Pancreatic Organoids: A Frontier Method for Investigating Pancreatic-Related Diseases
Abstract
1. Introduction
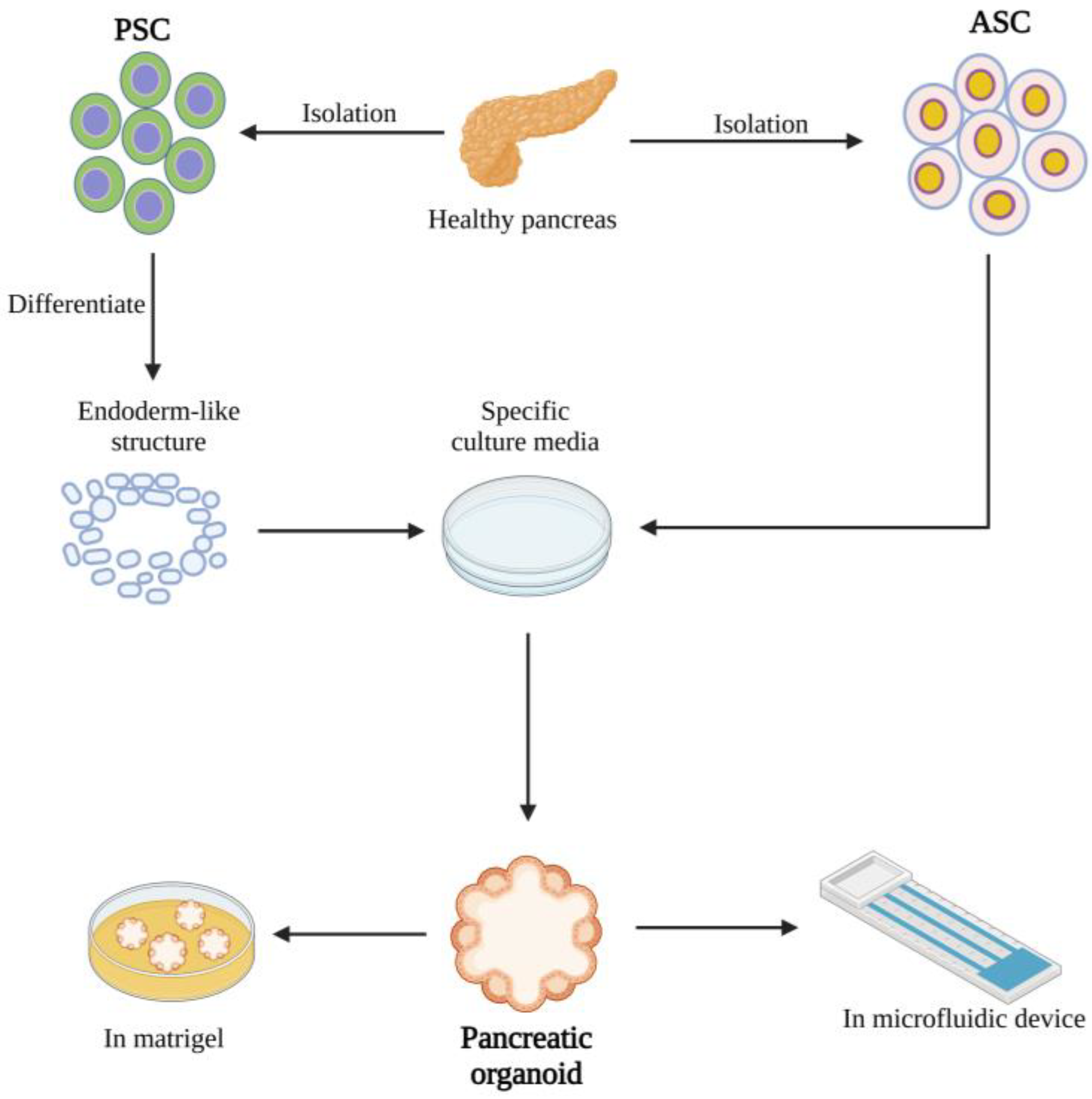
2. Creating Organoids
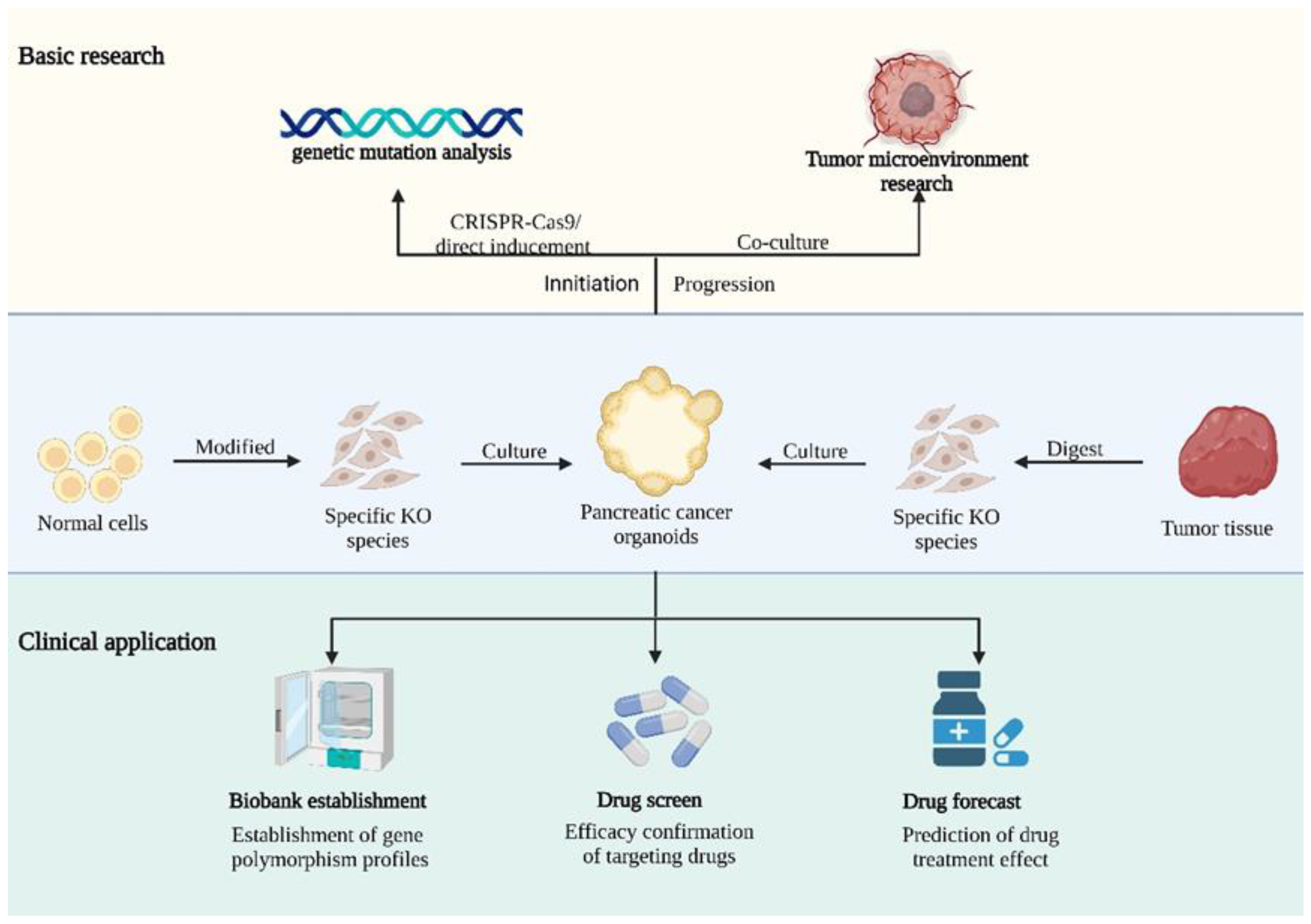
3. Applications of Organoids in Pancreatic Cancer
3.1. Cancer Initiation
3.2. Microenvironment
3.3. Drug Screening and Personalized Medicine
4. Diabetes
5. Other Applications
6. Limitations and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, T.; Shields, M.; Spaulding, C.; Principe, D.; Li, B.; Underwood, P.; Trevino, J.; Bentrem, D.; Munshi, H. Preclinical Models of Pancreatic Ductal Adenocarcinoma and Their Utility in Immunotherapy Studies. Cancers 2021, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.L.; Miller, A.L.; Yoon, K.J. Patient-Derived Xenograft Models of Pancreatic Cancer: Overview and Comparison with Other Types of Models. Cancers 2020, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.A.; Tiriac, H.; Clevers, H.; Tuveson, D.A. Modeling pancreatic cancer with organoids. Trends Cancer 2016, 2, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Swayden, M.; Soubeyran, P.; Iovanna, J. Upcoming Revolutionary Paths in Preclinical Modeling of Pancreatic Adenocarcinoma. Front. Oncol. 2019, 9, 1443. [Google Scholar] [CrossRef] [PubMed]
- Panoskaltsis, N.; McCarthy, N.E.; Knight, S.C. Myelopoiesis of acute inflammation: Lessons from TGN1412-induced cytokine storm. Cancer Immunol. Immunother. CII 2021, 70, 1155–1160. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer cell 2006, 9, 391–403. [Google Scholar] [CrossRef]
- Serebriiskii, I.; Castelló-Cros, R.; Lamb, A.; Golemis, E.A.; Cukierman, E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2008, 27, 573–585. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef]
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 4. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Barker, N.; Huch, M.; Kujala, P.; Van De Wetering, M.; Snippert, H.J.; Van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.J.; Van Es, J.H.; Van Den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Karthaus, W.R.; Iaquinta, P.J.; Drost, J.; Gracanin, A.; van Boxtel, R.; Wongvipat, J.; Dowling, C.M.; Gao, D.; Begthel, H.; Sachs, N.; et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014, 159, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Bonfanti, P.; Boj, S.F.; Sato, T.; Loomans, C.J.; Van De Wetering, M.; Sojoodi, M.; Li, V.S.; Schuijers, J.; Gracanin, A.; et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013, 32, 2708–2721. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Atteh, L.; Liu, P.; Mao, Y.; Meng, W.; Li, X. A pancreas tumor derived organoid study: From drug screen to precision medicine. Cancer Cell Int. 2021, 21, 398. [Google Scholar] [CrossRef]
