The Role of PKGIα and AMPK Signaling Interplay in the Regulation of Albumin Permeability in Cultured Rat Podocytes
Abstract
1. Introduction
2. Results
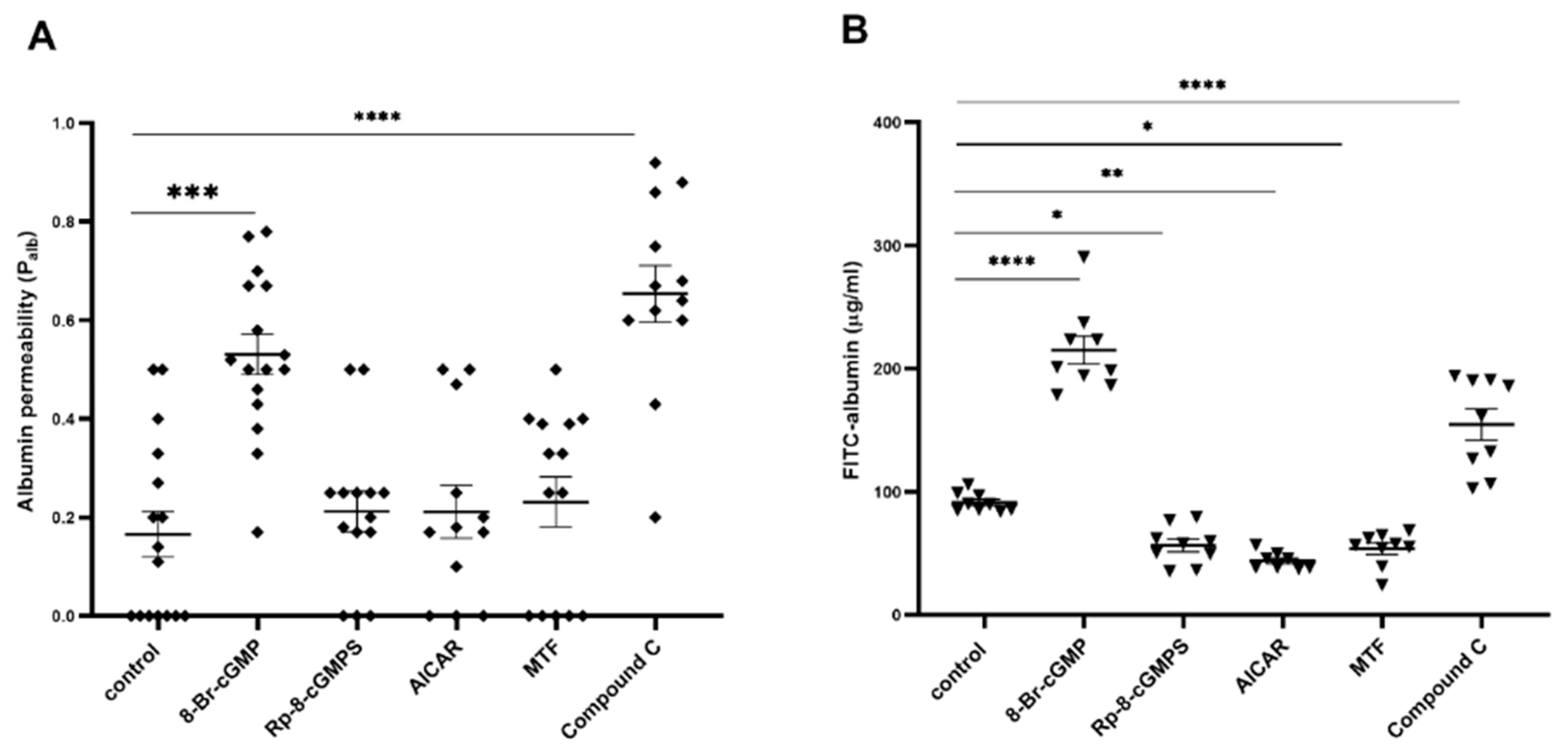
2.1. Role of PKGIα and AMPK in the Regulation of Filtration Barrier Permeability
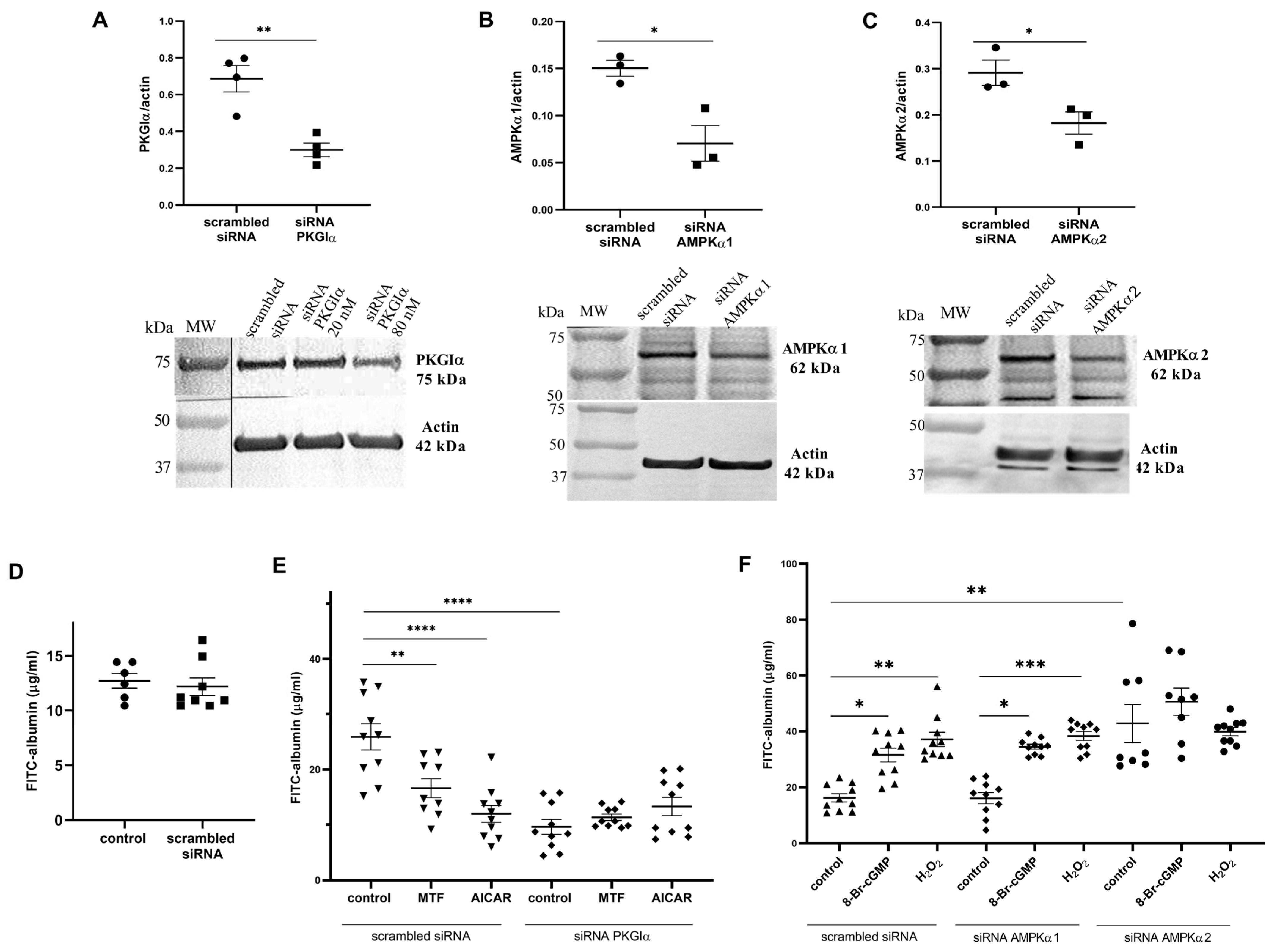
2.2. Downregulation of AMPK and PKGIα Affects Albumin Permeability across the Podocyte Monolayer
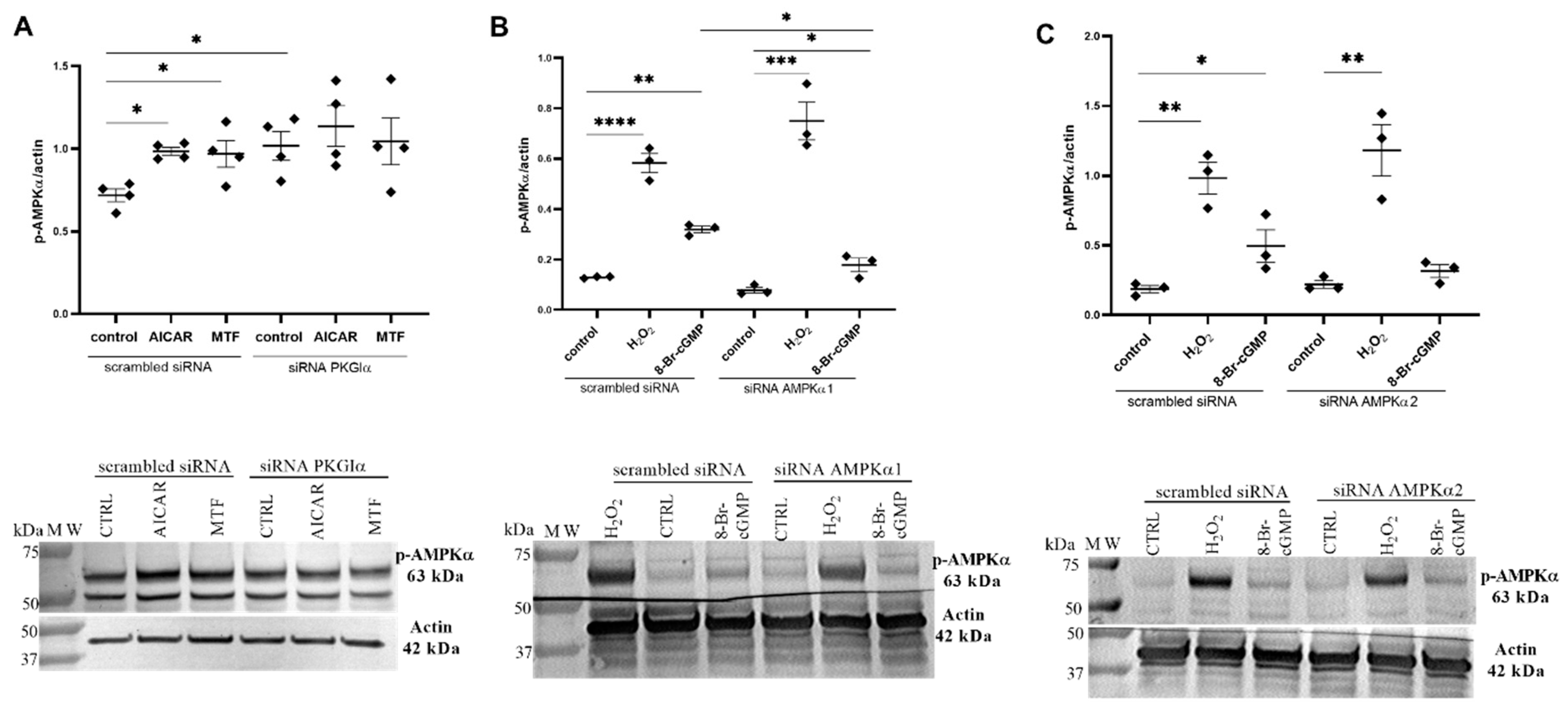
2.3. PKGIα Affects AMPK Activity in Podocytes
2.4. AMPKα Interacts with PKGIα in Cultured Rat Podocytes
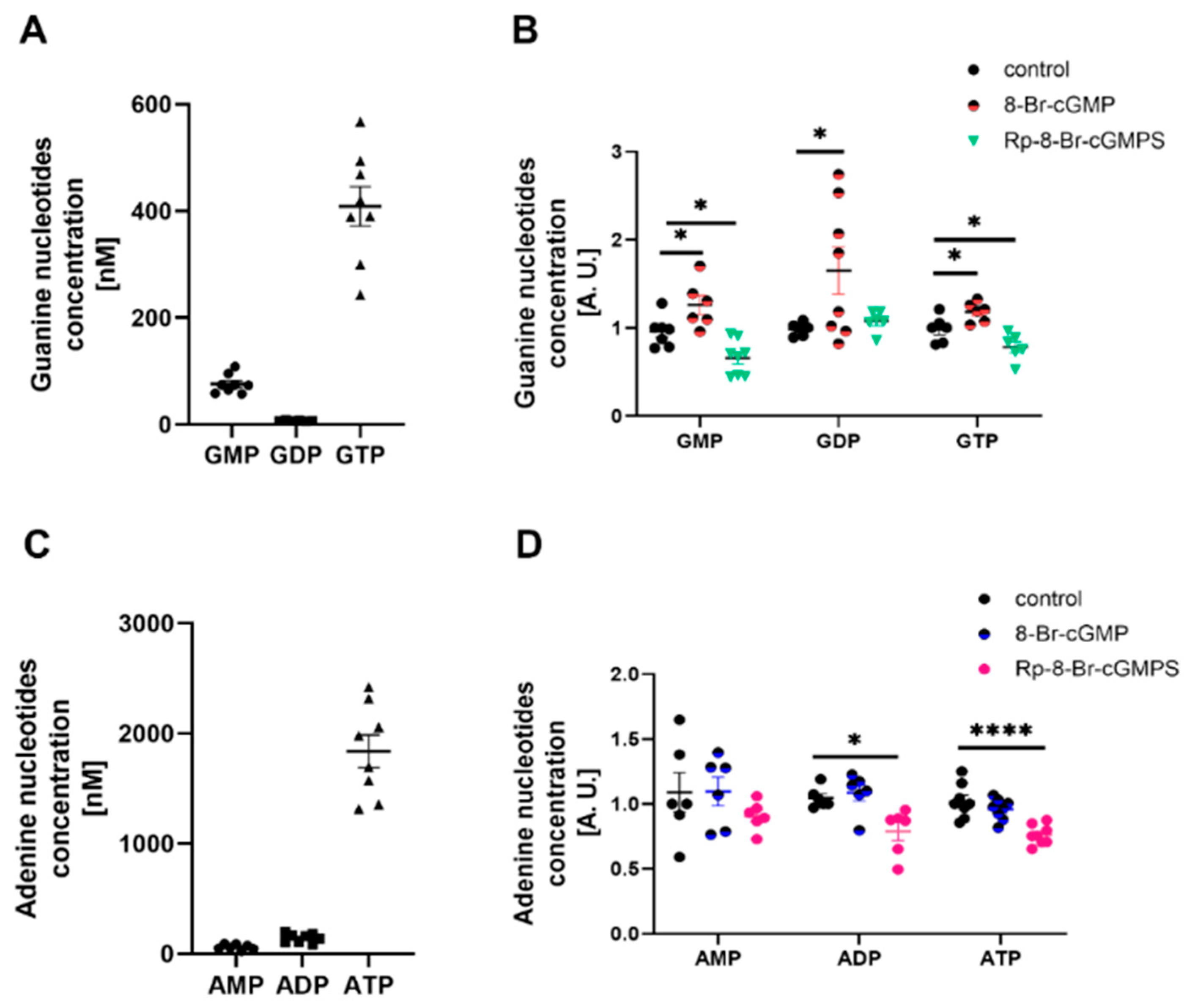
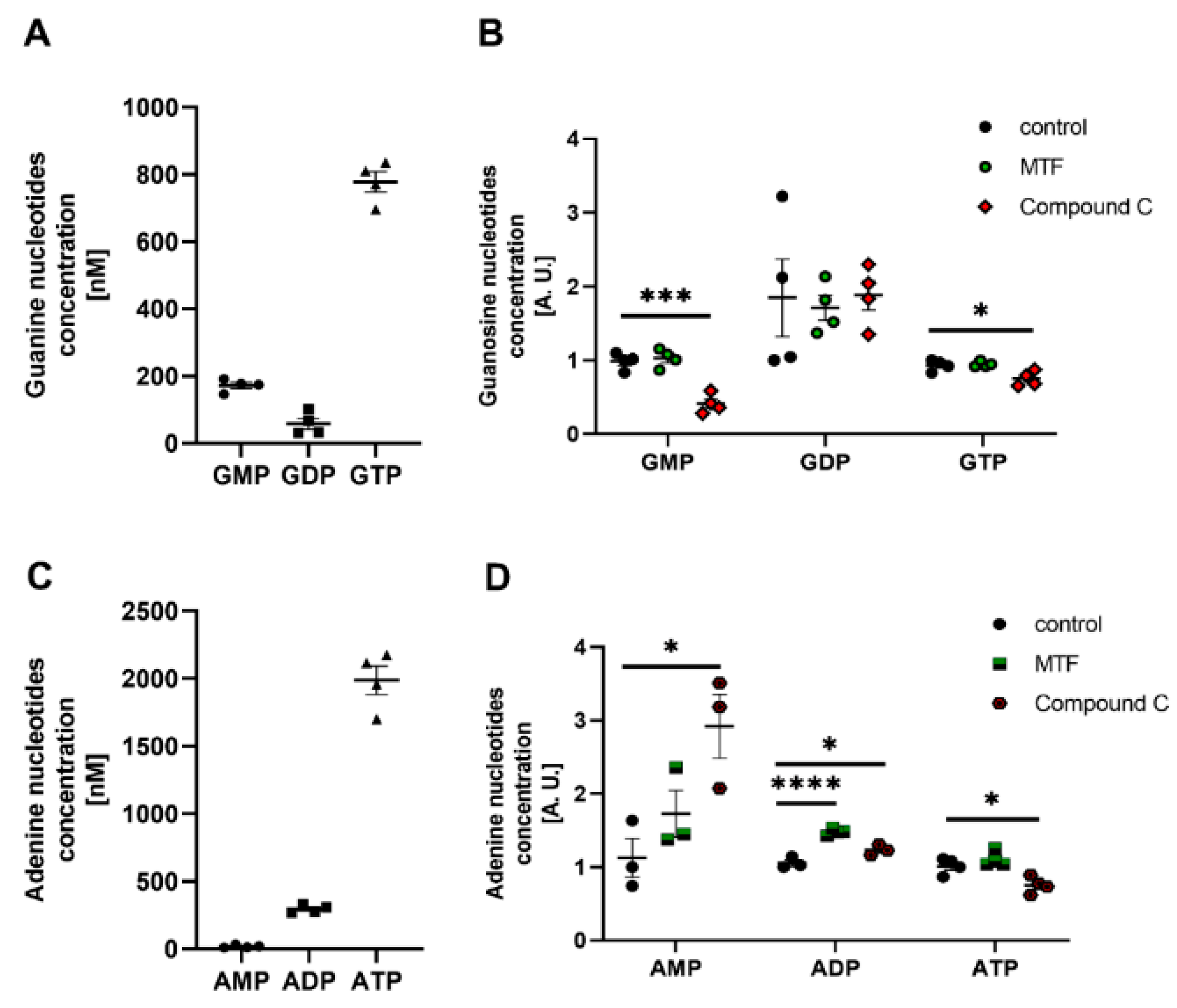
2.5. PKG and AMPK Modulators Affect Nucleotide Concentrations in Cultured Rat Podocytes
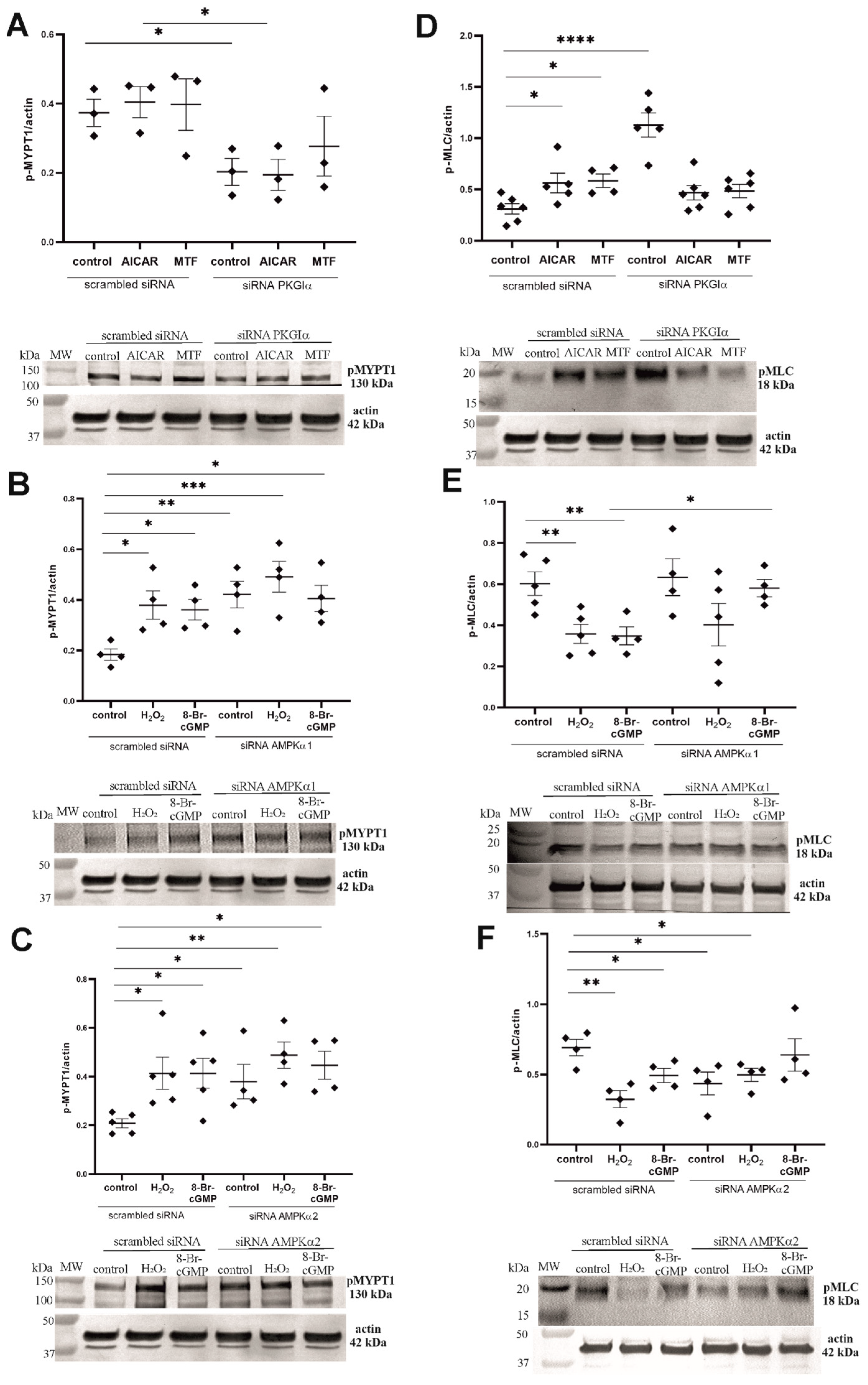
2.6. PKGIα and AMPK Mutually Regulate the Contractile Apparatus in Podocytes
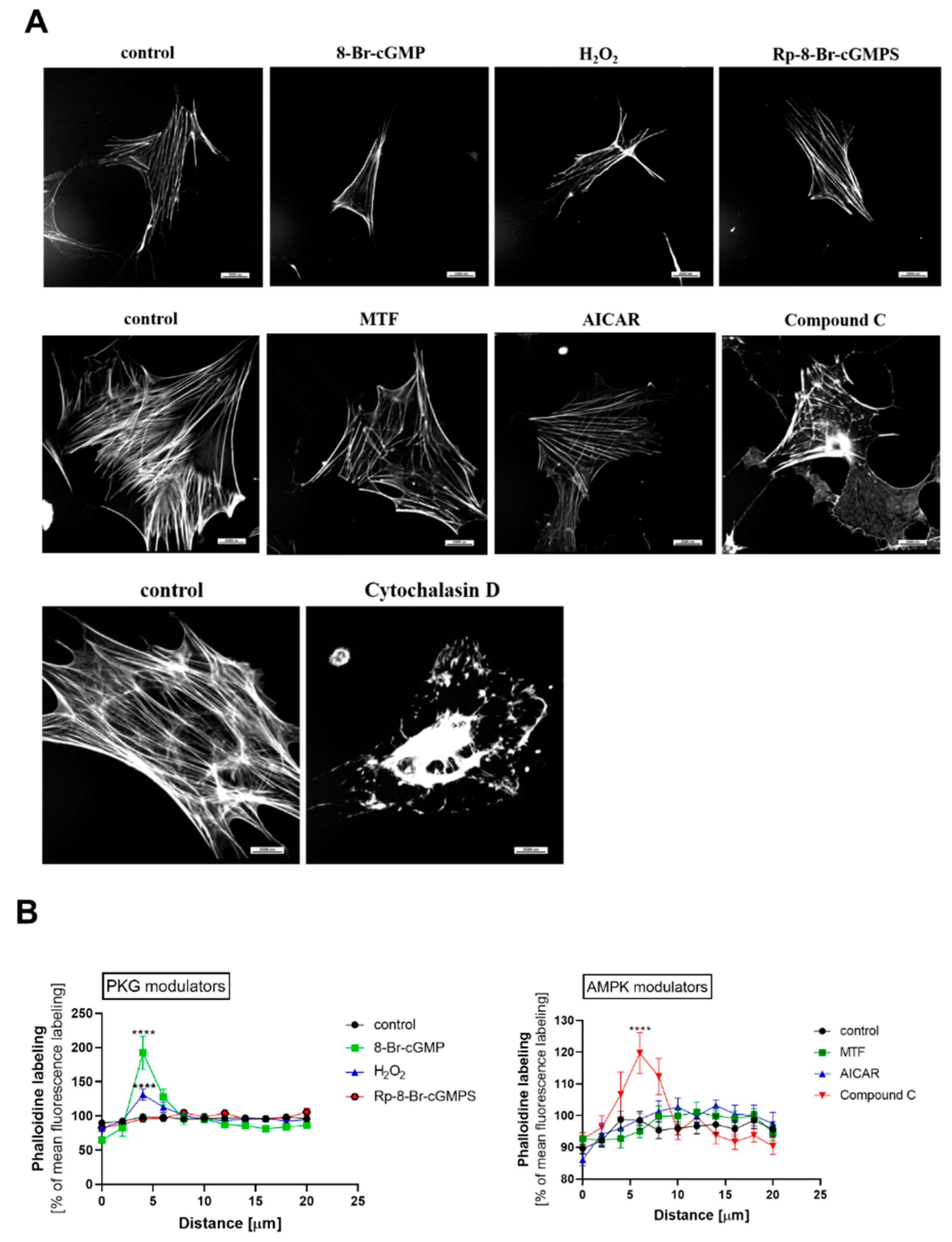
2.7. PKGIα and AMPK Affect Actin Cytoskeleton Architecture in an Antagonistic Manner
3. Discussion
4. Materials and Methods
4.1. Preparation and Culture of Rat Podocytes
4.2. Western Blot
4.3. Immunoprecipitation
4.4. siRNA Transfection
4.5. Immunofluorescence
4.6. Permeability Assay
4.7. Isolation of Rat Glomeruli
4.8. Glomerular Permeability to Albumin In Vitro
4.9. Extraction of Nucleotides and High-Performance Liquid Chromatography
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menon, M.C.; Chuang, P.Y.; He, C.J. The glomerular filtration barrier: Components and crosstalk. Int. J. Nephrol. 2012, 2012, 749010. [Google Scholar] [CrossRef] [PubMed]
- Pavenstädt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Pavenstädt, H. Roles of the podocyte in glomerular function. Am. J. Physiol. Ren. Physiol. 2000, 278, F173–F179. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Dylewski, J. Regulation of the actin cytoskeleton in podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef]
