Gain-of-Function p53N236S Mutation Drives the Bypassing of HRasV12-Induced Cellular Senescence via PGC–1α
Abstract
1. Introduction
2. Results
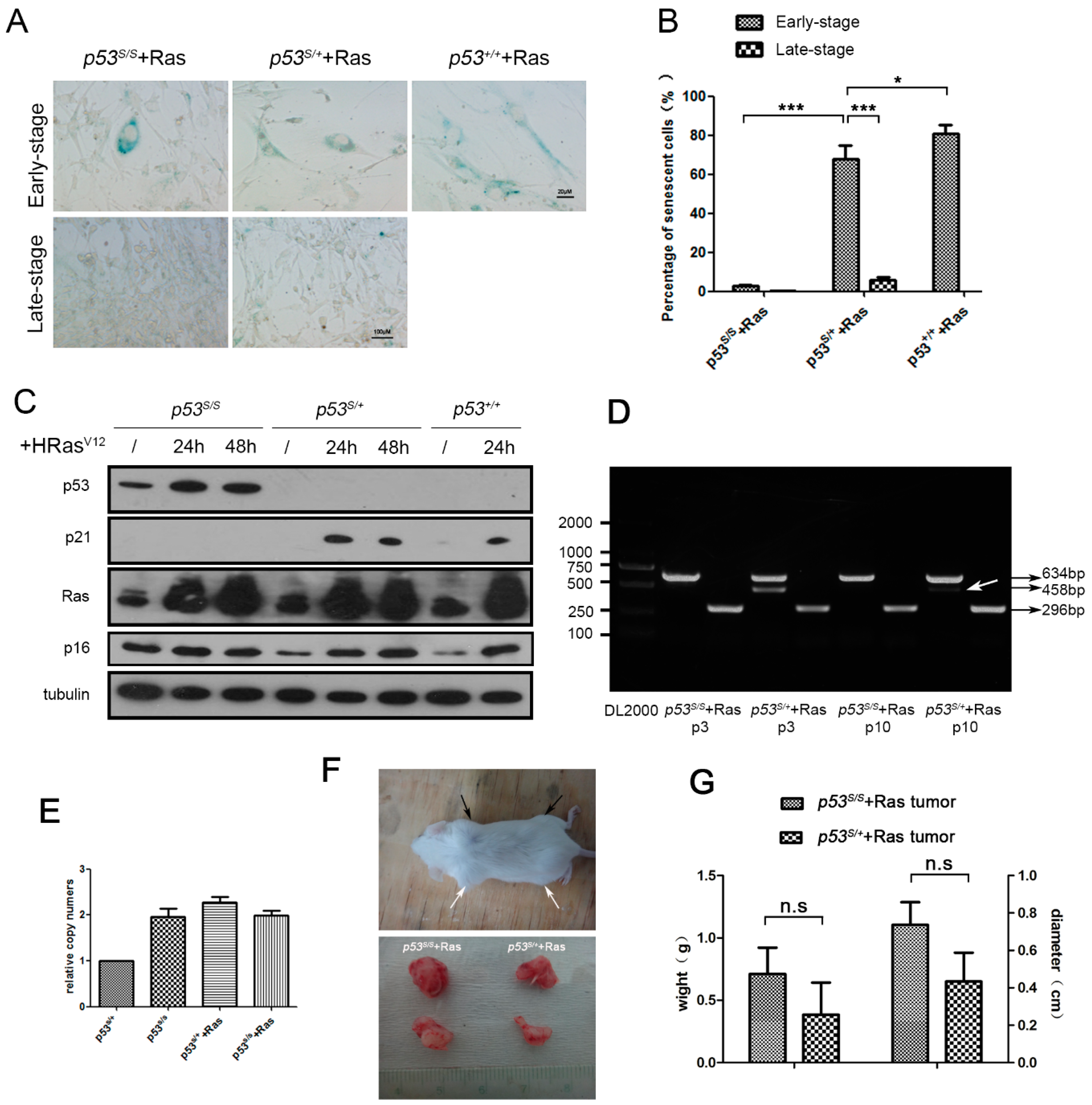
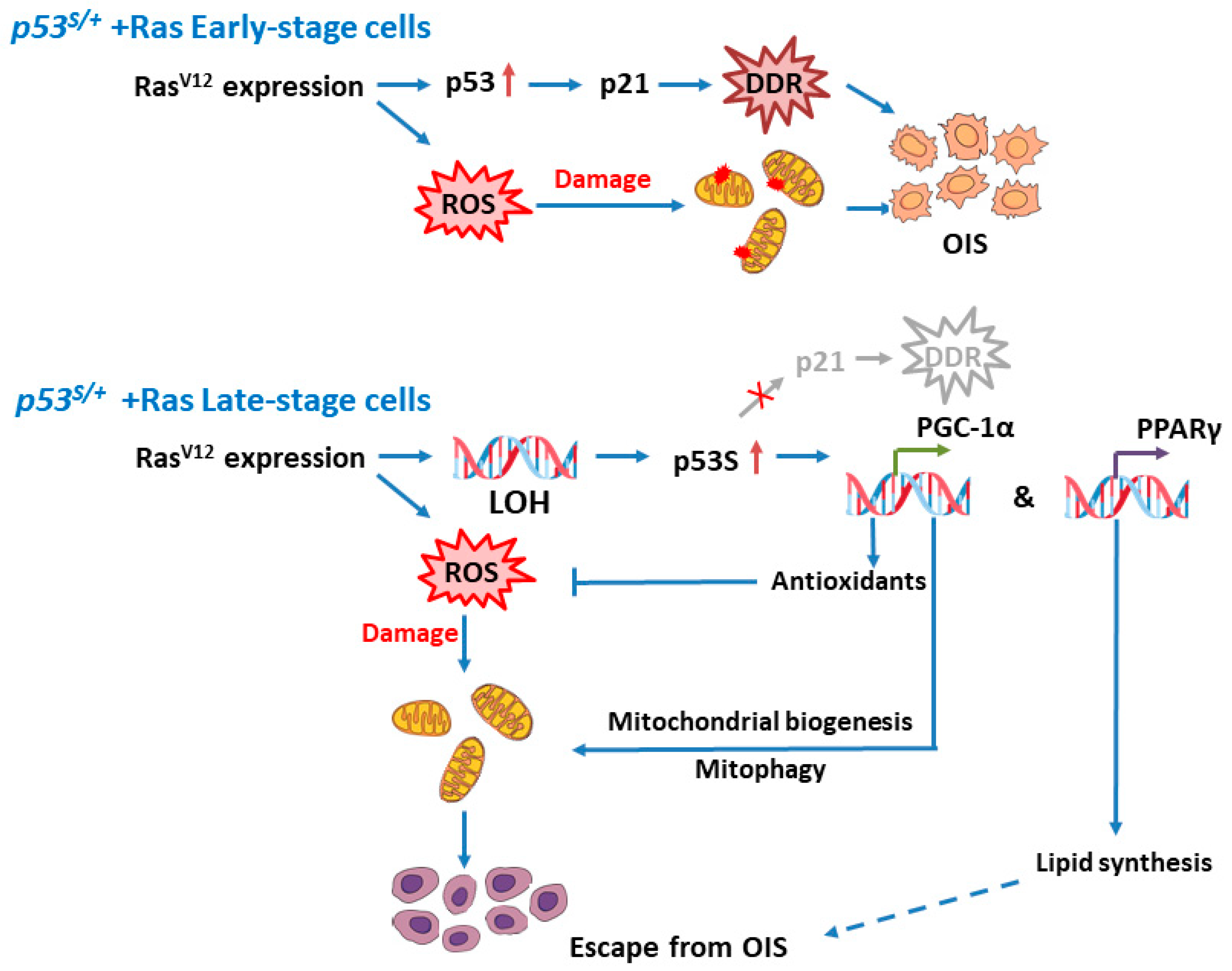
2.1. The p53S/+ MEFs Escape HRasV12-Induced Senescence and Develop LOH
