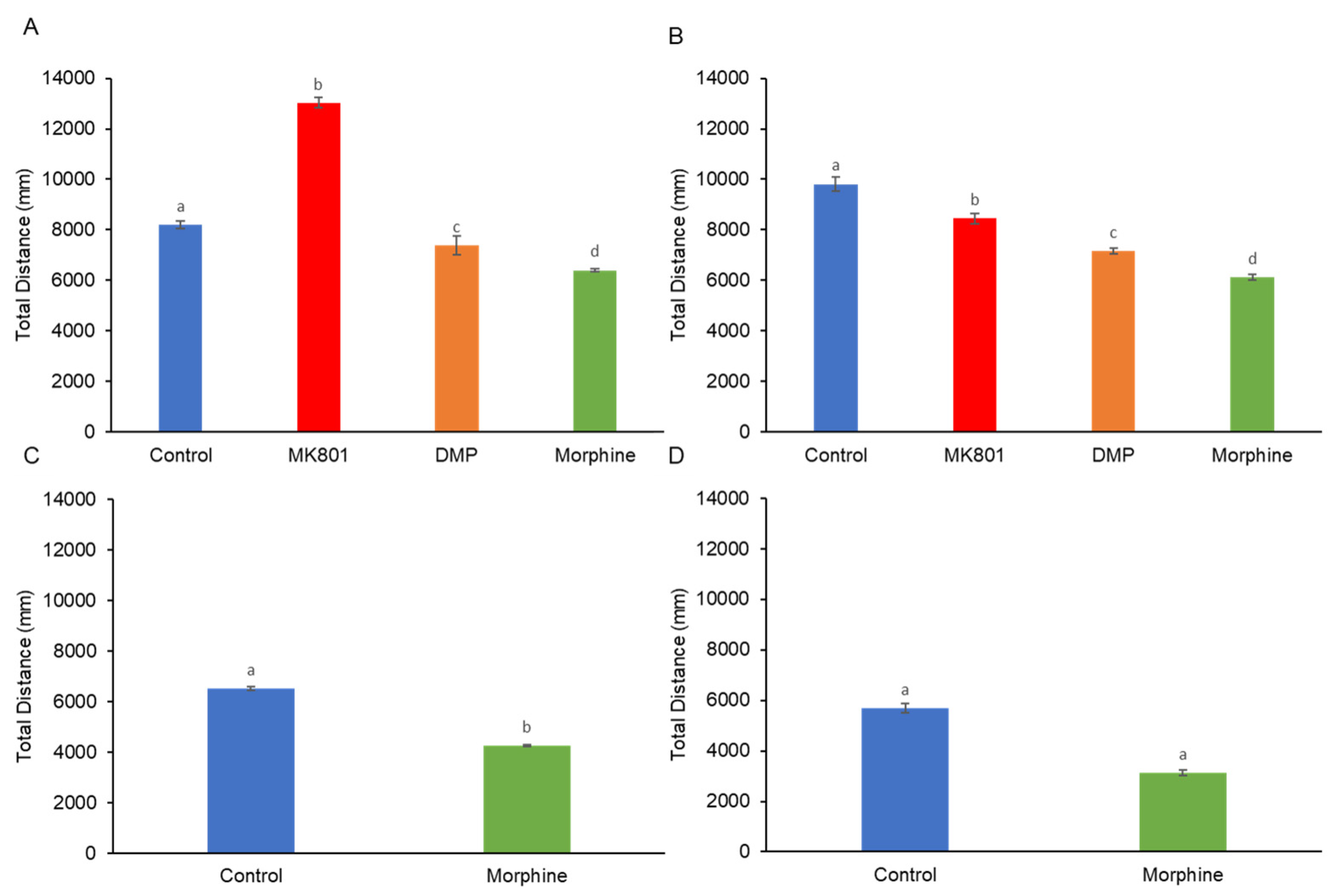
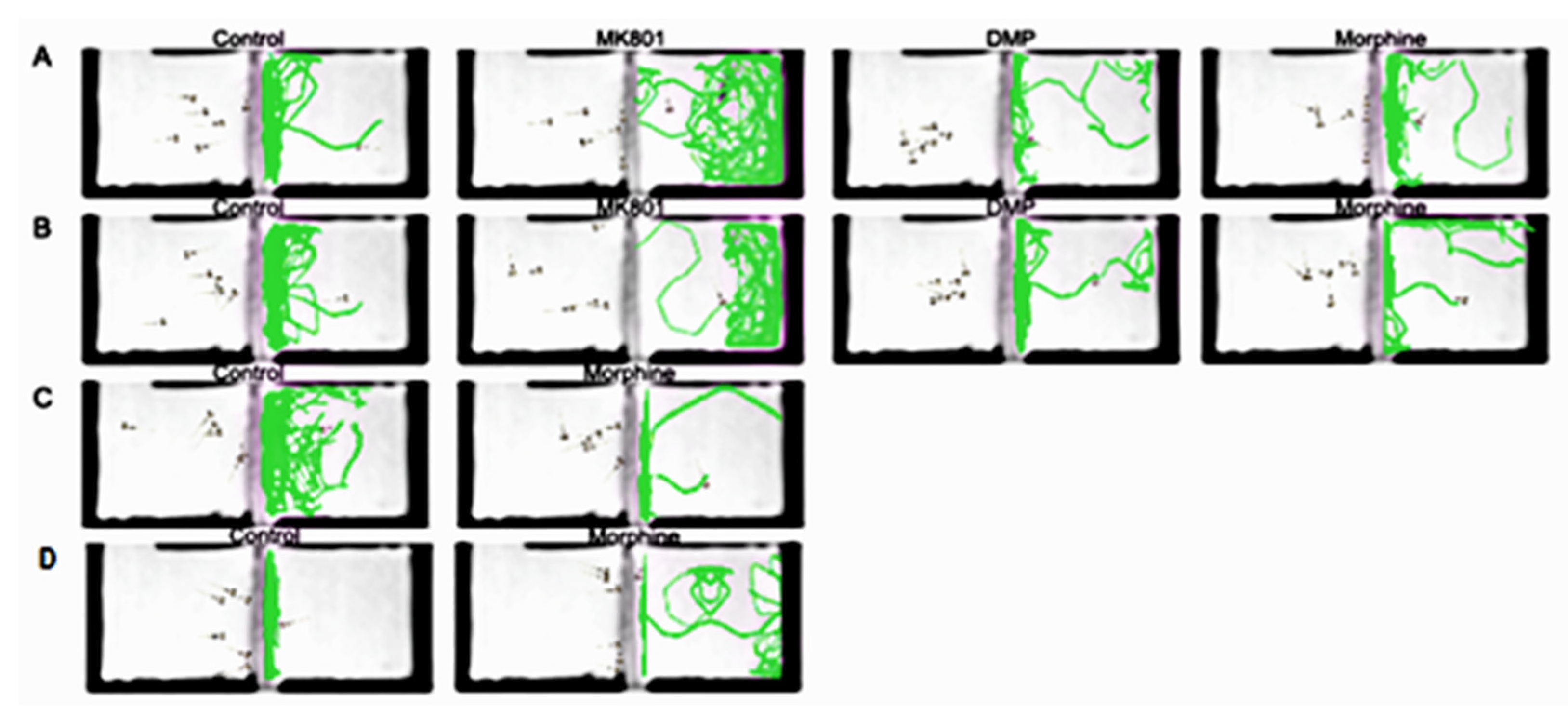
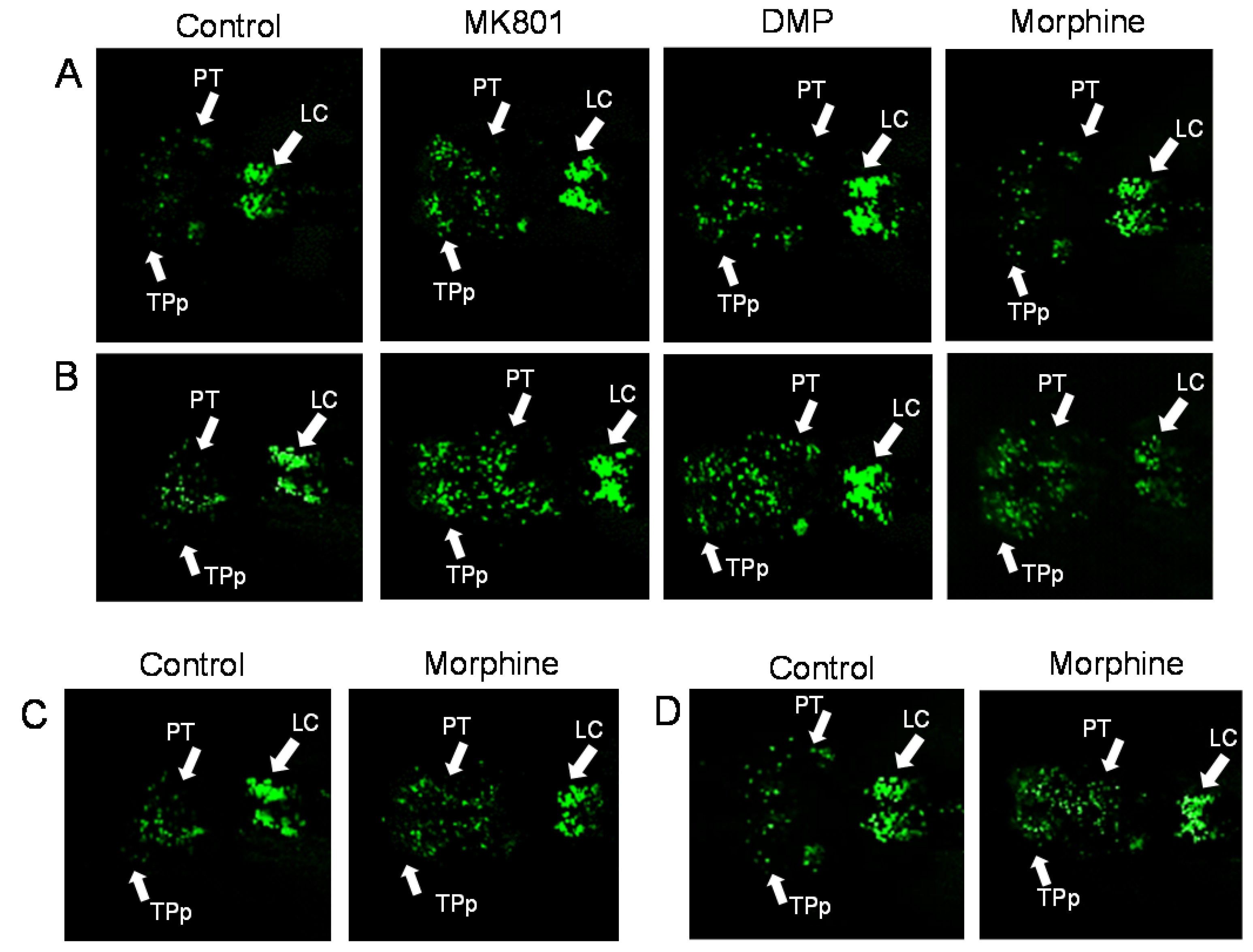
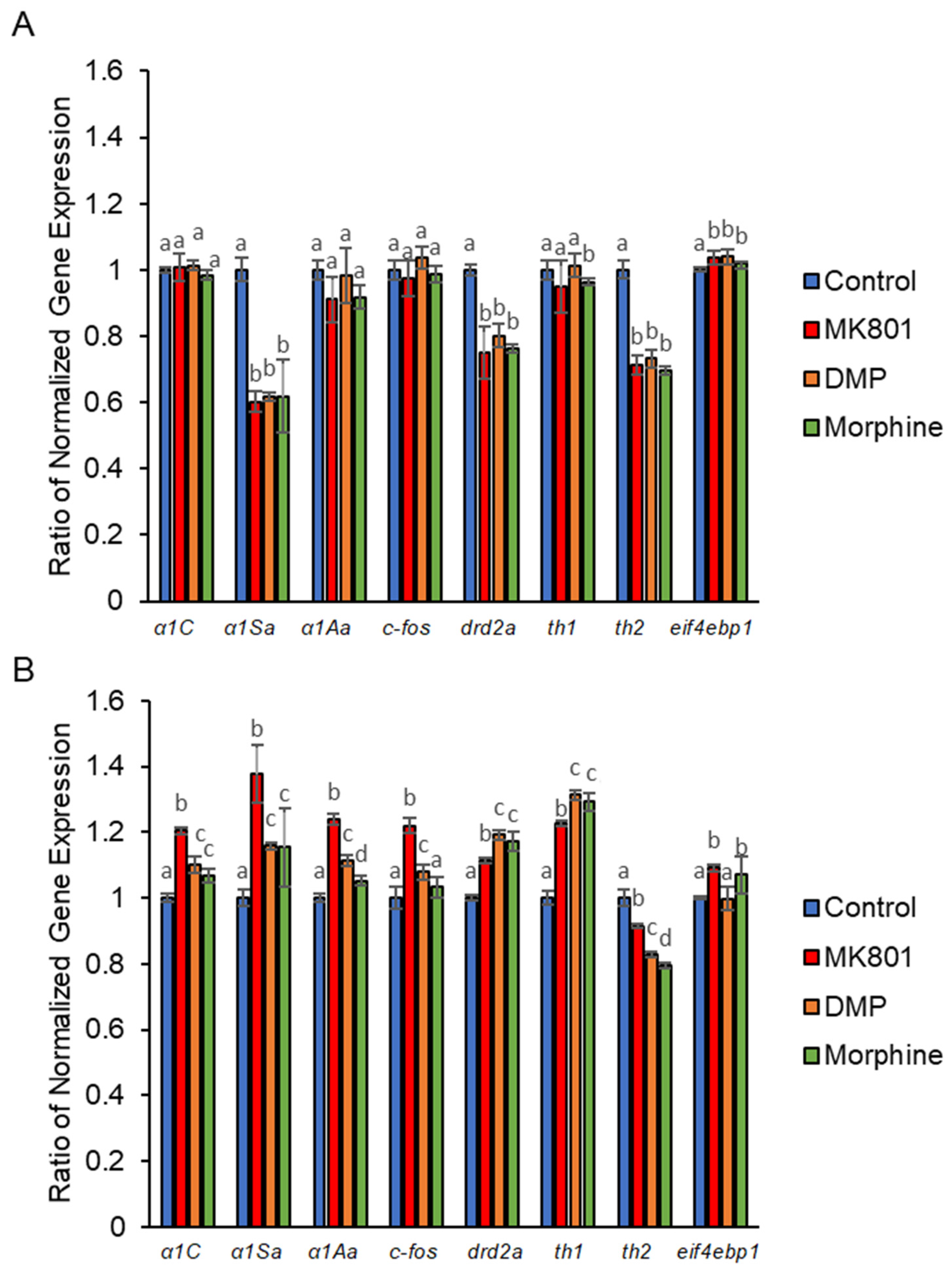
2. Results
3. Discussion
4. Materials and Methods
4.1. Zebrafish Strains and Housing Conditions
4.2. Drug Optimization and Treatments
4.3. Behavioral Analyses
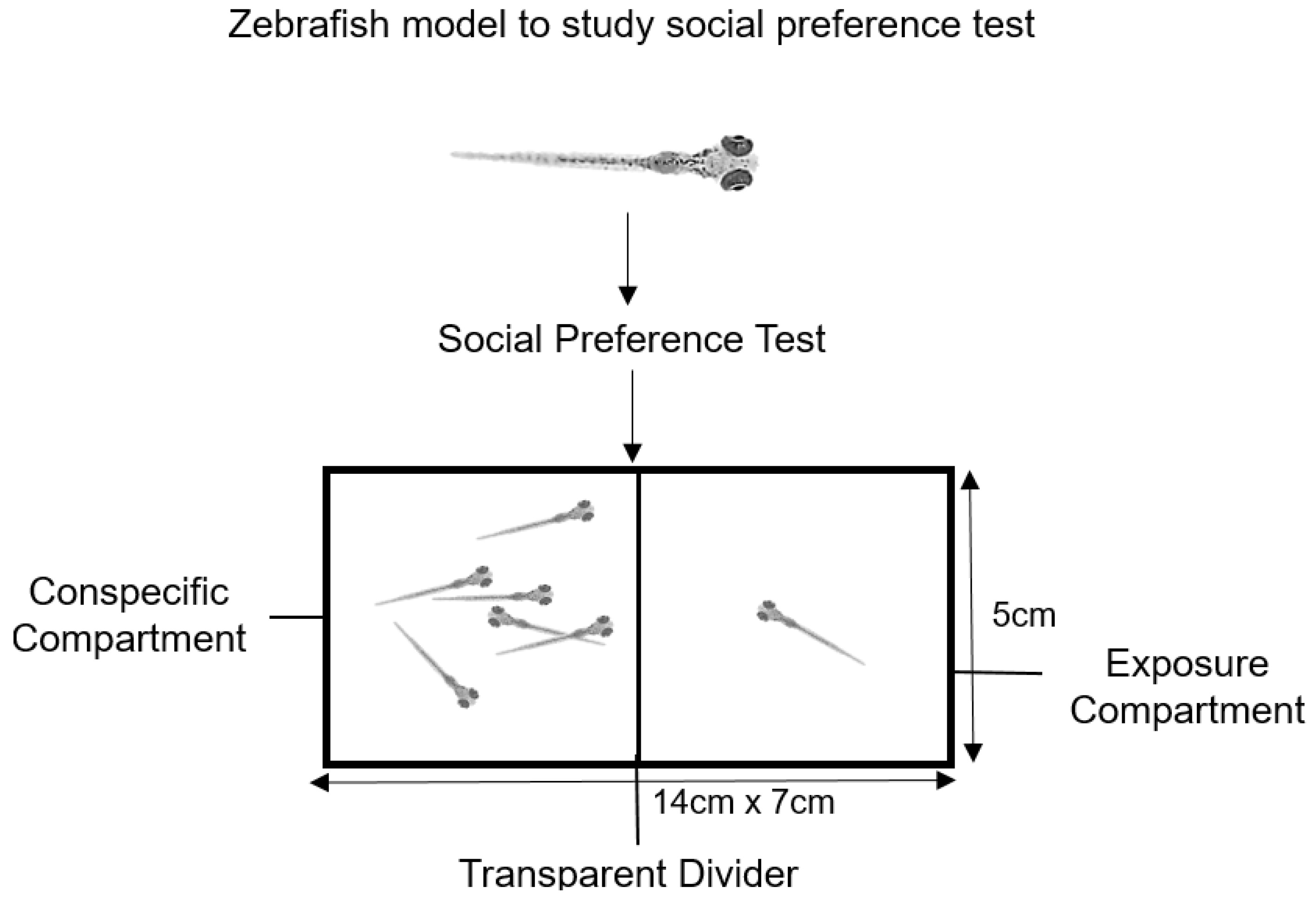
4.4. Social Preference
4.5. Gene Expression Analysis by Real-Time PCR
4.6. Total Protein Purification
4.7. Biochemical Assay
4.8. Confocal Microscopy
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Karam, E.G.; Yabroudi, P.F.; Melhem, N.M. Comorbidity of substance abuse and other psychiatric disorders in acute general psychiatric admissions: A study from Lebanon. Compr. Psychiatry 2002, 43, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D. Substance Use Disorders in Schizophrenia—Clinical Implications of Comorbidity. Schizophr. Bull. 2009, 35, 469–472. [Google Scholar] [CrossRef]
- Hartz, S.M.; Pato, C.N.; Medeiros, H.; Cavazos-Rehg, P.; Sobell, J.L.; Knowles, J.A.; Bierut, L.J.; Pato, M.T.; Genomic Psychiatry Cohort Consortium. Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry 2014, 71, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Helzer, J.E.; Pryzbeck, T.R. The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J. Stud. Alcohol 1988, 49, 219–224. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, P.A.; Mittleman, G. Schizophrenia and psychostimulant abuse: A review and re-analysis of clinical evidence. Psychopharmacology 1995, 121, 407–427. [Google Scholar] [CrossRef]
- Diwan, A.; Castine, M.; Pomerleau, C.S.; Meador-Woodruff, J.H.; Dalack, G.W. Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr. Res. 1998, 33, 113–118. [Google Scholar] [CrossRef]
- Di Forti, M.; Morgan, C.; Dazzan, P.; Pariante, C.M.; Mondelli, V.; Marques, T.R.; Handley, R.; Luzi, S.; Russo, M.; Paparelli, A.; et al. High-potency cannabis and the risk of psychosis. Br. J. Psychiatry 2009, 195, 488–491. [Google Scholar] [CrossRef]
- Moore, T.H.; Zammit, S.; Lingford-Hughes, A.; Barnes, T.R.; Jones, P.B.; Burke, M.; Lewis, G. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet 2007, 370, 319–328. [Google Scholar] [CrossRef]
- Gage, S.H.; Hickman, M.; Zammit, S. Association between cannabis and psychosis: Epidemiologic evidence. Biol. Psychiatry 2016, 79, 549–556. [Google Scholar] [CrossRef]
- Gage, S.H.; Jones, H.J.; Burgess, S.; Bowden, J.; Smith, G.D.; Zammit, S.; Munafo, M.R. Assessing causality in associations between cannabis use and schizophrenia risk: A two-sample Mendelian randomization study. Psychol. Med. 2017, 47, 971–980. [Google Scholar] [CrossRef]
- Kovasznay, B.; Fleischer, J.; Tanenberg-Karant, M.; Jandorf, L.; Miller, A.D.; Bromet, E. Substance use disorder and the early course of illness in schizophrenia and affective psychosis. Schizophr. Bull. 1997, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Addington, J.; Addington, D. Substance abuse and cognitive functioning in schizophrenia. J. Psychiatry Neurosci. 1997, 22, 99–104. [Google Scholar] [PubMed]
- Hunt, G.E.; Bergen, J.; Bashir, M. Medication compliance and comorbid substance abuse in schizophrenia: Impact on community survival 4 years after a relapse. Schizophr. Res. 2002, 54, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.S.; Wagner, H.R.; Swanson, J.W.; Stroup, T.S.; McEvoy, J.P.; Canive, J.M.; Miller, D.D.; Reimherr, F.; McGee, M.; Khan, A.; et al. Substance use in persons with schizophrenia: Baseline prevalence and correlates from the NIMH CATIE study. J. Nerv. Ment. Dis. 2006, 194, 164–172. [Google Scholar] [CrossRef]