- Surks, H.K. CGMP-dependent protein kinase I and smooth muscle relaxation. Circ. Res. 2007, 101, 1078–1080. [Google Scholar] [CrossRef]
- Hofmann, F. The biology of cyclic GMP-dependent protein kinases. J. Biol. Chem. 2005, 280, 1–4. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Madhani, M.; Cuello, F.; Charles, R.L.; Brennan, J.P.; Schröder, E.; Browning, D.D.; Eaton, P. Cysteine redox sensor in PKGIα enables oxidant-induced activation. Science 2007, 317, 1393–1397. [Google Scholar] [CrossRef]
- Butt, E.; Geiger, J.; Jarchau, T.; Lohmann, S.M.; Walter, U. The cGMP-dependent protein kinase: Gene, protein, and function. Neurochem. Res. 1993, 18, 27–42. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Mongue-Din, H.; Eaton, P.; Shah, A.M. Redox signaling in cardiac physiology and pathology. Circ. Res. 2012, 111, 1091–1106. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Kasztan, M.; Angielski, S.; Jankowski, M. Insulin increases glomerular filtration barrier permeability through dimerization of protein kinase G type Iα subunits. Biochim. Biophys. Acta 2013, 1832, 791–804. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Jankowski, M.; Kocbuch, K.; Angielski, S. Hydrogen peroxide induces dimerization of protein kinase G type Iα subunits and increases albumin permeability in cultured rat podocytes. J. Cell. Physiol. 2012, 227, 1004–1016. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Angielski, S.; Jankowski, M. High glucose increases glomerular filtration barrier permeability by activating protein kinase G type Iα subunits in a Nox4-dependent manner. Exp. Cell Res. 2014, 320, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Kasztan, M.; Angielski, S.; Jankowski, M. Intracellular calcium signaling regulates glomerular filtration barrier permeability: The role of the PKGIα-dependent pathway. FEBS Lett. 2016, 590, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Rogacka, D.; Audzeyenka, I.; Rachubik, P.; Rychłowski, M.; Kasztan, M.; Jankowski, M.; Angielski, S.; Piwkowska, A. Insulin increases filtration barrier permeability via TRPC6-dependent activation of PKGIα signaling pathways. Biochim. Biophys. Acta 2017, 1863, 1312–1325. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Rajamohan, F.; Reyes, A.R.; Frisbie, R.K.; Hoth, L.R.; Sahasrabudhe, P.; Magyar, R.; Landro, J.A.; Withka, J.M.; Caspers, N.L.; Calabrese, M.F.; et al. Probing the enzyme kinetics, allosteric modulation and activation of α1- and α2-subunit-containing AMP-activated protein kinase (AMPK) heterotrimeric complexes by pharmacological and physiological activators. Biochem. J. 2016, 473, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Mayer, F.V.; Sanders, M.J.; Gamblin, S.J. AMP-activated protein kinase: Nature’s energy sensor. Nat. Chem. Biol. 2011, 7, 512–518. [Google Scholar] [CrossRef]
- Willows, R.; Sanders, M.J.; Xiao, B.; Patel, B.R.; Martin, S.R.; Read, J.; Wilson, J.R.; Hubbard, J.; Gamblin, S.J.; Carling, D. Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem. J. 2017, 474, 3059–3073. [Google Scholar] [CrossRef]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef]
- Hurley, R.L.; Anderson, K.A.; Franzone, J.M.; Kemp, B.E.; Means, A.R.; Witters, L.A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005, 280, 29060–29066. [Google Scholar] [CrossRef]