- Blokzijl, F.; De Ligt, J.; Jager, M.; Sasselli, V.; Roerink, S.; Sasaki, N.; Huch, M.; Boymans, S.; Kuijk, E.; Prins, P.; et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 2016, 538, 260–264. [Google Scholar] [CrossRef]
- Tung, T.C.; Kü, S.H. Experimental studies on the development of the pronephric duct in anuran embryos. J. Anat. 1944, 78 Pt 1–2, 52–57. [Google Scholar]
- Marsee, A.; Roos, F.J.; Verstegen, M.M.; Roos, F.; Verstegen, M.; Clevers, H.; Vallier, L.; Takebe, T.; Huch, M.; Peng, W.C.; et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 2021, 28, 816–832. [Google Scholar] [CrossRef]
- Choi, J.-I.; Jang, S.I.; Hong, J.; Kim, C.H.; Kwon, S.S.; Park, J.S.; Lim, J.-B. Cancer-initiating cells in human pancreatic cancer organoids are maintained by interactions with endothelial cells. Cancer Lett. 2021, 498, 42–53. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, I.I.C.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015, 160, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Corsini, N.S.; Knoblich, J.A. Human organoids: New strategies and methods for analyzing human development and disease. Cell 2022, 185, 2756–2769. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef]
- Seino, T.; Kawasaki, S.; Shimokawa, M.; Tamagawa, H.; Toshimitsu, K.; Fujii, M.; Ohta, Y.; Matano, M.; Nanki, K.; Kawasaki, K.; et al. Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell 2018, 22, 454–467.e6. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.R.; Rempert, T.H.; Al-Hilal, T.A.; Wang, C.; Bhushan, A.; Bishehsari, F. Organ-Chip Models: Opportunities for Precision Medicine in Pancreatic Cancer. Cancers 2021, 13, 4487. [Google Scholar] [CrossRef] [PubMed]
- Saorin, G.; Caligiuri, I.; Rizzolio, F. Microfluidic organoids-on-a-chip: The future of human models. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Lau, H.C.H.; Kranenburg, O.; Xiao, H.; Yu, J. Organoid models of gastrointestinal cancers in basic and translational research. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 203–222. [Google Scholar] [CrossRef]
- Dennaoui, R.; Shrestha, H.; Wagner, K.-U. Models of pancreatic ductal adenocarcinoma. Cancer Metastasis Rev. 2021, 40, 803–818. [Google Scholar] [CrossRef]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Bhyravbhatla, N.; Kisling, A.; Li, X.; Batra, S.K.; Kumar, S. Cytokines chattering in pancreatic ductal adenocarcinoma tumor microenvironment. Semin. Cancer Biol. 2022, 86, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, T.; Maru, Y.; Izumiya, M.; Hoshi, D.; Kato, S.; Ochiai, M.; Hori, M.; Yamamoto, S.; Tatsuno, K.; Imai, T.; et al. Organoid-based ex vivo reconstitution of Kras-driven pancreatic ductal carcinogenesis. Carcinogenesis 2020, 41, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef]
