Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis
Abstract
1. Introduction
2. Fluoride as an Environmental Toxin
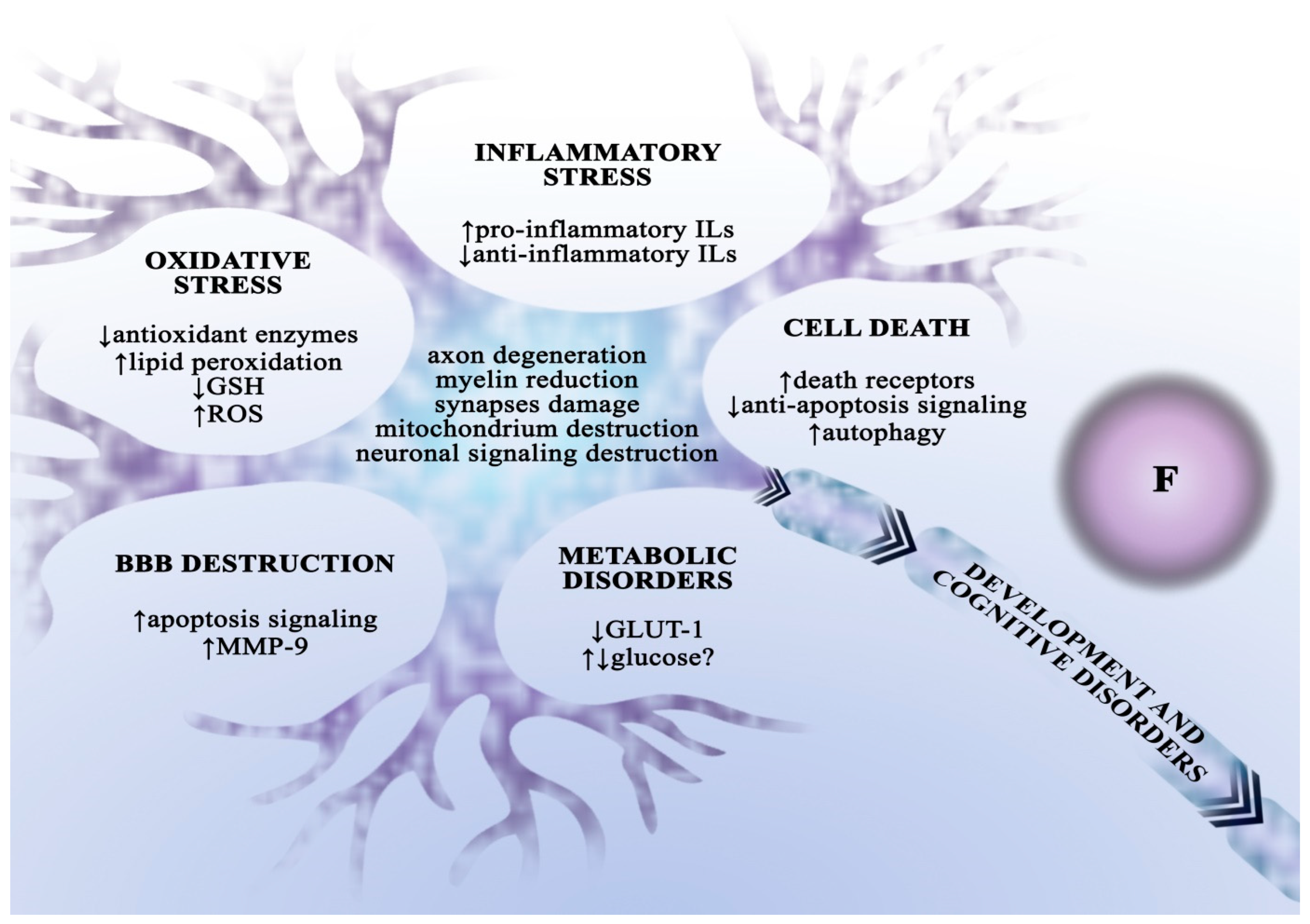
Fluoride Neurotoxicity
3. Gliomas
Description
4. Mechanisms of Drug Resistance in Glioblastoma
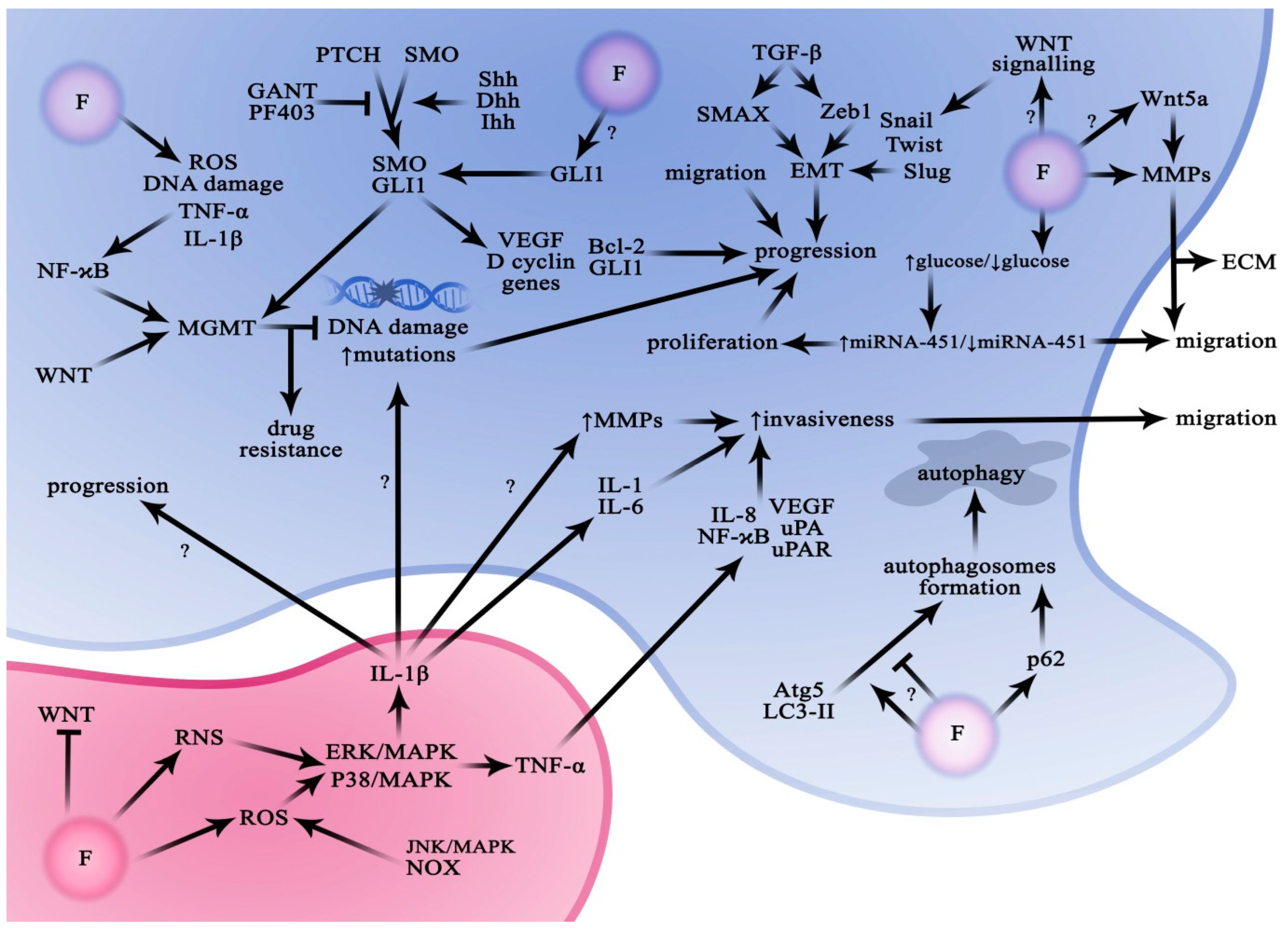
4.1. Hedgehog Signalling Pathway (HH/GLI1)
The Role of Fluoride in the Regulation of the Hedgehog Signalling Pathway
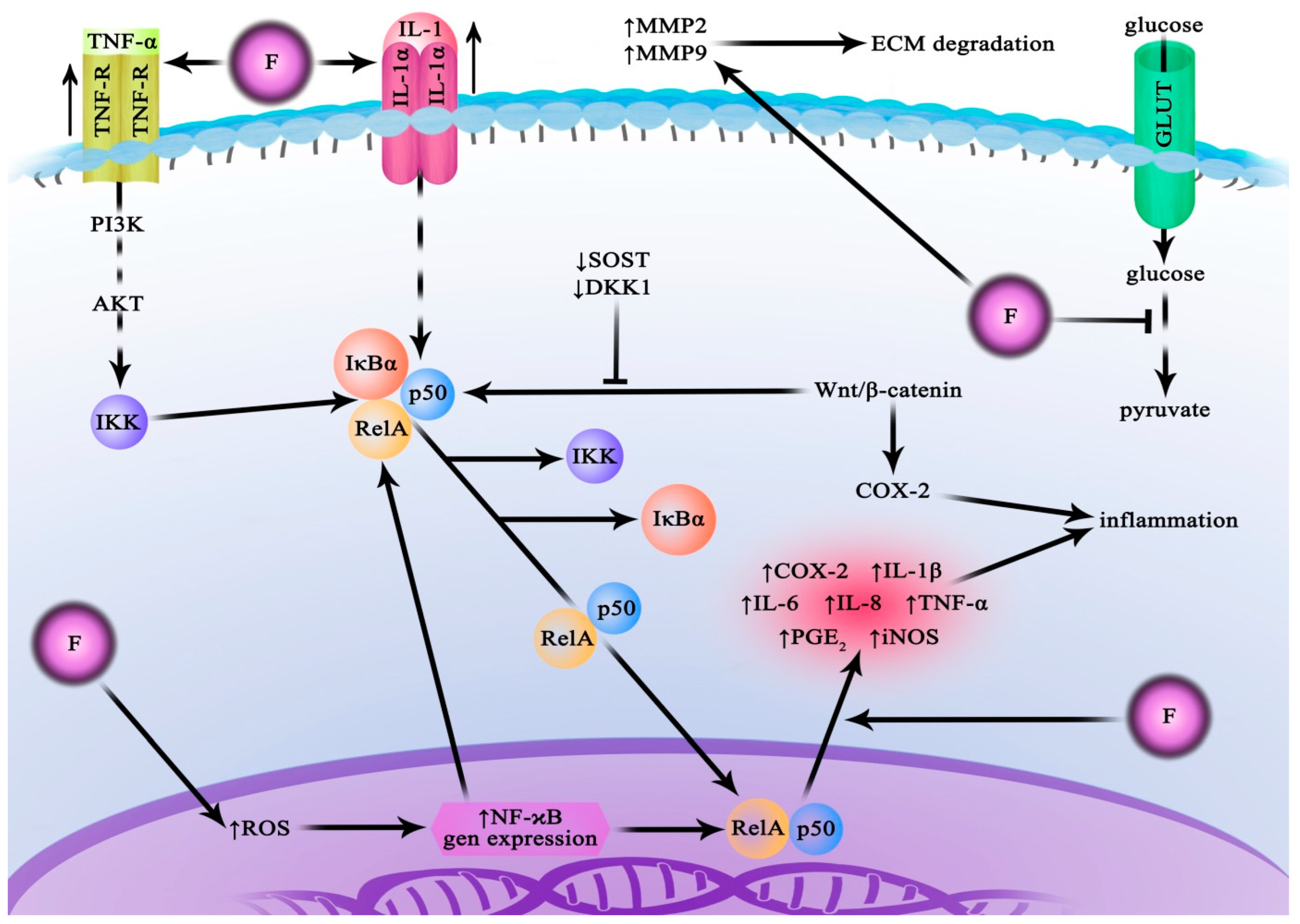
4.2. Nuclear Transcription Factor κB (NF-ĸB) Pathway
The Role of Fluoride in the Regulation of the NF-κB Pathway
4.3. Wingless/Int1 Trail (Wnt) Pathway
The Role of Fluoride in the Regulation of the Wingless/Int1 (Wnt) Pathway
4.4. Notch Signalling Pathway
The Role of Fluoride in the Regulation of the Notch Pathway
5. Autophagy—Its Role in the Pathogenesis of Cancer
The Role of Fluoride in Autophagy Regulation
6. Glioma Microenvironment
The Role of Fluoride in Modulating the Glioma Microenvironment
7. Involvement of Metalloproteinases in Glioma Development
The Role of Fluoride in the Regulation of Metalloproteinase Activity
8. Glial Defence Mechanisms against Metabolic Stress (Glucose)
The Role of Fluoride in the Regulation of Metabolic Stress (Glucose)
9. Involvement of Insulin and Insulin-like Growth Factor (IGF-1) in Glioma Development
The Role of Fluoride in the Regulation of Insulin and Insulin-like Growth Factor
10. The Role of Transforming Growth Factor β in Glioma Metabolism
Effects of Fluoride on TGF-β
11. The Role of Thyroid Hormones in Glioma Development
Effects of Fluoride on the Production of Thyroid Hormones
12. The Role of Glutamate in Gliomas
Effects of Fluoride on Glutamate Metabolism
13. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Elem. Res. 2002, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.; Cherian, M. Differential regulation of signal transduction pathways in wild type and mutated p53 breast cancer epithelial cells by copper and zinc. Arch. Biochem. Biophys. 2004, 423, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.; Manz, D.; Paul, B.; Blanchette-Farra, N.; Torti, F. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Coombs, M.R.P.; Grant, T.; Greenshields, A.L.; Arsenault, D.J.; Holbein, B.E.; Hoskin, D.W. Inhibitory effect of iron withdrawal by chelation on the growth of human and murine mammary carcinoma and fibrosarcoma cells. Exp. Mol. Pathol. 2015, 99, 262–270. [Google Scholar] [CrossRef]
- García, J.A.; Fernández, D.T.; Álvarez, E.A.; González, E.B.; Montes-Bayón, M.; Sanz-Medel, A. Iron speciation, ferritin concentrations and Fe: Ferritin ratios in different malignant breast cancer cell lines: On the search for cancer biomarkers. Metallomics 2016, 8, 1090–1096. [Google Scholar] [CrossRef]
- Stepien, M.; Jenab, M.; Freisling, H.; Becker, N.-P.; Czuban, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.-C.; Mancini, F.; et al. Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Carcinogenesis 2017, 38, 699–707. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef]
- Serna, J.; Bergwitz, C. Importance of Dietary Phosphorus for Bone Metabolism and Healthy Aging. Nutrients 2020, 12, 3001. [Google Scholar] [CrossRef]
- Phipps, O.; Brookes, M.; Al-Hassi, H. Iron deficiency, immunology, and colorectal cancer. Nutr. Rev. 2021, 79, 88–97. [Google Scholar] [CrossRef]
- Paganoni, R.; Lechel, A.; Spasic, M.V. Iron at the Interface of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 4097. [Google Scholar] [CrossRef]
- Cilliers, K.; Muller, C.J.F.; Page, B.J. Trace Element Concentration Changes in Brain Tumors: A Review. Anat. Rec. 2020, 303, 1293–1299. [Google Scholar] [CrossRef]
- Mulware, S.J. Comparative Trace Elemental Analysis in Cancerous and Noncancerous Human Tissues Using PIXE. J. Biophys. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Sohrabi, M.; Gholami, A.; Azar, M.H.; Yaghoobi, M.; Shahi, M.M.; Shirmardi, S.; Nikkhah, M.; Kohi, Z.; Salehpour, D.; Khoonsari, M.R.; et al. Trace Element and Heavy Metal Levels in Colorectal Cancer: Comparison Between Cancerous and Non-cancerous Tissues. Biol. Trace Elem. Res. 2018, 183, 1–8. [Google Scholar] [CrossRef]
