Nanomechanical Signatures in Glioma Cells Depend on CD44 Distribution in IDH1 Wild-Type but Not in IDH1R132H Mutant Early-Passage Cultures
Abstract
1. Introduction
2. Results
2.1. Cell Culture Establishing and Description
2.2. IDH1 R132H Is Characterized by Induced Rigidity and Stiffness but a Lower Proliferation Rate
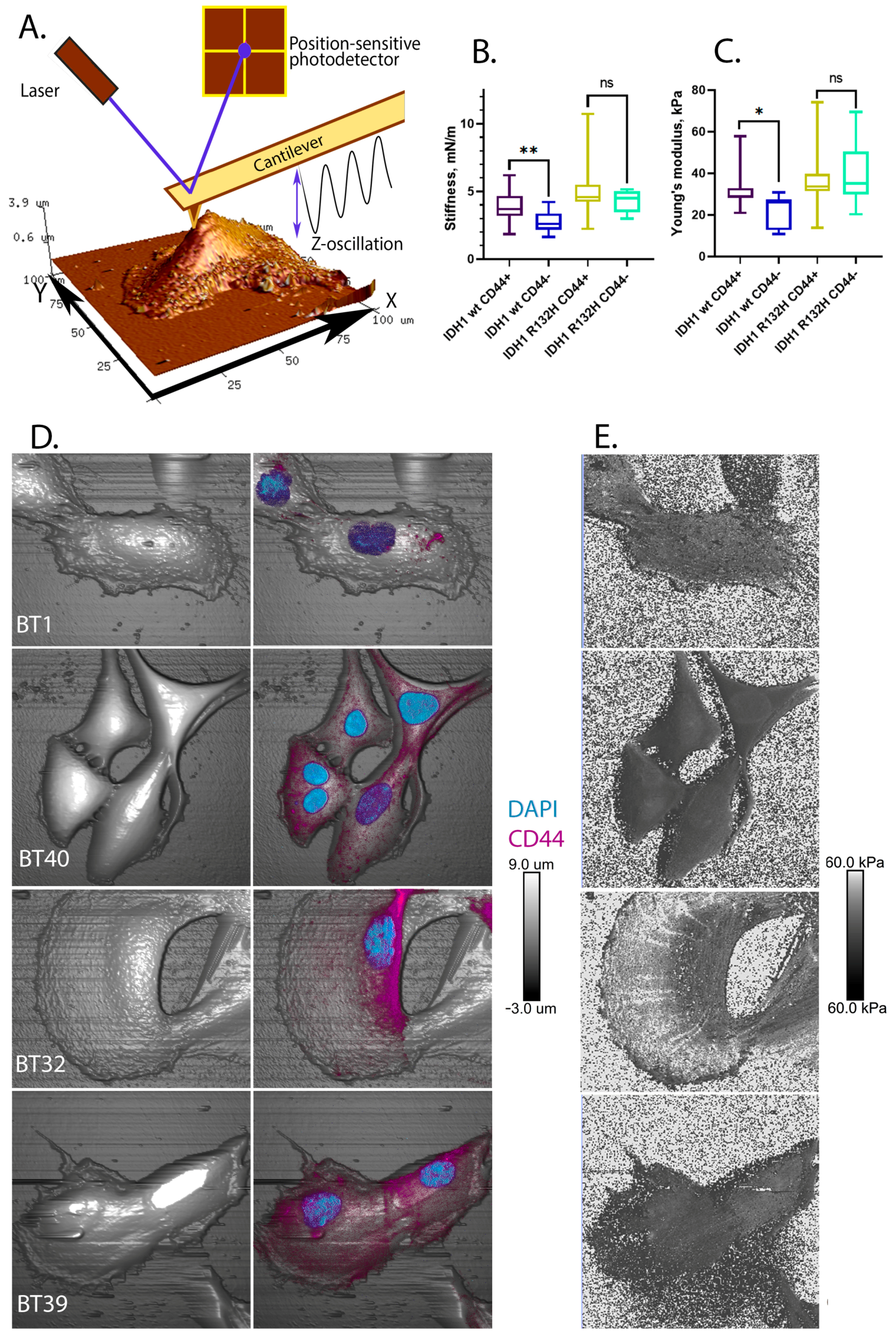
2.3. CD44+ Cells Are More Rigid Than CD44- Cells in IDH1 Wild-Type but Not in IDH1 R132H Mutant Gliomas
3. Discussion
4. Materials and Methods
4.1. Primary Cell Culture Establishment
4.2. Cell Proliferation Assay
4.3. Cell Culture Genotyping
4.4. The Immunocytochemistry (ICC) and Immunohistochemistry (IHC)
4.5. Protein Expression Quantification
4.6. Cell Morphology Analysis
4.7. Physical and Nanomechanical Cell Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO Guidelines on the Diagnosis and Treatment of Diffuse Gliomas of Adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M. Discrimination Between Normal and Cancerous Cells Using AFM. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Bastatas, L.; Martinez-Marin, D.; Matthews, J.; Hashem, J.; Lee, Y.J.; Sennoune, S.; Filleur, S.; Martinez-Zaguilan, R.; Park, S. AFM Nano-Mechanics and Calcium Dynamics of Prostate Cancer Cells with Distinct Metastatic Potential. Biochim. Biophys. Acta BBA Gen. Subj. 2012, 1820, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Gostek, J.; Prauzner-Bechcicki, S.; Nimmervoll, B.; Mayr, K.; Pabijan, J.; Hinterdorfer, P.; Chtcheglova, L.A.; Lekka, M. Nano-Characterization of Two Closely Related Melanoma Cell Lines with Different Metastatic Potential. Eur. Biophys. J. 2015, 44, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Weder, G.; Hendriks-Balk, M.C.; Smajda, R.; Rimoldi, D.; Liley, M.; Heinzelmann, H.; Meister, A.; Mariotti, A. Increased Plasticity of the Stiffness of Melanoma Cells Correlates with Their Acquisition of Metastatic Properties. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 141–148. [Google Scholar] [CrossRef]
- Shmelev, M.E.; Titov, S.I.; Belousov, A.S.; Farniev, V.M.; Zhmenia, V.M.; Lanskikh, D.V.; Penkova, A.O.; Kumeiko, V.V. Cell and Tissue Nanomechanics: From Early Development to Carcinogenesis. Biomedicines 2022, 10, 345. [Google Scholar] [CrossRef]
- Grimm, K.B.; Oberleithner, H.; Fels, J. Fixed Endothelial Cells Exhibit Physiologically Relevant Nanomechanics of the Cortical Actin Web. Nanotechnology 2014, 25, 215101. [Google Scholar] [CrossRef]
- Sharma, S.; Santiskulvong, C.; Bentolila, L.A.; Rao, J.; Dorigo, O.; Gimzewski, J.K. Correlative Nanomechanical Profiling with Super-Resolution F-Actin Imaging Reveals Novel Insights into Mechanisms of Cisplatin Resistance in Ovarian Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 757–766. [Google Scholar] [CrossRef]
- Kis, A.; Kasas, S.; Babić, B.; Kulik, A.J.; Benoît, W.; Briggs, G.A.D.; Schönenberger, C.; Catsicas, S.; Forró, L. Nanomechanics of Microtubules. Phys. Rev. Lett. 2002, 89, 248101. [Google Scholar] [CrossRef]
