Conformational Dynamics of Phytoglobin BvPgb1.2 from Beta vulgaris ssp. vulgaris
Abstract
1. Introduction
2. Results and Discussion
2.1. NMR Spectroscopy Provides Evidence for Dimerization of CN-BvPgb1.2
2.2. 15N Relaxation Confirms Dimerization and Identifies Flexible Regions
3. Materials and Methods
3.1. Protein Expression and Purification
3.1.1. Construction and Transformation of Expression Constructs
3.1.2. Expression of Triple-Labeled Protein in Minimal Media
3.1.3. Cell Harvesting and Protein Extraction
3.1.4. Protein Purification
3.2. Protein Sample Preparation
3.3. NMR Spectroscopy and Data Analysis
3.3.1. NMR Sample Preparation
3.3.2. Triple-Resonance NMR Experiments and Data Analysis for Backbone Assignment
3.3.3. 15N NMR Relaxation Experiments and Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, F.B.; Fago, A.; Weber, R. Hemoglobin Structure and Function. In Fish Phys; Perry, S., Tufts, B., Eds.; Academic Press: Cambridge, MA, USA, 1998; pp. 1–40. [Google Scholar]
- Vinogradov, S.N.; Moens, L. Diversity of globin function: Enzymatic, transport, storage, and sensing. J. Biol. Chem. 2008, 283, 8773–8777. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Eriksson, N.; Pin, P.A.; Kraft, T.; Dohm, J.C.; Minoche, A.E.; Himmelbauer, H.; Bülow, L. Differential expression patterns of non-symbiotic hemoglobins in sugar beet (Beta vulgaris ssp. vulgaris). Plant Cell Physiol. 2014, 55, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Puscas, C.; Miclea, P.; Naumova-Letia, G.; Dorneanu, S.; Podar, D.; Dissmeyer, N.; Silaghi-Dumitrescu, R. Redox control and autoxidation of class 1, 2 and 3 phytoglobins from Arabidopsis thaliana. Sci. Rep. 2018, 8, 13714. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J.; Rodríguez-Gacio, M.D.C. Non-symbiotic hemoglobins in the life of seeds. Phytochem 2013, 87, 7–15. [Google Scholar] [CrossRef]
- Athwal, N.S.; Alagurajan, J.; Andreotti, A.H.; Hargrove, M.S. Role of Reversible Histidine Coordination in Hydroxylamine Reduction by Plant Hemoglobins (Phytoglobins). Biochemistry 2016, 55, 5809–5817. [Google Scholar] [CrossRef]
- Garrocho-Villegas, V.; Gopalasubramaniam, S.; Arredondo-Peter, R. Plant hemoglobins: What we know six decades after their discovery. Gene 2007, 398, 78–85. [Google Scholar] [CrossRef]
- Hill, R.; Hargrove, M.; Arredondo-Peter, R. Phytoglobin: A novel nomenclature for plant globins accepted by the globin community at the 2014 XVIII conference on Oxygen-Binding and Sensing Proteins. F1000Research 2016, 5, 212. [Google Scholar] [CrossRef]
- Taylor, E.R.; Nie, X.Z.; MacGregor, A.W.; Hill, R.D. A cereal haemoglobin gene is expressed in seed and root tissues under anaerobic conditions. Plant Mol. Biol. 1994, 24, 853–862. [Google Scholar] [CrossRef]
- Dordas, C.; Hasinoff, B.B.; Igamberdiev, A.U.; Manac’H, N.; Rivoal, J.; Hill, R.D. Expression of a stress-induced hemolobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003, 35, 763–770. [Google Scholar] [CrossRef]
