Abstract
The exchange coupling, represented by the J parameter, is of tremendous importance in understanding the reactivity and magnetic behavior of open-shell molecular systems. In the past, it was the subject of theoretical investigations, but these studies are mostly limited to the interaction between metallic centers. The exchange coupling between paramagnetic metal ions and radical ligands has hitherto received scant attention in theoretical studies, and thus the understanding of the factors governing this interaction is lacking. In this paper, we use DFT, CASSCF, CASSCF/NEVPT2, and DDCI3 methods to provide insight into exchange interaction in semiquinonato copper(II) complexes. Our primary objective is to identify structural features that affect this magnetic interaction. We demonstrate that the magnetic character of Cu(II)-semiquinone complexes are mainly determined by the relative position of the semiquinone ligand to the Cu(II) ion. The results can support the experimental interpretation of magnetic data for similar systems and can be used for the in-silico design of magnetic complexes with radical ligands.
Keywords:
radicals; semiquinone; exchange coupling; DFT; ab initio; ferromagnetism; antiferromagnetism; broken symmetry 1. Introduction
Transition-metal complexes containing redox-active, proradical ligands have long been attracting the researcher’s attention because the distribution of electrons between the metal centers and such ligands provides unique electronic properties [1,2,3,4,5,6,7,8,9,10]. Extensive research has shown that the interplay of redox-active transition metal ions and pro-radical ligands plays an important role in biochemistry [11,12,13], with copper-containing enzymes being a prominent example [14,15,16]. For the copper complexes with catecholate ligands, which are an intermediate in the catalytic oxidation of catechols [17,18], the formation of semiquinonato copper complexes is a crucial step. These natural mechanisms inspired the design of catalytic systems with redox-active/radical ligands, including semiquinones [14,19,20,21,22].
Radical ligands also represent a growing field for another reason. They are increasingly important in the design of molecular materials that exhibit magnetic properties. Most ligands mediate antiferromagnetic interactions, but organic spin carriers can link metal centers providing high-spin molecules due to ferromagnetic or ferrimagnetic ordering [23,24,25,26]. Importantly, the exchange coupling between paramagnetic metal ions and radical ligands is usually much stronger than the superexchange interactions. The strength of exchange coupling is one of the critical parameters for single-molecule magnets. It determines the separation between the spin ground state and the spin excited states, which must be sufficiently large to maintain slow magnetization dynamics at elevated temperatures and shuts down fast quantum relaxation pathways. Therefore, it is hardly surprising that radical ligands are an important building block for such systems [27,28].
Another significant aspect of the interaction between transient metal ions and radicals is valence tautomerism, which is the ability to possess two or more isomeric forms that are connected by intramolecular electron transfer between the ligand and the metal [29,30,31,32,33]. Therefore, this feature can be applied, for instance, as a spin crossover to build magnetic switches [33], but it also occurs in enzymatic centers and tailors their reactivity [34].
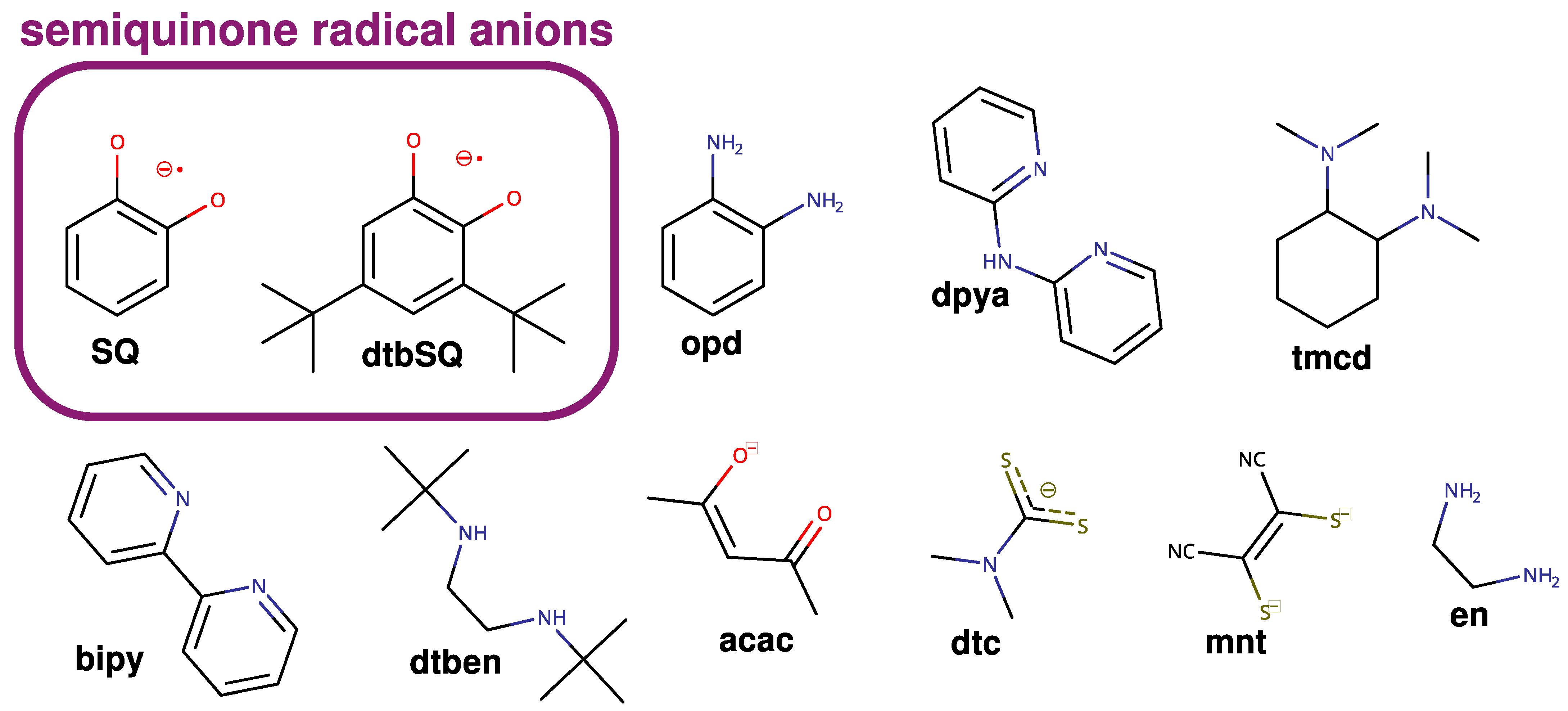
It is now well established that theoretical methods are an efficient multipurpose tool for chemistry, biochemistry, and material science. Recent scientific efforts have shown that they can help us understand various phenomena, for example, reaction mechanisms or reactivity [35,36,37,38], electron density distribution [39,40,41], and elusive structure of complex biochemical systems or radical centers [42,43,44], anticancer [35,45] and antioxidant properties [46,47,48,49,50,51], soot formation [52] or pesticide decomposition [53]. The exchange coupling between paramagnetic centers has been a subject of such investigations at the density functional theory (DFT) and ab initio levels, but these studies were mainly limited to the interaction between metallic centers [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. Theoretical studies on the exchange coupling between radical ligands and paramagnetic metal ions were performed [77,78,79,80,81]. However, in our opinion, a systematic understanding of how various structural and electronic features of such complexes affect exchange coupling is still lacking. It is surprising since exchange coupling is crucial for both magnetic properties and reactivity [82,83,84]. Therefore, this article discusses the case of semiquinonato copper(II) complexes, which have been studied experimentally for 40 years [18,85,86,87,88,89,90,91,92,93,94,95]. Our primary aim was to identify structural features that affect exchange coupling; however, a comparison with experimental data is also provided. In this investigation, we used broken-symmetry (BS) DFT and ab initio methods (CASSCF, CASSCF/NEVPT2, and DDCI3). The structures of all ligands used in this study are shown in Figure 1.
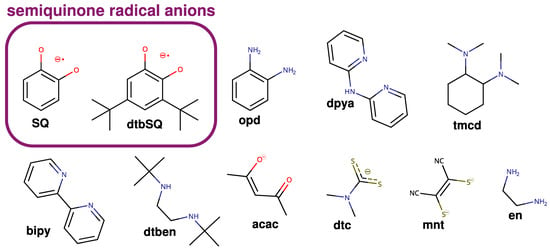
Figure 1.
Ligands were used in this study.
The d9 Cu(II) ion and semiquinonanto ligand have local spins SA and SB with SA = SB = ½. Their magnetic coupling leads to two states with total spin S = 1 or 0. The order and spacing of these two states can be modeled by the isotropic Heisenberg-Dirac-Van Vleck Hamiltonian:
where J is the exchange coupling constant that parametrizes the fictitious exchange interaction. It is positive for ferromagnetic (S = 1) and negative for antiferromagnetic (S = 0) coupling. According to the above Hamiltonian, the two spin states are spaced by 2J. All the experimental values of J used in this work were converted to fit this Hamiltonian.
2. Results and Discussion
2.1. General Considerations
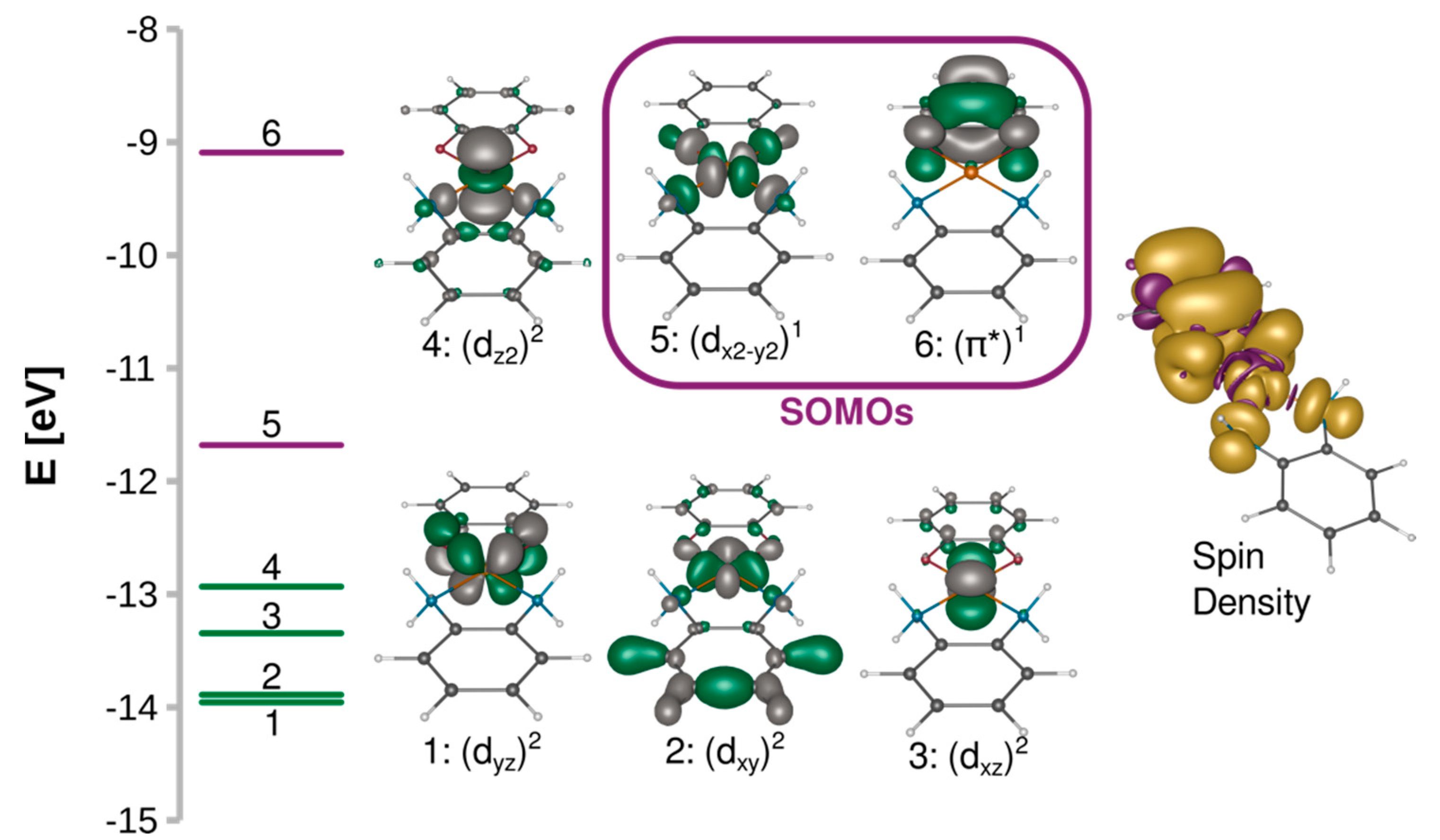
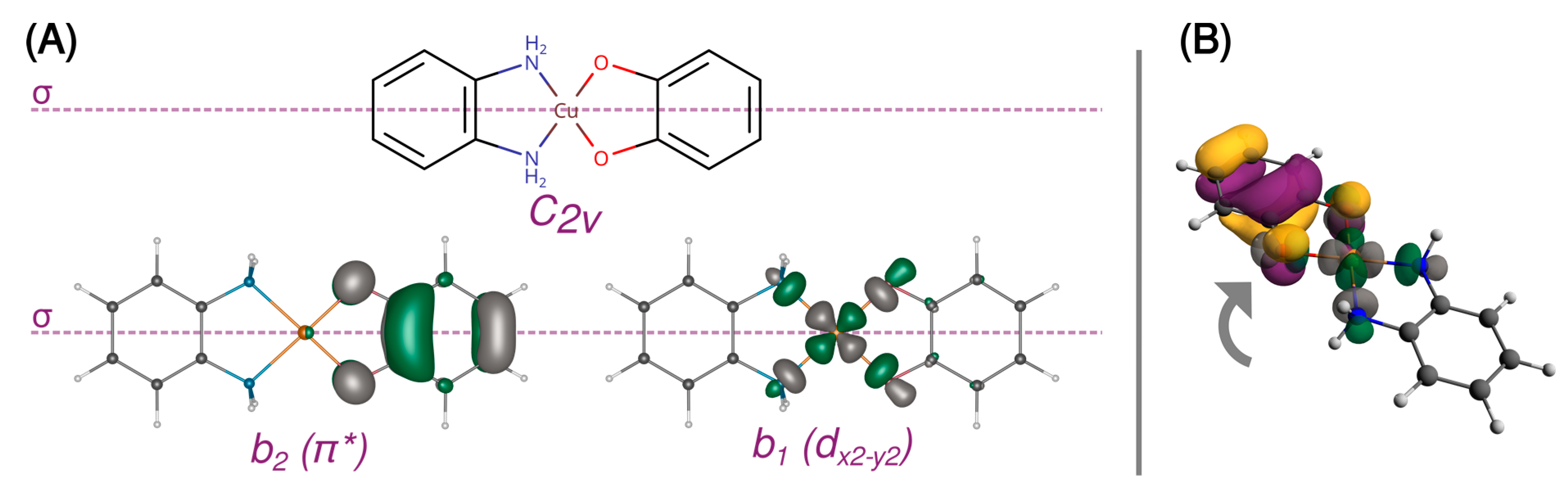
A 3d9 Cu(II) ion in the octahedral ligand field is subject to the Jahn–Teller distortion, which removes the degeneracy of the eg and t2g orbitals by a decrease in molecular symmetry. Typically, the 3d9 system becomes then an elongated octahedral, square pyramidal, or square planar complex with the unpaired electron occupying the molecular orbital with a strong dx2−y2 contribution. Figure 2 shows such split d orbitals for a model C2v square planar complex ([Cu(SQ)(opd)]+). The singly occupied (magnetic) molecular orbital (SOMO) of the copper(II) ion points along the Cu-N and Cu-O bonds in the basal plane. As shown in Figure 3A, it is antisymmetric with respect to the mirror plane σ, transforms as b1 in the C2v point group and is strongly σ-antibonding with respect to both ligands. When the CuN2O2 unit is planar, the two SOMOs are strictly orthogonal. The SOMO of Cu(II) is significantly delocalized toward the oxygen atoms of the o-semiquinone radical, whose SOMO has a significant density on the same oxygen atoms. Due to symmetry, the overlap density between two SOMOs has two equivalent positive and two negative lobes around each oxygen atom of the o-semiquinone (Figure 3B). Therefore, as explained by Kahn et al. [88], in a planar semiquinonato copper(II) complex, the total overlap between the two SOMOs should be zero, and the magnetic exchange interaction is expected to be ferromagnetic. Our BS B3LYP/def2-TZVP calculations for the model square planar [Cu(SQ)(opd)]+ complex confirm that the overlap integral is zero and the magnetic exchange coupling is ferromagnetic with a large singlet-triplet energy gap (J = 294 cm−1). The magnitude of the ferromagnetic coupling for this model agrees with the experimental observation for [Cu(dtbSQ)(dpya)(ClO4)] (140 cm−1), [Cu(dtbSQ)(bipy)(BF4)] (191 cm−1) and [Cu(dtbSQ)(dpya)(THF)2]+ (168 cm−1) [85].