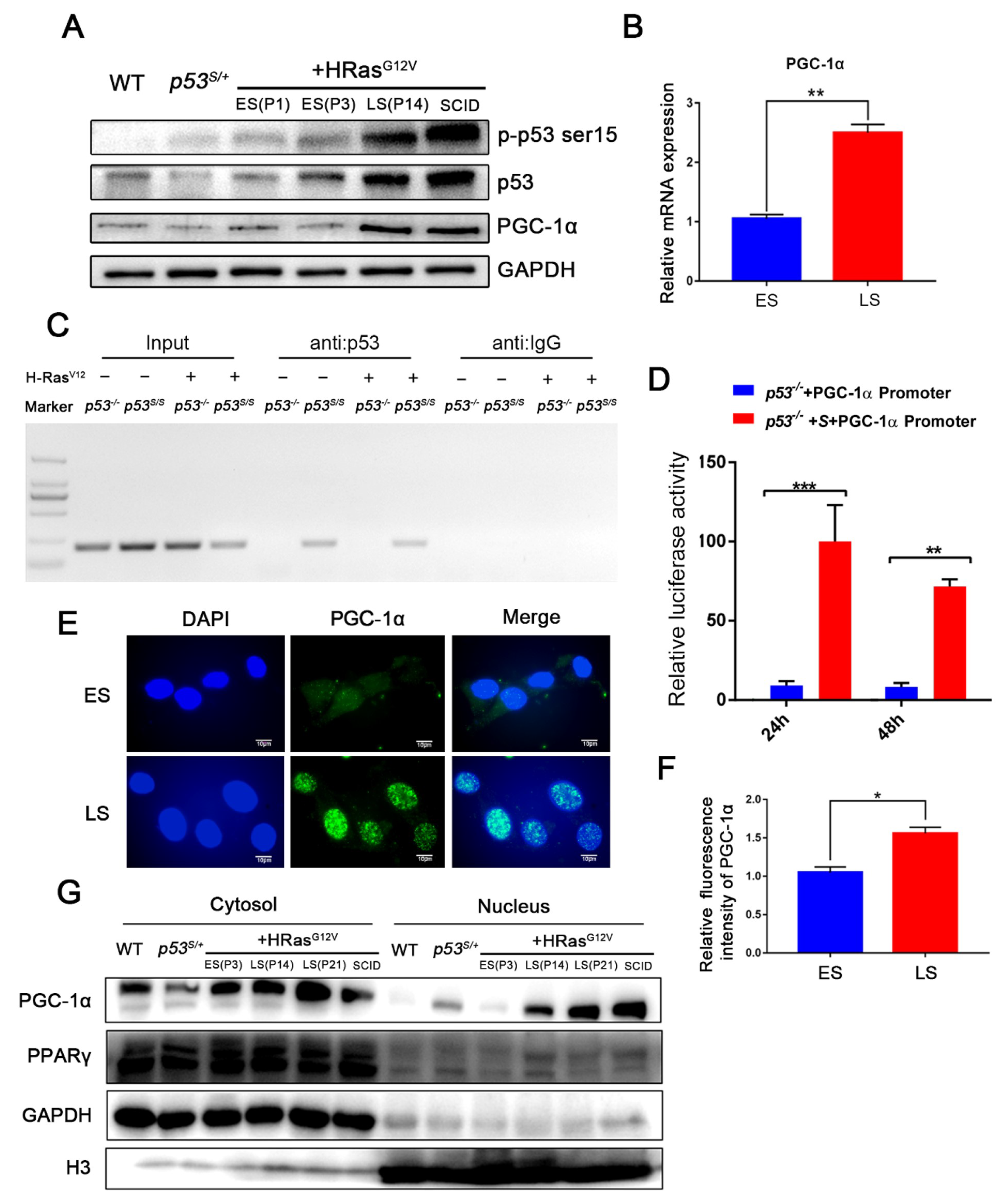
2.2. p53S Upregulates PGC–1α and Its Nuclear Translocation in Late-Stage p53S/++Ras Cells
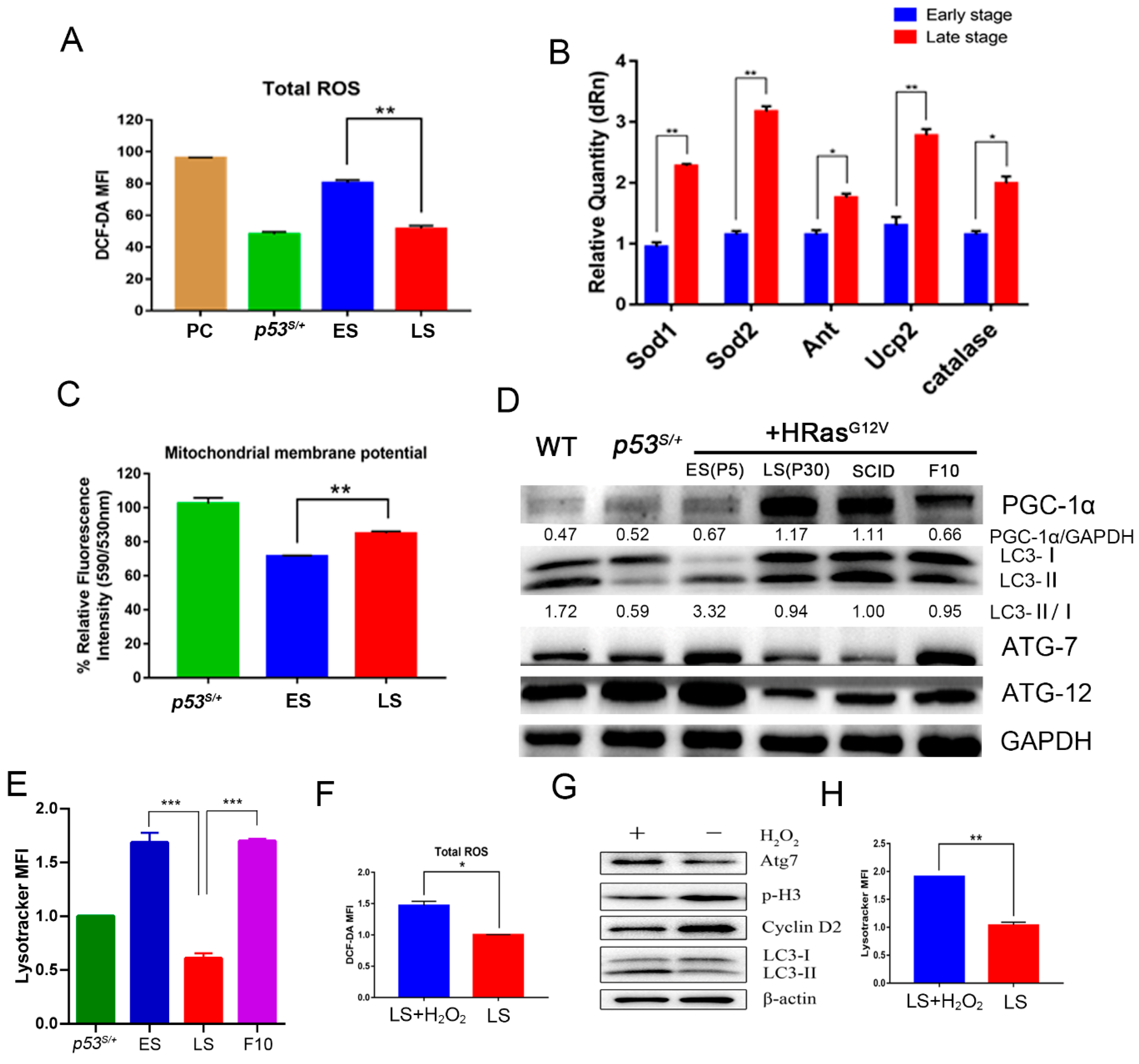
2.3. p53S Improves Mitochondrial Quality and Quantity in