- Li, X.; Nadauld, L.; Ootani, A.; Corney, D.C.; Pai, R.K.; Gevaert, O.; A Cantrell, M.; Rack, P.G.; Neal, J.T.; Chan, C.W.-M.; et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat. Med. 2014, 20, 769–777. [Google Scholar] [CrossRef]
- Roper, J.; Tammela, T.; Cetinbas, N.M.; Akkad, A.; Roghanian, A.; Rickelt, S.; Almeqdadi, M.; Wu, K.; Oberli, M.A.; Sánchez-Rivera, F.J.; et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 2017, 35, 569–576. [Google Scholar] [CrossRef]
- Maru, Y.; Tanaka, N.; Tatsumi, Y.; Nakamura, Y.; Itami, M.; Hippo, Y. Kras activation in endometrial organoids drives cellular transformation and epithelial-mesenchymal transition. Oncogenesis 2021, 10, 46. [Google Scholar] [CrossRef]
- Zafra, M.P.; Parsons, M.J.; Kim, J.; Alonso-Curbelo, D.; Goswami, S.; Schatoff, E.M.; Han, T.; Katti, A.; Fernandez, M.T.C.; Wilkinson, J.E.; et al. An In Vivo Kras Allelic Series Reveals Distinct Phenotypes of Common Oncogenic Variants. Cancer Discov. 2020, 10, 1654–1671. [Google Scholar] [CrossRef]
- Li, J.-T.; Yin, M.; Wang, D.; Wang, J.; Lei, M.-Z.; Zhang, Y.; Liu, Y.; Zhang, L.; Zou, S.-W.; Hu, L.-P.; et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2020, 22, 167–174. [Google Scholar] [CrossRef]
- Totaro, A.; Zhuang, Q.; Panciera, T.; Battilana, G.; Azzolin, L.; Brumana, G.; Gandin, A.; Brusatin, G.; Cordenonsi, M.; Piccolo, S. Cell phenotypic plasticity requires autophagic flux driven by YAP/TAZ mechanotransduction. Proc. Natl. Acad. Sci. USA 2019, 116, 17848–17857. [Google Scholar] [CrossRef]
- Park, J.; Eisenbarth, D.; Choi, W.; Kim, H.; Choi, C.; Lee, D.; Lim, D.-S. YAP and AP-1 Cooperate to Initiate Pancreatic Cancer Development from Ductal Cells in Mice. Cancer Res. 2020, 80, 4768–4779. [Google Scholar] [CrossRef] [PubMed]
- Lupo, F.; Piro, G.; Torroni, L.; Delfino, P.; Trovato, R.; Rusev, B.; Fiore, A.; Filippini, D.; De Sanctis, F.; Manfredi, M.; et al. Organoid-Transplant Model Systems to Study the Effects of Obesity on the Pancreatic Carcinogenesis in vivo. Front. Cell Dev. Biol. 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Ramsay, R.G.; Narasimhan, V.; Heriot, A.G.; De Wever, O. Targeting the Tumor Microenvironment in Colorectal Peritoneal Metastases. Trends Cancer 2020, 6, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Procacci, P.; Moscheni, C.; Sartori, P.; Sommariva, M.; Gagliano, N. Tumor-Stroma Cross-Talk in Human Pancreatic Ductal Adenocarcinoma: A Focus on the Effect of the Extracellular Matrix on Tumor Cell Phenotype and Invasive Potential. Cells 2018, 7, 158. [Google Scholar] [CrossRef]