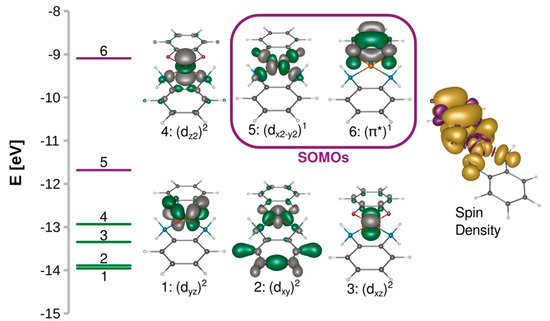
Figure 2.
Splitting of the quasi-restricted orbitals and their isosurfaces contoured at 0.05 a.u. and spin density isosurfaces contoured at 0.001 a.u. calculated at the B3LYP/def2-TZVP level for [Cu(SQ)(opd)]+.
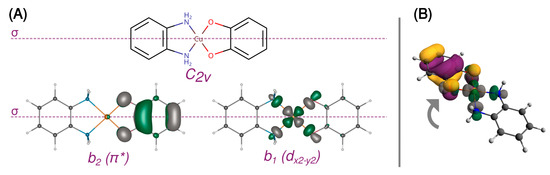
Figure 3.
Orthogonal magnetic orbitals in [Cu(SQ)(opd)]+ with the mirror plane σ (A) and the ineffective overlap between them (B). The isosurfaces were contoured at 0.05 a.u.
2.2. Performance of Computational Methods
Before performing a detailed analysis, it is reasonable to compare the performance of functionals, ab initio methods, and basis sets. Table 1 presents the values of the magnetic exchange coupling J predicted for various model complexes. The results of calculations for the models with various ligands in the Cu(II) axial position are given in Table S1.

Table 1.
Exchange coupling constants J (in cm−1) calculated with DFT and ab initio methods for model semiquinonato Cu(II) complexes.
BS DFT calculations can be accurate when compared to the experimentally determined J values, but selecting the XC functional is a delicate matter. The GGA functionals overestimate exchange coupling [54,55,56,57,58,59,60], and for this reason, we did not include them in this investigation. There are known cases in which the physical interpretation of J depends on the chosen XC functional, but, favorably, all the functionals we tested predicted qualitatively similar J values that reflect strong ferromagnetic interactions. In general, the functional B3LYP and TPSSh predict larger J constants in comparison with PBE0. For most molecular models, the results obtained by the three hybrid functionals are spread over a narrow range of 25 cm−1. For instance, the predicted values for [Cu(SQ)(opd)]+ at the B3LYP/def2-TZVP, TPSSh/def-TZVP and PBE0/def2-TZVP levels are 294, 276 and 271 cm−1, respectively. We observed the smallest differences of a few cm−1 for [Cu(SQ)(dtc)] and the largest, up to 70 cm−1, for the two model complexes with the opd ligand occupying the equatorial position and methanoate ([Cu(SQ)(opd)(HCOO)]) or chloride ion ([Cu(SQ)(opd)(Cl)]) in the axial position (Table S1).
The larger differences between the exchange coupling constants J should not be associated with negatively charged ligands occupying the axial position of Cu(II), as they are not observed for the complexes with tetrafluoroborate ([Cu(SQ)(opd)(BF4)]) and perchlorate anion ([Cu(SQ)(opd)(ClO4)]). Also, there is no evidence that the sulfur donor atoms reduce the spread, as indicated by the [Cu(SQ)(dtc)] model, as we did not observe this for [Cu(SQ)(mnt)]–.
Multireference calculations allow direct access to all spin states without the need for spin projection approximations. The CASSCF calculations with the minimal active space composed of two electrons in two SOMOs, labeled as CASSCF(2e,2o), predict ferromagnetic coupling but with a magnitude even over four times lower than the DFT methods. For example, the predicted J values at the B3LYP/def2-TZVP and CASSCF(2e,2o)/def2-TZVP theory level for [Cu(SQ)(opd)]+ are 274 and 74 cm−1, respectively.
An accepted strategy to improve reference wave functions and, thus, the magnetic couplings is to extend the minimal active space. Therefore, it was extended by the remaining 3d orbitals of copper(II) [CASSCF(10e,6o)], but the predicted J values remained unchanged. The active space was further expanded by four lone pairs that can undergo a bonding interaction with Cu(II) (these orbitals are shown in Figure S1). However, these CASSCF(18e,10o) calculations produced the J parameters comparable to those of CASSCF(2e,2o) and CASSCF(10e,6o). It becomes apparent that the CASSCF calculations with the minimal and extended active spaces are insufficient to quantitatively reproduce the magnitude of the exchange coupling observed experimentally. This underestimation of the ferromagnetic interaction can be attributed to the inability of the relatively small active space to capture the physics of the spin coupling and the inability of the method to recover dynamic electron correlation. These results reflect earlier observations for the antiferromagnetically coupled transition metal dimers [75,96,97].
To improve the methodology systematically, we included the dynamical correlation with the multiconfigurational reference perturbation (MRPT) using the n-electron valence state perturbation theory (NEVPT2) approach. Two different formulations of NEVPT2 were tested, that is, strongly contracted (SC-NEVPT2) and fully internally contracted NEVPT2 (FIC-NEVPT2). It is apparent from Table 1 that, regardless of the variant, NEVPT2 has a very limited effect on the predicted strength of the ferromagnetic coupling if applied to the CASSCF(2e,2o) wave function. This is clear in the case of [Cu(SQ)(opd)]+, for which the J parameter decreases by 1 cm−1 after applying the NEVPT2 correction. These results are in line with previous observations that the treatment of the electron correlation by the MRPT methods is limited in the minimal active space, and this leads to a systematic underestimation of the magnetic coupling [85,87].
The ferromagnetic coupling predicted by the NEVPT2 method for the CASSCF(10e,6o) wave function underwent a noticeable increase, especially well pronounced for [Cu(SQ)(dtc)]. The inclusion of the four lone-pair ligand orbitals in the active space [CASSCF(18e,10o)] was accompanied by a more significant increase in the ferromagnetic coupling. The predicted J values became closer to their experimental counterparts reported for [Cu(dtbSQ)(dpya)(ClO4)] (140 cm−1), [Cu(dtbSQ)(bipy)(BF4)] (191 cm−1) and [Cu(dtbSQ)(dpya)(THF)2]+ (161 cm−1). The SC-NEVPT2 and FIC-NEVPT2 variants produced very similar numerical results, but for the largest active space we used, these differences were slightly more pronounced, and FIC-NEVPT2 tended to predict slightly stronger ferromagnetic couplings in comparison with SC-NEVPT2.
As suggested by previous studies on the exchange interaction between paramagnetic metal ions [61,62,63,64,65,66,96], the potentially very accurate singlet-triplet energy gap, and thus the J constant, is obtained with the difference dedicated MRCI (DDCI3) even with the minimal active space. This computational technique, in concert with the def2-TZVP basis set, produces J = 173 cm−1 for the model complex [Cu(SQ)(opd)]+, and this prediction is in line with the values 140, 191 and 161 cm−1 that were experimentally determined for structurally similar [Cu(dtbSQ)(dpya)(ClO4)], [Cu(dtbSQ)(bipy)(BF4)] and [Cu(dtbSQ)(dpya)(THF)2]+, respectively [85]. The difference in accuracy between the result obtained at the minimal active space CASSCF(2e,2o)/NEVPT2 and DDCI3(2e,2o) calculations requires a brief comment. Regardless of the variant, NEVPT2 provides a perturbational estimate of the electron correlation effect beyond the minimal active space description. In contrast, DDCI accounts for electron correlation in a variational and, therefore, in principle, more precise manner. This has been discussed in detail by Malrieu et al. in their excellent review paper [96]. Regrettably, the DDCI3 calculations with extended active spaces were computationally too expensive to perform.