- Schmidt, L.M.; Hesse, M.; Lykke, J. The impact of substance use disorders on the course of schizophrenia—A 15-year follow-up study: Dual diagnosis over 15 years. Schizophr. Res. 2011, 130, 228–233. [Google Scholar] [CrossRef]
- Janssen, B.; Gaebel, W.; Haerter, M.; Komaharadi, F.; Lindel, B.; Weinmann, S. Evaluation of factors influencing medication compliance in inpatient treatment of psychotic disorders. Psychopharmacology 2006, 187, 229–236. [Google Scholar] [CrossRef]
- Strømme, M.F.; Mellesdal, L.S.; Bartz-Johannesen, C.A.; Kroken, R.A.; Krogenes, M.L.; Mehlum, L.; Johnsen, E. Use of benzodiazepines and antipsychotic drugs are inversely associated with acute readmission risk in schizophrenia. J. Clin. Psychopharmacol. 2022, 42, 37–42. [Google Scholar] [CrossRef]
- Goldstein, M.; Deutch, A.Y. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992, 6, 2413–2421. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; Rodenhiser, J.; Printz, D.; Zea-Ponce, Y.; Gil, R.; Kegeles, L.S.; Weiss, R.; Cooper, T.B.; Mann, J.J.; Van Heertum, R.L.; et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. USA 2000, 97, 8104–8109. [Google Scholar] [CrossRef]
- Abi-Dargham, A.; van de Giessen, E.; Slifstein, M.; Kegeles, L.S.; Laruelle, M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol. Psychiatry 2009, 65, 1091–1093. [Google Scholar] [CrossRef]
- Howes, O.O.; Fusar-Poli, P.; Bloomfield, M.; Selvaraj, S.; McGuire, P. From the prodrome to chronic schizophrenia: The neurobiology underlying psychotic symptoms and cognitive impairments. Curr. Pharm. Des. 2012, 18, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kambeitz, J.; Kim, E.; Stahl, D.; Slifstein, M.; Abi-Dargham, A.; Kapur, S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: Meta-analysis of imaging studies. Arch. Gen. Psychiatry 2012, 69, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Williams, M.; Ibrahim, K.; Leung, G.; Egerton, A.; McGuire, P.K.; Turkheimer, F. Midbrain dopamine function in schizophrenia and depression: A post-mortem and positron emission tomographic imaging study. Brain 2013, 136, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Manion, M.T.C.; Glasper, E.R.; Wang, K.H. A sex difference in mouse dopaminergic projections from the midbrain to basolateral amygdala. Biol. Sex Differ. 2022, 13, 75. [Google Scholar] [CrossRef]
- Radua, J.; Schmidt, A.; Borgwardt, S.; Heinz, A.; Schlagenhauf, F.; McGuire, P.; Fusar-Poli, P. Ventral striatal activation during reward processing in psychosis: A neurofunctional meta-analysis. JAMA Psychiatry 2015, 72, 1243–1251. [Google Scholar] [CrossRef]
- Panayi, M.C.; Boerner, T.; Jahans-Price, T.; Huber, A.; Sprengel, R.; Gilmour, G.; Sanderson, D.J.; Harrison, P.J.; Walton, M.E.; Bannerman, D.M. Glutamatergic dysfunction leads to a hyper-dopaminergic phenotype through deficits in short-term habituation: A mechanism for aberrant salience. Mol. Psychiatry 2022, 28, 579–587. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef]
- Koob, G.F.; Bloom, F.E. Cellular and molecular mechanisms of drug dependence. Science 1988, 242, 715–723. [Google Scholar] [CrossRef]