- Lin, M.; Gao, M.; Pandalai, P.K.; Cavnar, M.J.; Kim, J. An Organotypic Microcosm for the Pancreatic Tumor Microenvironment. Cancers 2020, 12, 811. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Popova, O.; Panchenkov, D.; Dyuzheva, T.; Ivanov, A. Pancreatic ductal adenocarcinoma: Tumor microenvironment and problems in the development of novel therapeutic strategies. Clin. Exp. Med. 2022. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Mostafa, A.M.R.H.; Morton, J.P.; Hawinkels, L.J.A.C.; Prakash, J. Translating complexity and heterogeneity of pancreatic tumor: 3D in vitro to in vivo models. Adv. Drug Deliv. Rev. 2021, 174, 265–293. [Google Scholar] [CrossRef]
- Tsai, S.; McOlash, L.; Palen, K.; Johnson, B.; Duris, C.; Yang, Q.; Dwinell, M.B.; Hunt, B.; Evans, D.B.; Gershan, J.; et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 2018, 18, 335. [Google Scholar] [CrossRef]
- Kadaba, R.; Birke, H.; Wang, J.; Hooper, S.; Andl, C.D.; Di Maggio, F.; Soylu, E.; Ghallab, M.; Bor, D.; Froeling, F.E.; et al. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J. Pathol. 2013, 230, 107–117. [Google Scholar] [CrossRef]
- Xiao, W.; Pahlavanneshan, M.; Eun, C.-Y.; Zhang, X.; DeKalb, C.; Mahgoub, B.; Knaneh-Monem, H.; Shah, S.; Sohrabi, A.; Seidlits, S.K.; et al. Matrix stiffness mediates pancreatic cancer chemoresistance through induction of exosome hypersecretion in a cancer associated fibroblasts-tumor organoid biomimetic model. Matrix Biol. Plus 2022, 14, 100111. [Google Scholar] [CrossRef]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef]
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 36, 1770–1778. [Google Scholar] [CrossRef]
- Sub-Tumor Microenvironments Influence PDAC Progression and Therapy Response. Cancer Discov. 2021, 11, 2959. [CrossRef]
- Grünwald, B.T.; Devisme, A.; Andrieux, G.; Vyas, F.; Aliar, K.; McCloskey, C.W.; Macklin, A.; Jang, G.H.; Denroche, R.; Romero, J.M.; et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 2021, 184, 5577–5592.e18. [Google Scholar] [CrossRef]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Feldmann, K.; Maurer, C.; Peschke, K.; Teller, S.; Schuck, K.; Steiger, K.; Engleitner, T.; Öllinger, R.; Nomura, A.; Wirges, N.; et al. Mesenchymal Plasticity Regulated by Prrx1 Drives Aggressive Pancreatic Cancer Biology. Gastroenterology 2021, 160, 346–361.e24. [Google Scholar] [CrossRef]
- Oni, T.E.; Biffi, G.; Baker, L.A.; Hao, Y.; Tonelli, C.; Somerville, T.D.; Deschênes, A.; Belleau, P.; Hwang, C.-I.; Sánchez-Rivera, F.J.; et al. SOAT1 promotes mevalonate pathway dependency in pancreatic cancer. J. Exp. Med. 2020, 217, e20192389. [Google Scholar] [CrossRef]