The choice of basis set can be important for the exchange coupling calculation. Selecting a fairly large basis set for paramagnetic metal ions while keeping reasonable ones for the other atoms is frequently recommended [69,98,99]. This has been shown to work well for systems without radical ligands. However, such an approach may lead to an unbalanced description of the spin density of radical ligand, and thus we used an equally accurate basis set on all atoms that allowed for carrying out the DDCI3(2e,2o) calculations for experimentally relevant models. Regardless of the method, the computationally efficient def2-SVP provides values noticeably, that is, up to 20%, different from def2-TZVP. In contrast, the cc-pVDZ and def2-TZVP basis sets produce quite similar results, especially in concert with the DDCI3(2e,2o) method. A good performance of cc-pVDZ in the J computations at the DDCI3(2e,2o) level was previously observed for the Cu(II) dinuclear complex with 4-N-(2′-pyridylimine)benzoic acid ligands [61].
2.3. Magnetostructural Correlations
In experimental studies, it is not straightforward to separate the effects of individual structural parameters on the exchange coupling constant. Conversely, molecular modeling offers the possibility of scanning J separately along the selected structural parameter. This was recently well demonstrated for the Mn(III) dinuclear complex [98].
To provide a detailed analysis of structural effects, we carried out a decomposition of the magnetic exchange coupling into its different physical mechanisms using BS DFT. For two S = ½ magnetic centers, the most important ones are the direct exchange (J0) and the kinetic exchange (ΔJKE) between the magnetic electrons and the spin (or core) polarization of the nonmagnetic electrons (ΔJCP):
J = J0 + ΔJKE + ΔJCP
This decomposition starts with the high-spin state (HS) calculated in the restricted open-shell formalism (RO). The direct exchange contribution J0 is calculated by flipping the spin of one magnetic orbital and the immediate calculation of the BS energy with all the orbitals frozen (without the SCF procedure). Then, only the magnetic orbitals are relaxed in the field of the frozen nonmagnetic orbitals, allowing the magnetic orbitals to delocalize from one center to the other. From this procedure, the kinetic exchange ΔJKE is obtained. Finally, to calculate the core polarization contribution, the core orbitals are relaxed in both the HS RO and unrestricted BS determinants, keeping the magnetic orbitals frozen. The details are well described in the literature [67,68,69]; the ΔJCP contribution was calculated according to ref. [68]. Regarding this decomposition, some limitations need to be acknowledged. In this step-by-step procedure, we start from the HS RO formalism, and all orbitals are never relaxed in the same SCF procedure. Thus, the final orbitals can slightly differ from the ones generated in the "standard" BS calculation. Therefore, the J parameter predicted according to Equation (2) can slightly deviate from the results of the standard BS calculation. A similar decomposition was also done with high-level quantum-mechanical methods by selectively considering classes of excitations in configuration interaction calculations [70], but the computational cost limits its applicability to small molecules.
For the optimized [Cu(SQ)(opd)]+ model, we found at the B3LYP/def2-TZVP theory level that J0 = 244 cm−1, ΔJKE = −1 cm−1 and ΔJCP = 22 cm−1. The insignificant ΔJKE contribution is consistent with the orthogonality of the magnetic orbitals dx2−y2 and π* discussed in Section 2.1 General Considerations. The quality of the decomposition can be assessed by comparing the total J value calculated according to Equation (2) with the value produced by the standard BS calculation. The sum of the three contributions is approximately 10% lower than the result of the standard BS B3LYP/def-TZVP calculations. This, along with the insignificant ΔJKE contribution, shows that the adapted decomposition scheme is reasonable. An interesting thing to note is that, for the model [Cu(SQ)(opd)]+, the spin polarization supports the ferromagnetic coupling (ΔJCP > 0). The magnitude of ΔJCP may seem low, but for the Cu(II) dinuclear complexes that exhibit significant magnetic exchange, this contribution was between –1 and +9 cm−1 [69].
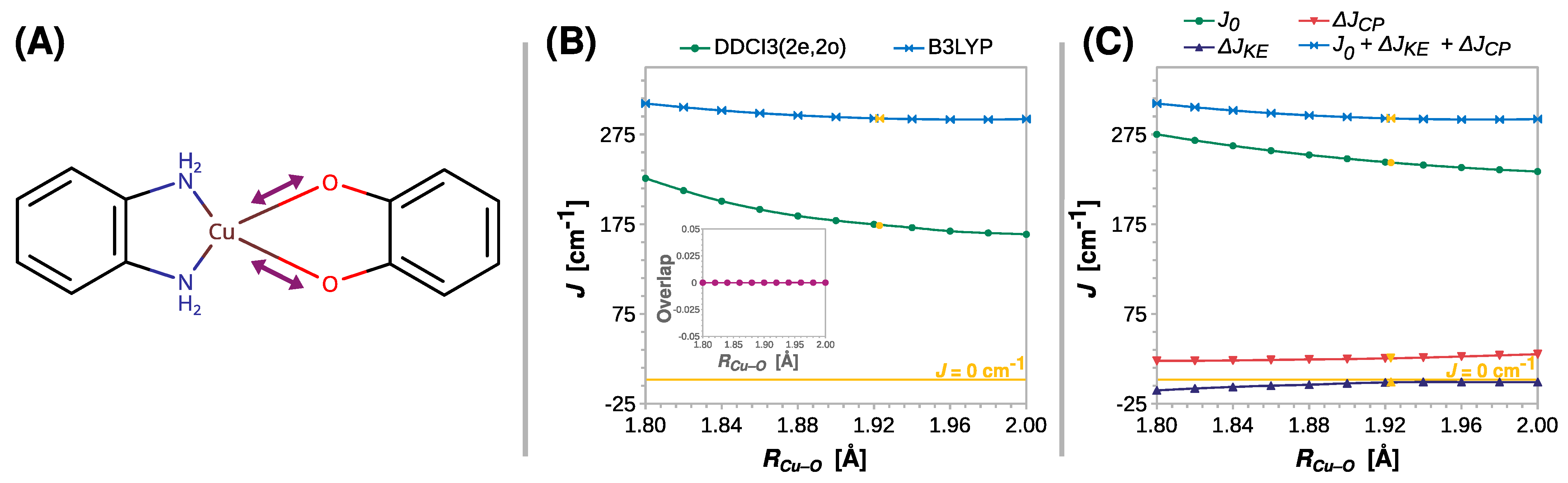
The length of the coordination bond is an important parameter that influences the properties of the coordination compounds. This inspired a search for the correlation between the exchange coupling constant J and the Cu–O distance (RCu–O). The results of the appropriate rigid scan are shown in Figure 4. Surprisingly, only a minor decrease in J was predicted by DFT with increasing RCu–O. The value of J fell from 310 to 292 cm−1 at the B3LYP theory level, but the weakening of the ferromagnetic coupling was more substantial when the DDCI3(2e,2o) approach was used. This ab initio method predicted a reduction in J by more than a fourth, that is, from 226 to 164 cm−1, strongly suggesting that RCu–O affects the coupling strength in semiquinonato Cu(II) complexes. The results of the scans performed at the CASSCF(2e,2o) and CASSCF(2e,2o)/NEVPT2 levels are shown in Figures S2–S5.
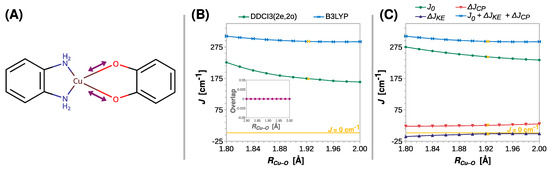
Figure 4.
Rigid scan of J along the Cu–O distance (RCu–O): schematic representation of the varied structural parameter (A); the parameter J predicted at the B3LYP and DDCI3(2e,2o) levels (B); decomposition of J at the B3LYP level (C). Inset in (B) shows the overlap of the magnetic orbitals calculated at the B3LYP level. All are calculated with the def2-TZVP basis set. For clarity, the line J = 0 cm−1 is shown as a reference. For the geometry optimized at the B3LYP/def2-TZVP theory level RCu–O = 1.925 Å (labeled with the yellow symbols).
Although the DFT methods underestimate the role of this structural parameter, the decomposition of J at the B3LYP level qualitatively revealed the reasons for the decrease in the ferromagnetic coupling. What stands out in Figure 4C is that the change in kinetic exchange ΔJKE with RCu–O is negligible, and the reduction in the ferromagnetic coupling stems from the decrease in direct exchange J0, which is, however, alleviated by the increasing ΔJCP contribution.
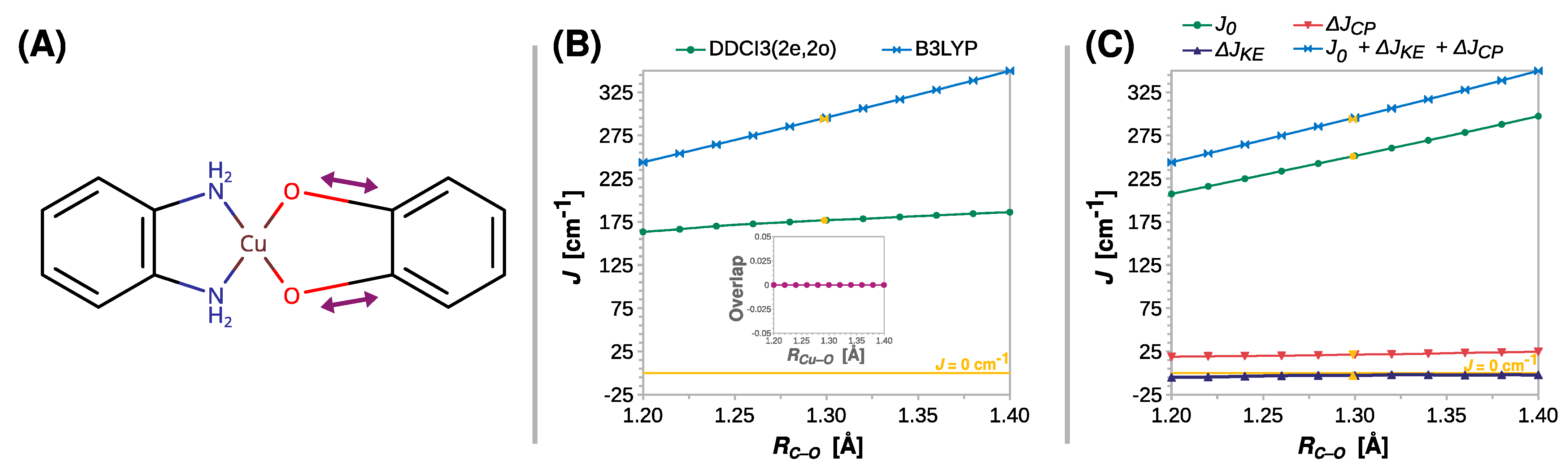
As shown previously in the studies on the EPR g tensor, the length of the C–O bond (RCu–O) can have a significant impact on the properties of semiquinone radicals [100,101] and, as shown in Figure 5, this is also true for the strength of exchange coupling in their Cu(II) complexes. Contrary to RCu–O, the functional B3LYP and DDCI3(2e,2o) showed that J increases as the C–O bond elongates. Compared to DDCI3(2e,2o), the functional B3LYP yielded a more significant rise in the J value. The breakdown of J at the B3LYP level exposed that the contribution of the direct exchange J0 dominates the observed increase, as ΔJKE remains insignificant and ΔJCP increases only slightly, that is, from 19 to 24 cm−1 (Figure 5C).
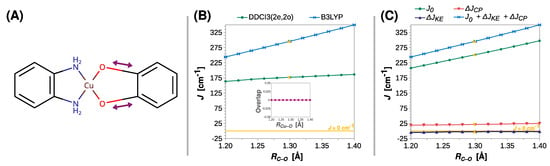
Figure 5.
Rigid scan of J along the C–O distance (RC–O): schematic representation of the varied structural parameter (A); the parameter J predicted at the B3LYP and DDCI3(2e,2o) levels and DDCI3(2e,2o) (B); decomposition of J at the B3LYP level (C). Inset in (B) shows the overlap of the magnetic orbitals calculated at the B3LYP level. All are calculated with the def2-TZVP basis set. For clarity, the line J = 0 cm−1 is shown as a reference. For the geometry optimized at the B3LYP/def2-TZVP theory level, RC–O = 1.294 Å (labeled with the yellow symbols).
For the optimized model [Cu(SQ)(opd)]+, two ligands are coplanar, but, as shown by the X-ray diffraction experiments [18,54,85,102,103], this is rarely the case. Therefore, the impact of divergence from coplanarity should be investigated. Let us start with the bending angle (Φ), which is shown schematically in Figure 6A.