- Kumar, P.; Kedaria, D.; Mahapatra, C.; Mohandas, M.; Chatterjee, K. A Designer Cell Culture Insert with a Nanofibrous Membrane toward Engineering an Epithelial Tissue Model Validated by Cellular Nanomechanics. Nanoscale Adv. 2021, 3, 4714–4725. [Google Scholar] [CrossRef]
- Svitkina, T.M. Ultrastructure of the Actin Cytoskeleton. Curr. Opin. Cell Biol. 2018, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Parrot, D.; Blümel, M.; Utermann, C.; Chianese, G.; Krause, S.; Kovalev, A.; Gorb, S.N.; Tasdemir, D. Mapping the Surface Microbiome and Metabolome of Brown Seaweed Fucus Vesiculosus by Amplicon Sequencing, Integrated Metabolomics and Imaging Techniques. Sci. Rep. 2019, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, C.; Chang, K.; Karnad, A.; Jagirdar, J.; Kumar, A.P.; Freeman, J.W. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin. Cancer Res. 2016, 22, 5592–5604. [Google Scholar] [CrossRef]
- Zanjani, L.S.; Madjd, Z.; Abolhasani, M.; Rasti, A.; Fodstad, O.; Andersson, Y.; Asgari, M. Increased Expression of CD44 Is Associated with More Aggressive Behavior in Clear Cell Renal Cell Carcinoma. Biomark. Med. 2018, 12, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a Tumor Biomarker and Therapeutic Target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S.; Tsukita, S. Ezrin/Radixin/Moesin (ERM) Proteins Bind to a Positively Charged Amino Acid Cluster in the Juxta-Membrane Cytoplasmic Domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [CrossRef]
- Stylianou, A.; Gkretsi, V.; Stylianopoulos, T. Transforming Growth Factor-β Modulates Pancreatic Cancer Associated Fibroblasts Cell Shape, Stiffness and Invasion. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1537–1546. [Google Scholar] [CrossRef]
- Osmulski, P.A.; Cunsolo, A.; Chen, M.; Qian, Y.; Lin, C.-L.; Hung, C.-N.; Mahalingam, D.; Kirma, N.B.; Chen, C.-L.; Taverna, J.A.; et al. Contacts with Macrophages Promote an Aggressive Nanomechanical Phenotype of Circulating Tumor Cells in Prostate Cancer. Cancer Res. 2021, 81, 4110–4123. [Google Scholar] [CrossRef]
- Farniev, V.M.; Shmelev, M.E.; Shved, N.A.; Gulaia, V.S.; Biktimirov, A.R.; Zhizhchenko, A.Y.; Kuchmizhak, A.A.; Kumeiko, V.V. Nanomechanical and Morphological AFM Mapping of Normal Tissues and Tumors on Live Brain Slices Using Specially Designed Embedding Matrix and Laser-Shaped Cantilevers. Biomedicines 2022, 10, 1742. [Google Scholar] [CrossRef]
- Cieśluk, M.; Pogoda, K.; Deptuła, P.; Werel, P.; Kułakowska, A.; Kochanowicz, J.; Mariak, Z.; Łysoń, T.; Reszeć, J.; Bucki, R. Nanomechanics and Histopathology as Diagnostic Tools to Characterize Freshly Removed Human Brain Tumors. Int. J. Nanomed. 2020, 15, 7509–7521. [Google Scholar] [CrossRef]