- Athwal, N.S.; Alagurajan, J.; Sturms, R.; Fulton, D.B.; Andreotti, A.H.; Hargrove, M.S. Electron self-exchange in hemoglobins revealed by deutero-hemin substitution. J. Inorg. Biochem. 2015, 150, 139–147. [Google Scholar] [CrossRef]
- Ohwaki, Y.; Kawagishi-Kobayashi, M.; Wakasa, K.; Fujihara, S.; Yoneyama, T. Induction of Class-1 Non-symbiotic Hemoglobin Genes by Nitrate, Nitrite and Nitric Oxide in Cultured Rice Cells. Plant Cell Physiol. 2005, 46, 324–331. [Google Scholar] [CrossRef]
- Eriksson, N.L.; Reeder, B.J.; Wilson, M.T.; Bülow, L. Sugar beet hemoglobins: Reactions with nitric oxide and nitrite reveal differential roles for nitrogen metabolism. Biochem. J. 2019, 476, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.D. Non-symbiotic haemoglobins—What’s happening beyond nitric oxide scavenging? AoB Plants 2012, 2012, Pls004. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hill, R.D.; Wally, O.S.; Dionisio, G.; Ayele, B.T.; Jami, S.K.; Stasolla, C. Hemoglobin Control of Cell Survival/Death Decision Regulates in Vitro Plant Embryogenesis. Plant Physiol. 2014, 165, 810–825. [Google Scholar] [CrossRef]
- Gupta, K.J.; Hebelstrup, K.H.; Mur, L.A.; Igamberdiev, A.U. Plant hemoglobins: Important players at the crossroads between oxygen and nitric oxide. FEBS Lett. 2011, 585, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B.; Watts, R.A.; Andersson, C.R.; Llewellyn, D.J.; Hargrove, M.S.; Olson, J.S.; Dennis, E.S.; Peacock, W.J. Two hemoglobin genes in Arabidopsis thaliana: The evolutionary origins of leghemoglobins. Proc. Natl. Acad. Sci. USA 1997, 94, 12230–12234. [Google Scholar] [CrossRef]
- Hoy, J.A.; Hargrove, M.S. The structure and function of plant hemoglobins. Plant Physiol. Biochem. 2008, 46, 371–379. [Google Scholar] [CrossRef]
- Gisbert, C.; Timoneda, A.; Porcel, R.; Ros, R.; Mulet, J.M. Overexpression of BvHb2, a Class 2 Non-Symbiotic Hemoglobin from Sugar Beet, Confers Drought-Induced Withering Resistance and Alters Iron Content in Tomato. Agronomy 2020, 10, 1754. [Google Scholar] [CrossRef]
- Ivarson, E.; Leiva-Eriksson, N.; Ahlman, A.; Kanagarajan, S.; Bülow, L.; Zhu, L.-H. Effects of Overexpression of WRI1 and Hemoglobin Genes on the Seed Oil Content of Lepidium campestre. Front. Plant Sci. 2017, 7, 2032. [Google Scholar] [CrossRef]
- Holmberg, N.; Lilius, G.; Bailey, J.E.; Bülow, L. Transgenic tobacco expressing Vitreoscilla hemoglobin exhibits enhanced growth and altered metabolite production. Nature Biotechnol. 1997, 15, 244–247. [Google Scholar] [CrossRef]
- Bülow, L. The metabolic effects of native and transgenic hemoglobins on plants. Trends Biotechnol. 1999, 17, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Groth, L.; Leiva-Eriksson, N.; Nyblom, M.; Bülow, L. Oxidative Implications of Substituting a Conserved Cysteine Residue in Sugar Beet Phytoglobin BvPgb 1.2. Antioxidants 2022, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Marion, D. An introduction to biological NMR spectroscopy. Mol. Cell. Proteom. 2013, 12, 3006–3025. [Google Scholar] [CrossRef] [PubMed]