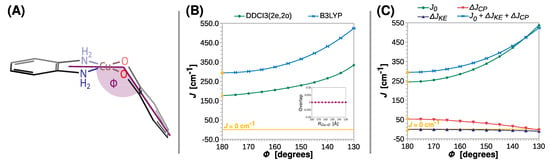
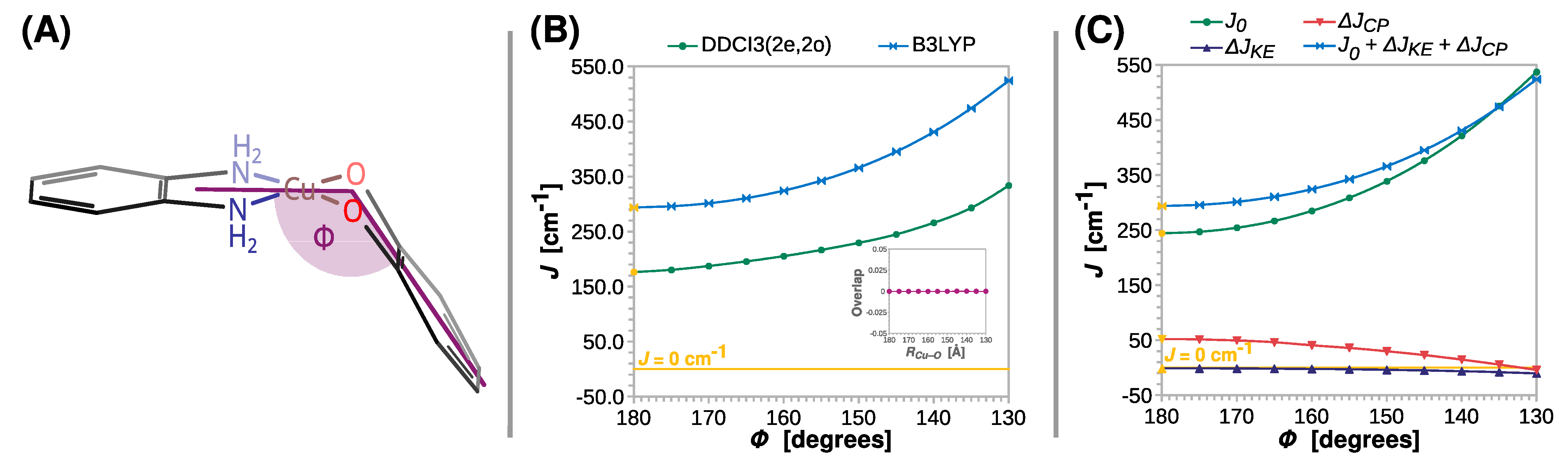
Figure 6.
Rigid scan of J along the bending angle between the N-Cu-N and O-Cu-O planes (Φ): schematic representation of the varied structural parameter (A); the parameter J predicted at the B3LYP and DDCI3(2e,2o) levels (B); decomposition of J at the B3LYP level (C). Inset in (B) shows the overlap of the magnetic orbitals calculated at the B3LYP level. All are calculated with the def2-TZVP basis set. For clarity, the line J = 0 cm−1 is shown as a reference. For the geometry optimized at the B3LYP/def2-TZVP theory level Φ = 0° (labeled with the yellow symbols).
Regardless of the computational method, the strength of ferromagnetic coupling increases gradually with Φ, which is reflected in the value of J going up from 294 to 524 cm−1 and from 176 to 334 cm−1 at the B3LYP and DDCI3(2e,2o) level, respectively. As for RCu–O and RC–O, the two magnetic orbitals stay orthogonal, and therefore the ΔJKE contribution remains insignificant; the change in J is dominated by the direct exchange J0. Interestingly, ΔJCP steadily decreases to a low point of approximately −2 cm−1 at Φ = 130° (a small antiferromagnetic contribution to J).
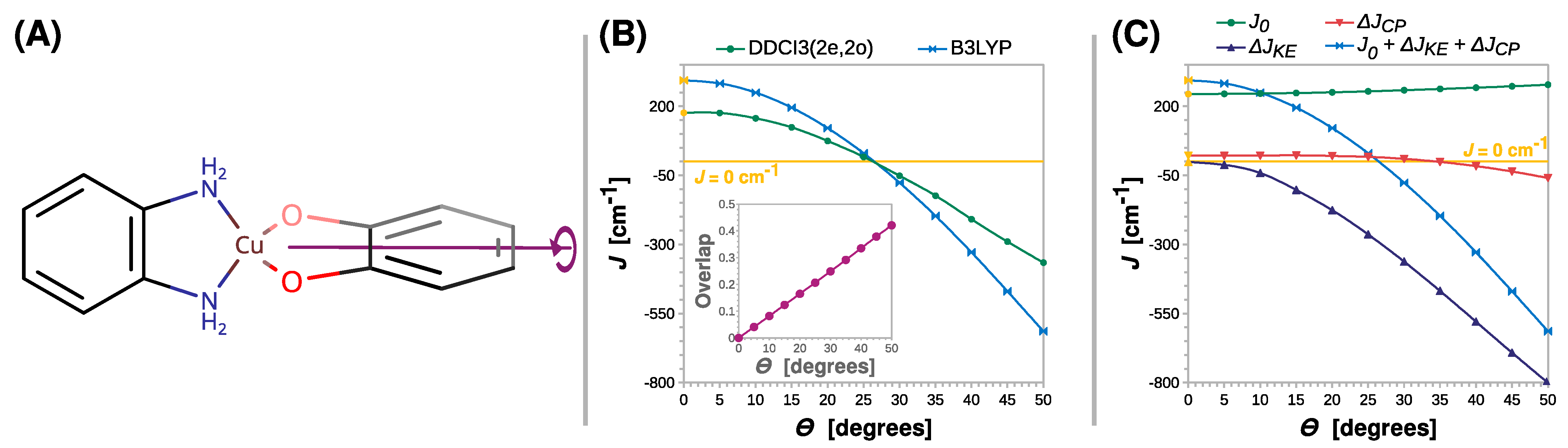
Next, we monitored the rhombic distortion of the Cu(II) coordination sphere. This was carried out by the rigid scan in which the intersection angle Θ between the N-Cu-N and O-Cu-O planes was varied. The twist of the SQ ligand out of the basal plane is schematically shown in Figure 7A. What is striking about Figure 7B is that, conversely to previously analyzed distortions, the twist of the radical ligand can clearly change the nature of magnetic coupling into antiferromagnetic (the J parameter becomes negative). The B3LYP and DDCI3(2e,2o) calculations for the simple model [Cu(SQ)(opd)]+ suggest that the crossover point lies at an angle of approximately 25°.
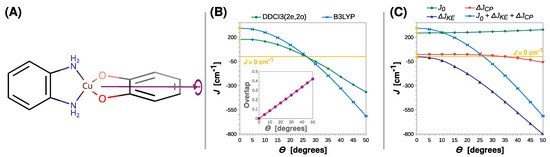
Figure 7.
Rigid scan of J along the twisting angle between the N-Cu-N and O-Cu-O planes (Θ): schematic representation of the varied structural parameter (A); the parameter J predicted at the B3LYP and DDCI3(2e,2o) levels (B); decomposition of J at the B3LYP level (C). Inset in (B) shows the overlap of the magnetic orbitals calculated at the B3LYP level. All are calculated with the def2-TZVP basis set. For clarity, the line J = 0 cm−1 is shown as a reference. For the geometry optimized at the B3LYP/def2-TZVP theory level Θ = 0° (labeled with the yellow symbols).
The change in the nature of magnetic interaction in the semiquinonato Cu(II) complexes can be triggered by the twist of the radical ligand and can be explained by the orthogonality of the copper dx2-y2 and the semiquinone π* magnetic orbitals. The elongation of the Cu–O or C–O bonds and the increase in bending angle Θ preserve the mirror plane σ, which is shown in Figure 3A. Therefore, as shown in the insets in Figure 4B, Figure 5B and Figure 6B, the overlap integral between the two magnetic orbitals remains zero; they stay orthogonal and promote the ferromagnetic coupling. However, upon the rhombic distortion, the minor plane σ vanishes, and the overlap integral between the two magnetic orbitals becomes non-zero and stabilizes the antiferromagnetic state. This is because the overlap improves bonding in the antiferromagnetic state and destabilizes the triplet state because of the Pauli repulsion between two unpaired electrons. The inset in Figure 7B shows that the overlap integral between the magnetic orbitals increases linearly with Θ.
A wider understanding of the crossover between ferro- and antiferromagnetic coupling in the semiquinonato Cu(II) complexes can be developed by the decomposition of J into the three contributions. The most important finding of this analysis is that with the increase in Θ, the kinetic exchange ΔJKE rapidly becomes negative and cancels the positive contribution of the direct exchange J0. After the crossover point, ΔJKE dominates the magnetic coupling. The interesting feature is that the twist of the radical ligand increases J0 by 34 cm−1, but this effect can only slightly alleviate rising ΔJKE. Furthermore, the rhombic distortion influence ΔJCP. The ferromagnetic contribution from the core polarization decreases, and starting from Θ = 35°, it becomes negative and thus stabilizes the antiferromagnetic state.
2.4. Comparison with Experiment
Rather than aiming at reproducing experimental values, in the present study, our goal was to provide a reference set of correlations between structural parameters and magnetic properties in semiquinonato Cu(II) complexes. In doing so, we emphasized obtaining qualitative insight through the examination of the mechanisms of magnetic coupling, particularly as revealed through the decomposition of J into J0, ΔJKE and ΔJCP. Nevertheless, a comparison with the experimental results is always reasonable to perform, even if the environmental effects are neglected in the computations. The results of the calculations carried out for the systems that have been realized synthetically [18,85] are given in Table 2, and the molecular units used in these calculations are shown in Figure 8. To avoid introducing additional errors or bias in our comparison of methods, the counterions or solvent molecules were not removed if they occupied the axial position. Ferromagnetic coupling is an exceedingly common situation in complexes comprising Cu(II) and semiquinone radicals; therefore, only one complex represents a case of antiferromagnetic coupling, that is, [Cu(dtbSQ)(dtben)]+.

Table 2.
Exchange coupling constants J (in cm−1) were calculated with DFT and ab initio methods for the semiquinonato Cu(II) complexes that were characterized experimentally.
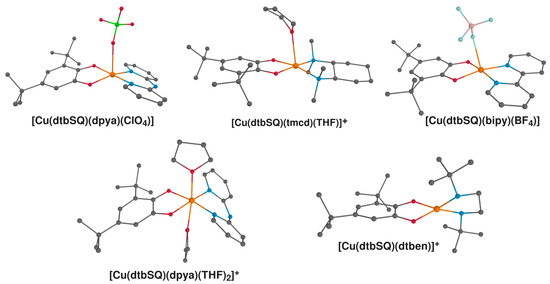
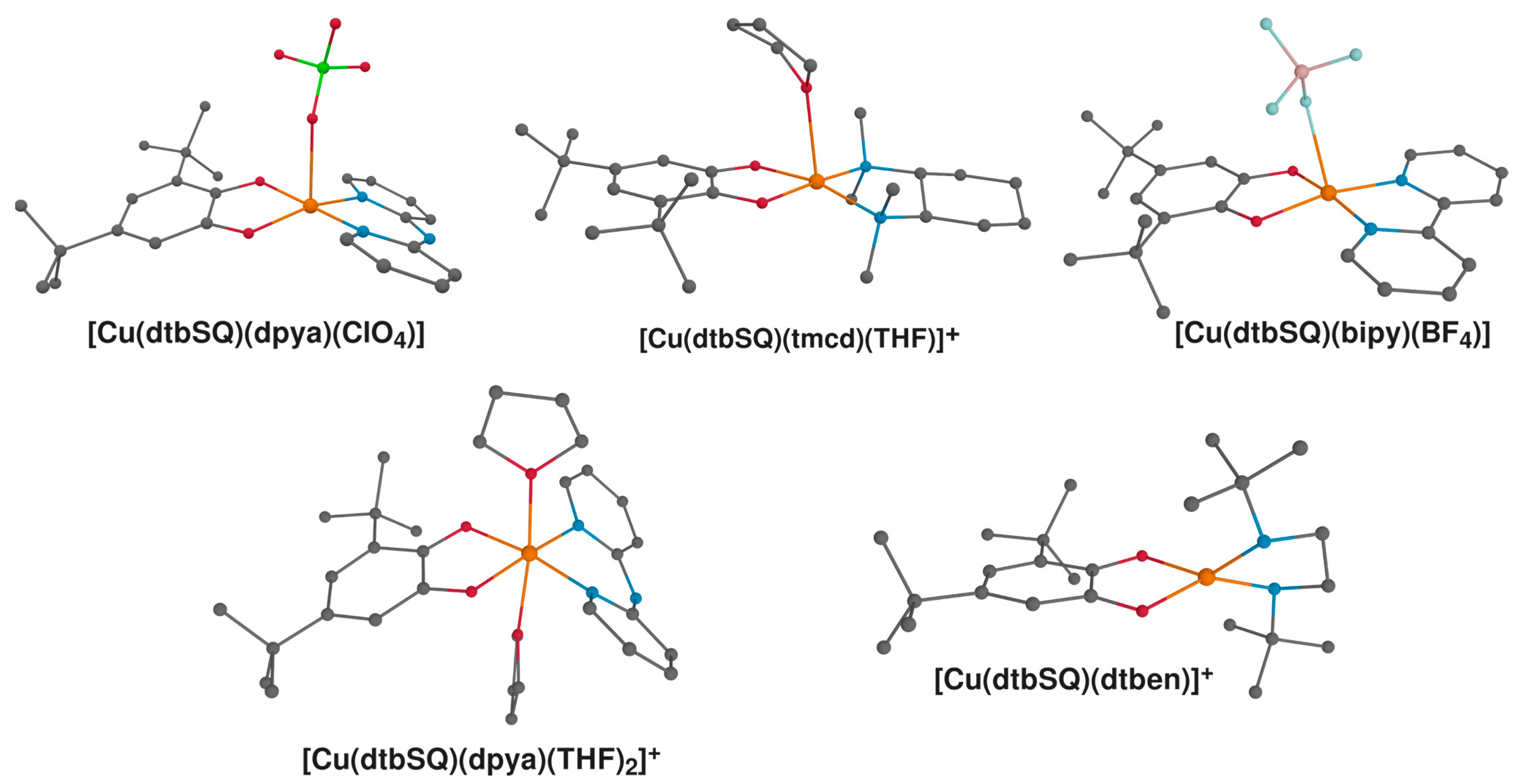
Figure 8.