- Nelson, J.K.; Thin, M.Z.; Evan, T.; Howell, S.; Wu, M.; Almeida, B.; Legrave, N.; Koenis, D.S.; Koifman, G.; Sugimoto, Y.; et al. USP25 promotes pathological HIF-1-driven metabolic reprogramming and is a potential therapeutic target in pancreatic cancer. Nat. Commun. 2022, 13, 2070. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Matsumoto, R.; Masamune, A. Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology. Front. Physiol. 2022, 13, 865105. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Moriyama, T.; Ohuchida, K.; Sheng, N.; Iwamoto, C.; Shindo, K.; Shirahane, K.; Ikenaga, N.; Nagai, S.; Nakata, K.; et al. N-acetyl cysteine induces quiescent-like pancreatic stellate cells from an active state and attenuates cancer-stroma interactions. J. Exp. Clin. Cancer Res. CR 2021, 40, 133. [Google Scholar] [CrossRef] [PubMed]
- Upadhrasta, S.; Zheng, L. Strategies in Developing Immunotherapy for Pancreatic Cancer: Recognizing and Correcting Multiple Immune “Defects” in the Tumor Microenvironment. J. Clin. Med. 2019, 8, 1472. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Holokai, L.; Chakrabarti, J.; Lundy, J.; Croagh, D.; Adhikary, P.; Richards, S.S.; Woodson, C.; Steele, N.; Kuester, R.; Scott, A.; et al. Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 3816. [Google Scholar] [CrossRef]
- Bishehsari, F.; Zhang, L.; Barlass, U.; Preite, N.Z.; Turturro, S.; Najor, M.S.; Shetuni, B.B.; Zayas, J.P.; Mahdavinia, M.; Abukhdeir, A.M.; et al. KRAS mutation and epithelial-macrophage interplay in pancreatic neoplastic transformation. Int. J. Cancer 2018, 143, 1994–2007. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, T.T.; Zimmerman, J.W.; Suri, R.; Zlomke, H.; Ivey, G.D.; Szabolcs, A.; Shubert, C.R.; Cameron, J.L.; Burns, W.R.; Lafaro, K.J.; et al. Precision Medicine in Pancreatic Cancer: Patient-Derived Organoid Pharmacotyping Is a Predictive Biomarker of Clinical Treatment Response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 3296–3307. [Google Scholar] [CrossRef]
- Tiriac, H.; Plenker, D.; Baker, L.A.; Tuveson, D.A. Organoid models for translational pancreatic cancer research. Curr. Opin. Genet. Dev. 2019, 54, 7–11. [Google Scholar] [CrossRef]
- Demyan, L.; Habowski, A.N.; Plenker, D.; King, D.A.; Standring, O.J.; Tsang, C.B.; Surin, L.S.; Rishi, A.; Crawford, J.M.; Boyd, J.; et al. Pancreatic Cancer Patient-derived Organoids Can Predict Response to Neoadjuvant Chemotherapy. Ann. Surg. 2022, 276, 450–462. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Liu, T.P.; Chou, C.J.; Chen, H.Y.; Lee, K.H.; Yang, P.M. Integration of Bioinformatics Resources Reveals the Therapeutic Benefits of Gemcitabine and Cell Cycle Intervention in SMAD4-Deleted Pancreatic Ductal Adenocarcinoma. Genes 2019, 10, 766. [Google Scholar] [CrossRef]