- Rachubik, P.; Szrejder, M.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Angielski, S.; Piwkowska, A. The TRPC6-AMPK pathway is involved in insulin-dependent cytoskeleton reorganization and glucose uptake in cultured rat podocytes. Cell. Physiol. Biochem. 2018, 51, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signaling pathway coordinates cell growth, autophagy, and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, S.; Caridi, B.; Karatsai, E.; Burgoyne, T.; Sarker, M.H.; Turowski, P. AMP-activated protein kinase is a key regulator of acute neurovascular permeability. J. Cell Sci. 2021, 134, jcs253179. [Google Scholar] [CrossRef]
- Schubert, K.M.; Qiu, J.; Blodow, S.; Wiedenmann, M.; Lubomirov, L.T.; Pfitzer, G.; Pohl, U.; Schneider, H. The AMP-related kinase (AMPK) induces Ca2+-independent dilation of resistance arteries by interfering with actin filament formation. Circ. Res. 2017, 121, 149–161. [Google Scholar] [CrossRef]
- Bultot, L.; Horman, S.; Neumann, D.; Walsh, M.P.; Hue, L.; Rider, M.H. Myosin light chains are not a physiological substrate of AMPK in the control of cell structure changes. FEBS Lett. 2009, 583, 25–28. [Google Scholar] [CrossRef]
- Rogacka, D.; Piwkowska, A.; Audzeyenka, I.; Angielski, S.; Jankowski, M. Involvement of the AMPK-PTEN pathway in insulin resistance induced by high glucose in cultured rat podocytes. Int. J. Biochem. Cell Biol. 2014, 51, 120–130. [Google Scholar] [CrossRef]
- Rogacka, D.; Piwkowska, A.; Audzeyenka, I.; Angielski, S.; Jankowski, M. SIRT1-AMPK crosstalk is involved in high glucose-dependent impairment of insulin responsiveness in primary rat podocytes. Exp. Cell Res. 2016, 349, 328–338. [Google Scholar] [CrossRef]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta 2020, 1866, 165610. [Google Scholar] [CrossRef]