Structures of semiquinonato Cu(II) complexes characterized experimentally that were included in this study: [Cu(dtbSQ)(dpya)(ClO4)] [87], [Cu(dtbSQ)(tmcd)(THF)] [18], [Cu(dtbSQ)(tmcd)(THF)] [85]; [Cu(dtbSQ)(bipy)(BF4)] [85], [Cu(dtbSQ)(dpya)(THF)2]+ [85] and [Cu(dtbSQ)(dtben)]+ [18]. Hydrogen atoms were omitted for clarity; Cu: orange; C: grey; N: blue; O: red; B: pale pink; Cl: green; F: pale blue.
As seen in Table 2, for all experimental complexes, the DFT methods overestimate the strength of magnetic coupling but correctly predict the sign of J. Regardless of the functional, for the complex [Cu(dtbSQ)(dpya)(ClO4)], the J is overestimated more than twice compared to the experimental counterpart. The agreement between DFT and experiment is significantly better for [Cu(dtbSQ)(bipy)(BF4)], [Cu(dtbSQ)(dpya)(THF)2]+ and antiferromagnetically coupled [Cu(dtbSQ)(dtben)]+, but the overestimation is still noticeable. Among the functionals tested, PBE0 provides results that are closer to the experiment, but it is not a significant improvement over B3LYP or TPSSh. The J values computed at the DFT level using the def2-TZVP are superior to those provided by def2-SVP, but the differences between the def2-TZVP and cc-pVDZ basis set are less apparent.
In contrast to DFT and regardless of the active space size, the CASSCF approach produced J parameters that are underestimated noticeably in comparison with experimentally determined values. For the minimal (2e,2o) active space, the SC- and FIC-NEVPT2 methods did not correct the predicted J parameter, except for the case of antiferromagnetic coupling for [Cu(dtbSQ)(dtben)]+. This is consistent with the results of the rigid scan along the twist angle Θ at the CASSCF(2e,2o)/SC-NEVPT2 theory level (Figure S5), which confirmed that even with the minimal active space, the perturbative correction provides significant improvement when the magnetic coupling between the radical ligand and Cu(II) ion becomes antiferromagnetic.
For the ferromagnetically coupled complexes, the extension of the active space to (10e,6o) did not have a significant impact on the results of the NEVPT2 calculations. However, the inclusion of the four lone pairs in the active space [(18e,10o)] clearly increased the predicted strength of the ferromagnetic coupling and thus improved the agreement between theoretical and experimental J values. The agreement was very good for [Cu(dtbSQ)(dpya)(ClO4)] and good for [Cu(dtbSQ)(dpya)(THF)2]+ if the basis cc-pVDZ was used in the calculations. The extension of the active space decreased the strength of the antiferromagnetic coupling for [Cu(dtbSQ)(dtben)]+ (the J parameter became less negative) and thus slightly worsened the agreement between theory and experiment. This showed that the prediction of J at the CASSCF(2e,2o)/NEVPT2 level for [Cu(dtbSQ)(dtben)]+ benefited from errors cancelations.
For the DDCI3(2e,2o) method, the computations with def2-TZVP become prohibitively expensive. However, our test calculations for smaller systems, which are summarized in Table 1 and discussed in Section 2.2 Performance of Computational Methods, showed that at this ab initio theory level, results obtained with def2-TZVP and cc-pVDZ should be comparable. Looking at Table 2, it is immediately apparent that the DDCI3(2e,2o)/cc-pVDZ calculations well reproduced the experimental values of J, with a somewhat larger discrepancy observed only for [Cu(dtbSQ)(dpya)(ClO4)]. This again shows that the variational approach to dynamic correlation at the DDCI(2e,2o) level is more accurate than the perturbational corrections for the CASSCF wave function.
The antiferromagnetically coupled complex [Cu(dtbSQ)(dtben)]+ requires a short comment. Using the rigid scan approach, we have been able to demonstrate that the increase in the intersection angle Θ between the N-Cu-N and O-Cu-O planes can switch the magnetic coupling from ferromagnetic to antiferromagnetic, which can become significant for large Θ. Our conclusion from the scan of J along Θ stays in line with the strong antiferromagnetic coupling determined for [Cu(dtbSQ)(dtben)]+ from the temperature dependence of the NMR chemical shifts [18]. For [Cu(dtbSQ)(dtben)]+, the intersection angle amounts to 51°, which reflects a strong rhombic distortion. Moreover, this ferromagnetic coupling was well reproduced in our computations for this complex, especially well at the DDCI3/ccpVD theory level.
3. Methods and Materials
We performed all computations using ORCA 4.2.1 [104,105]. Geometry optimizations were carried out at the DFT level with the hybrid functional B3LYP [106,107,108], def2-TZVP basis set [109] and the D3 dispersion corrections proposed by Grimme [110]. The accuracy of the DFT-optimized structures is generally excellent to good compared to X-ray diffraction data [54,102,103], although a systematic deviation is always expected compared to experimental data at finite temperatures [111]. Following previous practice, the high-spin state (HS) was assumed for optimizations [60,71,72]. For systems that have been realized synthetically, the structures were taken from the Cambridge Structural Database [112], and only the positions of hydrogen atoms were optimized after the noncoordinated counterions and solvent molecules were removed, and hydrogens were added where necessary. These structures are shown in Figure 8 with references in the description. All the optimized structures are given in the Supplementary Materials in XYZ format.
Subsequently, single-point calculations were performed to obtain the final energies of the S = 1 and S = 0 states. In these calculations, the basis sets def2-SVP [109], def2-TZVP [109] and correlation-consistent cc-pVDZ [75,96,97] were used. At the DFT level, we used the hybrid functional B3LYP [106,107,108], PBE0 [113] and TPSSh [114] and followed the standard broken symmetry (BS) DFT procedure using the FlipSpin feature of ORCA to generate the initial guess for the BS calculation. All BS solutions were verified for correctness by examining the spin populations to confirm the expected number of unpaired electrons at the Cu(II) and semiquinone sites. Exchange coupling constants J were computed with the Yamaguchi formula [115,116] that covers weak to strong exchange coupling by scaling the energy difference between the high-spin and broken-symmetry solutions according to their spin expectation values:
The overlap integrals between two SOMOs were obtained at the BS B3LYP/def2-TZVP theory level by applying the corresponding orbital transformation [117].
Multireference CASSCF calculations were carried out in a state-averaged manner. For the minimal active space that describes singlet-triplet splitting, that is, two electrons in two SOMOs [CASSCF(2e,2o)], the initial orbitals were quasi-restricted DFT orbitals [118]. Two larger active spaces were tested. The first was constructed by adding the remaining four 3d orbitals of copper(II) [CASSCF(10e,6o)], and the second also included four lone pairs of ligands that can undergo bonding interaction with Cu(II) (these orbitals are shown in Figure S1) [CASSCF(18e,10o)]. The initial orbitals for CASSCF(10e,6o) and CASSCF(18e,10o) were obtained by the localization of the internal orbitals from CASSCF(2e,2o) using the Pipek−Mezey localization algorithm [119]. An overview of CASSCF can be found in ref. [120]. Two variants of the internally contracted N-electron valence state perturbation theory (NEVPT2) were adopted to apply the dynamical correlation to the CASSCF wave function, that is, strongly contracted NEVPT2 (SC-NEVPT2) and a fully internally contracted NEVPT2 (FIC-NEVPT2) [121,122,123] FIC-NEVPT2 is frequently referred to as partially contracted NEVPT2 (PC-NEVPT2).
The magnetic coupling constants J were also evaluated by means of difference dedicated configuration interaction with excitations up to three degrees of freedom (DDCI3). The DDCI3 approach is essentially MRCI with singles and doubles, where the two-hole two-particle excitations are omitted [124,125]. For the DDCI3 calculations, the CASSCF(2e,2o) reference wavefunctions were used with the same active space, and hence these calculations were referred to as DDCI3(2e,2o). This method with minimal active space was demonstrated to provide highly accurate J values for magnetic systems while remaining computationally feasible [61,96,125,126,127,128,129,130]. In the case of the ab initio methods, the value of J was calculated as follows:
For hybrid DFT, very tight energy convergence criteria (VeryTightSCF), enhanced integration grids (Grid6) and the chain-of-spheres approximation to exact exchange (COSX) [131] with increased grid settings (GridX6) were employed. The auxiliary def2/J basis sets were used in combination with def2-SVP and def2-TZVP, while for cc-pVDZ, the auxiliary basis set was generated using an automatic procedure (AutoAux). For the CASSCF calculations, we used the conventional integral storage and the RI approximation for the integral transformation and the Fock matrix construction (RIJK) to speed up the calculations [132,133]. Thus, the appropriate auxiliary basis sets for correlation calculations were employed, that is, def2/JK [134] for def2-SVP and def2-TZVP and the AutoAux procedure for cc-pVDZ. In the DDCI3 calculations, all single excitations were included, and the thresholds Tsel and Tpre, defined elsewhere [135,136], were set to 10−6 and 10−4, respectively. These parameters can affect the computed J values, and thus they were validated (Tables S2–S4). As for CASSCF, the RI approximation for the integral transformation was used with the same correlation fitting basis sets.
We used Marvin for drawing chemical structures (version 21.2.0, ChemAxon https://www.chemaxon.com accessed on 15 February 2023). 3-D visualizations were done with the Gabedit 2.5.0 [137] (Figure 2, Figure 3A and Figure 8) and Avogadro 1.2 [138] (Figure 3B) software, both in combination with the PovRay ray-tracing program (version 3.7, http://www.povray.org/ accessed on 15 February 2023).
4. Conclusions
In this study, we theoretically analyzed the exchange coupling for a set of copper(II) complexes with semiquinone radicals. We used three hybrid DFT functionals, CASSCF with NEVPT2 for dynamical correlation and DDCI3. Rather than aiming at reproducing experimental values, in the present study, our objective was to uncover correlations between structural parameters and exchange coupling. We showed that the elongation of the Cu–O bonds and the rhombical distortion that twists the radical ligand decrease the ferromagnetic coupling, while the elongation of the C–O bond and bending of the radical ligand out of planarity increase it. From the inspected structural modifications, the twist of the radical ligand out of the basal plane deserves special attention. It can easily switch the magnetic interaction from ferromagnetic to antiferromagnetic and, thus, is the most suitable for tuning magnetic exchange couplings.
To provide more comprehensive insight into structural effects, we carried out a decomposition of the magnetic exchange coupling into three different contributions, that is, the direct exchange (J0), the kinetic exchange (ΔJKE) and the spin (or core) polarization of the nonmagnetic electrons (ΔJCP). The bending of the semiquinone ligand out of the complex planarity and the elongation of the Cu–O and C–O bonds correspond to the changes in J0. The twist of the radical ligand differed from these three modifications, as it enhanced ΔJKE, which is an antiferromagnetic contribution. We showed that, for semiquinonoato Cu(II) complexes, this structural modification and the increase in ΔJKE correlates with the vanishing orthogonality between two orbitals occupied by the unpaired electrons.