- Tu, Q.; Liu, X.; Yao, X.; Li, R.; Liu, G.; Jiang, H.; Li, K.; Chen, Q.; Huang, X.; Chang, Q.; et al. RETSAT associates with DDX39B to promote fork restarting and resistance to gemcitabine based chemotherapy in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. CR 2022, 41, 274. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. New Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Farshadi, E.A.; Chang, J.; Sampadi, B.; Doukas, M.; Land, F.; Sijde, F.; Vietsch, E.E.; Pothof, J.; Koerkamp, B.G.; van Eijck, C.H. Organoids Derived from Neoadjuvant FOLFIRINOX Patients Recapitulate Therapy Resistance in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 6602–6612. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschenes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, K.; Bailey, P.; Springfeld, C.; Roth, S.; Kurilov, R.; Brors, B.; Gress, T.; Buchholz, M.; An, J.; et al. Clinical Impact of Molecular Subtyping of Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 2805. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, Y.; Yuan, Q.; Tang, S.; Guo, S.; Zhang, Y.; He, J.; Zhang, X.; Han, M.; Liu, Z.; et al. Integrated profiling of human pancreatic cancer organoids reveals chromatin accessibility features associated with drug sensitivity. Nat. Commun. 2022, 13, 2169. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Dey, P.; Vaz, A.P.; Bhaumik, S.R.; Ponnusamy, M.P.; Batra, S.K. PD2/PAF1 at the Crossroads of the Cancer Network. Cancer Res. 2018, 78, 313–319. [Google Scholar] [CrossRef]
- Karmakar, S.; Rauth, S.; Nallasamy, P.; Perumal, N.; Nimmakayala, R.K.; Leon, F.; Gupta, R.; Barkeer, S.; Venkata, R.C.; Raman, V.; et al. RNA Polymerase II-Associated Factor 1 Regulates Stem Cell Features of Pancreatic Cancer Cells, Independently of the PAF1 Complex, via Interactions With PHF5A and DDX3. Gastroenterology 2020, 159, 1898–1915.e6. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Inkielewicz-Stepniak, I. Pancreatic Cancer and Platelets Crosstalk: A Potential Biomarker and Target. Front. Cell Dev. Biol. 2021, 9, 749689. [Google Scholar] [CrossRef]
- Yeo, D.; Giardina, C.; Saxena, P.; Rasko, J.E.J. The next wave of cellular immunotherapies in pancreatic cancer. Mol. Ther. Oncolytics 2022, 24, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Koikawa, K.; Kibe, S.; Suizu, F.; Sekino, N.; Kim, N.; Manz, T.D.; Pinch, B.J.; Akshinthala, D.; Verma, A.; Gaglia, G.; et al. Targeting Pin1 renders pancreatic cancer eradicable by synergizing with immunochemotherapy. Cell 2021, 184, 4753–4771.e27. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhuo, Q.; Ye, Z.; Xu, X.; Ji, S. Organoid model: A new hope for pancreatic cancer treatment? Biochim. Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188466. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.-O.; Wessel, C.R.; Leary, D.A.-O.; Wang, C.; Bhushan, A.A.-O.; Bishehsari, F.A.-O. Patient-derived pancreatic cancer-on-a-chip recapitulates the tumor microenvironment. Microsyst. Nanoeng. 2022, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Z.; Song, E.; Xu, T. Islet organoid as a promising model for diabetes. Protein Cell 2022, 13, 239–257. [Google Scholar] [CrossRef]