- Cao, X.; Luo, T.; Luo, X.; Tang, Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 2014, 37, 803–810. [Google Scholar] [CrossRef]
- Rachubik, P.; Szrejder, M.; Audzeyenka, I.; Rogacka, D.; Rychłowski, M.; Angielski, S.; Piwkowska, A. The PKGIα/VASP pathway is involved in insulin- and high glucose-dependent regulation of albumin permeability in cultured rat podocytes. J. Biochem. 2020, 168, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, Q.; Coughlan, K.; Viollet, B.; Moriasi, C.; Zou, M.H. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am. J. Pathol. 2013, 182, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Huang, Q.; Yuan, Y.; Granger, H.J. VEGF induces NO-dependent hyperpermeability in coronary venules. Am. J. Physiol. 1996, 271, H2735–H2739. [Google Scholar] [CrossRef] [PubMed]
- Rudyk, O.; Phinikaridou, A.; Prysyazhna, O.; Burgoyne, J.R.; Botnar, R.M.; Eaton, P. Protein kinase G oxidation is a major cause of injury during sepsis. Proc. Natl. Acad. Sci. USA 2013, 110, 9909–9913. [Google Scholar] [CrossRef]
- Lieberthal, W.; Tang, M.; Zhang, L.; Viollet, B.; Patel, V.; Levine, J.S. Susceptibility to ATP depletion of primary proximal tubular cell cultures derived from mice lacking either the α1 or the α2 isoform of the catalytic domain of AMPK. BMC Nephrol. 2013, 14, 251. [Google Scholar] [CrossRef]
- Mahboubi, H.; Barisé, R.; Stochaj, U. 5’-AMP-activated protein kinase alpha regulates stress granule biogenesis. Biochim. Biophys. Acta 2015, 1853, 1725–1737. [Google Scholar] [CrossRef]