Although it was not the primary aim of our work, we conducted computations for the complexes that have been synthesized. All the methods tested correctly predict the ground spin state of the systems. The hybrid functionals overestimate, and CASSCF underestimates the values of J, even if the active space is extended to (18e,10o). We should note that for the complexes with ferromagnetic coupling between Cu(II) and semiquinone, the NEVPT2 correction noticeably improves the results if the calculations are done with relatively large (18e,10o) active space. In the case of antiferromagnetic coupling observed for [Cu(dtbSQ)(dtben)]+, the NEVPT2 correction improves the predicted J parameter even in combination with the minimal (2e,2o) active space. In summary, to obtain qualitative estimates of J, it is sufficient to perform DFT or CASSCF calculations with minimal active space with just the magnetic orbitals and the unpaired electrons. However, to obtain more quantitative numbers, one should add the remaining 3d and ligands lone pair orbitals to the active space and apply the NEVPT2 correction. However, the highly accurate predictions of J can be made at the DDCI3 level with minimal active space. For DFT methods, we found that PBE0 was slightly better than B3LYP and TPSSh.
We believe that our results improve the understanding of the exchange coupling between Cu(II) ions and semiquinone radicals and can be helpful in the experimental interpretation of magnetic data for such systems, and through generalization, they can be extended to other complexes of paramagnetic ions, with π-type radical ligands. The comprehensive analysis of the magnetic exchange we provided in this paper can be used for in silico design of molecular compounds with selected magnetic properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24044001/s1.
Author Contributions
Conceptualization, M.W.; methodology, M.W.; investigation, A.Z. and M.W.; writing—original draft preparation, M.W. and A.Z.; writing—review and editing, M.W. and A.Z.; visualization, A.Z. and M.W.; supervision, M.W.; funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research project is supported by the program „Excellence initiative—research university" for the years 2020–2026 for the University of Wrocław. The computations were performed using computers and software licenses of the Wrocław Centre for Networking and Supercomputing (Grant No. 47).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the current study are available in the article and in the associated Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pierpont, C.G.; Buchanan, R.M. Transition Metal Complexes of O-Benzoquinone, o-Semiquinone, and Catecholate Ligands. Coord. Chem. Rev. 1981, 38, 45–87. [Google Scholar] [CrossRef]
- Pierpont, C.G.; Lange, C.W. The Chemistry of Transition Metal Complexes Containing Catechol and Semiquinone Ligands. Prog. Inorg. Chem. 2007, 41, 331–442. [Google Scholar]
- Mederos, A.; Domínguez, S.; Hernández-Molina, R.; Sanchiz, J.; Brito, F. Coordinating Ability of Phenylenediamines. Coord. Chem. Rev. 1999, 193–195, 913–939. [Google Scholar] [CrossRef]
- Pierpont, C. Unique Properties of Transition Metal Quinone Complexes of the MQ3 Series. Coord. Chem. Rev. 2001, 219–221, 415–433. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Cherkasov, V.K.; Abakumov, G.A. Transition Metal Complexes with Bulky 4,6-Di-Tert-Butyl-N-Aryl(Alkyl)-o-Iminobenzoquinonato Ligands: Structure, EPR and Magnetism. Coord. Chem. Rev. 2009, 253, 291–324. [Google Scholar] [CrossRef]
- Kaim, W. Manifestations of Noninnocent Ligand Behavior. Inorg. Chem. 2011, 50, 9752–9765. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Beyer, K.; Filippou, V.; Záliš, S. Charge and Spin Coupling in Copper Compounds with Hemilabile Noninnocent Ligands—Ambivalence in Three Dimensions. Coord. Chem. Rev. 2018, 355, 173–179. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, A.K.; Barman, S.K.; Saha, A.; Mukherjee, R. Valence Tautomerism and Delocalization in Transition Metal Complexes of o-Amidophenolates and Other Redox-Active Ligands. Some Recent Results. Coord. Chem. Rev. 2020, 414, 213240. [Google Scholar] [CrossRef]
- Ershova, I.V.; Piskunov, A.V.; Cherkasov, V.K. Complexes of Diamagnetic Cations with Radical Anion Ligands. Russ. Chem. Rev. 2020, 89, 1157–1183. [Google Scholar] [CrossRef]
- Pashanova, K.I.; Poddel’sky, A.I.; Piskunov, A.v. Complexes of “Late” Transition Metals of the 3d Row Based on Functionalized o-Iminobenzoquinone Type Ligands: Interrelation of Molecular and Electronic Structure, Magnetic Behaviour. Coord. Chem. Rev. 2022, 459, 214399. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-Innocent Ligands in Bioinorganic Chemistry—An Overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]
- Heims, F.; Ray, K. Multiple Spin Scenarios in Transition-Metal Complexes Involving Redox Non-Innocent Ligands. In Spin States in Biochemistry and Inorganic Chemistry; John Wiley & Sons, Ltd.: Oxford, UK, 2015; pp. 229–262. [Google Scholar]
- Stubbe, J.; van der Donk, W.A. Protein Radicals in Enzyme Catalysis. Chem. Rev. 1998, 98, 705–762. [Google Scholar] [CrossRef] [PubMed]
- Storr, T.; Verma, P.; Pratt, R.C.; Wasinger, E.C.; Shimazaki, Y.; Stack, T.D.P. Defining the Electronic and Geometric Structure of One-Electron Oxidized Copper−Bis-Phenoxide Complexes. J. Am. Chem. Soc. 2008, 130, 15448–15459. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.W. Free Radical Catalysis by Galactose Oxidase. Chem. Rev. 2003, 103, 2347–2364. [Google Scholar] [CrossRef]
- Kaim, W. The Chemistry and Biochemistry of the Copper–Radical Interaction. Dalton Trans. 2003, 761–768. [Google Scholar] [CrossRef]
- Kodera, M.; Kawata, T.; Kano, K.; Tachi, Y.; Itoh, S.; Kojo, S. Mechanism for Aerobic Oxidation of 3,5-Di-Tert-Butylcatechol to 3,5-Di-Tert-Butyl-o-Benzoquinone Catalyzed by Di-μ-Hydroxo-Dicopper(II) Complexes of Peralkylated Ethylelnediamine Ligands. Bull. Chem. Soc. Jpn 2003, 76, 1957–1964. [Google Scholar] [CrossRef]
- Verma, P.; Weir, J.; Mirica, L.; Stack, T.D.P. Tale of a Twist: Magnetic and Optical Switching in Copper(II) Semiquinone Complexes. Inorg. Chem. 2011, 50, 9816–9825. [Google Scholar] [CrossRef]
- Liu, J.; Lorraine, S.C.; Dolinar, B.S.; Hoover, J.M. Aerobic Oxidation Reactivity of Well-Defined Cobalt(II) and Cobalt(III) Aminophenol Complexes. Inorg. Chem. 2022, 61, 6008–6016. [Google Scholar] [CrossRef]
- Lecarme, L.; Kochem, A.; Chiang, L.; Moutet, J.; Berthiol, F.; Philouze, C.; Leconte, N.; Storr, T.; Thomas, F. Electronic Structure and Reactivity of One-Electron-Oxidized Copper(II) Bis(Phenolate)–Dipyrrin Complexes. Inorg. Chem. 2018, 57, 9708–9719. [Google Scholar] [CrossRef]
- Lyaskovskyy, V.; de Bruin, B. Redox Non-Innocent Ligands: Versatile New Tools to Control Catalytic Reactions. ACS Catal. 2012, 2, 270–279. [Google Scholar] [CrossRef]
- Sutradhar, M.; Pombeiro, A.J.L.; da Silva, J.A.L. Water Oxidation with Transition Metal Catalysts with Non-Innocent Ligands and Its Mechanisms. Coord. Chem. Rev. 2021, 439, 213911. [Google Scholar] [CrossRef]
- Vostrikova, K.E. High-Spin Molecules Based on Metal Complexes of Organic Free Radicals. Coord. Chem. Rev. 2008, 252, 1409–1419. [Google Scholar] [CrossRef]
- Preuss, K.E. Metal-Radical Coordination Complexes of Thiazyl and Selenazyl Ligands. Coord. Chem. Rev. 2015, 289–290, 49–61. [Google Scholar] [CrossRef]
- Matsuoka, R.; Yoshimoto, T.; Kitagawa, Y.; Kusamoto, T. Structural and Magnetic Studies on Nickel(II) and Cobalt(II) Complexes with Polychlorinated Diphenyl(4-Pyridyl)Methyl Radical Ligands. Molecules 2021, 26, 5596. [Google Scholar] [CrossRef]
- Ito, S.; Yoshitake, T.; Ishida, T. Ferromagnetic 2p-2p and 4f-2p Couplings in a Macrocycle from Two Biradicals and Two Gadolinium(III) Ions. Molecules 2022, 27, 4930. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 149–176. [Google Scholar] [CrossRef]
- Chakarawet, K.; Harris, T.D.; Long, J.R. Semiquinone Radical-Bridged M2 (M = Fe, Co, Ni) Complexes with Strong Magnetic Exchange Giving Rise to Slow Magnetic Relaxation. Chem. Sci. 2020, 11, 8196–8203. [Google Scholar] [CrossRef]
- Himmel, H.J. Valence Tautomerism in Copper Coordination Chemistry. Inorg. Chim. Acta 2018, 481, 56–68. [Google Scholar] [CrossRef]
- Sundaresan, S.; Diehl, M.; Carrella, L.M.; Rentschler, E. Triggering of Valence Tautomeric Transitions in Dioxolene-Based Cobalt Complexes Influenced by Ligand Substituents, Co-Ligands, and Anions. Magnetochemistry 2022, 8, 109. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Kondo, M.; Fujita, T.; Namiki, K.; Murata, M.; Nishihara, H. Thioacetyl-Terminated Ferrocene-Anthraquinone Conjugates: Synthesis, Photo- and Electrochemical Properties Triggered by Protonation-Induced Intramolecular Electron Transfer. Molecules 2010, 15, 150–163. [Google Scholar] [CrossRef]
- Fleming, C.; Chung, D.; Ponce, S.; Brook, D.J.R.; DaRos, J.; Das, R.; Ozarowski, A.; Stoian, S.A. Valence Tautomerism in a Cobalt-Verdazyl Coordination Compound. Chem. Commun. 2020, 56, 4400–4403. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular Magnetism, Quo Vadis? A Historical Perspective from a Coordination Chemist Viewpoint☆. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Kishishita, S.; Okajima, T.; Kim, M.; Yamaguchi, H.; Hirota, S.; Suzuki, S.; Kuroda, S.; Tanizawa, K.; Mure, M. Role of Copper Ion in Bacterial Copper Amine Oxidase: Spectroscopic and Crystallographic Studies of Metal-Substituted Enzymes. J. Am. Chem. Soc. 2003, 125, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, H.; Zhou, J.; Wang, Y.; Yan, H.; Jin, R.; Tang, Y. Evodiamine and Rutaecarpine as Potential Anticancer Compounds: A Combined Computational Study. Int. J. Mol. Sci. 2022, 23, 11513. [Google Scholar] [CrossRef]
- Muzalevskiy, V.M.; Sizova, Z.A.; Nechaev, M.S.; Nenajdenko, V.G. Acid-Switchable Synthesis of Trifluoromethylated Triazoles and Isoxazoles via Reaction of CF3-Ynones with NaN3: DFT Study of the Reaction Mechanism. Int. J. Mol. Sci. 2022, 23, 14522. [Google Scholar] [CrossRef]
- Huang, H.; Hu, G.; Hu, C.; Fan, X. Enhanced Hydrogen Evolution Reactivity of T’-Phase Tungsten Dichalcogenides (WS2, WSe2, and WTe2) Materials: A DFT Study. Int. J. Mol. Sci. 2022, 23, 11727. [Google Scholar] [CrossRef]
- Krupka, K.M.; Pocheć, M.; Panek, J.J.; Jezierska, A. Comprehensive Empirical Model of Substitution—Influence on Hydrogen Bonding in Aromatic Schiff Bases. Int. J. Mol. Sci. 2022, 23, 12439. [Google Scholar] [CrossRef]