- Bax, A. Multidimensional nuclear magnetic resonance methods for protein studies. Curr. Opin. Struct. Biol. 1994, 4, 738–744. [Google Scholar] [CrossRef]
- Bax, A.; Grzesiek, S. Methodological advances in protein NMR. Acc. Chem. Res. 1993, 26, 131–138. [Google Scholar] [CrossRef]
- Kay, L.E.; Gardner, K. Solution NMR spectroscopy beyond 25 kDa. Curr. Opin. Struct. Biol. 1997, 7, 722–731. [Google Scholar] [CrossRef]
- Clore, G.M.; Gronenborn, A. Determining the structures of large proteins and protein complexes by NMR. Trends Biotechnol. 1998, 16, 22–34. [Google Scholar] [CrossRef]
- Wüthrich, K.; Hochmann, J.; Keller, R.; Wagner, G.; Brunori, M.; Giacometti, C. 1H NMR relaxation in high-spin ferrous hemoproteins. J. Magn. Reson. 1975, 19, 111–113. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Marconi, S.; Pierattelli, R. 1H-NMR study of reduced heme proteins myoglobin and cytochrome P450. Eur. J. Biochem. 1993, 215, 431–437. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, M.; Gudanis, D.; Gracz, H.; Findenegg, G.H.; Gdaniec, Z.; Franzen, S. Dynamics of dehaloperoxidase-hemoglobin A derived from NMR relaxation spectroscopy and molecular dynamics simulation. J. Inorg. Biochem. 2018, 181, 65–73. [Google Scholar] [CrossRef]
- Laine, J.M.; Amat, M.; Morgan, B.R.; Royer, J.W.E.; Massi, F. Insight into the Allosteric Mechanism of Scapharca Dimeric Hemoglobin. Biochemistry 2014, 53, 7199–7210. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, M.S.; Brucker, E.A.; Stec, B.; Sarath, G.; Arredondo-Peter, R.; Klucas, R.V.; Olson, J.S.; Phillips, G.N. Crystal structure of a nonsymbiotic plant hemoglobin. Structure 2000, 8, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Sturms, R.; Kakar, S.; Trent, J., III; Hargrove, M.S. Trema and Parasponia Hemoglobins Reveal Convergent Evolution of Oxygen Transport in Plants. Biochemistry 2010, 49, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.; Sturms, R.; Tiffany, A.; Nix, J.C.; DiSpirito, A.A.; Hargrove, M.S. Crystal structures of Parasponia and Trema hemoglobins: Differential heme coordination is linked to quaternary structure. Biochemistry 2011, 50, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Astegno, A.; Conter, C.; Bertoldi, M.; Dominici, P. Structural Insights into the Heme Pocket and Oligomeric State of Non-Symbiotic Hemoglobins from Arabidopsis thaliana. Biomolecules 2020, 10, 1615. [Google Scholar] [CrossRef]
- Savard, P.-Y.; Daigle, R.; Morin, S.; Sebilo, A.; Meindre, F.; Lagüe, P.; Guertin, M.; Gagné, S.M. Structure and Dynamics of Mycobacterium tuberculosis Truncated Hemoglobin N: Insights from NMR Spectroscopy and Molecular Dynamics Simulations. Biochemistry 2011, 50, 11121–11130. [Google Scholar] [CrossRef]