- Rogacka, D.; Rachubik, P.; Audzeyenka, I.; Szrejder, M.; Kulesza, T.; Myślińska, D.; Angielski, S.; Piwkowska, A. Enhancement of cGMP-dependent pathway activity ameliorates hyperglycemia-induced decrease in SIRT1-AMPK activity in podocytes: Impact on glucose uptake and podocyte function. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119362. [Google Scholar] [CrossRef]
- Bogachus, L.D.; Turcotte, L.P. Genetic downregulation of AMPK-α isoforms uncovers the mechanism by which metformin decreases FA uptake and oxidation in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2010, 299, C1549–C1561. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Murphy, B.A.; Fakira, K.A.; Song, Z.; Beuve, A.; Routh, V.H. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am. J. Physiol. Cell Physiol. 2009, 297, C750–C758. [Google Scholar] [CrossRef]
- Choi, S.L.; Kim, S.J.; Lee, K.T.; Kim, J.; Mu, J.; Birnbaum, M.J.; Soo Kim, S.; Ha, J. The regulation of AMP-activated protein kinase by H2O2. Biochem. Biophys. Res. Commun. 2001, 287, 92–97. [Google Scholar] [CrossRef]
- Rodríguez, C.; Contreras, C.; Sáenz-Medina, J.; Muñoz, M.; Corbacho, C.; Carballido, J.; García-Sacristán, A.; Hernandez, M.; López, M.; Rivera, L.; et al. Activation of the AMP-related kinase (AMPK) induces renal vasodilatation and downregulates Nox-derived reactive oxygen species (ROS) generation. Redox Biol. 2020, 34, 101575. [Google Scholar] [CrossRef]
- Ramnanan, C.J.; McMullen, D.C.; Groom, A.G.; Storey, K.B. The regulation of AMPK signaling in a natural state of profound metabolic rate depression. Mol. Cell. Biochem. 2010, 335, 91–105. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Stefańska, K.; Zieliński, M.; Sakowska, J.; Jankowiak, M.; Trzonkowski, P.; Marek-Trzonkowska, N.; Kwiatkowski, S. Podocytes: The most vulnerable renal cells in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5051. [Google Scholar] [CrossRef]