- Domagała, M.; Jabłoński, M.; Dubis, A.T.; Zabel, M.; Pfitzner, A.; Palusiak, M. Testing of Exchange-Correlation Functionals of DFT for a Reliable Description of the Electron Density Distribution in Organic Molecules. Int. J. Mol. Sci. 2022, 23, 14719. [Google Scholar] [CrossRef]
- Witwicki, M.; Walencik, P.K.; Jezierska, J. How Accurate Is Density Functional Theory in Predicting Spin Density? An Insight from the Prediction of Hyperfine Coupling Constants. J. Mol. Model. 2020, 26, 10. [Google Scholar] [CrossRef]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.; Perdew, J.P.; Lyssenko, K.A. Density Functional Theory Is Straying from the Path toward the Exact Functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef]
- Wojtkowiak, K.; Michalczyk, M.; Zierkiewicz, W.; Jezierska, A.; Panek, J.J. Chalcogen Bond as a Factor Stabilizing Ligand Conformation in the Binding Pocket of Carbonic Anhydrase IX Receptor Mimic. Int. J. Mol. Sci. 2022, 23, 13701. [Google Scholar] [CrossRef] [PubMed]
- Witwicki, M.; Jezierska, J. Protonated O-Semiquinone Radical as a Mimetic of the Humic Acids Native Radicals: A DFT Approach to the Molecular Structure and EPR Properties. Geochim. Cosmochim. Acta 2012, 86, 384–391. [Google Scholar] [CrossRef]
- Witwicki, M.; Lewińska, A.; Ozarowski, A. O-Semiquinone Radical Anion Isolated as an Amorphous Porous Solid. Phys. Chem. Chem. Phys. 2021, 23, 17408–17419. [Google Scholar] [CrossRef] [PubMed]
- Ritacco, I.; Russo, N.; Sicilia, E. DFT Investigation of the Mechanism of Action of Organoiridium(III) Complexes As Anticancer Agents. Inorg. Chem. 2015, 54, 10801–10810. [Google Scholar] [CrossRef] [PubMed]
- Lewińska, A.; Kulbacka, J.; Domżał-Kędzia, M.; Witwicki, M. Antiradical Properties of N-Oxide Surfactants—Two in One. Int. J. Mol. Sci. 2021, 22, 8040. [Google Scholar] [CrossRef]
- Amić, A.; Mastiľák Cagardová, D. DFT Study of the Direct Radical Scavenging Potency of Two Natural Catecholic Compounds. Int. J. Mol. Sci. 2022, 23, 14497. [Google Scholar] [CrossRef]
- Jerzykiewicz, M.; Ćwieląg-Piasecka, I.; Witwicki, M.; Jezierski, A. EPR Spin Trapping and DFT Studies on Structure of Active Antioxidants in Biogycerol. Chem. Phys. Lett. 2010, 497, 135–141. [Google Scholar] [CrossRef]
- Amić, A.; Cagardová, D.M. Mactanamide and Lariciresinol as Radical Scavengers and Fe2+ Ion Chelators—A DFT Study. Phytochemistry 2022, 204, 113442. [Google Scholar] [CrossRef]
- Amić, A.; Milenković, D.; Marković, Z.; Cagardová, D.; Rodríguez-Guerra Pedregal, J.; Dimitrić Marković, J.M. Impact of the Phenolic O–H vs. C-Ring C–H Bond Cleavage on the Antioxidant Potency of Dihydrokaempferol. New J. Chem. 2021, 45, 7977–7986. [Google Scholar] [CrossRef]
- Amić, A.; Lučić, B.; Stepanić, V.; Marković, Z.; Marković, S.; Dimitrić Marković, J.M.; Amić, D. Free Radical Scavenging Potency of Quercetin Catecholic Colonic Metabolites: Thermodynamics of 2H+/2e− Processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef]
- Cong, Y.; Zhai, Y.; Chen, X.; Li, H. The Accuracy of Semi-Empirical Quantum Chemistry Methods on Soot Formation Simulation. Int. J. Mol. Sci. 2022, 23, 13371. [Google Scholar] [CrossRef] [PubMed]
- Ćwieląg-Piasecka, I.; Witwicki, M.; Jerzykiewicz, M.; Jezierska, J. Can Carbamates Undergo Radical Oxidation in the Soil Environment? A Case Study on Carbaryl and Carbofuran. Environ. Sci. Technol. 2017, 51, 14124–14134. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Prediction of Molecular Properties and Molecular Spectroscopy with Density Functional Theory: From Fundamental Theory to Exchange-Coupling. Coord. Chem. Rev. 2009, 253, 526–563. [Google Scholar] [CrossRef]
- Sikdar, Y.; Modak, R.; Bose, D.; Banerjee, S.; Bieńko, D.; Zierkiewicz, W.; Bieńko, A.; das Saha, K.; Goswami, S. Doubly Chloro Bridged Dimeric Copper(II) Complex: Magneto-Structural Correlation and Anticancer Activity. Dalton Trans. 2015, 44, 8876–8888. [Google Scholar] [CrossRef]
- Baffert, C.; Orio, M.; Pantazis, D.A.; Duboc, C.; Blackman, A.G.; Blondin, G.; Neese, F.; Deronzier, A.; Collomb, M.-N. Trinuclear Terpyridine Frustrated Spin System with a MnIV3O4 Core: Synthesis, Physical Characterization, and Quantum Chemical Modeling of Its Magnetic Properties. Inorg. Chem. 2009, 48, 10281–10288. [Google Scholar] [CrossRef]
- Orio, M.; Pantazis, D.A.; Petrenko, T.; Neese, F. Magnetic and Spectroscopic Properties of Mixed Valence Manganese (III,IV) Dimers: A Systematic Study Using Broken Symmetry Density Functional Theory. Inorg. Chem. 2009, 48, 7251–7260. [Google Scholar] [CrossRef]
- Singh, S.K.; Tibrewal, N.K.; Rajaraman, G. Density Functional Studies on Dinuclear {NiIIGdIII} and Trinuclear {NiIIGdIIINiII} Complexes: Magnetic Exchange and Magneto-Structural Maps. Dalton Trans. 2011, 40, 10897. [Google Scholar] [CrossRef]
- Rajaraman, G.; Totti, F.; Bencini, A.; Caneschi, A.; Sessoli, R.; Gatteschi, D. Density Functional Studies on the Exchange Interaction of a Dinuclear Gd(Iii)–Cu(Ii) Complex: Method Assessment, Magnetic Coupling Mechanism and Magneto-Structural Correlations. Dalton Trans. 2009, 17, 3153–3161. [Google Scholar] [CrossRef]
- Pantazis, D.A.; Krewald, V.; Orio, M.; Neese, F. Theoretical Magnetochemistry of Dinuclear Manganese Complexes: Broken Symmetry Density Functional Theory Investigation on the Influence of Bridging Motifs on Structure and Magnetism. Dalton Trans. 2010, 39, 4959. [Google Scholar] [CrossRef]
- Buvaylo, E.A.; Kokozay, V.N.; Makhankova, V.G.; Melnyk, A.K.; Korabik, M.; Witwicki, M.; Skelton, B.W.; Vassilyeva, O.Y. Synthesis, Characterization, and Magnetic Properties of a Series of Copper(II) Chloride Complexes of Pyridyliminebenzoic Acids. Eur. J. Inorg. Chem. 2018, 2018, 1603–1619. [Google Scholar] [CrossRef]
- Oms, O.; Rota, J.; Norel, L.; Calzado, C.J.; Rousselière, H.; Train, C.; Robert, V. Beyond Kahn’s Model: Substituent and Heteroatom Influence on Exchange Interaction in a Metal-Verdazyl Complex. Eur. J. Inorg. Chem. 2010, 2010, 5373–5378. [Google Scholar] [CrossRef]
- Zueva, E.M.; Petrova, M.M.; Shamsieva, A.V.; Trigulova, K.R.; Musina, E.I.; Fayzullin, R.R.; Bogomyakov, A.S.; Ovcharenko, V.I.; Karasik, A.A. Insight into the Influence of Terminal Ligands on Magnetic Exchange Coupling in a Series of Dimeric Copper(II) Acetate Adducts. Int. J. Quantum Chem. 2020, 120, e26145. [Google Scholar] [CrossRef]
- Bolvin, H.; Wagner, F.R. Case of a Strong Antiferromagnetic Exchange Coupling Induced by Spin Polarization of a Mn–Mn Partial Single Bond. Inorg. Chem. 2012, 51, 7112–7118. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Pohlhammer, N.; Urzúa-Leiva, R.; Páez-Hernández, D.; Cárdenas-Jirón, G. Spin-Filter Transport and Magnetic Properties in a Binuclear Cu(II) Expanded Porphyrin Based Molecular Junction. Dalton Trans. 2019, 48, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Maurice, R.; Sivalingam, K.; Ganyushin, D.; Guihéry, N.; de Graaf, C.; Neese, F. Theoretical Determination of the Zero-Field Splitting in Copper Acetate Monohydrate. Inorg. Chem. 2011, 50, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Coulaud, E.; Malrieu, J.-P.; Guihéry, N.; Ferré, N. Additive Decomposition of the Physical Components of the Magnetic Coupling from Broken Symmetry Density Functional Theory Calculations. J. Chem. Theory Comput. 2013, 9, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Ferré, N.; Guihéry, N.; Malrieu, J.-P. Spin Decontamination of Broken-Symmetry Density Functional Theory Calculations: Deeper Insight and New Formulations. Phys. Chem. Chem. Phys. 2015, 17, 14375–14382. [Google Scholar] [CrossRef]
- David, G.; Wennmohs, F.; Neese, F.; Ferré, N. Chemical Tuning of Magnetic Exchange Couplings Using Broken-Symmetry Density Functional Theory. Inorg. Chem. 2018, 57, 12769–12776. [Google Scholar] [CrossRef]
- Terencio, T.; Bastardis, R.; Suaud, N.; Maynau, D.; Bonvoisin, J.; Malrieu, J.P.; Calzado, C.J.; Guihéry, N. Physical Analysis of the Through-Ligand Long-Distance Magnetic Coupling: Spin-Polarization versus Anderson Mechanism. Phys. Chem. Chem. Phys. 2011, 13, 12314. [Google Scholar] [CrossRef]
- Pantazis, D.A. Assessment of Double-Hybrid Density Functional Theory for Magnetic Exchange Coupling in Manganese Complexes. Inorganics 2019, 7, 57. [Google Scholar] [CrossRef]
- Teyar, B.; Belkhiri, L.; Costuas, K.; Boucekkine, A.; Meyer, K. Electronic Structure and Magnetic Properties of Dioxo-Bridged Diuranium Complexes with Diamond-Core Structural Motifs: A Relativistic DFT Study. Inorg. Chem. 2016, 55, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-C.; Zheng, Y.-Z. Methods and Models of Theoretical Calculation for Single-Molecule Magnets. Magnetochemistry 2021, 7, 107. [Google Scholar] [CrossRef]
- Fujii, T.; Kitagawa, Y.; Ikenaga, K.; Tada, H.; Era, I.; Nakano, M. Theoretical Study on Magnetic Interaction in Pyrazole-Bridged Dinuclear Metal Complex: Possibility of Intramolecular Ferromagnetic Interaction by Orbital Counter-Complementarity. Magnetochemistry 2020, 6, 10. [Google Scholar] [CrossRef]
- Pantazis, D.A. Meeting the Challenge of Magnetic Coupling in a Triply-Bridged Chromium Dimer: Complementary Broken-Symmetry Density Functional Theory and Multireference Density Matrix Renormalization Group Perspectives. J. Chem. Theory Comput. 2019, 15, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Benediktsson, B.; Bjornsson, R. Analysis of the Geometric and Electronic Structure of Spin-Coupled Iron–Sulfur Dimers with Broken-Symmetry DFT: Implications for FeMoco. J. Chem. Theory Comput. 2022, 18, 1437–1457. [Google Scholar] [CrossRef]
- Jung, J.; Puget, M.; Cador, O.; Bernot, K.; Calzado, C.J.; le Guennic, B. Analysis of the Magnetic Exchange Interactions in Yttrium(III) Complexes Containing Nitronyl Nitroxide Radicals. Inorg. Chem. 2017, 56, 6788–6801. [Google Scholar] [CrossRef]
- Zapata-Rivera, J.; Calzado, C.J. Magnetostructural Relationships in [Ni(Dmit) 2]—Radical Anions. Dalton Trans. 2021, 50, 6620–6630. [Google Scholar] [CrossRef]
- Starikova, A.A.; Minkin, V.I. Adducts of Transition Metal Complexes with Redox-Active Ligands: The Structure and Spin-State-Switching Rearrangements. Russ. Chem. Rev. 2018, 87, 1049–1079. [Google Scholar] [CrossRef]
- Minkin, V.I.; Starikova, A.A. Molecular Design of the Valence Tautomeric Mixed-Ligand Adducts of CoII Diketonates with Redox-Active Ligands. Mendeleev Commun. 2015, 25, 83–92. [Google Scholar] [CrossRef]
- Kaim, W.; Paretzki, A. Interacting Metal and Ligand Based Open Shell Systems: Challenges for Experiment and Theory. Coord. Chem. Rev. 2017, 344, 345–354. [Google Scholar] [CrossRef]