- Andrási, E.; Suhajda, M.; Sáray, I.; Bezúr, L.; Ernyei, L.; Réffy, A. Concentration of elements in human brain: Glioblastoma multiforme. Sci. Total Environ. 1993, 139–140, 399–402. [Google Scholar] [CrossRef]
- Chandra, S.; Parker, D.J.; Barth, R.F.; Pannullo, S.C. Quantitative imaging of magnesium distribution at single-cell resolution in brain tumors and infiltrating tumor cells with secondary ion mass spectrometry (SIMS). J. Neuro Oncol. 2015, 127, 33–41. [Google Scholar] [CrossRef]
- Floriańczyk, B.; Kaczmarczyk, R.; Osuchowski, J.; Trojanowski, T. Metallothionein and manganese concentrations in brain tumors. J. Pre Clin. Clin. Res. 2007, 1, 89–91. [Google Scholar]
- Wandzilak, A.; Czyzycki, M.; Radwanska, E.; Adamek, D.; Geraki, K.; Lankosz, M. X-ray fluorescence study of the concentration of selected trace and minor elements in human brain tumours. Spectrochim. Acta Part B At. Spectrosc. 2015, 114, 52–57. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Shinwari, N. Levels of Cadmium, Lead, and Mercury in Human Brain Tumors. Biol. Trace Elem. Res. 2001, 79, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Zhou, Y.; Lu, H.; Lu, W.; Zhou, M.; Wang, Y.; Tan, M. Concentration of rare earth elements, As, and Th in human brain and brain tumors, determined by neutron activation analysis. Biol. Trace Elem. Res. 1996, 53, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.-F. Physiology of Blood–Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghosh, S. Flouride and Brain: A Review. Int. J. Pharm. Sci. Res. 2020, 11, 2011–2017. [Google Scholar]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 1–17. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; de Blank, P.M.; Finlay, J.L.; Gurney, J.G.; McKean-Cowdin, R.; Stearns, D.S.; Wolff, J.E.; Liu, M.; Wolinsky, Y.; et al. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol. 2016, 18 (Suppl. 1), i1–i50. [Google Scholar] [CrossRef]
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives; World Health Organization: Geneva, Switzerland, 2021.
- Patel, S.; Bhatnagar, A.; Wear, C.; Osiro, S.; Gabriel, A.; Kimball, D.; John, A.; Fields, P.J.; Tubbs, R.S.; Loukas, M. Are pediatric brain tumors on the rise in the USA? Significant incidence and survival findings from the SEER database analysis. Childs Nerv. Syst. 2014, 30, 147–154. [Google Scholar] [CrossRef]
- Nakamoto, T.; Rawls, H.R. Fluoride Exposure in Early Life as the Possible Root Cause of Disease In Later Life. J. Clin. Pediatr. Dent. 2018, 42, 325–330. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Sørensen, M.; Carstensen, H.; Jensen, T.; Bernhardtsen, T.; Gjerris, F.; Schmiegelow, K. Increasing incidence of childhood tumours of the central nervous system in Denmark, 1980–1996. Br. J. Cancer 2006, 95, 416–422. [Google Scholar] [CrossRef]
- Smith, M.A.; Freidlin, B.; Ries, L.A.G.; Simon, R. Trends in Reported Incidence of Primary Malignant Brain Tumors in Children in the United States. Gynecol. Oncol. 1998, 90, 1269–1277. [Google Scholar] [CrossRef]
- Jha, S.; Mishra, V.; Sharma, D.; Damodaran, T. Fluoride in the Environment and Its Metabolism in Humans. In Reviews of Environmental Contamination and Toxicology Volume 211; Whitacre, D., Ed.; Springer New York: New York, NY, USA, 2011; pp. 121–142. [Google Scholar]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; E Petersen, P.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Community Dent Health 2016, 33, 69–99. [Google Scholar] [PubMed]
- World Health Organization. Preventing Disease through Healthy Environments: Inadequate or Excess Fluoride: A Major Public Health Concern; World Health Organization: Geneva, Switzerland, 2019.