- Shou, H.; Wu, J.; Tang, N.; Wang, B. Calcification-Based Cancer Diagnosis and Therapy. ChemMedChem 2022, 17, e202100339. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; McGee, K.P.; Arani, A.; Lake, D.S.; Glaser, K.J.; Manduca, A.; Parney, I.F.; Ehman, R.L.; Huston, J. MR Elastography Analysis of Glioma Stiffness and IDH1-Mutation Status. AJNR Am. J. Neuroradiol. 2018, 39, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Hohmann, T.; Güttler, A.; Petrenko, M.; Ostheimer, C.; Hohmann, U.; Bache, M.; Dehghani, F.; Vordermark, D. Radiosensitization and a Less Aggressive Phenotype of Human Malignant Glioma Cells Expressing Isocitrate Dehydrogenase 1 (IDH1) Mutant Protein: Dissecting the Mechanisms. Cancers 2019, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.; Hukku, B. Cell Line Characterization and Authentication. Methods Cell Biol. 1998, 57, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Okawa, S.; Gagrica, S.; Blin, C.; Ender, C.; Pollard, S.M.; Krijgsveld, J. Proteome and Secretome Characterization of Glioblastoma-Derived Neural Stem Cells. Stem Cells 2017, 35, 967–980. [Google Scholar] [CrossRef]
- Komori, T. Grading of Adult Diffuse Gliomas According to the 2021 WHO Classification of Tumors of the Central Nervous System. Lab. Investig. 2022, 102, 126–133. [Google Scholar] [CrossRef]
- Osborn, A.G.; Louis, D.N.; Poussaint, T.Y.; Linscott, L.L.; Salzman, K.L. The 2021 World Health Organization Classification of Tumors of the Central Nervous System: What Neuroradiologists Need to Know. Am. J. Neuroradiol. 2022, 43, 928–937. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Ofori-Amanfo, G.K.; Leidinger, M.R.; Goeken, J.A.; Khanna, R.; Sieren, J.C.; Darbro, B.W.; Quelle, D.E.; Weimer, J.M. Immunohistochemical Markers for Prospective Studies in Neurofibromatosis-1 Porcine Models. J. Histochem. Cytochem. 2017, 65, 607–618. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.L.; Boyle, A.M.; Budge, K.M.; Safadi, F.F.; Richardson, J.R. The Glycoprotein GPNMB Attenuates Astrocyte Inflammatory Responses through the CD44 Receptor. J. Neuroinflammation 2018, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Kurachi, M.; Shibasaki, K.; Okano-Uchida, T.; Ishizaki, Y. CD44-Positive Cells Are Candidates for Astrocyte Precursor Cells in Developing Mouse Cerebellum. Cerebellum 2012, 11, 181–193. [Google Scholar] [CrossRef]
- Konopka, A.; Zeug, A.; Skupien, A.; Kaza, B.; Mueller, F.; Chwedorowicz, A.; Ponimaskin, E.; Wilczynski, G.M.; Dzwonek, J. Cleavage of Hyaluronan and CD44 Adhesion Molecule Regulate Astrocyte Morphology via Rac1 Signalling. PLoS ONE 2016, 11, e0155053. [Google Scholar] [CrossRef]
- Al-Rekabi, Z.; Fura, A.M.; Juhlin, I.; Yassin, A.; Popowics, T.E.; Sniadecki, N.J. Hyaluronan-CD44 Interactions Mediate Contractility and Migration in Periodontal Ligament Cells. Cell Adhes. Migr. 2019, 13, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Kwak, H.-J.; Lee, S.-H. Role of Hyaluronan in Glioma Invasion. Cell Adhes. Migr. 2008, 2, 202–207. [Google Scholar] [CrossRef]