- Koob, G.F. Neural mechanisms of drug reinforcement. Ann. N. Y. Acad. Sci. 1992, 654, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Logan, J.; Gatley, S.J.; Wong, C.; Hitzemann, R.; Pappas, N.R. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2receptors. J. Pharmacol. Exp. Ther. 1999, 291, 409–415. [Google Scholar] [PubMed]
- Drevets, W.C.; Gautier, C.; Price, J.C.; Kupfer, D.J.; Kinahan, P.E.; Grace, A.A.; Price, J.L.; Mathis, C.A. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol. Psychiatry 2001, 49, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Mandelkern, M.A.; Olmstead, R.E.; Allen-Martinez, Z.; Scheibal, D.; Abrams, A.L.; Costello, M.R.; Farahi, J.; Saxena, S.; Monterosso, J.; et al. Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology 2009, 34, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Boileau, I.; Assaad, J.M.; Pihl, R.O.; Benkelfat, C.; Leyton, M.; Diksic, M.; Tremblay, R.E.; Dagher, A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 2003, 49, 226–231. [Google Scholar] [CrossRef]
- Bossong, M.G.; Van Berckel, B.N.; Boellaard, R.; Zuurman, L.; Schuit, R.C.; Windhorst, A.D.; van Gerven, J.; Ramsey, N.F.; Lammertsma, A.A.; Kahn, R.S. Δ9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 2009, 34, 759–766. [Google Scholar] [CrossRef]
- Wise, R.A. Brain reward circuitry: Insights from unsensed incentives. Neuron 2002, 36, 229–240. [Google Scholar] [CrossRef]
- Owesson-White, C.A.; Ariansen, J.; Stuber, G.D.; Cleaveland, N.A.; Cheer, J.F.; Mark Wightman, R.; Carelli, R.M. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur. J. Neurosci. 2009, 30, 1117–1127. [Google Scholar] [CrossRef]
- Björklund, A.; Lindvall, O. Dopamine-containing systems in the CNS. In Handbook of Chemical Neuroanatomy Vol. 2: Classical Transmitters in the CNS; Björklund, A., Hokfelt, T., Eds.; Elsevier: Toronto, ON, Canada, 1984; pp. 55–122. [Google Scholar]
- Blenau, W.; Baumann, A. Molecular and pharmacological properties of insect biogenic amine receptors: Lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. Physiol. 2001, 48, 13–38. [Google Scholar] [CrossRef]
- Roeder, T. Biochemistry and molecular biology of receptors for biogenic amines in locusts. Microsc. Res. Tech. 2002, 56, 237–247. [Google Scholar] [CrossRef]
- Perrone-Capano, C.; Tino, A.; di Porzio, U. Target cells modulate dopamine transporter gene expression during brain development. Neuroreport 1994, 5, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hasegawa, H. Pharmacological intervention of brain neurotransmission affects exercise capacity. In Physical Activity and the Aging Brain; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 53–64. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Matteson, D.R. Two distinct populations of calcium channels in a clonal line of pituitary cells. Science 1985, 227, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J. Gen. Physiol. 1985, 86, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Borgkvist, A.; Mosharov, E.V.; Sulzer, D. Calcium currents regulate dopamine autoreceptors. Brain 2014, 137, 2113–2115. [Google Scholar] [CrossRef]
- Heyes, S.; Pratt, W.S.; Rees, E.; Dahimene, S.; Ferron, L.; Owen, M.J.; Dolphin, A.C. Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog. Neurobiol. 2015, 134, 36–54. [Google Scholar] [CrossRef]
- Zamponi, G.W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W.A. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Hall, J.; Trent, S.; Thomas, K.L.; O’Donovan, M.C.; Owen, M.J. Genetic risk for schizophrenia: Convergence on synaptic pathways involved in plasticity. Biol. Psychiatry 2015, 77, 52–58. [Google Scholar] [CrossRef]