Late-Stage p53S/++Ras Cells via PGC–1α
2.4. PGC–1α Downregulates Autophagy Levels in Late-Stage Cells by Reducing ROS Levels
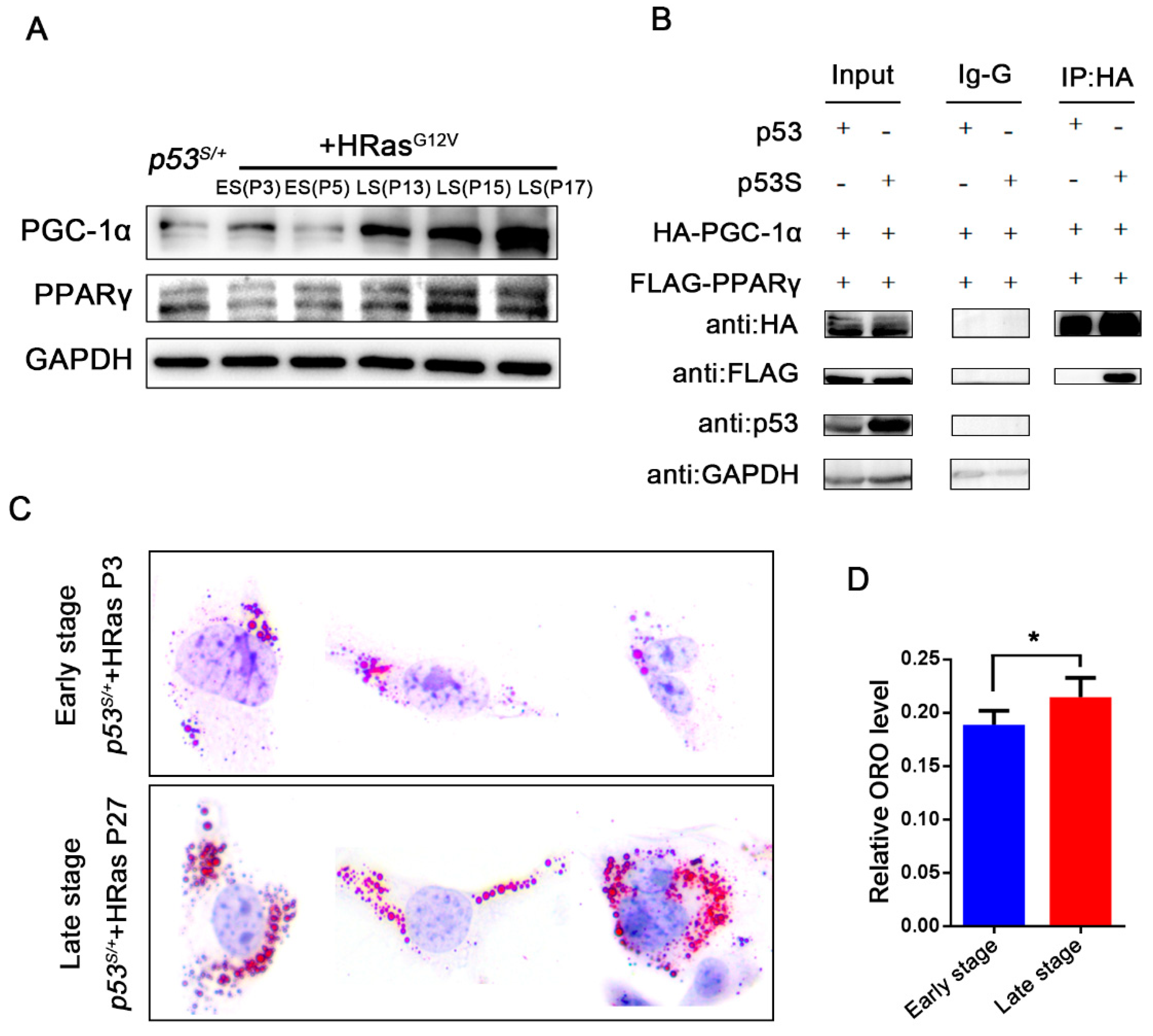
2.5. p53S Regulates the Interaction between PGC–1α and PPARγ
3. Discussion
4. Materials and Methods
4.1. Mice and Tumor Cells Harvest
4.2. Cell Lines and Constructs
4.3. Western Blot Analysis
4.4. SA-β-Gal Staining
4.5. Oil Red O Staining
4.6. Disruption of the PGC–1α Gene through CRISPR/Cas9
- PCR primers #1: 5′-TTTCTTGCTTTCCCTTTTTCTG-3′ and 5′-ACCCCTATCCTCCCCACTAATA-3′,
- PCR primers #2: 5′-TTGATGCACTGACAGATGGAG-3′ and 5′-ACAGAATGGGCAAATCTAGGAA-3′,
- PCR primers #3: 5′-TCAACCCACTCATGTCTTCTGT-3′ and 5′-TACTAGAGACGGCTCTTCTGCC-3′,
- PCR primers #4: 5′-CCAGATCTTCCTGAACTTGACC-3′ and 5′-CTCCCCATACATCAGTCAGACA-3′.