- Vithanage, M.; Bhattacharya, P. Fluoride in the environment: Sources, distribution and defluoridation. Environ. Chem. Lett. 2015, 13, 131–147. [Google Scholar] [CrossRef]
- Bombik, E.; Bombik, A.; Rymuza, K. The influence of environmental pollution with fluorine compounds on the level of fluoride in soil, feed and eggs of laying hens in Central Pomerania, Poland. Environ. Monit. Assess. 2020, 192, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarian, M.; Ghanbarian, M.; Tabatabaie, T.; Ghanbarian, M.; Ghadiri, S.-K. Distributing and assessing fluoride health risk in urban drinking water resources in Fars Province, Iran, using the geographical information system. Environ. Geochem. Health 2021, 44, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Jaudenes, J.R.; Gutiérrez, J.; Paz, S.; Rubio, C.; Hardisson, A. Fluoride Risk Assessment from Consumption of Different Foods Commercialized in a European Region. Appl. Sci. 2020, 10, 6582. [Google Scholar] [CrossRef]
- Riddell, J.; Malin, A.; McCague, H.; Flora, D.; Till, C. Urinary Fluoride Levels among Canadians with and without Community Water Fluoridation. Int. J. Environ. Res. Public Health 2021, 18, 6203. [Google Scholar] [CrossRef]
- Strunecka, A.; Strunecky, O. Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Appl. Sci. 2020, 10, 7100. [Google Scholar] [CrossRef]
- Medjedovic, E.; Medjedovic, S.; Deljo, D.; Sukalo, A. Impact of Fluoride on Dental Health Quality. Mater. Socio Medica 2015, 27, 395–398. [Google Scholar] [CrossRef]
- Chen, L.; Kuang, P.; Liu, H.; Wei, Q.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Sodium Fluoride (NaF) Induces Inflammatory Responses Via Activating MAPKs/NF-κB Signaling Pathway and Reducing Anti-inflammatory Cytokine Expression in the Mouse Liver. Biol. Trace Elem. Res. 2019, 189, 157–171. [Google Scholar] [CrossRef]
- Refsnes, M.; Skuland, T.; Schwarze, P.; Lag, M.; Ovrevik, J. Differential NF-κB and MAPK activation underlies fluoride- and TPA-mediated CXCL8 (IL-8) induction in lung epithelial cells. JIR 2014, 7, 169. [Google Scholar] [CrossRef]
- Pan, X.; Yan, W.; Qiu, B.; Liao, Y.; Liao, Y.; Wu, S.; Ming, J.; Zhang, A. Aberrant DNA methylation of Cyclind-CDK4-p21 is associated with chronic fluoride poisoning. Chem. Interact. 2019, 315, 108875. [Google Scholar] [CrossRef]
- Aulestia, F.J.; Groeling, J.; Bomfim, G.H.S.; Costiniti, V.; Manikandan, V.; Chaloemtoem, A.; Concepcion, A.R.; Li, Y.; Ii, L.E.W.; Idaghdour, Y.; et al. Fluoride exposure alters Ca2+ signaling and mitochondrial function in enamel cells. Sci. Signal. 2020, 13, eaay0086. [Google Scholar] [CrossRef]
- Nagendra, A.H.; Bose, B.; Shenoy, P.S. Recent advances in cellular effects of fluoride: An update on its signalling pathway and targeted therapeutic approaches. Mol. Biol. Rep. 2021, 48, 5661–5673. [Google Scholar] [CrossRef]
- Jianjie, C.; Wenjuan, X.; Jinling, C.; Jie, S.; Ruhui, J.; Meiyan, L. Fluoride caused thyroid endocrine disruption in male zebrafish (Danio rerio). Aquat. Toxicol. 2016, 171, 48–58. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Y.; Chen, J.; Yan, H.; Li, M.; Wang, J. Fluoride exposure changed the structure and the expressions of Y chromosome related genes in testes of mice. Chemosphere 2016, 161, 292–299. [Google Scholar] [CrossRef]
- Han, H.; Sun, Z.; Luo, G.; Wang, C.; Wei, R.; Wang, J. Fluoride exposure changed the structure and the expressions of reproductive related genes in the hypothalamus–pituitary–testicular axis of male mice. Chemosphere 2015, 135, 297–303. [Google Scholar] [CrossRef]
- Iano, F.G.; Ferreira, M.C.; Quaggio, G.B.; Fernandes, M.S.; Oliveira, R.C.; Ximenes, V.F.; Buzalaf, M.A.R. Effects of chronic fluoride intake on the antioxidant systems of the liver and kidney in rats. J. Fluor. Chem. 2014, 168, 212–217. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Sun, L.; Li, M.; Li, B.; Sun, D. Assessment of relationship on excess fluoride intake from drinking water and carotid atherosclerosis development in adults in fluoride endemic areas, China. Int. J. Hyg. Environ. Health 2014, 217, 413–420. [Google Scholar] [CrossRef]
- Liu, P.; Li, R.; Tian, X.; Zhao, Y.; Li, M.; Wang, M.; Ying, X.; Yuan, J.; Xie, J.; Yan, X.; et al. Co-exposure to fluoride and arsenic disrupts intestinal flora balance and induces testicular autophagy in offspring rats. Ecotoxicol. Environ. Saf. 2021, 222, 112506. [Google Scholar] [CrossRef]
- Raina, R.; Baba, N.A.; Verma, P.K.; Sultana, M.; Singh, M. Hepatotoxicity Induced by Subchronic Exposure of Fluoride and Chlorpyrifos in Wistar Rats: Mitigating Effect of Ascorbic Acid. Biol. Trace Elem. Res. 2015, 166, 157–162. [Google Scholar] [CrossRef]
- Song, C.; Fu, B.; Zhang, J.; Zhao, J.; Yuan, M.; Peng, W.; Zhang, Y.; Wu, H. Sodium fluoride induces nephrotoxicity via oxidative stress-regulated mitochondrial SIRT3 signaling pathway. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Gao, Q.; Wu, C.-X.; Guan, Z.-Z. Alterations of nAChRs and ERK1/2 in the brains of rats with chronic fluorosis and their connections with the decreased capacity of learning and memory. Toxicol. Lett. 2010, 192, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Reddy, K.; Kumar, K. Neurodegenerative Changes in Different Regions of Brain, Spinal Cord and Sciatic Nerve of Rats Treated with Sodium Fluoride. J. Med. Allied Sci. 2011, 1, 30–35. [Google Scholar]
- Wu, C.; Gu, X.; Ge, Y.; Jianhai, Z.; Wang, J. Effects of high fluoride and arsenic on brain biochemical indexes and learning-memory in rats. Fluoride 2006, 39, 274–279. [Google Scholar]
- Qing-Feng, S.; Ying-Peng, X.; Tian-Tong, X. Matrix metalloproteinase-9 and p53 involved in chronic fluorosis induced blood-brain barrier damage and neurocyte changes. Arch. Med. Sci. 2019, 15, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Opydo, J.; Borysewicz-Lewickaa, M. Transplacental passage of fluoride in pregnant Polish women assessed on the basis of fluoride concentrations in maternal and cord blood plasma. Fluoride 2007, 40, 46–50. [Google Scholar]
- Niu, Q.; Chen, J.; Xia, T.; Li, P.; Zhou, G.; Xu, C.; Zhao, Q.; Dong, L.; Zhang, S.; Wang, A. Excessive ER stress and the resulting autophagic flux dysfunction contribute to fluoride-induced neurotoxicity. Environ. Pollut. 2018, 233, 889–899. [Google Scholar] [CrossRef]
- Bartos, M.; Gumilar, F.; Gallegos, C.E.; Bras, C.; Dominguez, S.; Cancela, L.M.; Minetti, A. Effects of Perinatal Fluoride Exposure on Short- and Long-Term Memory, Brain Antioxidant Status, and Glutamate Metabolism of Young Rat Pups. Int. J. Toxicol. 2019, 38, 405–414. [Google Scholar] [CrossRef]
- Kupnicka, P.; Listos, J.; Tarnowski, M.; Kolasa-Wołosiuk, A.; Wąsik, A.; Łukomska, A.; Barczak, K.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Fluoride Affects Dopamine Metabolism and Causes Changes in the Expression of Dopamine Receptors (D1R and D2R) in Chosen Brain Structures of Morphine-Dependent Rats. Int. J. Mol. Sci. 2020, 21, 2361. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Xue, X.; Niu, R.; Wang, J. Maternal fluoride exposure during gestation and lactation decreased learning and memory ability, and glutamate receptor mRNA expressions of mouse pups. Hum. Exp. Toxicol. 2018, 37, 87–93. [Google Scholar] [CrossRef]
- Lopes, G.O.; Ferreira, M.K.M.; Davis, L.; Bittencourt, L.O.; Aragão, W.A.B.; Dionizio, A.; Buzalaf, M.A.R.; Crespo-Lopez, M.E.; Maia, C.S.F.; Lima, R.R. Effects of Fluoride Long-Term Exposure over the Cerebellum: Global Proteomic Profile, Oxidative Biochemistry, Cell Density, and Motor Behavior Evaluation. Int. J. Mol. Sci. 2020, 21, 7297. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, S.; Liu, H.; Guan, Z.; Zeng, Q.; Zhang, C.; Lei, R.; Xia, T.; Wang, Z.; Yang, L.; et al. Low Glucose Utilization and Neurodegenerative Changes Caused by Sodium Fluoride Exposure in Rat’s Developmental Brain. Neuromol. Med. 2014, 16, 94–105. [Google Scholar] [CrossRef]
- Rogalska, A.; Kuter, K.; Żelazko, A.; Głogowska-Gruszka, A.; Świętochowska, E.; Nowak, P. Fluoride Alteration of [3H]Glucose Uptake in Wistar Rat Brain and Peripheral Tissues. Neurotox. Res. 2017, 31, 436–443. [Google Scholar] [CrossRef]
- Guan, Z.-Z.; Wang, Y.-N.; Xiao, K.-Q.; Dai, D.-Y.; Chen, Y.-H.; Liu, J.-L.; Sindelar, P.; Dallner, G. Influence of Chronic Fluorosis on Membrane Lipids in Rat Brain. Neurotoxicol. Teratol. 1998, 20, 537–542. [Google Scholar] [CrossRef]
- Adedara, I.; Olabiyi, B.; Ojuade, T.; Idris, U.; Onibiyo, E.; Farombi, E. Taurine reverses sodium fluoride-mediated increase in inflammation, caspase-3 activity, and oxidative damage along the brain-pituitary-gonadal axis in male rats. Can. J. Physiol. Pharmacol. 2017, 95, 1019–1029. [Google Scholar] [CrossRef]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Jakubczyk, K.; Tarnowski, M.; Lubkowska, A.; Baranowska-Bosiacka, I.; Styburski, D.; Skórka-Majewicz, M.; Maciejewska, D.; et al. Chronic Exposure to Fluoride Affects GSH Level and NOX4 Expression in Rat Model of This Element of Neurotoxicity. Biomolecules 2020, 10, 422. [Google Scholar] [CrossRef]