- Moon, A.L.; Haan, N.; Wilkinson, L.S.; Thomas, K.L.; Hall, J. CACNA1C: Association with psychiatric disorders, behavior, and neurogenesis. Schizophr. Bull. 2018, 44, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Dao, D.T.; Terrillion, C.E.; Arad, M.; Smith, R.J.; Soldatov, N.M.; Gould, T.D. CACNA1C (Cav1. 2) in the pathophysiology of psychiatric disease. Prog. Neurobiol. 2012, 99, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Guo, S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin. Drug Discov. 2009, 4, 715–726. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Zebrafish get connected: Investigating neurotransmission targets and alterations in chemical toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef]
- Rink, E.; Wullimann, M.F. Development of the catecholaminergic system in the early zebrafish brain: An immunohistochemical study. Dev. Brain Res. 2002, 137, 89–100. [Google Scholar] [CrossRef]
- Wise, R.A. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 1996, 19, 319–340. [Google Scholar] [CrossRef]
- Berridge, K.C. From prediction error to incentive salience: Mesolimbic computation of reward motivation. Eur. J. Neurosci. 2012, 35, 1124–1143. [Google Scholar] [CrossRef] [PubMed]
- Juckel, G.; Schlagenhauf, F.; Koslowski, M.; Filonov, D.; Wüstenberg, T.; Villringer, A.; Knutson, B.; Kienast, T.; Gallinat, J.; Wrase, J.; et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology 2006, 187, 222–228. [Google Scholar] [CrossRef]
- Wolf, D.H.; Satterthwaite, T.D.; Kantrowitz, J.J.; Katchmar, N.; Vandekar, L.; Elliott, M.A.; Ruparel, K. Amotivation in schizophrenia: Integrated assessment with behavioral, clinical, and imaging measures. Schizophr. Bull. 2014, 40, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.; Lizardi-Ortiz, J.E.; Ramos, M.; De Mei, C.; Hopf, F.W.; Iaccarino, C.; Halbout, B.; Jacobsen, J.; Kinoshita, C.; Welter, M.; et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J. Neurosci. 2012, 32, 9023–9034. [Google Scholar] [CrossRef]
- Stelzel, C.; Basten, U.; Montag, C.; Reuter, M.; Fiebach, C.J. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J. Neurosci. 2010, 30, 14205–14212. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Y.; Zhang, G.; Jin, C.; Zhou, Z.; Cheng, Z.; Yuan, G. Correlation of DRD2 mRNA expression levels with deficit syndrome severity in chronic schizophrenia patients receiving clozapine treatment. Oncotarget 2017, 8, 86515–86526. [Google Scholar] [CrossRef]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Boehmler, W.; Obrecht-Pflumio, S.; Canfield, V.; Thisse, C.; Thisse, B.; Levenson, R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev. Dyn. 2004, 230, 481–493. [Google Scholar] [CrossRef]
- Micheletti, G.; Lannes, B.; Haby, C.; Borrelli, E.; Kempf, E.; Warter, J.M.; Zwiller, J. Chronic administration of NMDA antagonists induces D2 receptor synthesis in rat striatum. Mol. Brain Res. 1992, 14, 363–368. [Google Scholar] [CrossRef]
- Lannes, B.; Bernard, V.; Bloch, B.; Micheletti, G. Chronic treatment with dizocilpine maleate increases the number of striatal neurons expressing the D2 receptor gene. Neuroscience 1995, 65, 431–438. [Google Scholar] [CrossRef]
- Healy, J.D.; Meador-Woodruff, H.J. Differential regulation, by MK801, of dopamine receptor gene expression in rat nigrostriatal and mesocorticolimbic systems. Brain Res. 1996, 708, 38–44. [Google Scholar] [CrossRef]