4.7. Immunofluorescence and Microscopy Analysis
4.8. Real-Time PCR
- PGC–1α forward: 5′-AGCCGTGACCACTGACAACGAG-3′, reverse: 5′-GCTGCATGGTTCTGAGTGCTAAG-3′;
- Sod1 forward: 5′-CAAGCGGTGAACCAGTTGTG-3′, reverse: 5′-TGAGGTCCTGCACTGGTAC-3′;
- Sod2 forward: 5′-GCCTGCACTGAAGTTCAATG-3′, reverse: 5′-ATCTGTAAGCGACCTTGCTC-3′;
- Ucp2 forward: 5′-CAGGTCACTGTGCCCTTACCAT, reverse: 5′-CACTACGTTCCAGGATCCCAAG-3′;
- Ant forward: 5′-TTCCTGGCAGGTGGCATCG-3′, reverse: 5′-GGATTCTCACGACACAATCAATG-3′; and
- Catalase forward: 5′-ACCCTCTTATACCAGTTGGC-3′, reverse: 5′-GCATGCACATGGGGCCATCA-3′.
4.9. Chromatin Immunoprecipitation
4.10. ROS Detection
4.11. JC-10
4.12. ATP Detection Assay
4.13. MitoTrackerTM Green FM Staining
4.14. LysoTrackerTM Red DND-99 Staining
4.15. Co-IP Experiments
4.16. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alternative lengthening of telomere |
| ChIP-on-Chip | Immunoprecipitation on chip |
| DDR | DNA damage response |
| DN | Dominant negative |
| ER | Endoplasmic reticulum |
| ES cells | Early-stage p53S/++Ras cells, senescent cells |
| GOF | Gain of function |
| IF | Immunofluorescence staining |
| LFL | Li–Fraumeni like |
| LFS | Li–Fraumeni syndrome |
| LOF | Loss of function |
| LOH | Loss of heterozygosity |
| LS cells | Late-stage p53S/++Ras cells, which bypassed the OIS |
| MEFs | Mouse embryonic fibroblasts |
| OIS | Oncogene-induced senescence |
| p53S | p53N236S |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDH | Pyruvate dehydrogenase |
| PGC–1α | PPARγ coactivator |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| ROS | Reactive oxygen species |
| SCID | Severe combined immune deficiency |
| WT | Wild type |
References
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ko, L.J.; Prives, C. p53: Puzzle and paradigm. Genes Dev. 1996, 10, 1054–1072. [Google Scholar] [CrossRef] [PubMed]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef]
- Weisz, L.; Oren, M.; Rotter, V. Transcription regulation by mutant p53. Oncogene 2007, 26, 2202–2211. [Google Scholar] [CrossRef]
- Oren, M.; Rotter, V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2010, 2, a001107. [Google Scholar] [CrossRef]
- Brosh, R.; Rotter, V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer 2009, 9, 701–713. [Google Scholar] [CrossRef]
- Hussain, S.P.; Harris, C.C. Molecular epidemiology of human cancer: Contribution of mutation spectra studies of tumor suppressor genes. Cancer Res. 1998, 58, 4023–4037. [Google Scholar]
- Prives, C.; Hall, P.A. The p53 pathway. J. Pathol. 1999, 187, 112–126. [Google Scholar] [CrossRef]
- Dai, C.; Gu, W. p53 post-translational modification: Deregulated in tumorigenesis. Trends Mol. Med. 2010, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Deng, G.; Chen, K.M.; Chui, R.M.; Haughney, P.C.; Narayan, P. P53 tumour-suppressor gene mutations are mainly localised on exon 7 in human primary and metastatic prostate cancer. Br. J. Cancer 1996, 74, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. p53 function and dysfunction. Cell 1992, 70, 523–526. [Google Scholar] [CrossRef]
- Jia, S.; Zhao, L.; Tang, W.; Luo, Y. The Gain of Function of p53 Mutant p53S in Promoting Tumorigenesis by Cross-talking with H-RasV12. Int. J. Biol. Sci. 2012, 8, 596–605. [Google Scholar] [CrossRef]