- Shuhua, X.; Ziyou, L.; Ling, Y.; Fei, W.; Sun, G. A Role of Fluoride on Free Radical Generation and Oxidative Stress in BV-2 Microglia Cells. Mediat. Inflamm. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Yan, N.; Liu, Y.; Liu, S.; Cao, S.; Wang, F.; Wang, Z.; Xi, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460. [Google Scholar] [CrossRef]
- Liu, X.-L.; Li, C.-C.; Liu, K.-J.; Cui, C.-Y.; Zhang, Y.-Z.; Liu, Y. The Influence of Fluoride on the Expression of Inhibitors of Wnt/β-Catenin Signaling Pathway in Rat Skin Fibroblast Cells. Biol. Trace Elem. Res. 2012, 148, 117–121. [Google Scholar] [CrossRef]
- Xu, B.; Xu, Z.; Xia, T.; He, P.; Gao, P.; He, W.; Zhang, M.; Guo, L.; Niu, Q.; Wang, A. Effects of the Fas/Fas-L pathway on fluoride-induced apoptosis in SH-SY5Y cells. Environ. Toxicol. 2011, 26, 86–92. [Google Scholar] [CrossRef]
- Tu, W.; Zhang, Q.; Liu, Y.; Han, L.; Wang, Q.; Chen, P.; Zhang, S.; Wang, A.; Zhou, X. Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2018, 347, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Tang, S.; Yang, L.; Niu, Q.; Chen, J.; Xia, T.; Wang, S.; Wang, M.; Zhao, Q.; Liu, L.; et al. Effects of long-term fluoride exposure on cognitive ability and the underlying mechanisms: Role of autophagy and its association with apoptosis. Toxicol. Appl. Pharmacol. 2019, 378, 114608. [Google Scholar] [CrossRef] [PubMed]
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.; Basu, N.; Peterson, K.; Ettinger, A.; Wright, R.; Zhang, Z.; et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xiang, J.; Dong, Y.-T.; Xu, Y.; Li, Y.; Song, H.; Zeng, X.-X.; Ran, L.-Y.; Hong, W.; Guan, Z.-Z. Exposure to fluoride aggravates the impairment in learning and memory and neuropathological lesions in mice carrying the APP/PS1 double-transgenic mutation. Alzheimer’s Res. Ther. 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Farmus, L.; Till, C.; Green, R.; Hornung, R.; Mier, E.A.M.; Ayotte, P.; Muckle, G.; Lanphear, B.P.; Flora, D.B. Critical windows of fluoride neurotoxicity in Canadian children. Environ. Res. 2021, 200, 111315. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef]
- Russ, T.C.; Killin, L.O.J.; Hannah, J.; Batty, G.; Deary, I.J.; Starr, J.M. Aluminium and fluoride in drinking water in relation to later dementia risk. Br. J. Psychiatry 2020, 216, 29–34. [Google Scholar] [CrossRef]
- Malin, A.J.; Till, C. Exposure to fluoridated water and attention deficit hyperactivity disorder prevalence among children and adolescents in the United States: An ecological association. Environ. Health 2015, 14, 17. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Nadei, O. Inorganic fluoride and functions of brain. Crit. Rev. Toxicol. 2020, 50, 28–46. [Google Scholar] [CrossRef]
- Broadbent, J.M.; Thomson, W.M.; Ramrakha, S.; Moffitt, T.E.; Zeng, J.; Page, L.A.F.; Poulton, R. Community Water Fluoridation and Intelligence: Prospective Study in New Zealand. Am. J. Public Health 2015, 105, 72–76. [Google Scholar] [CrossRef]
- Saeed, M.; Malik, R.N.; Kamal, A. Fluorosis and cognitive development among children (6–14 years of age) in the endemic areas of the world: A review and critical analysis. Environ. Sci. Pollut. Res. 2020, 27, 2566–2579. [Google Scholar] [CrossRef]
- Till, C.; Green, R. Controversy: The evolving science of fluoride: When new evidence doesn’t conform with existing beliefs. Pediatr. Res. 2020, 90, 1093–1095. [Google Scholar] [CrossRef]
- Habib, A.; Hoppe, M.; Beiriger, J.; Kodavali, C.V.; Edwards, L.; Zinn, P.O. Letter: Glioblastoma Cell of Origin. Stem Cell Rev. Rep. 2022, 18, 691–693. [Google Scholar] [CrossRef]
- Claus, E.B.; Walsh, K.M.; Wiencke, J.K.; Molinaro, A.M.; Wiemels, J.L.; Schildkraut, J.M.; Bondy, M.L.; Berger, M.; Jenkins, R.; Wrensch, M. Survival and low-grade glioma: The emergence of genetic information. Neurosurg. Focus 2015, 38, E6. [Google Scholar] [CrossRef]
- Kanderi, T.; Gupta, V. Glioblastoma Multiforme. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Stupp, R.; Mason, W.; van den Bent, M.; Weller, M.; Fisher, B.; Taphoorn, M.; Belanger, K.; Brandes, A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Arora, A.; Somasundaram, K. Glioblastoma vs temozolomide: Can the red queen race be won? Cancer Biol. Ther. 2019, 20, 1083–1090. [Google Scholar] [CrossRef]
- Alfonso, J.C.L.; Talkenberger, K.; Seifert, M.; Klink, B.; Hawkins-Daarud, A.; Swanson, K.R.; Hatzikirou, H.; Deutsch, A. The biology and mathematical modelling of glioma invasion: A review. J. R. Soc. Interface 2017, 14, 20170490. [Google Scholar] [CrossRef]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef]
- Manini, I.; Caponnetto, F.; Bartolini, A.; Ius, T.; Mariuzzi, L.; Di Loreto, C.; Beltrami, A.; Cesselli, D. Role of Microenvironment in Glioma Invasion: What We Learned from In Vitro Models. Int. J. Mol. Sci. 2018, 19, 147. [Google Scholar] [CrossRef]
- Koh, I.; Cha, J.; Park, J.; Choi, J.; Kang, S.-G.; Kim, P. The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.P.; Sells, B.E.; Haque, S.J.; Chakravarti, A. Tumor Heterogeneity in Glioblastomas: From Light Microscopy to Molecular Pathology. Cancers 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.-d.-M.; Bonavia, R.; Seoane, J. Glioblastoma Multiforme: A Look Inside Its Heterogeneous Nature. Cancers 2014, 6, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef]
- Feldheim, J.; Kessler, A.F.; Monoranu, C.M.; Ernestus, R.-I.; Löhr, M.; Hagemann, C. Changes of O6-Methylguanine DNA Methyltransferase (MGMT) Promoter Methylation in Glioblastoma Relapse—A Meta-Analysis Type Literature Review. Cancers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Wang, K.; Pan, L.; Che, X.; Cui, D.; Li, C. Sonic Hedgehog/GLI1 signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurol. Res. 2010, 32, 975–980. [Google Scholar] [CrossRef]
- Avci, N.; Ebrahimzadeh-Pustchi, S.; Akay, Y.; Esquenazi, Y.; Tandon, N.; Zhu, J.-J.; Akay, M. NF-κB inhibitor with Temozolomide results in significant apoptosis in glioblastoma via the NF-κB(p65) and actin cytoskeleton regulatory pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Latour, M.; Her, N.-G.; Kesari, S.; Nurmemmedov, E. WNT Signaling as a Therapeutic Target for Glioblastoma. Int. J. Mol. Sci. 2021, 22, 8428. [Google Scholar] [CrossRef]
- D’Amico, M.; De Amicis, F. Aberrant Notch signaling in gliomas: A potential landscape of actionable converging targets for combination approach in therapies resistance. Cancer Drug Resist. 2022, 5, 939–953. [Google Scholar] [CrossRef]
- Choudhry, Z.; Rikani, A.A.; Choudhry, A.M.; Tariq, S.; Zakaria, F.; Asghar, M.W.; Sarfraz, M.; Haider, K.; Shafiq, A.A.; Mobassarah, N.J. Sonic hedgehog signalling pathway: A complex network. Ann. Neurosci. 2014, 21, 28–31. [Google Scholar] [CrossRef]
- Carpenter, R.; Lo, H.-W. Hedgehog pathway and GLI1 isoforms in human cancer. Discov. Med. 2012, 13, 105–113. [Google Scholar]
- Zhu, H.; Lo, H.-W. The Human Glioma-Associated Oncogene Homolog 1 (GLI1) Family of Transcription Factors in Gene Regulation and Diseases. Curr. Genom. 2010, 11, 238–245. [Google Scholar] [CrossRef]
- Pietrobono, S.; Gagliardi, S.; Stecca, B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019, 10, 556. [Google Scholar] [CrossRef]
- Xie, J.; Aszterbaum, M.; Zhang, X.; Bonifas, J.; Zachary, C.; Epstein, E.; McCormick, F. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 9255–9259. [Google Scholar] [CrossRef]
- Zhu, H.; Carpenter, R.L.; Han, W.; Lo, H.-W. The GLI1 splice variant TGLI1 promotes glioblastoma angiogenesis and growth. Cancer Lett. 2014, 343, 51–61. [Google Scholar] [CrossRef]
- Doheny, D.; Sirkisoon, S.; Carpenter, R.; Aguayo, N.; Regua, A.; Anguelov, M.; Manore, S.; Arrigo, A.; Jalboush, S.; Wong, G.; et al. Combined inhibition of JAK2-STAT3 and SMO-GLI1/tGLI1 pathways suppresses breast cancer stem cells, tumor growth, and metastasis. Oncogene 2020, 39, 6589–6605. [Google Scholar] [CrossRef]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Doheny, D.; Zhu, D.; Aguayo, N.R.; Xing, F.; Chan, M.; Ruiz, J.; Metheny-Barlow, L.J.; et al. TGLI1 transcription factor mediates breast cancer brain metastasis via activating metastasis-initiating cancer stem cells and astrocytes in the tumor microenvironment. Oncogene 2020, 39, 64–78. [Google Scholar] [CrossRef]
- Wang, K.; Chen, D.; Qian, Z.; Cui, D.; Gao, L.; Lou, M. Hedgehog/Gli1 signaling pathway regulates MGMT expression and chemoresistance to temozolomide in human glioblastoma. Cancer Cell Int. 2017, 17, 117. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Zhao, S.; Yao, K.; Sun, Y.; Li, Y.; Chen, L.; Li, R.; Zhai, X.; Zhang, J.; et al. GANT61, a GLI inhibitor, sensitizes glioma cells to the temozolomide treatment. J. Exp. Clin. Cancer Res. 2016, 35, 1–14. [Google Scholar] [CrossRef]