- Helman, A.; Melton, D.A. A Stem Cell Approach to Cure Type 1 Diabetes. Cold Spring Harb. Perspect. Biol. 2021, 13, a035741. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef]
- Turner, M.; Leslie, S.; Martin, N.; Peschanski, M.; Rao, M.; Taylor, C.J.; Trounson, A.; Turner, D.; Yamanaka, S.; Wilmut, I. Toward the development of a global induced pluripotent stem cell library. Cell Stem Cell 2013, 13, 382–384. [Google Scholar] [CrossRef]
- Yoshihara, E.; O’Connor, C.; Gasser, E.; Wei, Z.; Oh, T.G.; Tseng, T.W.; Wang, D.; Cayabyab, F.; Dai, Y.; Yu, R.T.; et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 2020, 586, 606–611. [Google Scholar] [CrossRef]
- Lebreton, F.; Hanna, R.; Wassmer, C.H.; Bellofatto, K.; Perez, L.; Othenin-Girard, V.; de Tejada, B.M.; Cohen, M.; Berishvili, E. Mechanisms of Immunomodulation and Cytoprotection Conferred to Pancreatic Islet by Human Amniotic Epithelial Cells. Stem Cell Rev. Rep. 2022, 18, 346–359. [Google Scholar] [CrossRef]
- Lebreton, F.; Lavallard, V.; Bellofatto, K.; Bonnet, R.; Wassmer, C.H.; Perez, L.; Kalandadze, V.; Follenzi, A.; Boulvain, M.; Kerr-Conte, J.; et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat. Commun. 2019, 10, 4491. [Google Scholar] [CrossRef]
- Lebreton, F.; Bellofatto, K.; Wassmer, C.H.; Perez, L.; Lavallard, V.; Parnaud, G.; Cottet-Dumoulin, D.; Kerr-Conte, J.; Pattou, F.; Bosco, D.; et al. Shielding islets with human amniotic epithelial cells enhances islet engraftment and revascularization in a murine diabetes model. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, C.-H.; Lebreton, F.; Bellofatto, K.; Perez, L.; Cottet-Dumoulin, D.; Andres, A.; Bosco, D.; Berney, T.; Othenin-Girard, V.; De Tejada, B.M.; et al. Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2021, 35, 10214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nair, G.G.; Russ, H.A.; Belair, C.D.; Li, M.L.; Shveygert, M.; Hebrok, M.; Blelloch, R. LIN28B Impairs the Transition of hESC-Derived β Cells from the Juvenile to Adult State. Stem Cell Rep. 2020, 14, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Meng, H.; Lin, J.; Ji, W.; Xu, T.; Liu, H. Pancreatic islet organoids-on-a-chip: How far have we gone? J. Nanobiotechnol. 2022, 20, 308. [Google Scholar] [CrossRef]
- Molnár, R.; Madácsy, T.; Varga, Á.; Németh, M.; Katona, X.; Görög, M.; Molnár, B.; Fanczal, J.; Rakonczay, Z., Jr.; Hegyi, P.; et al. Mouse pancreatic ductal organoid culture as a relevant model to study exocrine pancreatic ion secretion. Lab. Investig. A J. Tech. Methods Pathol. 2020, 100, 84–97. [Google Scholar] [CrossRef]