- Wang, B.; Dan, J.; Li, H.; Hou, J.; Shi, M.; Singh, S.K.; Chang, J.T.; Luo, Y. The transcription and expression profile of p53(N236S) mutant reveals new aspects of gain of function for mutant p53. FEBS Lett. 2018, 592, 3183–3197. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Timpson, P.; Karim, S.A.; Ridgway, R.A.; Athineos, D.; Doyle, B.; Jamieson, N.B.; Oien, K.A.; Lowy, A.M.; Brunton, V.G.; et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 246–251. [Google Scholar] [CrossRef]
- Kim, M.P.; Li, X.Q.; Deng, J.Y.; Zhang, Y.; Dai, B.B.; Allton, K.L.; Hughes, T.G.; Siangco, C.; Augustine, J.J.; Kang, Y.A.; et al. Oncogenic KRAS Recruits an Expansive Transcriptional Network through Mutant p53 to Drive Pancreatic Cancer Metastasis. Cancer Discov. 2021, 11, 2094–2111. [Google Scholar] [CrossRef]
- Klimovich, B.; Merle, N.; Neumann, M.; Elmshäuser, S.; Nist, A.; Mernberger, M.; Kazdal, D.; Stenzinger, A.; Timofeev, O.; Stiewe, T. p53 partial loss-of-function mutations sensitize to chemotherapy. Oncogene 2022, 41, 1011–1023. [Google Scholar] [CrossRef]
- Baker, S.J.; Preisinger, A.C.; Jessup, J.M.; Paraskeva, C.; Markowitz, S.; Willson, J.K.; Hamilton, S.; Vogelstein, B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990, 50, 7717–7722. [Google Scholar]
- Olmos, Y.; Valle, I.; Borniquel, S.; Tierrez, A.; Soria, E.; Lamas, S.; Monsalve, M. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J. Biol. Chem. 2009, 284, 14476–14484. [Google Scholar] [CrossRef]
- Elgendy, M.; Sheridan, C.; Brumatti, G.; Martin, S.J. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol. Cell 2011, 42, 23–35. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Et Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mui, E.; Galbraith, L.; Patel, R.; Tan, E.H.; Salji, M.; Rust, A.G.; Repiscak, P.; Hedley, A.; Markert, E.; et al. Sleeping Beauty screen reveals Pparg activation in metastatic prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Baitei, E.Y.; Al-Rijjal, R.A.; Parhar, R.S.; Al-Mohanna, F.A.; Kimura, S.; Pritchard, C.; Binessa, H.A.; Alzahrani, A.S.; Al-Khalaf, H.H.; et al. TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene 2016, 35, 1909–1918. [Google Scholar] [CrossRef]
- Kussie, P.H.; Gorina, S.; Marechal, V.; Elenbaas, B.; Moreau, J.; Levine, A.J.; Pavletich, N.P. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 1996, 274, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, Z.N.; Fojo, T.; Blagosklonny, M.V. Complementation of two mutant p53: Implications for loss of heterozygosity in cancer. FEBS Lett. 2005, 579, 2231–2235. [Google Scholar] [CrossRef]
- Nishida, N.; Fukuda, Y.; Kokuryu, H.; Toguchida, J.; Yandell, D.W.; Ikenega, M.; Imura, H.; Ishizaki, K. Role and mutational heterogeneity of the p53 gene in hepatocellular carcinoma. Cancer Res. 1993, 53, 368–372. [Google Scholar]
- Varley, J.M.; Thorncroft, M.; McGown, G.; Appleby, J.; Kelsey, A.M.; Tricker, K.J.; Evans, D.G.; Birch, J.M. A detailed study of loss of heterozygosity on chromosome 17 in tumours from Li-Fraumeni patients carrying a mutation to the TP53 gene. Oncogene 1997, 14, 865–871. [Google Scholar] [CrossRef]
- Forslund, A.; Kressner, U.; Lonnroth, C.; Andersson, M.; Lindmark, G.; Lundholm, K. P53 mutations in colorectal cancer assessed in both genomic DNA and cDNA as compared to the presence of p53 LOH. Int. J. Oncol. 2002, 21, 409–415. [Google Scholar] [CrossRef]
- Fenoglio-Preiser, C.M.; Wang, J.; Stemmermann, G.N.; Noffsinger, A. TP53 and gastric carcinoma: A review. Hum. Mutat. 2003, 21, 258–270. [Google Scholar] [CrossRef]