- Ji, M.; Wang, L.; Chen, J.; Xue, N.; Wang, C.; Lai, F.; Wang, R.; Yu, S.; Jin, J.; Chen, X. CAT3, a prodrug of 13a(S)-3-hydroxyl-6,7-dimethoxyphenanthro[9,10-b]-indolizidine, circumvents temozolomide-resistant glioblastoma via the Hedgehog signaling pathway, independently of O6-methylguanine DNA methyltransferase expression. Onco Targets Ther. 2018, 11, 3671–3684. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhao, Z.; Xu, H. Preliminary Analysis of MicroRNAs Expression Profiling in MC3T3-E1 Cells Exposed to Fluoride. Biol. Trace Elem. Res. 2017, 176, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yu, Y.; Deng, C. Protein and mRNA expression of Shh, Smo and Gli1 and inhibition by cyclopamine in hepatocytes of rats with chronic fluorosis. Toxicol. Lett. 2014, 225, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yu, Y.; Chen, R. Role of hedgehog signaling pathway on cartilage tissue damage in chronic fluorosis rats. Chin. J. Public Health 2018, 34, 241–245. [Google Scholar]
- Deng, C.; Xu, L.; Zhang, Y.; Zhao, L.; Linghu, Y.; Yu, Y. The value of the hedgehog signal in osteoblasts in fluoride-induced bone-tissue injury. J. Orthop. Surg. Res. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Sun, S.-C. Non-canonical NF-κB signaling pathway. Cell Res. 2011, 21, 71–85. [Google Scholar] [CrossRef]
- Bhat, K.; Balasubramaniyan, V.; Vaillant, B.; Ezhilarasan, R.; Hummelink, K.; Hollingsworth, F.; Wani, K.; Heathcock, L.; James, J.; Goodman, L.; et al. Mesenchymal Differentiation Mediated by NF-κB Promotes Radiation Resistance in Glioblastoma. Cancer Cell 2013, 24, 331–346. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D.; Narasimamurthy, R.; Xia, Y.; Myskiw, C.; Soda, Y.; Verma, I. Targeting NF-κB in glioblastoma: A therapeutic approach. Sci. Adv. 2016, 2, e1501292. [Google Scholar] [CrossRef]
- Gray, G.; McFarland, B.; Nozell, S.; Benveniste, E. NF-κB and STAT3 in glioblastoma: Therapeutic targets coming of age. Expert Rev. Neurother. 2014, 14, 1293–1306. [Google Scholar] [CrossRef]
- McFarland, B.; Gray, G.; Nozell, S.; Hong, S.; Benveniste, E. Activation of the NF-κB Pathway by the STAT3 Inhibitor JSI-124 in Human Glioblastoma Cells. Mol. Cancer Res. 2013, 11, 494–505. [Google Scholar] [CrossRef]
- Raychaudhuri, B.; Han, Y.; Lu, T.; Vogelbaum, M. Aberrant constitutive activation of nuclear factor kappaB in glioblastoma multiforme drives invasive phenotype. J. Neuro Oncol. 2007, 85, 39–47. [Google Scholar] [CrossRef]
- Fianco, G.; Mongiardi, M.; Levi, A.; De Luca, T.; Desideri, M.; Trisciuoglio, D.; Del Bufalo, D.; Cinà, I.; Di Benedetto, A.; Mottolese, M.; et al. Caspase-8 contributes to angiogenesis and chemotherapy resistance in glioblastoma. Elife 2017, 6, e22593. [Google Scholar] [CrossRef]
- Jiang, L.; Song, L.; Wu, J.; Yang, Y.; Zhu, X.; Hu, B.; Cheng, S.-Y.; Li, M. Bmi-1 promotes glioma angiogenesis by activating NF-κB signaling. PLoS ONE 2013, 8, e55527. [Google Scholar] [CrossRef]
- Ritchie, C.; Giordano, A.; Khalili, K. Integrin involvement in glioblastoma multiforme: Possible regulation by NF-kappaB. J. Cell Physiol. 2000, 184, 214–221. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Shi, Y.; Han, Y.; Liang, C.; Feng, Z.; Zheng, H.; Eng, M.; Wang, J. Fluoride-Induced Autophagy via the Regulation of Phosphorylation of Mammalian Targets of Rapamycin in Mice Leydig Cells. J. Agric. Food Chem. 2017, 65, 8966–8976. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Wang, P.; Yan, Y.-J.; Li, Y.; Guan, M.-W.; Yu, J.-J.; Wang, X.-D. IL-33 enhances glioma cell migration and invasion by upregulation of MMP2 and MMP9 via the ST2-NF-κB pathway. Oncol. Rep. 2017, 38, 2033–2042. [Google Scholar] [CrossRef]
- Liu, T.; Ma, W.; Xu, H.; Huang, M.; Zhang, D.; He, Z.; Zhang, L.; Brem, S.; O’Rourke, D.; Gong, Y.; et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 2018, 9, 3439. [Google Scholar] [CrossRef]
- Yamini, B. NF-κB, Mesenchymal Differentiation and Glioblastoma. Cells 2018, 7, 125. [Google Scholar] [CrossRef]
- Soubannier, V.; Stifani, S. NF-κB Signalling in Glioblastoma. Biomedicines 2017, 5, 29. [Google Scholar] [CrossRef]
- Kim, Y.; Varn, F.S.; Park, S.-H.; Yoon, B.W.; Park, H.R.; Lee, C.; Verhaak, R.G.W.; Paek, S.H. Perspective of mesenchymal transformation in glioblastoma. Acta Neuropathol. Commun. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Yu, X.; Wang, M.; Zuo, J.; Wahafu, A.; Mao, P.; Li, R.; Wu, W.; Xie, W.; Wang, J. Nuclear factor I A promotes temozolomide resistance in glioblastoma via activation of nuclear factor κB pathway. Life Sci. 2019, 236, 116917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; JIia, L.; Jin, X.; Liu, Q.; Cao, W.; Gao, X.; Yang, M.; Sun, B. NF-κB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol. Lett. 2015, 9, 2586–2590. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ruiz-Ontañon, P.; Vazquez-Barquero, A.; Moris, F.; Fernandez-Luna, J.L. The NFκB pathway: A therapeutic target in glioblastoma. Oncotarget 2011, 2, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Baśkiewicz-Masiuk, E.; Machaliński, B.; Rybicka, M.; Gutowska, I.; Bober, J.; Grymula, K.; Dziedziejko, V.; Chlubek, D. Sodium Fluoride Enhancement Of Monocyte Differentiation Via Nuclear Factor Κb Mechanism. Fluoride 2005, 38, 297–306. [Google Scholar]
- Tian, Y.; Huo, M.; Li, G.; Li, Y.; Wang, J. Regulation of LPS-induced mRNA expression of pro-inflammatory cytokines via alteration of NF-κB activity in mouse peritoneal macrophages exposed to fluoride. Chemosphere 2016, 161, 89–95. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Xiong, Y.; Zou, X.; Liu, Z. Comparative study of p38 MAPK signal transduction pathway of peripheral blood mononuclear cells from patients with coal-combustion-type fluorosis with and without high hair selenium levels. Int. J. Hyg. Environ. Health 2010, 213, 381–386. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride induces renal inflammatory responses by activating NF-κB signaling pathway and reducing anti-inflammatory cytokine expression in mice. Oncotarget 2017, 8, 80192–80207. [Google Scholar] [CrossRef]
- Deng, H.; Kuang, P.; Cui, H.; Luo, Q.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride induces apoptosis in mouse splenocytes by activating ROS-dependent NF-κB signaling. Oncotarget 2017, 8, 114428–114441. [Google Scholar] [CrossRef]
- Sana, S.; Ghosh, S.; Das, N.; Sarkar, S.; Mandal, A. Vesicular melatonin efficiently downregulates sodium fluoride-induced rat hepato- and broncho-TNF-α, TGF-β expressions, and associated oxidative injury: A comparative study of liposomal and nanoencapsulated forms. Int. J. Nanomed. 2017, 12, 4059–4071. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, S.K.; Kumar, K.; Trivedi, R.; Godbole, M.M. Simultaneous Exposure of Excess Fluoride and Calcium Deficiency Alters VDR, CaR, and Calbindin D 9 k mRNA Levels in Rat Duodenal Mucosa. Calcif. Tissue Int. 2004, 75, 313–320. [Google Scholar] [CrossRef]
- Sun, J.; Kong, J.; Duan, Y.; Szeto, F.; Liao, A.; Madara, J.; Li, Y. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E315–E322. [Google Scholar] [CrossRef]
- Łukomska, A.; Baranowska-Bosiacka, I.; Dec, K.; Pilutin, A.; Tarnowski, M.; Jakubczyk, K.; Żwierełło, W.; Skórka-Majewicz, M.; Chlubek, D.; Gutowska, I. Changes in Gene and Protein Expression of Metalloproteinase-2 and -9 and their Inhibitors TIMP2 and TIMP3 in Different Parts of Fluoride-Exposed Rat Brain. Int. J. Mol. Sci. 2020, 22, 391. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, W.-J.; Xu, X.-H.; Zhang, Z.-G. Effect of fluoride on calcium ion concentration and expression of nuclear transcription factor kappa-B ρ65 in rat hippocampus. Exp. Toxicol. Pathol. 2011, 63, 407–411. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Xia, T.; He, P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons. Toxicol. Lett. 2008, 179, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, T.; Zhang, J.; Mao, Q.; Li, S.; Xiong, W.; Qiu, Y.; Xie, Q.; Ge, J. Notch1 promotes glioma cell migration and invasion by stimulating β-catenin and NF-κB signaling via AKT activation. Cancer Sci. 2012, 103, 181–190. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.-K.; Ahn, S.H.; Lee, J.; Nam, D.-H. WNT signaling in glioblastoma and therapeutic opportunities. Lab. Investig. 2016, 96, 137–150. [Google Scholar] [CrossRef]
- Kahlert, U.D.; Maciaczyk, D.; Doostkam, S.; Orr, B.A.; Simons, B.; Bogiel, T.; Reithmeier, T.; Prinz, M.; Schubert, J.; Niedermann, G.; et al. Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 2012, 325, 42–53. [Google Scholar] [CrossRef]
- Basu, S.; Cheriyamundath, S.; Ben-Ze’Ev, A. Cell–cell adhesion: Linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis. F1000Research 2018, 7, 1488. [Google Scholar] [CrossRef]
- Zhang, M.; Atkinson, R.L.; Rosen, J.M. Selective targeting of radiation-resistant tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3522–3527. [Google Scholar] [CrossRef]
- Semenov, M.V.; Habas, R.; MacDonald, B.T.; He, X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell 2007, 131, 1378. [Google Scholar] [CrossRef]