- Nair, V.D.; Savelli, J.E.; Mishra, R.K. Modulation of dopamine D2 receptor expression by an NMDA receptor antagonist in rat brain. J. Mol. Neurosci. 1998, 11, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Carlsson, A. Interactions between glutamatergic and monoaminergic system within the basal ganglia-implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990, 13, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. The depolarization block hypothesis of neuroleptic action: Implications for the etiology and treatment of schizophrenia. J. Neural Transm. Suppl. 1992, 36, 91–131. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, D.; Núñez, C.; Laorden, M.L.; Milanés, M.V. Regulation of dopaminergic markers expression in response to acute and chronic morphine and to morphine withdrawal. Addict. Biol. 2016, 21, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.; Kratochwil, C.F.; Ryu, S.; Schweitzer, J.; Driever, W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol. 2010, 518, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Mahler, J.; Schweitzer, J.; Driever, W. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 2010, 518, 423–438. [Google Scholar] [CrossRef]
- Semenova, S.A.; Chen, Y.C.; Zhao, X.; Rauvala, H.; Panula, P. The tyrosine hydroxylase 2 (TH2) system in zebrafish brain and stress activation of hypothalamic cells. Histochem. Cell Biol. 2014, 142, 619–633. [Google Scholar] [CrossRef]
- McPherson, A.D.; Barrios, J.P.; Luks-Morgan, S.J.; Manfredi, J.P.; Bonkowsky, J.L.; Douglass, A.D.; Dorsky, R.I. Motor behavior mediated by continuously generated dopaminergic neurons in the zebrafish hypothalamus recovers after cell ablation. Curr. Biol. 2016, 26, 263–269. [Google Scholar] [CrossRef]
- Barrios, J.P.; Wang, W.C.; England, R.; Reifenberg, E.; Douglass, A.D. Hypothalamic dopamine neurons control sensorimotor behavior by modulating brainstem premotor nuclei in zebrafish. Curr. Biol. 2020, 30, 4606–4618. [Google Scholar] [CrossRef]
- Gao, Y.J.; Ji, R.R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef]
- Vishnoi, S.; Raisuddin, S.; Parvez, S. Modulatory effects of an NMDAR partial agonist in MK-801-induced memory impairment. Neuroscience 2015, 311, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, L.I.; Bolaños, C.A.; Choi, K.H.; Russo, S.J.; Edwards, S.; Ulery, P.G.; Wallace, D.L.; Self, D.W.; Nestler, E.J.; Barrot, M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci. 2005, 21, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Kaufling, J.; Waltisperger, E.; Bourdy, R.; Valera, A.; Veinante, P.; Freund-Mercier, M.J.; Barrot, M. Pharmacological recruitment of the GABAergic tail of the ventral tegmental area by acute drug exposure. Br. J. Pharmacol. 2010, 161, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.D.; Duman, R.S.; Nestler, E.J. Induction of the c-fos proto-oncogene during opiate withdrawal in the locus coeruleus and other regions of the rat brain. Brain Res. 1990, 525, 256–266. [Google Scholar] [CrossRef]
- Stornetta, R.L.; Norton, F.E.; Guyenet, P.G. Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res. 1993, 624, 19–28. [Google Scholar] [CrossRef]
- Rasmussen, K.; Brodsky, M.; Inturrisi, C.E. NMDA antagonists and clonidine block c-Fos expression during morphine withdrawal. Synapse 1995, 20, 68–74. [Google Scholar] [CrossRef]