- Lee, M.K.; Sabapathy, K. The R246S hot-spot p53 mutant exerts dominant-negative effects in embryonic stem cells in vitro and in vivo. J. Cell Sci. 2008, 121, 1899–1906. [Google Scholar] [CrossRef]
- Jackson, E.L.; Olive, K.P.; Tuveson, D.A.; Bronson, R.; Crowley, D.; Brown, M.; Jacks, T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005, 65, 10280–10288. [Google Scholar] [CrossRef]
- Olive, K.P.; Tuveson, D.A.; Ruhe, Z.C.; Yin, B.; Willis, N.A.; Bronson, R.T.; Crowley, D.; Jacks, T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 2004, 119, 847–860. [Google Scholar] [CrossRef]
- Lee, A.C.; Fenster, B.E.; Ito, H.; Takeda, K.; Bae, N.S.; Hirai, T.; Yu, Z.X.; Ferrans, V.J.; Howard, B.H.; Finkel, T. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 1999, 274, 7936–7940. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, J.; Zheng, L.; Meissl, K.; Chaneton, B.; Selivanov, V.A.; Mackay, G.; van der Burg, S.H.; Verdegaal, E.M.E.; Cascante, M.; Shlomi, T.; et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 2013, 498, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Chen, T.; Wanagat, J.; Laflamme, M.; Marcinek, D.J.; Emond, M.J.; Ngo, C.P.; Prolla, T.A.; Rabinovitch, P.S. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 2010, 9, 536–544. [Google Scholar] [CrossRef]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Muller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef]
- Moslehi, J.; DePinho, R.A.; Sahin, E. Telomeres and mitochondria in the aging heart. Circ. Res. 2012, 110, 1226–1237. [Google Scholar] [CrossRef]
- Zhu, B.K.; Ferry, C.H.; Markell, L.K.; Blazanin, N.; Glick, A.B.; Gonzalez, F.J.; Peters, J.M. The Nuclear Receptor Peroxisome Proliferator-activated Receptor-beta/delta (PPAR beta/delta) Promotes Oncogene-induced Cellular Senescence through Repression of Endoplasmic Reticulum Stress. J. Biol. Chem. 2014, 289, 20102–20119. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zong, H.; Hou, K.; Zhang, Y.; Zhang, R.; Zhao, D.; Guo, X.; Luo, Y.; Jia, S. p53N236S Activates Autophagy in Response to Hypoxic Stress Induced by DFO. Genes 2022, 13, 763. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, K.; Guo, Y.; Guo, X.; Hou, K.; Hou, J.; Luo, Y.; Liu, J.; Jia, S. Gain-of-Function p53N236S Mutation Drives the Bypassing of HRasV12-Induced Cellular Senescence via PGC–1α. Int. J. Mol. Sci. 2023, 24, 3790. https://doi.org/10.3390/ijms24043790
Yang H, Zhang K, Guo Y, Guo X, Hou K, Hou J, Luo Y, Liu J, Jia S. Gain-of-Function p53N236S Mutation Drives the Bypassing of HRasV12-Induced Cellular Senescence via PGC–1α. International Journal of Molecular Sciences. 2023; 24(4):3790. https://doi.org/10.3390/ijms24043790
Chicago/Turabian StyleYang, Hao, Ke Zhang, Yusheng Guo, Xin Guo, Kailong Hou, Jing Hou, Ying Luo, Jing Liu, and Shuting Jia. 2023. "Gain-of-Function p53N236S Mutation Drives the Bypassing of HRasV12-Induced Cellular Senescence via PGC–1α" International Journal of Molecular Sciences 24, no. 4: 3790. https://doi.org/10.3390/ijms24043790
APA StyleYang, H., Zhang, K., Guo, Y., Guo, X., Hou, K., Hou, J., Luo, Y., Liu, J., & Jia, S. (2023). Gain-of-Function p53N236S Mutation Drives the Bypassing of HRasV12-Induced Cellular Senescence via PGC–1α. International Journal of Molecular Sciences, 24(4), 3790. https://doi.org/10.3390/ijms24043790