- Rao, T.; Kühl, M. An updated overview on Wnt signaling pathways: A prelude for more. Circ. Res. 2010, 106, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Visioli, A.; Giani, F.; Trivieri, N.; Palumbo, O.; Restelli, S.; Dezi, F.; Mazza, T.; Fusilli, C.; Legnani, F.; et al. Wnt5a Drives an Invasive Phenotype in Human Glioblastoma Stem-like Cells. Cancer Res. 2017, 77, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Kamino, M.; Kishida, M.; Kibe, T.; Ikoma, K.; Iijima, M.; Hirano, H.; Tokudome, M.; Koriyama, C.; Kishida, S.; Chen, L.; et al. Wnt-5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci. 2011, 102, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Wickström, M.; Dyberg, C.; Milosevic, J.; Einvik, C.; Calero, R.; Sveinbjörnsson, B.; Sandén, E.; Darabi, A.; Siesjö, P.; Kool, M.; et al. Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat. Commun. 2015, 6, 8904. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, L.-D.; Liu, H.; Li, H.-H.; Ren, C.; Zhang, P.; Guo, K.-T.; Zhang, H.-X.; Geng, D.-Q.; Zhang, C.-Y. Fluoride Induces Neuroinflammation and Alters Wnt Signaling Pathway in BV2 Microglial Cells. Inflammation 2017, 40, 1123–1130. [Google Scholar] [CrossRef]
- Zeng, Q.B.; Xu, Y.Y.; Tu, C.L.; Yu, X.; Yang, J.; Hong, F. [Biological exposure limits caused by co exposure to fluoride and arsenic based on Wnt signaling pathway]. Ying Yong Sheng Tai Xue Bao 2019, 30, 37–42. [Google Scholar]
- Luo, K.; Qin, Y.; Ouyang, T.; Wang, X.; Zhang, A.; Luo, P.; Pan, X. let-7c-5p regulates CyclinD1 in fluoride-mediated osteoblast proliferation and activation. Toxicol. Sci. 2021, 182, 275–287. [Google Scholar] [CrossRef]
- Peng, L.; Li, Y.; Shusterman, K.; Kuehl, M.; Gibson, C. Wnt-RhoA signaling is involved in dental enamel development. Eur. J. Oral Sci. 2011, 119 (Suppl. 1), 41–49. [Google Scholar] [CrossRef]
- Shusterman, K.; Gibson, C.; Li, Y.; Healey, M.; Peng, L. Wnt-RhoA Signaling Pathways in Fluoride-Treated Ameloblast-Lineage Cells. CTO 2014, 199, 159–168. [Google Scholar] [CrossRef]
- Nadei, O.V.; Khvorova, I.A.; Agalakova, N.I. Cognitive Decline of Rats with Chronic Fluorosis Is Associated with Alterations in Hippocampal Calpain Signaling. Biol. Trace Elem. Res. 2020, 197, 495–506. [Google Scholar] [CrossRef]
- Mathieu, P.; Adami, P.V.M.; Morelli, L. Notch signaling in the pathologic adult brain. Biomol. Concepts 2013, 4, 465–476. [Google Scholar] [CrossRef]
- Takebe, N.; Nguyen, D.; Yang, S.X. Targeting Notch signaling pathway in cancer: Clinical development advances and challenges. Pharmacol. Ther. 2013, 141, 140–149. [Google Scholar] [CrossRef]
- Kanamori, M.; Kawaguchi, T.; Nigro, J.M.; Feuerstein, B.G.; Berger, M.S.; Miele, L.; Pieper, R.O. Contribution of Notch signaling activation to human glioblastoma multiforme. J. Neurosurg. 2007, 106, 417–427. [Google Scholar] [CrossRef]
- Biswas, S.; Rao, C.M. Epigenetics in cancer: Fundamentals and Beyond. Pharmacol. Ther. 2017, 173, 118–134. [Google Scholar] [CrossRef]
- Yi, G.-Z.; Huang, G.; Guo, M.; Zhang, X.; Wang, H.; Deng, S.; Li, Y.; Xiang, W.; Chen, Z.; Pan, J.; et al. Acquired temozolomide resistance in MGMT-deficient glioblastoma cells is associated with regulation of DNA repair by DHC2. Brain 2019, 142, 2352–2366. [Google Scholar] [CrossRef]
- Bazzoni, R.; Bentivegna, A. Role of Notch Signaling Pathway in Glioblastoma Pathogenesis. Cancers 2019, 11, 292. [Google Scholar] [CrossRef]
- Han, N.; Hu, G.; Shi, L.; Long, G.; Yang, L.; Xi, Q.; Guo, Q.; Wang, J.; Dong, Z.; Zhang, M. Notch1ablation radiosensitizes glioblastoma cells. Oncotarget 2017, 8, 88059–88068. [Google Scholar] [CrossRef]
- Xing, Z.-Y.; Sun, L.-G.; Guo, W.-J. Elevated expression of Notch-1 and EGFR induced apoptosis in glioblastoma multiforme patients. Clin. Neurol. Neurosurg. 2015, 131, 54–58. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, X.; He, Y.; Zhang, J.; Huang, H.; Bian, W.; Chilufya, M.M.; Zhao, Y.; Han, J. Progress of Signaling Pathways, Stress Pathways and Epigenetics in the Pathogenesis of Skeletal Fluorosis. Int. J. Mol. Sci. 2021, 22, 11932. [Google Scholar] [CrossRef]
- Allen, E.A.; Baehrecke, E.H. Autophagy in animal development. Cell Death Differ. 2020, 27, 903–918. [Google Scholar] [CrossRef]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef] [PubMed]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.; Cabodevilla, A.G.; Simpson, J.; Gammoh, N. Interplay of autophagy, receptor tyrosine kinase signalling and endocytic trafficking. Essays Biochem. 2017, 61, 597–607. [Google Scholar] [PubMed]
- Barrow-McGee, R.; Kishi, N.; Joffre, C.; Ménard, L.; Hervieu, A.; Bakhouche, B.; Noval, A.; Mai, A.; Guzmán, C.; Robbez-Masson, L.; et al. Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat. Commun. 2016, 7, 11942. [Google Scholar] [CrossRef]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.; Kakiuchi, M.; Gupta, S.; Sohn, A.; Mukhopadhyay, S.; Lin, E.; Parker, S.; et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 2020, 581, 100–105. [Google Scholar] [CrossRef]
- Gu, X.; Han, D.; Chen, W.; Zhang, L.; Lin, Q.; Gao, J.; Fanning, S.; Han, B. SIRT1-mediated FoxOs pathways protect against apoptosis by promoting autophagy in osteoblast-like MC3T3-E1 cells exposed to sodium fluoride. Oncotarget 2016, 7, 65218–65230. [Google Scholar] [CrossRef]
- Li, X.; Meng, L.; Wang, F.; Hu, X.; Yu, Y. Sodium fluoride induces apoptosis and autophagy via the endoplasmic reticulum stress pathway in MC3T3-E1 osteoblastic cells. Mol. Cell. Biochem. 2019, 454, 77–85. [Google Scholar] [CrossRef]
- Xu, L.; Deng, C.; Zhang, Y.; Zhao, L.; Linghu, Y.; Yu, Y. Expression of Autophagy-Related Factors LC3A and Beclin 1 and Apoptosis-Related Factors Bcl-2 and BAX in Osteoblasts Treated With Sodium Fluoride. Front. Physiol. 2021, 12, 603848. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Mehmood, K.; Hussain, R.; Abbas, R.Z.; Javed, M.T.; Chang, Y.-F.; Hu, L.; Pan, J.; Li, Y.; et al. Long-term exposure to the fluoride blocks the development of chondrocytes in the ducks: The molecular mechanism of fluoride regulating autophagy and apoptosis. Ecotoxicol. Environ. Saf. 2021, 217, 112225. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, R.; Li, D.; Qiao, T.; Guo, X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem. Interact. 2021, 349, 109659. [Google Scholar] [CrossRef]
- Suzuki, M.; Bartlett, J.D. Sirtuin1 and autophagy protect cells from fluoride-induced cell stress. Biochim. Biophys. Acta 2014, 1842, 245–255. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, Y.; Zhang, K.-Q.; Li, J. [In vivo and in vitro experimental study on the effect of fluoride-induced autophagy in rat HAT-7 cell line]. Shanghai Kou Qiang Yi Xue 2016, 25, 426–430. [Google Scholar]
- Urut, F.; DeDe, S.; Yuksek, V.; Cetin, S.; Usta, A.; Taspinar, M. In Vitro Evaluation of the Apoptotic, Autophagic, and Necrotic Molecular Pathways of Fluoride. Biol. Trace Elem. Res. 2021, 199, 3700–3706. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, Z.; Niu, R.; Manthari, R.K.; Yuan, M.; Yang, K.; Cheng, M.; Gong, Z.; Wang, J. Effect of arsenic and/or fluoride gestational exposure on renal autophagy in offspring mice. Chemosphere 2020, 241, 124861. [Google Scholar] [CrossRef]
- Tian, X.; Xie, J.; Chen, X.; Dong, N.; Feng, J.; Gao, Y.; Tian, F.; Zhang, W.; Qiu, Y.; Niu, R.; et al. Deregulation of autophagy is involved in nephrotoxicity of arsenite and fluoride exposure during gestation to puberty in rat offspring. Arch. Toxicol. 2019, 94, 749–760. [Google Scholar] [CrossRef]
- Kuang, P.; Deng, H.; Liu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Sodium fluoride induces splenocyte autophagy via the mammalian targets of rapamycin (mTOR) signaling pathway in growing mice. Aging 2018, 10, 1649–1665. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, J.; Manthari, R.K.; Wang, J. Fluoride induces apoptosis and autophagy through the IL-17 signaling pathway in mice hepatocytes. Arch. Toxicol. 2018, 92, 3277–3289. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, S.; Chen, W.; Quan, C.; Duan, P.; Huang, W.; Wang, A.; Yang, K. [Effects of fluoride on autophagy level in human neuroblastoma SH-SY5Y cells]. Wei Sheng Yan Jiu 2017, 46, 472–480. [Google Scholar]
- Zhang, C.; Huo, S.; Fan, Y.; Gao, Y.; Yang, Y.; Sun, D. Autophagy May Be Involved in Fluoride-Induced Learning Impairment in Rats. Biol. Trace Elem. Res. 2020, 193, 502–507. [Google Scholar] [CrossRef]
- Coniglio, S.; Miller, I.; Symons, M.; Segall, J.E. Coculture Assays to Study Macrophage and Microglia Stimulation of Glioblastoma Invasion. J. Vis. Exp. 2016, 116, e53990. [Google Scholar] [CrossRef] [PubMed]
- Prionisti, I.; Buhler, L.H.; Walker, P.R.; Jolivet, R.B. Harnessing Microglia and Macrophages for the Treatment of Glioblastoma. Front. Pharmacol. 2019, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.-Z.; Xu, S.-L.; Xin, Y.-H.; Yu, S.-C.; Ping, Y.-F.; Chen, L.; Xiao, H.-L.; Wang, B.; Yi, L.; Wang, Q.-L.; et al. Tumor-Associated Microglia/Macrophages Enhance the Invasion of Glioma Stem-like Cells via TGF-β1 Signaling Pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.T.; McDonald, K.L.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: Implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A. Interleukins in Cancer Biology: Their Heterogeneous Role; Elsevier/AP, Academic Press is an Imprint of Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2014. [Google Scholar]
- Yan, L.; Liu, S.; Wang, C.; Wang, F.; Song, Y.; Yan, N.; Xi, S.; Liu, Z.; Sun, G. JNK and NADPH Oxidase Involved in Fluoride-Induced Oxidative Stress in BV-2 Microglia Cells. Mediat. Inflamm. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jin, P.; Wang, X.; Zhou, Q.; Lin, X.; Xi, S. Fluoride activates microglia, secretes inflammatory factors and influences synaptic neuron plasticity in the hippocampus of rats. Neurotoxicology 2018, 69, 108–120. [Google Scholar] [CrossRef]