- Song, X.J.; Yuan, Y.; Simplaceanu, V.; Sahu, S.C.; Ho, N.T.; Ho, C. A Comparative NMR Study of the Polypeptide Backbone Dynamics of Hemoglobin in the Deoxy and Carbonmonoxy Forms. Biochemistry 2007, 46, 6795–6803. [Google Scholar] [CrossRef]
- Volkman, B.F.; Alam, S.L.; Satterlee, J.D.; Markley, J.L. Solution Structure and Backbone Dynamics of Component IV Glycera dibranchiata Monomeric Hemoglobin−CO. Biochemistry 1998, 37, 10906–10919. [Google Scholar] [CrossRef]
- Fushman, D.; Varadan, R.; Assfalg, M.; Walker, O. Determining domain orientation in macromolecules by using spin-relaxation and residual dipolar coupling measurements. Prog. Nucl. Magn. Reson. Spectrosc. 2004, 44, 189–214. [Google Scholar] [CrossRef]
- Wang, D.; Kreutzer, U.; Chung, Y.; Jue, T. Myoglobin and hemoglobin rotational diffusion in the cell. Biophys. J. 1997, 73, 2764–2770. [Google Scholar] [CrossRef]
- Mukhi, N.; Dhindwal, S.; Uppal, S.; Kumar, P.; Kaur, J.; Kundu, S. X-Ray crystallographic structural characteristics of Arabidopsis hemoglobin I and their functional implications. Biochim Biophys Acta Proteins and Proteomics. 2013, 1834, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Silkstone, R.S.; Silkstone, G.; Baath, J.A.; Rajagopal, B.; Nicholls, P.; Reeder, B.J.; Ronda, L.; Bulow, L.; Cooper, C.E. The βLys66Tyr Variant of Human Hemoglobin as a Component of a Blood Substitute. In Oxygen Transport to Tissue XXXVII; Springer: New York, NY, USA, 2016; Volume 876, pp. 455–460. [Google Scholar]
- Reeder, B.J.; Grey, M.; Silaghi-Dumitrescu, R.-L.; Svistunenko, D.A.; Bülow, L.; Cooper, C.E.; Wilson, M.T. Tyrosine residues as redox cofactors in human hemoglobin: Implications for engineering nontoxic blood substitutes. J. Biol. Chem. 2008, 283, 30780–30787. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.; Ahrné, E.; Goldie, K.N.; Schmidt, A.; Grzesiek, S. Deuterium induces a distinctive Escherichia coli proteome that correlates with the reduction in growth rate. J. Biol. Chem. 2018, 294, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Jaravine, V.A.; Orekhov, V.Y. Targeted acquisition for real-time NMR spectroscopy. J. Am. Chem. Soc. 2006, 128, 13421–13426. [Google Scholar] [CrossRef]
- Kazimierczuk, K.; Orekhov, V.Y. Accelerated NMR spectroscopy by using compressed sensing. Angew. Chem. Int. Ed. 2011, 50, 5556–5559. [Google Scholar] [CrossRef]
- Mayzel, M.; Rosenlöw, J.; Isaksson, L.; Orekhov, V.Y. Time-resolved multidimensional NMR with non-uniform sampling. J. Biomol. NMR 2014, 58, 129–139. [Google Scholar] [CrossRef]
- Vranken, W.; Boucher, W.; Stevens, T.J.; Fogh, R.H.; Pajon, A.; Llinas, M.; Ulrich, E.L.; Markley, J.L.; Ionides, J.; Laue, E.D. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins: Struct. Funct. Bioinform. 2005, 59, 687–696. [Google Scholar] [CrossRef]
- Zhu, G.; Xia, Y.; Nicholson, L.K.; Sze, K.H. Protein dynamics measurements by TROSY-based NMR experiments. J. Magn. Reson. 2000, 143, 423–426. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biol. NMR. 1995, 6, 277–293. [Google Scholar] [CrossRef]