- Dong, Q.; Li, Q.; Wang, M.; Hu, J.; Dai, J.; Niu, L.; Yuan, G.; Pan, Y. Elevated CD44 Expression Predicts Poor Prognosis in Patients with Low-grade Glioma. Oncol. Lett. 2019, 18, 3698–3704. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, K.; Wang, Z.; Zhao, M.; Deng, Y.; Ji, W.; Zou, Y.; Qian, C.; Liu, Y.; Xiao, H.; et al. CD44-Mediated Poor Prognosis in Glioma Is Associated with M2-Polarization of Tumor-Associated Macrophages and Immunosuppression. Front. Surg. 2021, 8, 775194. [Google Scholar] [CrossRef]
- Si, D.; Yin, F.; Peng, J.; Zhang, G. High Expression of CD44 Predicts a Poor Prognosis in Glioblastomas. Cancer Manag. Res. 2020, 12, 769–775. [Google Scholar] [CrossRef]
- Nishikawa, M.; Inoue, A.; Ohnishi, T.; Kohno, S.; Ohue, S.; Matsumoto, S.; Suehiro, S.; Yamashita, D.; Ozaki, S.; Watanabe, H.; et al. Significance of Glioma Stem-Like Cells in the Tumor Periphery That Express High Levels of CD44 in Tumor Invasion, Early Progression, and Poor Prognosis in Glioblastoma. Stem Cells Int. 2018, 2018, 5387041. [Google Scholar] [CrossRef]
- Hassn Mesrati, M.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Sinkala, M. Cancer Stem Cell Marker CD44 Plays Multiple Key Roles in Human Cancers: Immune Suppression/Evasion, Drug Resistance, Epithelial-Mesenchymal Transition, and Metastasis. OMICS 2021, 25, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A Subcellular Map of the Human Proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Y.; Liang, J.; Gao, S.; Cai, X.; Yu, Y.; Qi, Z.; Li, J.; Xie, Y.; Wang, Z. Association of Glioma CD44 Expression with Glial Dynamics in the Tumour Microenvironment and Patient Prognosis. Comput. Struct. Biotechnol. J. 2022, 20, 5203–5217. [Google Scholar] [CrossRef] [PubMed]
- Hassn Mesrati, M.; Behrooz, A.B.; Abuhamad, A.Y.; Syahir, A. Understanding Glioblastoma Biomarkers: Knocking a Mountain with a Hammer. Cells 2020, 9, 1236. [Google Scholar] [CrossRef]
- Gulaia, V.; Shmelev, M.; Romanishin, A.; Shved, N.; Farniev, V.; Goncharov, N.; Biktimirov, A.; Vargas, I.L.; Khodosevich, K.; Kagansky, A.; et al. Single-Nucleus Transcriptomics of IDH1- and TP53-Mutant Glioma Stem Cells Displays Diversified Commitment on Invasive Cancer Progenitors. Sci. Rep. 2022, 12, 18975. [Google Scholar] [CrossRef]
- Bralten, L.B.C.; Kloosterhof, N.K.; Balvers, R.; Sacchetti, A.; Lapre, L.; Lamfers, M.; Leenstra, S.; de Jonge, H.; Kros, J.M.; Jansen, E.E.W.; et al. IDH1 R132H Decreases Proliferation of Glioma Cell Lines in Vitro and in Vivo. Ann. Neurol. 2011, 69, 455–463. [Google Scholar] [CrossRef]
- Lin, L.; Cai, J.; Tan, Z.; Meng, X.; Li, R.; Li, Y.; Jiang, C. Mutant IDH1 Enhances Temozolomide Sensitivity via Regulation of the ATM/CHK2 Pathway in Glioma. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2021, 53, 367–377. [Google Scholar] [CrossRef]
- Oraiopoulou, M.-E.; Tzamali, E.; Tzedakis, G.; Vakis, A.; Papamatheakis, J.; Sakkalis, V. In Vitro/In Silico Study on the Role of Doubling Time Heterogeneity among Primary Glioblastoma Cell Lines. Biomed. Res. Int. 2017, 2017, 8569328. [Google Scholar] [CrossRef]