- Faul, C.; Asanuma, K.; Yanagida-Asanuma, E.; Kim, K.; Mundel, P. Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007, 17, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Kasztan, M.; Angielski, S.; Jankowski, M. Insulin increases glomerular filtration barrier permeability through PKGIα-dependent mobilization of BKCa channels in cultured rat podocytes. Biochim. Biophys. Acta 2015, 1852, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Isotani, E.; Huang, J.; Ding, H.; Stull, J.T.; Kamm, K.E. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am. J. Physiol. Cell Physiol. 2008, 295, C358–C364. [Google Scholar] [CrossRef] [PubMed]
- Sit, S.T.; Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 2011, 124, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Audzeyenka, I.; Rogacka, D.; Rachubik, P.; Typiak, M.; Rychłowski, M.; Angielski, S.; Piwkowska, A. The PKGIα-Rac1 pathway is a novel regulator of insulin-dependent glucose uptake in cultured rat podocytes. J. Cell. Physiol. 2021, 236, 4655–4668. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Laroux, F.S.; Coe, L.L.; Morise, Z.; Kawachi, S.; Bauer, P.; Grisham, M.B.; Specian, R.D.; Carter, P.; Jennings, S.; et al. Interferon-γ and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc. Res. 2001, 61, 130–143. [Google Scholar] [CrossRef]