- Sánchez-Catalán, M.J.; Faivre, F.; Yalcin, I.; Muller, M.A.; Massotte, D.; Majchrzak, M.; Barrot, M. Response of the tail of the ventral tegmental area to aversive stimuli. Neuropsychopharmacology 2017, 42, 638–648. [Google Scholar] [CrossRef]
- Chatterjee, D.; Tran, S.; Shams, S.; Gerlai, R.A. Simple method for immunohistochemical staining of zebrafish brain sections for c-fos protein expression. Zebrafish 2015, 12, 414–420. [Google Scholar] [CrossRef]
- Wulaer, B.; Kunisawa, K.; Tanabe, M.; Yanagawa, A.; Saito, K.; Mouri, A.; Nabeshima, T. Pharmacological blockade of dopamine D1- or D2-receptor in the prefrontal cortex induces attentional impairment in the object-based attention test through different neuronal circuits in mice. Mol. Brain 2021, 14, 43. [Google Scholar] [CrossRef]
- Surmeier, D.J. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol. 2007, 6, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Bito, H.; Deisseroth, K.; Tsien, R.W. CREB phosphorylation and dephosphorylation: A Ca2+-and stimulus duration–dependent switch for hippocampal gene expression. Cell 1996, 87, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Dolmetsch, R.E.; Pajvani, U.; Fife, K.; Spotts, J.M.; Greenberg, M.E. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 2001, 294, 333–339. [Google Scholar] [CrossRef]
- Weick, J.P.; Groth, R.D.; Isaksen, A.L.; Mermelstein, P.G. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J. Neurosci. 2003, 23, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Maximov, A.; Fu, Y.; Xu, F.; Tang, T.S.; Tkatch, T.; Surmeier, D.J.; Bezprozvanny, I. Association of CaV1. 3 L-type calcium channels with Shank. J. Neurosci. 2005, 25, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Graef, I.A.; Mermelstein, P.G.; Stankunas, K.; Neilson, J.R.; Deisseroth, K.; Tsien, R.W.; Crabtree, G.R. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature 1999, 401, 703–708. [Google Scholar] [CrossRef]
- Stanley, E.F. PresyNaptic calcium channels: Why is P selected before N? Biophys. J. 2015, 108, 451–452. [Google Scholar] [CrossRef]
- Stanley, E.F. The nanophysiology of fast transmitter release. Trends Neurosci. 2016, 39, 183–197. [Google Scholar] [CrossRef]
- Pliakas, A.M.; Carlson, R.R.; Neve, R.L.; Konradi, C.; Nestler, E.J.; Carlezon, W.A. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J. Neurosci. 2001, 21, 7397–7403. [Google Scholar] [CrossRef]
- Newton, S.S.; Thome, J.; Wallace, T.L.; Shirayama, Y.; Schlesinger, L.; Sakai, N.; Chen, J.; Neve, R.; Nestler, E.J.; Duman, R.S. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J. Neurosci. 2002, 22, 10883–10890. [Google Scholar] [CrossRef]
- Thiagarajan, S.K.; Mok, S.Y.; Ogawa, S.; Parhar, I.S.; Tang, P.Y. Receptor-mediated AKT/PI3K signalling and behavioural alterations in zebrafish larvae reveal association between schizophrenia and opioid use disorder. Int. J. Mol. Sci. 2022, 23, 4715. [Google Scholar] [CrossRef]
- Kessi, M.; Chen, B.; Peng, J.; Yan, F.; Yang, L.; Yin, F. Calcium channelopathies and intellectual disability: A systematic review. Orphanet J. Rare Dis. 2021, 16, 219. [Google Scholar] [CrossRef]
- Kramer, P.; Bressan, P. Our (mother’s) mitochondria and our mind. Perspect. Psychol. Sci. 2018, 13, 88–100. [Google Scholar] [CrossRef]
- Splawski, I.; Timothy, K.W.; Sharpe, L.M.; Decher, N.; Kumar, P.; Bloise, R.; Napolitano, C.; Schwartz, P.J.; Joseph, R.M.; Condouris, K.; et al. CaV1. 2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 2004, 119, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Timothy, K.W.; Decher, N.; Kumar, P.; Sachse, F.B.; Beggs, A.H.; Sanguinetti, M.C.; Keating, M.T. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 8089–8096. [Google Scholar] [CrossRef] [PubMed]