- Wang, J.; Yue, B.; Zhang, X.; Guo, X.; Sun, Z.; Niu, R. Effect of exercise on microglial activation and transcriptome of hippocampus in fluorosis mice. Sci. Total Environ. 2021, 760, 143376. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Cuny, E.; Loiseau, H.; Penchet, G.; Ellie, E.; Arsaut, J.; Vital, A.; Vincendeau, P.; Demotes-Mainard, J. Association of elevated glial expression of interleukin-1β with improved survival in patients with glioblastomas multiforme. J. Neurosurg. 2002, 96, 294–301. [Google Scholar] [CrossRef]
- Sharma, V.; Dixit, D.; Ghosh, S.; Sen, E. COX-2 regulates the proliferation of glioma stem like cells. Neurochem. Int. 2011, 59, 567–571. [Google Scholar] [CrossRef]
- Sarkar, S.; Yong, V.W. Inflammatory cytokine modulation of matrix metalloproteinase expression and invasiveness of glioma cells in a 3-dimensional collagen matrix. J. Neuro Oncol. 2009, 91, 157–164. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Bryce, N.; Adams, S.; Braidy, N.; Konayagi, M.; McDonald, K.L.; Teo, C.; Guillemin, G.; Grewal, T.; Munoz, L. p38 MAPK inhibitors attenuate pro-inflammatory cytokine production and the invasiveness of human U251 glioblastoma cells. J. Neuro Oncol. 2012, 109, 35–44. [Google Scholar] [CrossRef]
- Sakuma, S.; Sawamura, Y.; Tada, M.; Aida, T.; Abe, H.; Suzuki, K.; Taniguchi, N. Responses of human glioblastoma cells to human natural tumor necrosis factor-alpha: Susceptibility, mechanism of resistance and cytokine production studies. J. Neuro Oncol. 1993, 15, 197–208. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, B.; Lee, Y.; Jeong, J.; Kang, S.; Choi, J.; Yang, Y.; Lee, D.; Roh, G.; Kim, H.; et al. Resveratrol Reduces TNF-α-induced U373MG Human Glioma Cell Invasion through Regulating NF-κB Activation and uPA/uPAR Expression. Anticancer. Res. 2011, 31, 4223–4230. [Google Scholar]
- Nabors, L.; Suswam, E.; Huang, Y.; Yang, X.; Johnson, M.; King, P. Tumor Necrosis Factor α Induces Angiogenic Factor Up-Regulation in Malignant Glioma Cells: A Role for RNA Stabilization and HuR. Cancer Res. 2003, 63, 4181–4187. [Google Scholar]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol. Rep. 2017, 37, 1907–1913. [Google Scholar] [CrossRef]
- Chopra, S.; Overall, C.M.; Dufour, A. Matrix metalloproteinases in the CNS: Interferons get nervous. Cell. Mol. Life Sci. 2019, 76, 3083–3095. [Google Scholar] [CrossRef]
- Mu, N.; Gu, J.; Liu, N.; Xue, X.; Shu, Z.; Zhang, K.; Huang, T.; Chu, C.; Zhang, W.; Gong, L.; et al. PRL-3 is a potential glioblastoma prognostic marker and promotes glioblastoma progression by enhancing MMP7 through the ERK and JNK pathways. Theranostics 2018, 8, 1527–1539. [Google Scholar] [CrossRef]
- Sincevičiūtė, R.; Vaitkienė, P.; Urbanavičiūtė, R.; Steponaitis, G.; Tamašauskas, A.; Skiriutė, D. MMP2 is associated with glioma malignancy and patient outcome. Int. J. Clin. Exp. Pathol. 2018, 11, 3010–3018. [Google Scholar]
- Zhang, H.; Ma, Y.; Wang, H.; Xu, L.; Yu, Y. MMP-2 expression and correlation with pathology and MRI of glioma. Oncol. Lett. 2019, 17, 1826–1832. [Google Scholar] [PubMed]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef] [PubMed]
- Slompo, C.; Buzalaf, C.P.; Damante, C.A.; Martins, G.M.; Hannas, A.R.; Buzalaf, M.A.R.; Oliveira, R.C. Fluoride modulates preosteoblasts viability and matrix metalloproteinases-2 and -9 activities. Braz. Dent. J. 2012, 23, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Tan, P.; Liu, J.; Zhao, J.; Zhou, B. The MMP-9/TIMP-1 System is Involved in Fluoride-Induced Reproductive Dysfunctions in Female Mice. Biol. Trace Elem. Res. 2017, 178, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Quadri, J.A.; Sarwar, S.; Pinky; Kar, P.; Singh, S.; Mallick, S.R.; Arava, S.; Nag, T.C.; Roy, T.S.; Shariff, A. Fluoride induced tissue hypercalcemia, IL-17 mediated inflammation and apoptosis lead to cardiomyopathy: Ultrastructural and biochemical findings. Toxicology 2018, 406–407, 44–57. [Google Scholar] [CrossRef]
- Ichikawa, T.; Otani, Y.; Kurozumi, K.; Date, I. Phenotypic Transition as a Survival Strategy of Glioma. Neurol. Med. Chir. 2016, 56, 387–395. [Google Scholar] [CrossRef]
- Höring, E.; Harter, P.; Seznec, J.; Schittenhelm, J.; Bühring, H.-J.; Bhattacharyya, S.; von Hattingen, E.; Zachskorn, C.; Mittelbronn, M.; Naumann, U. The “go or grow” potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic stress. Acta Neuropathol. 2012, 124, 83–97. [Google Scholar] [CrossRef]
- Godlewski, J.; Nowicki, M.; Bronisz, A.; Nuovo, G.; Palatini, J.; De Lay, M.; Van Brocklyn, J.; Ostrowski, M.; Chiocca, E.; Lawler, S. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol. Cell 2010, 37, 620–632. [Google Scholar] [CrossRef]
- Godlewski, J.; Bronisz, A.; O Nowicki, M.; Chiocca, E.A.; Lawler, S. microRNA-451: A conditional switch controlling glioma cell proliferation and migration. Cell Cycle 2010, 9, 2742–2748. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, K.; Krepel, S.; Tang, P.; Gong, W.; Zhang, M.; Liang, W.; Trivett, A.; Zhou, M.; Wang, J.M. High Glucose Promotes Human Glioblastoma Cell Growth by Increasing the Expression and Function of Chemoattractant and Growth Factor Receptors. Transl. Oncol. 2019, 12, 1155–1163. [Google Scholar] [CrossRef]
- Pain, G. Fluoride Causes Diabetes 2018 Update. 2018. Available online: https://www.researchgate.net/publication/328249196_Fluoride_Causes_Diabetes_2018_Update (accessed on 8 January 2023).
- Fluegge, K. Community water fluoridation predicts increase in age-adjusted incidence and prevalence of diabetes in 22 states from 2005 and 2010. J. Water Health 2016, 14, 864–877. [Google Scholar] [CrossRef]
- Trevizol, J.S.; Buzalaf, N.R.; Dionizio, A.; Delgado, A.Q.; Cestari, T.M.; Bosqueiro, J.R.; Magalhães, A.C.; Buzalaf, M.A.R. Effects of low-level fluoride exposure on glucose homeostasis in female NOD mice. Chemosphere 2020, 254, 126602. [Google Scholar] [CrossRef]
- Verma, R.; Sherlin, D.G. Sodium fluoride-induced hypoproteinemia and hypoglycemia in parental and F1-generation rats and amelioration by vitamins. Food Chem. Toxicol. 2002, 40, 1781–1788. [Google Scholar] [CrossRef]
- Lombarte, M.; Fina, B.L.; Lupo, M.; Buzalaf, M.A.; Rigalli, A. Physical exercise ameliorates the toxic effect of fluoride on the insulin–glucose system. J. Endocrinol. 2013, 218, 99–103. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Ren, L.-Q.; Li, X.-N.; Wu, N.; Li, G.-S.; Liu, Q.-Y.; Xu, H. Effect of Fluoride on Insulin Level of Rats and Insulin Receptor Expression in the MC3T3-E1 Cells. Biol. Trace Elem. Res. 2012, 150, 297–305. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, Y.; Sinyuk, M.; Loganathan, S.; Thompson, R.C.; Sarkaria, J.N.; Chen, W.; Lathia, J.D.; Mobley, B.C.; Clark, S.W.; et al. Insulin-mediated signaling promotes proliferation and survival of glioblastoma through Akt activation. Neuro Oncol. 2015, 18, 48–57. [Google Scholar] [CrossRef]
- Hakuno, F.; Takahashi, S.-I. 40 YEARS OF IGF1: IGF1 receptor signaling pathways. J. Mol. Endocrinol. 2018, 61, T69–T86. [Google Scholar] [CrossRef]
- Schlenska-Lange, A.; Knüpfer, H.; Lange, T.J.; Kiess, W.; Knüpfer, M. Cell proliferation and migration in glioblastoma multiforme cell lines are influenced by insulin-like growth factor I in vitro. Anticancer. Res. 2008, 28, 1055–1060. [Google Scholar]
- Tirrò, E.; Massimino, M.; Romano, C.; Martorana, F.; Pennisi, M.S.; Stella, S.; Pavone, G.; Di Gregorio, S.; Puma, A.; Tomarchio, C.; et al. Prognostic and Therapeutic Roles of the Insulin Growth Factor System in Glioblastoma. Front. Oncol. 2021, 10, 3301. [Google Scholar] [CrossRef]
- Lobo, J.; Leite, A.; Pereira, H.; Fernandes, M.; Peres-Buzalaf, C.; Sumida, D.; Rigalli, A.; Buzalaf, M. Low-Level Fluoride Exposure Increases Insulin Sensitivity in Experimental Diabetes. J. Dent. Res. 2015, 94, 990–997. [Google Scholar] [CrossRef]
- Lupo, M.; Buzalaf, M.A.R.; Rigalli, A. Effect of Fluoridated Water on Plasma Insulin Levels and Glucose Homeostasis in Rats with Renal Deficiency. Biol. Trace Elem. Res. 2010, 140, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, N.; Mithal, A.; Gupta, S.; Godbole, M. Reversible impairment of glucose tolerance in patients with endemic fluorosis Fluoride Collaborative Study Group. Diabetologia 1993, 36, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Garetto, L.P.; Dunipace, A.J.; Zhang, W.; Wilson, M.E.; Grynpas, M.D.; Chachra, D.; McClintock, R.; Peacock, M.; Stookey, G.K. Fluoride Treatment Increased Serum IGF-1, Bone Turnover, and Bone Mass, but Not Bone Strength, in Rabbits. Calcif. Tissue Int. 1997, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gutowska, I.; Baranowska-Bosiacka, I.; Goschorska, M.; Kolasa, A.; Łukomska, A.; Jakubczyk, K.; Dec, K.; Chlubek, D. Fluoride as a factor initiating and potentiating inflammation in THP1 differentiated monocytes/macrophages. Toxicol. Vitr. 2015, 29, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Riches, D.; Winston, B.; Rose, D.; Young, S.; Noble, P.; Lake, F.; Henson, P. Divergence in macrophage insulin-like growth factor-I (IGF-I) synthesis induced by TNF-alpha and prostaglandin E2. J. Immunol. 1995, 155, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.; Liu, N.; Sun, Z.; Jiang, Y.; Jiang, T.; Xv, M.; Jia, L.; Tu, Y.; Wang, L. TGF-β Signaling Promotes Glioma Progression Through Stabilizing Sox9. Front. Immunol. 2021, 11, 3773. [Google Scholar] [CrossRef]