- Angyal, D.; Bijvelds, M.J.C.; Bruno, M.J.; Peppelenbosch, M.P.; de Jonge, H.R. Bicarbonate Transport in Cystic Fibrosis and Pancreatitis. Cells 2021, 11, 54. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Singh, V.K.; Yadav, D.; Garg, P.K. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA 2019, 322, 2422–2434. [Google Scholar] [CrossRef]
- Hohwieler, M.; Illing, A.; Hermann, P.C.; Mayer, T.; Stockmann, M.; Perkhofer, L.; Eiseler, T.; Antony, J.S.; Müller, M.; Renz, S.; et al. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 2017, 66, 473–486. [Google Scholar] [CrossRef]
- Madácsy, T.; Varga, Á.; Papp, N.; Tél, B.; Pallagi, P.; Szabó, V.; Kiss, A.; Fanczal, J.; Rakonczay, Z., Jr.; Tiszlavicz, L.; et al. Impaired regulation of PMCA activity by defective CFTR expression promotes epithelial cell damage in alcoholic pancreatitis and hepatitis. Cell. Mol. Life Sci. CMLS 2022, 79, 265. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Gong, Y.-F.; Zhuang, H.-K.; Zhou, Z.-X.; Huang, S.-Z.; Zou, Y.-P.; Huang, B.; Sun, Z.-H.; Zhang, C.-Z.; Tang, Y.-Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef] [PubMed]
- April-Monn, S.L.; Wiedmer, T.; Skowronska, M.; Maire, R.; Schiavo Lena, M.; Trippel, M.; Di Domenico, A.; Muffatti, F.; Andreasi, V.; Capurso, G.; et al. Three-Dimensional Primary Cell Culture: A Novel Preclinical Model for Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2021, 111, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Toshimitsu, K.; Matano, M.; Fujita, M.; Fujii, M.; Togasaki, K.; Ebisudani, T.; Shimokawa, M.; Takano, A.; Takahashi, S.; et al. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell 2020, 183, 1420–1435.e21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, N.; Zhu, Y. Pancreatic Organoids: A Frontier Method for Investigating Pancreatic-Related Diseases. Int. J. Mol. Sci. 2023, 24, 4027. https://doi.org/10.3390/ijms24044027
Liu Y, Li N, Zhu Y. Pancreatic Organoids: A Frontier Method for Investigating Pancreatic-Related Diseases. International Journal of Molecular Sciences. 2023; 24(4):4027. https://doi.org/10.3390/ijms24044027
Chicago/Turabian StyleLiu, Yuxiang, Nianshuang Li, and Yin Zhu. 2023. "Pancreatic Organoids: A Frontier Method for Investigating Pancreatic-Related Diseases" International Journal of Molecular Sciences 24, no. 4: 4027. https://doi.org/10.3390/ijms24044027
APA StyleLiu, Y., Li, N., & Zhu, Y. (2023). Pancreatic Organoids: A Frontier Method for Investigating Pancreatic-Related Diseases. International Journal of Molecular Sciences, 24(4), 4027. https://doi.org/10.3390/ijms24044027