- Gillis, J.; Burashnikov, E.; Antzelevitch, C.; Blaser, S.; Gross, G.; Turner, L.; Babul-Hirji, R.; Chitayat, D. Long QT, syndactyly, joint contractures, stroke and novel CACNA1C mutation: Expanding the spectrum of Timothy syndrome. Am. J. Med. Genet. A 2012, 158, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, A.; Mader, S.; Pause, A.; Sonenberg, N. Repression of cap-dependent translation by 4E-binding protein 1: Competition with p220 for binding to eukaryotic initiation factor-4E. EMBO J. 1995, 14, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Mostaid, M.S.; Lee, T.T.; Chana, G.; Sundram, S.; Shannon Weickert, C.; Pantelis, C.; Everall, I.; Bousman, C. Peripheral transcription of NRG-ErbB pathway genes are upregulated in treatment-resistant schizophrenia. Front. Psychiatry 2017, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Duan, X.; Liu, C.Y.; Jang, M.H.; Guo, J.U.; Pow-anpongkul, N.; Kang, E.; Song, H.; Ming, G.L. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 2009, 63, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, W.; Huang, S.; Song, J.; Kim, J.Y.; Tian, X.; Kang, E.; Sano, Y.; Liu, C.; Balaji, J.; et al. mTOR Inhibition ameliorates cognitive and affective deficits caused by Disc1 knockdown in adult-born dentate granule neurons. Neuron 2013, 77, 647–654. [Google Scholar] [CrossRef]
- Gururajan, A.; van den Buuse, M. Is the mTOR-signalling cascade disrupted in Schizophrenia? J. Neurochem. 2014, 129, 377–387. [Google Scholar] [CrossRef] [PubMed]
- English, J.A.; Fan, Y.; Föcking, M.; Lopez, L.M.; Hryniewiecka, M.; Wynne, K.; Dicker, P.; Matigian, N.; Cagney, G.; Mackay-Sim, A.; et al. Reduced protein synthesis in schizophrenia patient-derived olfactory cells. Transl. Psychiatry 2015, 5, e663. [Google Scholar] [CrossRef] [PubMed]
- Boehmler, W.; Carr, T.; Thisse, C.; Thisse, B.; Canfield, V.A.; Levenson, R. D4 Dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007, 6, 155–166. [Google Scholar] [CrossRef]
- Sterling, M.E.; Chang, G.Q.; Karatayev, O.; Chang, S.Y.; Leibowitz, S.F. Effects of embryonic ethanol exposure at low doses on neuronal development, voluntary ethanol consumption and related behaviors in larval and adult zebrafish: Role of hypothalamic orexigenic peptides. Behav. Brain Res. 2016, 304, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef]
- Becker, A.; Peters, B.; Schroeder, H.; Mann, T.; Huether, G.; Grecksch, G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2003, 27, 687–700. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar]
- Haverinen, J.; Hassinen, M.; Dash, S.N.; Vornanen, M. Expression of calcium channel transcripts in the zebrafish heart: Dominance of T-type channels. J. Exp. Biol. 2018, 221, jeb179226. [Google Scholar] [CrossRef]
- Keow, J. Physiological and Behavioral Changes in a Rotenone Model of Dopamine Neurotoxicity and Neurodegeneration in Zebrafish. Doctoral Dissertation, University of Ottawa, Ottawa, ON, Canada, September 2016. [Google Scholar] [CrossRef]
- Barcellos, H.H.; Koakoski, G.; Chaulet, F.; Kirsten, K.S.; Kreutz, L.C.; Kalueff, A.V.; Barcellos, L.J. The effects of auditory enrichment on zebrafish behavior and physiology. PeerJ 2018, 6, e5162. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sundvik, M.; Rozov, S.; Priyadarshini, M.; Panula, P. MANF regulates dopaminergic neuron development in larval zebrafish. Dev. Biol. 2012, 370, 237–249. [Google Scholar] [CrossRef]