- Rodón, L.; Gonzàlez-Juncà, A.; Inda, M.d.M.; Sala-Hojman, A.; Martínez-Sáez, E.; Seoane, J. Active CREB1 promotes a malignant TGFβ2 autocrine loop in glioblastoma. Cancer Discov. 2014, 4, 1230–1241. [Google Scholar] [CrossRef]
- Liu, X.-L.; Song, J.; Liu, K.-J.; Wang, W.-P.; Xu, C.; Zhang, Y.-Z.; Liu, Y. Role of inhibition of osteogenesis function by Sema4D/Plexin-B1 signaling pathway in skeletal fluorosis in vitro. J. Huazhong Univ. Sci. Technol. 2015, 35, 712–715. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Xu, H. Fluoride Regulate Osteoblastic Transforming Growth Factor-β1 Signaling by Mediating Recycling of the Type I Receptor ALK5. PLoS ONE 2017, 12, e0170674. [Google Scholar]
- Zhao, Y.; Li, Y.; Gao, Y.; Yuan, M.; Manthari, R.K.; Wang, J.; Wang, J. TGF-β1 acts as mediator in fluoride-induced autophagy in the mouse osteoblast cells. Food Chem. Toxicol. 2018, 115, 26–33. [Google Scholar] [CrossRef]
- Ouyang, T.; Qin, Y.; Luo, K.; Han, X.; Yu, C.; Zhang, A.; Pan, X. miR-486-3p regulates CyclinD1 and promotes fluoride-induced osteoblast proliferation and activation. Environ. Toxicol. 2021, 36, 1817–1828. [Google Scholar] [CrossRef]
- Lütfioğlu, M.; Sakallıoğlu, E.; Sakallıoğlu, U.; Gülbahar, M.; Muğlalı, M.; Baş, B.; Aksoy, A. Excessıve fluorıde ıntake alters the MMP-2, TIMP-1 and TGF-β levels of perıodontal soft tıssues: An experımental study ın rabbits. Clin. Oral Investig. 2012, 16, 1563–1570. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wang, J.; Cheng, M.; Wang, J. Interleukin 17A deficiency alleviates fluoride-induced testicular injury by inhibiting the immune response and apoptosis. Chemosphere 2020, 263, 128178. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, R.; Sun, Y.-P.; Lee, T.-J.; Shah, G. Antiproliferative action of calcitonin on lactotrophs of the rat anterior pituitary gland: Evidence for the involvement of transforming growth factor beta 1 in calcitonin action. Endocrinology 2003, 144, 2164–2171. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Lin, S.; Huang, Y.; Zhao, X.; Zhang, M.; Xia, Y.; Fang, X.; Wang, J.; Hwang, S.-A.; et al. Change of urinary fluoride and bone metabolism indicators in the endemic fluorosis areas of southern china after supplying low fluoride public water. BMC Public Health 2013, 13, 156. [Google Scholar] [CrossRef]
- Shashi, A.; Singla, S. Parathyroid function in osteofluorosis. World J. Med. Sci. 2013, 8, 67–73. [Google Scholar]
- Zhang, X.; Zhang, Y.; Xi, S.; Cheng, G.; Guo, X. [The effect of different fluoride concentrations on the expression of transforming growth factor-beta1 in ameloblast of rat incisor]. Hua Xi Kou Qiang Yi Xue Za Zhi 2012, 30, 434–438. [Google Scholar]
- Suzuki, M.; Shin, M.; Simmer, J.; Bartlett, J. Fluoride Affects Enamel Protein Content via TGF-β1-mediated KLK4 Inhibition. J. Dent. Res. 2014, 93, 1022–1027. [Google Scholar] [CrossRef]
- Luo, P.-P.; Xu, H.-S. Effect of overdose fluoride on the expression of TGF-β3 in rat incisor. Shanghai Kou Qiang Yi Xue 2018, 27, 22–24. [Google Scholar]
- Nauman, P. Thyroid hormones in the central nervous system (CNS) and their effect on neoplasm formation, particularly on the development and course of glioblastoma multiforme—Research hypothesis. Endokrynol. Pol. 2015, 66, 444–459. [Google Scholar] [CrossRef]
- Davis, P.J.; Lin, H.-Y.; Thangirala, S.; Yalcin, M.; Tang, H.-Y.; Hercbergs, A.; Leith, J.T.; Luidens, M.K.; Ashur-Fabian, O.; Incerpi, S.; et al. Nanotetrac targets integrin αvβ3 on tumor cells to disorder cell defense pathways and block angiogenesis. Onco Targets Ther. 2014, 7, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Tomonaga, D.; Kalashnikova, A.; Furuya, F.; Akimoto, N.; Ifuku, M.; Okuno, Y.; Beppu, K.; Fujita, K.; Katafuchi, T.; et al. Effects of 3,3’,5-triiodothyronine on microglial functions. Glia 2015, 63, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Moeller, L.C.; Dumitrescu, A.M.; Refetoff, S. Cytosolic Action of Thyroid Hormone Leads to Induction of Hypoxia-Inducible Factor-1α and Glycolytic Genes. Mol. Endocrinol. 2005, 19, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Ashur-Fabian, O.; Blumenthal, D.; Bakon, M.; Nass, D.; Davis, P.; Hercbergs, A. Long-term response in high-grade optic glioma treated with medically induced hypothyroidism and carboplatin: A case report and review of the literature. Anticancer Drugs 2013, 24, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Wippel, C.; Starzer, A.M.; Ballarini, N.; Wolpert, F.; Bergen, E.; Wolf, P.; Steindl, A.; Widhalm, G.; Gatterbauer, B.; et al. Hypothyroidism correlates with favourable survival prognosis in patients with brain metastatic cancer. Eur. J. Cancer 2020, 135, 150–158. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.; Di Liegro, I. Involvement of Thyroid Hormones in Brain Development and Cancer. Cancers 2021, 13, 2693. [Google Scholar] [CrossRef]
- Kheradpisheh, Z.; Mirzaei, M.; Mahvi, A.H.; Mokhtari, M.; Azizi, R.; Fallahzadeh, H.; Ehrampoush, M.H. Impact of Drinking Water Fluoride on Human Thyroid Hormones: A Case- Control Study. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Singh, N.; Verma, K.G.; Verma, P.; Sidhu, G.K.; Sachdeva, S. A comparative study of fluoride ingestion levels, serum thyroid hormone & TSH level derangements, dental fluorosis status among school children from endemic and non-endemic fluorosis areas. Springerplus 2014, 3, 7. [Google Scholar]
- Zhang, S.; Zhang, X.; Liu, H.; Qu, W.; Guan, Z.; Zeng, Q.; Jiang, C.; Gao, H.; Zhang, C.; Lei, R.; et al. Modifying Effect of COMT Gene Polymorphism and a Predictive Role for Proteomics Analysis in Children’s Intelligence in Endemic Fluorosis Area in Tianjin, China. Toxicol. Sci. 2015, 144, 238–245. [Google Scholar] [CrossRef]
- Uller, R.P.; Van Herle, A.J.; Chopra, I.J. Comparison of Alterations in Circulating Thyroglobulin, Triiodothyronine and Thyroxine in Response to Exogenous (Bovine) and Endogenous (Human) Thyrotropin. J. Clin. Endocrinol. Metab. 1973, 37, 741–745. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A neurocentric perspective on glioma invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- de Groot, J.; Sontheimer, H. Glutamate and the Biology of Gliomas. Glia 2011, 59, 1181–1189. [Google Scholar] [CrossRef]
- Piao, Y.; Lu, L.; de Groot, J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro Oncol. 2009, 11, 260–273. [Google Scholar] [CrossRef]
- Colman, H.; Zhang, L.; Sulman, E.P.; McDonald, J.M.; Shooshtari, N.L.; Rivera, A.; Popoff, S.; Nutt, C.L.; Louis, D.N.; Cairncross, J.G.; et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010, 12, 49–57. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Li, Y.-L.; Wang, H.-Y.; Zhu, M.; Guo, D.; Wang, G.-L.; Gao, Y.-T.; Yang, Z.; Li, T.; Yang, C.-Y.; et al. Propofol inhibits invasion and proliferation of C6 glioma cells by regulating the Ca2+ permeable AMPA receptor-system xc− pathway. Toxicol. In Vitro 2017, 44, 57–65. [Google Scholar] [CrossRef]
- Yang, C.; Xia, Z.; Li, T.; Chen, Y.; Zhao, M.; Sun, Y.; Ma, J.; Wu, Y.; Wang, X.; Wang, P.; et al. Antioxidant Effect of Propofol in Gliomas and Its Association With Divalent Metal Transporter 1. Front. Oncol. 2020, 10, 590931. [Google Scholar] [CrossRef]
- Beretta, F.; Bassani, S.; Binda, E.; Verpelli, C.; Bello, L.; Galli, R.; Passafaro, M. The GluR2 subunit inhibits proliferation by inactivating Src-MAPK signalling and induces apoptosis by means of caspase 3/6-dependent activation in glioma cells. Eur. J. Neurosci. 2009, 30, 25–34. [Google Scholar] [CrossRef]
- Salmaggi, A.; Corno, C.; Maschio, M.; Donzelli, S.; D’Urso, A.; Perego, P.; Ciusani, E. Synergistic Effect of Perampanel and Temozolomide in Human Glioma Cell Lines. J. Pers. Med. 2021, 11, 390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Gutowska, I. Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis. Int. J. Mol. Sci. 2023, 24, 1558. https://doi.org/10.3390/ijms24021558
Żwierełło W, Maruszewska A, Skórka-Majewicz M, Gutowska I. Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis. International Journal of Molecular Sciences. 2023; 24(2):1558. https://doi.org/10.3390/ijms24021558
Chicago/Turabian StyleŻwierełło, Wojciech, Agnieszka Maruszewska, Marta Skórka-Majewicz, and Izabela Gutowska. 2023. "Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis" International Journal of Molecular Sciences 24, no. 2: 1558. https://doi.org/10.3390/ijms24021558
APA StyleŻwierełło, W., Maruszewska, A., Skórka-Majewicz, M., & Gutowska, I. (2023). Fluoride in the Central Nervous System and Its Potential Influence on the Development and Invasiveness of Brain Tumours—A Research Hypothesis. International Journal of Molecular Sciences, 24(2), 1558. https://doi.org/10.3390/ijms24021558