- Ahlner, A.; Carlsson, M.; Jonsson, B.-H.; Lundström, P. PINT: A software for integration of peak volumes and extraction of relaxation rates. J. Biol. NMR. 2013, 56, 191–202. [Google Scholar] [CrossRef]
- De la Torre, J.G.; Huertas, M.; Carrasco, B. HYDRONMR: Prediction of NMR relaxation of globular proteins from atomic-level structures and hydrodynamic calculations. J. Magn. Reson. 2000, 147, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, M.W.; Schuyler, A.D.; Gryk, M.R.; Moraru, I.I.; Romero, P.R.; Ulrich, E.L.; Eghbalnia, H.R.; Livny, M.; Delaglio, F.; Hoch, J.C. NMRbox: A Resource for Biomolecular NMR Computation. Biophys. J. 2017, 112, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.; Varadan, R.; Fushman, D. Fushman, Efficient and accurate determination of the overall rotational diffusion tensor of a molecule from 15N relaxation data using computer program ROTDIF. J. Magn. Reson. 2004, 168, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Berlin, K.; Longhini, A.; Dayie, T.K.; Fushman, D. Deriving quantitative dynamics information for proteins and RNAs using ROTDIF with a graphical user interface. J. Biol. NMR. 2013, 57, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comp. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]

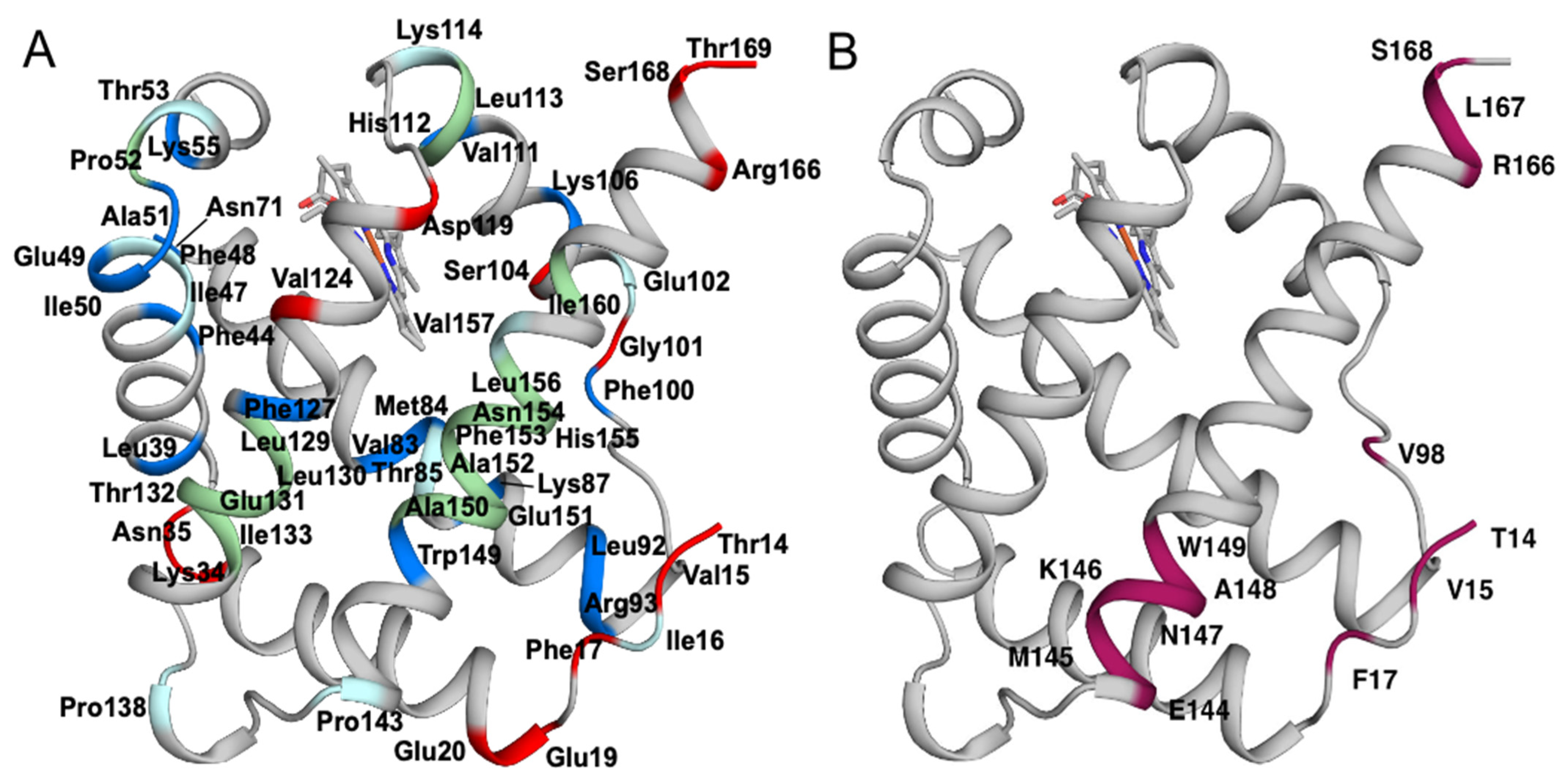

| Partial assignments | N5 P9 I16 I47 F48 T53 P67 T85 E102 K114 P138 P143 V157 |
| Missing assignments | M1 S2 F3 T4 P52 H112 L113 L129 L130 E131 T132 I133 A150 E151 A152 F153 N154 H155 L156 I160 P171 |
| Resides with more than one conformation | V6 N7 Y8 A10 S11 D12 G13 T14 V15 F17 V98 E144 M145 K146 N147 A148 W149 R166 L167 S168 |
| τc (ns) | Anisotropy | Rhombicity | |
|---|---|---|---|
| ROTDIF | 22.8 | 0.84 | 0.63 |
| Monomer (pdbID: 7ZOS) | 11.3 | 0.83 | 0.40 |
| Dimer (pdbID: 7Z1U) | 28.9 | 1.44 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, S.; Stenström, O.; Akke, M.; Bülow, L. Conformational Dynamics of Phytoglobin BvPgb1.2 from Beta vulgaris ssp. vulgaris. Int. J. Mol. Sci. 2023, 24, 3973. https://doi.org/10.3390/ijms24043973
Christensen S, Stenström O, Akke M, Bülow L. Conformational Dynamics of Phytoglobin BvPgb1.2 from Beta vulgaris ssp. vulgaris. International Journal of Molecular Sciences. 2023; 24(4):3973. https://doi.org/10.3390/ijms24043973
Chicago/Turabian StyleChristensen, Simon, Olof Stenström, Mikael Akke, and Leif Bülow. 2023. "Conformational Dynamics of Phytoglobin BvPgb1.2 from Beta vulgaris ssp. vulgaris" International Journal of Molecular Sciences 24, no. 4: 3973. https://doi.org/10.3390/ijms24043973
APA StyleChristensen, S., Stenström, O., Akke, M., & Bülow, L. (2023). Conformational Dynamics of Phytoglobin BvPgb1.2 from Beta vulgaris ssp. vulgaris. International Journal of Molecular Sciences, 24(4), 3973. https://doi.org/10.3390/ijms24043973