- Biedermann, J.; Preussler, M.; Conde, M.; Peitzsch, M.; Richter, S.; Wiedemuth, R.; Abou-El-Ardat, K.; Krüger, A.; Meinhardt, M.; Schackert, G.; et al. Mutant IDH1 Differently Affects Redox State and Metabolism in Glial Cells of Normal and Tumor Origin. Cancers 2019, 11, 2028. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W. Hyaluronan-Mediated CD44 Activation of RhoGTPase Signaling and Cytoskeleton Function Promotes Tumor Progression. Semin. Cancer Biol. 2008, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Koochekpour, S.; Pilkington, G.J.; Merzak, A. Hyaluronic Acid/CD44H Interaction Induces Cell Detachment and Stimulates Migration and Invasion of Human Glioma Cells in Vitro. Int. J. Cancer 1995, 63, 450–454. [Google Scholar] [CrossRef]
- Delpech, B.; Maingonnat, C.; Girard, N.; Chauzy, C.; Maunoury, R.; Olivier, A.; Tayot, J.; Creissard, P. Hyaluronan and Hyaluronectin in the Extracellular Matrix of Human Brain Tumour Stroma. Eur. J. Cancer 1993, 29A, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.G.; Barwick, B.G.; Jin, G.; Rago, C.; Kapoor-Vazirani, P.; Powell, D.R.; Chi, J.-T.; Bigner, D.D.; Vertino, P.M.; Yan, H. A Heterozygous IDH1R132H/WT Mutation Induces Genome-Wide Alterations in DNA Methylation. Genome Res. 2012, 22, 2339–2355. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Janczyk, P.Ł.; Zhang, Y.; Liu, A.; Shi, X.; Singh, S.; Facemire, L.; Kubow, K.; Li, Z.; Jia, Y.; et al. A Cytoskeleton Regulator AVIL Drives Tumorigenesis in Glioblastoma. Nat. Commun. 2020, 11, 3457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, Y.; Li, A.; Celiku, O.; Han, S.; Qian, M.; Yang, C. MTORC2/Rac1 Pathway Predisposes Cancer Aggressiveness in IDH1-Mutated Glioma. Cancers 2020, 12, 787. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Irani, K.; Moldovan, N.I.; Finkel, T.; Goldschmidt-Clermont, P.J. The Actin Cytoskeleton Reorganization Induced by Rac1 Requires the Production of Superoxide. Antioxid. Redox Signal 1999, 1, 29–43. [Google Scholar] [CrossRef]
- Filić, V.; Marinović, M.; Faix, J.; Weber, I. A Dual Role for Rac1 GTPases in the Regulation of Cell Motility. J. Cell Sci. 2012, 125, 387–398. [Google Scholar] [CrossRef]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Pellegrino, P.; Rizzello, L.; Rinaldi, R. Tailoring Cell Morphomechanical Perturbations Through Metal Oxide Nanoparticles. Nanoscale Res. Lett. 2019, 14, 109. [Google Scholar] [CrossRef]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef]
- Esmaeili, M.; Hamans, B.C.; Navis, A.C.; van Horssen, R.; Bathen, T.F.; Gribbestad, I.S.; Leenders, W.P.; Heerschap, A. IDH1 R132H Mutation Generates a Distinct Phospholipid Metabolite Profile in Glioma. Cancer Res. 2014, 74, 4898–4907. [Google Scholar] [CrossRef] [PubMed]