- Savin, V.J.; Sharma, R.; Lovell, H.B.; Welling, D.J. Measurement of albumin reflection coefficient with isolated rat glomeruli. J. Am. Soc. Nephrol. 1992, 3, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, R.T.; Lachno, D.R.; Ledingham, S.J.; Yacoub, M.H. Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in hearts for transplantation. J. Chromatogr. 1990, 527, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zur Nedden, S.; Eason, R.; Doney, A.S.; Frenguelli, B.G. An ion-pair reversed-phase HPLC method for determination of fresh tissue adenine nucleotides avoiding freeze-thaw degradation of ATP. Anal. Biochem. 2009, 388, 108–114. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Szrejder, M.; Topolewska, A.; Rychłowski, M.; Piwkowska, A. The Role of PKGIα and AMPK Signaling Interplay in the Regulation of Albumin Permeability in Cultured Rat Podocytes. Int. J. Mol. Sci. 2023, 24, 3952. https://doi.org/10.3390/ijms24043952
Rachubik P, Rogacka D, Audzeyenka I, Szrejder M, Topolewska A, Rychłowski M, Piwkowska A. The Role of PKGIα and AMPK Signaling Interplay in the Regulation of Albumin Permeability in Cultured Rat Podocytes. International Journal of Molecular Sciences. 2023; 24(4):3952. https://doi.org/10.3390/ijms24043952
Chicago/Turabian StyleRachubik, Patrycja, Dorota Rogacka, Irena Audzeyenka, Maria Szrejder, Anna Topolewska, Michał Rychłowski, and Agnieszka Piwkowska. 2023. "The Role of PKGIα and AMPK Signaling Interplay in the Regulation of Albumin Permeability in Cultured Rat Podocytes" International Journal of Molecular Sciences 24, no. 4: 3952. https://doi.org/10.3390/ijms24043952
APA StyleRachubik, P., Rogacka, D., Audzeyenka, I., Szrejder, M., Topolewska, A., Rychłowski, M., & Piwkowska, A. (2023). The Role of PKGIα and AMPK Signaling Interplay in the Regulation of Albumin Permeability in Cultured Rat Podocytes. International Journal of Molecular Sciences, 24(4), 3952. https://doi.org/10.3390/ijms24043952