- Sundvik, M.; Puttonen, H.; Semenova, S.; Panula, P. The bullies are the leaders of the next generation: Inherited aminergic neurotransmitter system changes in socially dominant zebrafish, Danio rerio. Behav. Brain Res. 2021, 409, 113309. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Gao, L.; Han, Q.; Li, A.; Yu, H.; Liu, D.; Pang, Q. GSK-3β at the crossroads in regulating protein synthesis and lipid deposition in zebrafish. Cells 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Senay, E.C.; Adams, E.H.; Geller, A.; Inciardi, J.A.; Munoz, A.; Schnoll, S.H.; Woody, G.E.; Cicero, T.J. Physical dependence on Ultram®(tramadol hydrochloride): Both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003, 69, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Ronneberger, O.; Ryu, S.; Nitschke, R.; Driever, W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 2011, 2, 171. [Google Scholar] [CrossRef]





| Protein | Gene | Primer Sequences (5′ → 3′) | GenBank Accession No | References |
|---|---|---|---|---|
| Cav1.2 | α1C | F: ACGGAGTCACTCTCCGACAC | XM_009300335 | [120] |
| R: AGAGAGGGCACAGGCTGATA | ||||
| Cav1.1a | α1Sa | F: TCTATAGGCGTGCTGGAGGT | NM_001146150 | [120] |
| R: GCTATCTGCGAGCTGTAGGG | ||||
| Cav2.1a | α1Aa | F: TGCTACCCAGCCACATGATA | ENSDARG00000037905 | [120] |
| R: TGGTAGAGAGTGAGGGTAAA | ||||
| Dopamine receptor D2a | drd2a | F: TGGTACTCCGGAAAAGACG | NM_183068 | [121] |
| R: ACTCGGGATGGGTGCATTTC | ||||
| c-FOS | c-fos | F:GCAGAGCATTGGCAGGAG | DQ003339 | [122] |
| R: CCCTTCGGATTCTCCTTTTCT | ||||
| Tyrosine hydroxylase 1 | th1 | F: GACGGAAGATGATCGGAGACA | XM_682702.1 | [123] |
| R: CCGCCATGTTCCGATTTCT | ||||
| Tyrosine hydroxylase 2 | th2 | F: CTCCAGAAGAGAATGCCACATG | NM_001001829.1 | [124] |
| R: ACGTTCACTCTCCAGCTGAGTG | ||||
| Eukaryotic translation initiation factor 4E-binding protein | eif4ebp1 | F: AACGACAAGGTGCAAAGAC | NM_199645 | [125] |
| R: GTGGTTGGAATTGCCTGACT | ||||
| Beta actin (β-actin) | actb1 | F: AAGCTGTGACCCACCTCACG | AF057040 | [126] |
| R:GGCTTTGCACATACCGGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiagarajan, S.K.; Mok, S.Y.; Ogawa, S.; Parhar, I.S.; Tang, P.Y. Integrative Roles of Dopamine Pathway and Calcium Channels Reveal a Link between Schizophrenia and Opioid Use Disorder. Int. J. Mol. Sci. 2023, 24, 4088. https://doi.org/10.3390/ijms24044088
Thiagarajan SK, Mok SY, Ogawa S, Parhar IS, Tang PY. Integrative Roles of Dopamine Pathway and Calcium Channels Reveal a Link between Schizophrenia and Opioid Use Disorder. International Journal of Molecular Sciences. 2023; 24(4):4088. https://doi.org/10.3390/ijms24044088
Chicago/Turabian StyleThiagarajan, Siroshini K., Siew Ying Mok, Satoshi Ogawa, Ishwar S. Parhar, and Pek Yee Tang. 2023. "Integrative Roles of Dopamine Pathway and Calcium Channels Reveal a Link between Schizophrenia and Opioid Use Disorder" International Journal of Molecular Sciences 24, no. 4: 4088. https://doi.org/10.3390/ijms24044088
APA StyleThiagarajan, S. K., Mok, S. Y., Ogawa, S., Parhar, I. S., & Tang, P. Y. (2023). Integrative Roles of Dopamine Pathway and Calcium Channels Reveal a Link between Schizophrenia and Opioid Use Disorder. International Journal of Molecular Sciences, 24(4), 4088. https://doi.org/10.3390/ijms24044088