- Ansari, A.; Ansari, M.; Singha, A.; Rajaraman, G. Interplay of Electronic Cooperativity and Exchange Coupling in Regulating the Reactivity of Diiron(IV)-Oxo Complexes towards C−H and O−H Bond Activation. Chem. Eur. J. 2017, 23, 10110–10125. [Google Scholar] [CrossRef]
- Chen, P.; Solomon, E.I. O2 Activation by Binuclear Cu Sites: Noncoupled versus Exchange Coupled Reaction Mechanisms. Proc. Natl. Acad. Sci. USA 2004, 101, 13105–13110. [Google Scholar] [CrossRef] [PubMed]
- Weldon, B.T.; Wheeler, D.E.; Kirby, J.P.; McCusker, J.K. Bimolecular Electron and Energy Transfer Reactivity of Exchange-Coupled Dinuclear Iron(III) Complexes. Inorg. Chem. 2001, 40, 6802–6812. [Google Scholar] [CrossRef]
- Davidson, R.A.; Hao, J.; Rheingold, A.L.; Miller, J.S. High Spin Ground State Copper(II) and Nickel(II) Complexes Possessing the 3,5-Di-Tert-Butyl-1,2-Semiquinonate Radical Anion. Polyhedron 2017, 133, 348–357. [Google Scholar] [CrossRef]
- Harmalker, S.; Jones, S.E.; Sawyer, D.T. Electrochemical and Spectroscopic Studies of 3,5-Di-Tert-Butylcatecholato and 3,5-Di-Tert-Butyl-o-Semiquinonato Complexes of Copper(II). Inorg. Chem. 1983, 22, 2790–2794. [Google Scholar] [CrossRef]
- Thompson, J.S.; Calabrese, J.C. Synthesis, Spectroscopy, and Structures of Copper(II)-3,5-Di-Tert-Butyl-o-Semiquinone Complexes. Inorg. Chem. 1985, 24, 3167–3171. [Google Scholar] [CrossRef]
- Kahn, O.; Prins, R.; Reedijk, J.; Thompson, J.S. Orbital Symmetries and Magnetic Interaction between Copper(II) Ions and the o-Semiquinone Radical. Magnetic Studies of (Di-2-Pyridylamine)(3,5-Di-Tert-Butyl-o-Semiquinonato)Copper(II) Perchlorate and Bis(Bis(3,5-Di-Tert-Butyl-o-Semiquinonato)Copper(II)). Inorg. Chem. 1987, 26, 3557–3561. [Google Scholar] [CrossRef]
- Dooley, D.M.; McGuirl, M.A.; Brown, D.E.; Turowski, P.N.; Mclntire, W.S.; Knowles, P.F. A Cu(I)-Semiquinone State in Substrate-Reduced Amine Oxidases. Nature 1991, 349, 262–264. [Google Scholar] [CrossRef]
- Rall, J.; Kaim, W. Ligand-Controlled Oxidation State Ambivalence in Copper–Quinone Complexes. Replacement of N-Donor by S-Donor Ligands Favours the Copper(I)–Semiquinone over the Copper(II)–Catecholate Form. J. Chem. Soc. Faraday Trans. 1994, 90, 2905–2908. [Google Scholar] [CrossRef]
- Caneschi, A.; Dei, A.; Lee, H.; Shultz, D.A.; Sorace, L. Ferromagnetically Coupled Bis(Semiquinone) Ligand Enforces High-Spin Ground States in Bis-Metal Complexes. Inorg. Chem. 2001, 40, 408–411. [Google Scholar] [CrossRef]
- Dei, A.; Gatteschi, D.; Pardi, L.; Barra, A.L.; Brunel, L.C. Millimeter Band EPR Spectra Reveal Large Zero-Field Splittings in Copper(II)—Semiquinonato Complexes. Chem. Phys. Lett. 1990, 175, 589–592. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Cherkasov, V.K.; Nevodchikov, V.I.; Kuropatov, V.A.; Yee, G.T.; Pierpont, C.G. Magnetic Properties and Redox Isomerism for 4,4‘-Bis(Semiquinone) Complexes of Copper. Inorg. Chem. 2001, 40, 2434–2436. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Verani, C.N.; Bill, E.; Bothe, E.; Weyhermüller, T.; Wieghardt, K. Electronic Structure of Bis(0-Iminobenzosemiquinonato)Metal Complexes (Cu, Ni, Pd). The Art of Establishing Physical Oxidation States in Transition-Metal Complexes Containing Radical Ligands. J. Am. Chem. Soc. 2001, 123, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.I.; Gorelik, E.V.; Fokin, S.V.; Romanenko, G.V.; Ikorskii, V.N.; Krashilina, A.V.; Cherkasov, V.K.; Abakumov, G.A. Ligand Effects on the Ferro- to Antiferromagnetic Exchange Ratio in Bis(o-Semiquinonato)Copper(II). J. Am. Chem. Soc. 2007, 129, 10512–10521. [Google Scholar] [CrossRef]
- Malrieu, J.P.; Caballol, R.; Calzado, C.J.; de Graaf, C.; Guihéry, N. Magnetic Interactions in Molecules and Highly Correlated Materials: Physical Content, Analytical Derivation, and Rigorous Extraction of Magnetic Hamiltonians. Chem. Rev. 2014, 114, 429–492. [Google Scholar] [CrossRef]
- Roemelt, M.; Krewald, V.; Pantazis, D.A. Exchange Coupling Interactions from the Density Matrix Renormalization Group and N -Electron Valence Perturbation Theory: Application to a Biomimetic Mixed-Valence Manganese Complex. J. Chem. Theory Comput. 2018, 14, 166–179. [Google Scholar] [CrossRef]
- Buchanan, J.K.; Dais, T.N.; Plieger, P.G. Computational Studies of the Magneto-Structural Correlations in a Manganese Dimer with Jahn–Teller Distortions. Phys. Chem. Chem. Phys. 2022, 24, 4407–4414. [Google Scholar] [CrossRef]
- Razquin-Bobillo, L.; Pajuelo-Corral, O.; Artetxe, B.; Zabala-Lekuona, A.; Choquesillo-Lazarte, D.; Rodríguez-Diéguez, A.; San Sebastian, E.; Cepeda, J. Combined Experimental and Theoretical Investigation on the Magnetic Properties Derived from the Coordination of 6-Methyl-2-Oxonicotinate to 3d-Metal Ions. Dalton Trans. 2022, 51, 9780–9792. [Google Scholar] [CrossRef]
- Witwicki, M.; Jerzykiewicz, M.; Jaszewski, A.R.; Jezierska, J.; Ozarowski, A. Influence of Pb(II) Ions on the EPR Properties of the Semiquinone Radicals of Humic Acids and Model Compounds: High Field EPR and Relativistic DFT Studies. J. Phys. Chem. A 2009, 113, 14115–14122. [Google Scholar] [CrossRef]
- Ciofini, I.; Reviakine, R.; Arbuznikov, A.; Kaupp, M. Solvent Effects on G-Tensors of Semiquinone Radical Anions: Polarizable Continuum versus Cluster Models. Theor. Chem. Acc. 2004, 111, 132–140. [Google Scholar] [CrossRef]
- Neese, F.; Ames, W.; Christian, G.; Kampa, M.; Liakos, D.G.; Pantazis, D.A.; Roemelt, M.; Surawatanawong, P.; Shengfa, Y.E. Dealing with Complexity in Open-Shell Transition Metal Chemistry from a Theoretical Perspective: Reaction Pathways, Bonding, Spectroscopy, And Magnetic Properties. Adv. Inorg. Chem. 2010, 62, 301–349. [Google Scholar]
- Witwicki, M. Theoretical Characterisation of Phosphinyl Radicals and Their Magnetic Properties: G Matrix. ChemPhysChem. 2015, 16, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Ashvar, C.S.; Chabalowski, C.F.; Frisch, M.J. Theoretical Calculation of Vibrational Circular Dichroism Spectra. Faraday Discuss 1994, 99, 103. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta–Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takahara, Y.; Fueno, T. Ab-Initio Molecular Orbital Studies of Structure and Reactivity of Transition Metal-OXO Compounds. In Applied Quantum Chemistry; Springer: Dordrecht, The Netherlands, 1986; pp. 155–184. [Google Scholar]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab Initio Computations of Effective Exchange Integrals for H–H, H–He–H and Mn2O2 Complex: Comparison of Broken-Symmetry Approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
- Neese, F. Definition of Corresponding Orbitals and the Diradical Character in Broken Symmetry DFT Calculations on Spin Coupled Systems. J. Phys. Chem. Solids 2004, 65, 781–785. [Google Scholar] [CrossRef]
- Neese, F. Importance of Direct Spin−Spin Coupling and Spin-Flip Excitations for the Zero-Field Splittings of Transition Metal Complexes: A Case Study. J. Am. Chem. Soc. 2006, 128, 10213–10222. [Google Scholar] [CrossRef]
- Pipek, J.; Mezey, P.G. A Fast Intrinsic Localization Procedure Applicable for ab initio and Semiempirical Linear Combination of Atomic Orbital Wave Functions. J. Chem. Phys. 1989, 90, 4916–4926. [Google Scholar] [CrossRef]
- Olsen, J. The CASSCF Method: A Perspective and Commentary. Int. J. Quantum Chem. 2011, 111, 3267–3272. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.-P. Introduction of N-Electron Valence States for Multireference Perturbation Theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.-P. N-Electron Valence State Perturbation Theory: A Fast Implementation of the Strongly Contracted Variant. Chem. Phys. Lett. 2001, 350, 297–305. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.-P. N-Electron Valence State Perturbation Theory: A Spinless Formulation and an Efficient Implementation of the Strongly Contracted and of the Partially Contracted Variants. J. Chem. Phys. 2002, 117, 9138–9153. [Google Scholar] [CrossRef]
- Miralles, J.; Castell, O.; Caballol, R.; Malrieu, J.-P. Specific CI Calculation of Energy Differences: Transition Energies and Bond Energies. Chem. Phys. 1993, 172, 33–43. [Google Scholar] [CrossRef]
- Miralles, J.; Daudey, J.-P.; Caballol, R. Variational Calculation of Small Energy Differences. The Singlet-Triplet Gap in [Cu2Cl6]2−. Chem. Phys. Lett. 1992, 198, 555–562. [Google Scholar] [CrossRef]
- Calzado, C.J.; Sanz, J.F.; Malrieu, J.P. Accurate Ab Initio Determination of Magnetic Interactions and Hopping Integrals in La2−xSrxCuO4 Systems. J. Chem. Phys. 2000, 112, 5158–5167. [Google Scholar] [CrossRef]
- Vancoillie, S.; Chalupský, J.; Ryde, U.; Solomon, E.I.; Pierloot, K.; Neese, F.; Rulíšek, L. Multireference Ab Initio Calculations of g Tensors for Trinuclear Copper Clusters in Multicopper Oxidases. J. Phys. Chem. B 2010, 114, 7692–7702. [Google Scholar] [CrossRef]
- Lunghi, A.; Totti, F. The Role of Anisotropic Exchange in Single Molecule Magnets: A CASSCF/NEVPT2 Study of the Fe4 SMM Building Block [Fe2(OCH3)2(Dbm)4] Dimer. Inorganics 2016, 4, 28. [Google Scholar] [CrossRef]
- Bajaj, A.; Ali, M.E. First-Principle Design of Blatter’s Diradicals with Strong Ferromagnetic Exchange Interactions. J. Phys. Chem. C 2019, 123, 15186–15194. [Google Scholar] [CrossRef]
- Zapata-Rivera, J.; Calzado, C. Light-Induced Control of the Spin Distribution on Cu–Dithiolene Complexes: A Correlated Ab Initio Study. Molecules 2019, 24, 1088. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree–Fock and Hybrid DFT Calculations. A ‘Chain-of-Spheres’ Algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Weigend, F.; Kattannek, M.; Ahlrichs, R. Approximated Electron Repulsion Integrals: Cholesky Decomposition versus Resolution of the Identity Methods. J. Chem. Phys. 2009, 130, 164106. [Google Scholar] [CrossRef]
- Kollmar, C.; Sivalingam, K.; Helmich-Paris, B.; Angeli, C.; Neese, F. A Perturbation-based Super-CI Approach for the Orbital Optimization of a CASSCF Wave Function. J. Comput. Chem. 2019, 40, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Hartree–Fock Exchange Fitting Basis Sets for H to Rn. J. Comput. Chem. 2008, 29, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. A Spectroscopy Oriented Configuration Interaction Procedure. J. Chem. Phys. 2003, 119, 9428–9443. [Google Scholar] [CrossRef]
- Neese, F. Sum-over-States Based Multireferenceab Initio Calculation of EPR Spin Hamiltonian Parameters for Transition Metal Complexes. A Case Study. Magn. Reson. Chem. 2004, 42, S187–S198. [Google Scholar] [CrossRef]
- Allouche, A.-R. Gabedit-A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).