- Narkilahti, S.; Rajala, K.; Pihlajamäki, H.; Suuronen, R.; Hovatta, O.; Skottman, H. Monitoring and Analysis of Dynamic Growth of Human Embryonic Stem Cells: Comparison of Automated Instrumentation and Conventional Culturing Methods. Biomed. Eng. Online 2007, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A Simple Method for Quantitating Confocal Fluorescent Images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chew, G.; Devapragash, N.; Loh, J.Z.; Huang, K.Y.; Guo, J.; Liu, S.; Tan, E.L.S.; Chen, S.; Tee, N.G.Z.; et al. The E3 Ubiquitin Ligase WWP2 Regulates Pro-Fibrogenic Monocyte Infiltration and Activity in Heart Fibrosis. Nat. Commun. 2022, 13, 7375. [Google Scholar] [CrossRef] [PubMed]
- Mullin, N.; Hobbs, J.K. A Non-Contact, Thermal Noise Based Method for the Calibration of Lateral Deflection Sensitivity in Atomic Force Microscopy. Rev. Sci. Instrum. 2014, 85, 113703. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of Contact Deformations on the Adhesion of Particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]



| Cell Culture | Diagnosis | Gender | Tumor Sample IHC Assay Results | IDH1 R132H Mutation Status |
|---|---|---|---|---|
| BT1 | Grade IV glioblastoma | female | GFAP up to 90% cells | wild-type |
| Synaptophysin up to 40% cells | ||||
| KI 67 up to 15% cells | ||||
| P53 up to 60% cells | ||||
| BT40 | Grade IV glioblastoma | female | GFAP up to 80% cells | wild-type |
| Synaptophysin up to 40% cells | ||||
| KI 67 up to 10% cells | ||||
| P53 up to 60% cells | ||||
| BT32 | Grade III astrocytoma | male | GFAP up to 100% cells | mutant-type |
| Synaptophysin up to 100% cells | ||||
| Vimentin—negative | ||||
| KI 67 up to 10% cells | ||||
| P53 up to 50% cells | ||||
| BT39 | Grade III astrocytoma | female | Synaptophysin up to 50% cells | mutant-type |
| Vimentin—negative | ||||
| KI 67 up to 10% cells | ||||
| P53 up to 50% cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmelev, M.E.; Farniev, V.M.; Shved, N.A.; Kumeiko, V.V. Nanomechanical Signatures in Glioma Cells Depend on CD44 Distribution in IDH1 Wild-Type but Not in IDH1R132H Mutant Early-Passage Cultures. Int. J. Mol. Sci. 2023, 24, 4056. https://doi.org/10.3390/ijms24044056
Shmelev ME, Farniev VM, Shved NA, Kumeiko VV. Nanomechanical Signatures in Glioma Cells Depend on CD44 Distribution in IDH1 Wild-Type but Not in IDH1R132H Mutant Early-Passage Cultures. International Journal of Molecular Sciences. 2023; 24(4):4056. https://doi.org/10.3390/ijms24044056
Chicago/Turabian StyleShmelev, Mikhail E., Vladislav M. Farniev, Nikita A. Shved, and Vadim V. Kumeiko. 2023. "Nanomechanical Signatures in Glioma Cells Depend on CD44 Distribution in IDH1 Wild-Type but Not in IDH1R132H Mutant Early-Passage Cultures" International Journal of Molecular Sciences 24, no. 4: 4056. https://doi.org/10.3390/ijms24044056
APA StyleShmelev, M. E., Farniev, V. M., Shved, N. A., & Kumeiko, V. V. (2023). Nanomechanical Signatures in Glioma Cells Depend on CD44 Distribution in IDH1 Wild-Type but Not in IDH1R132H Mutant Early-Passage Cultures. International Journal of Molecular Sciences, 24(4), 4056. https://doi.org/10.3390/ijms24044056






