Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit
Abstract
1. Introduction
2. Results
2.1. Experiment 1—Selection of the Most Appropriate Melatonin Treatment
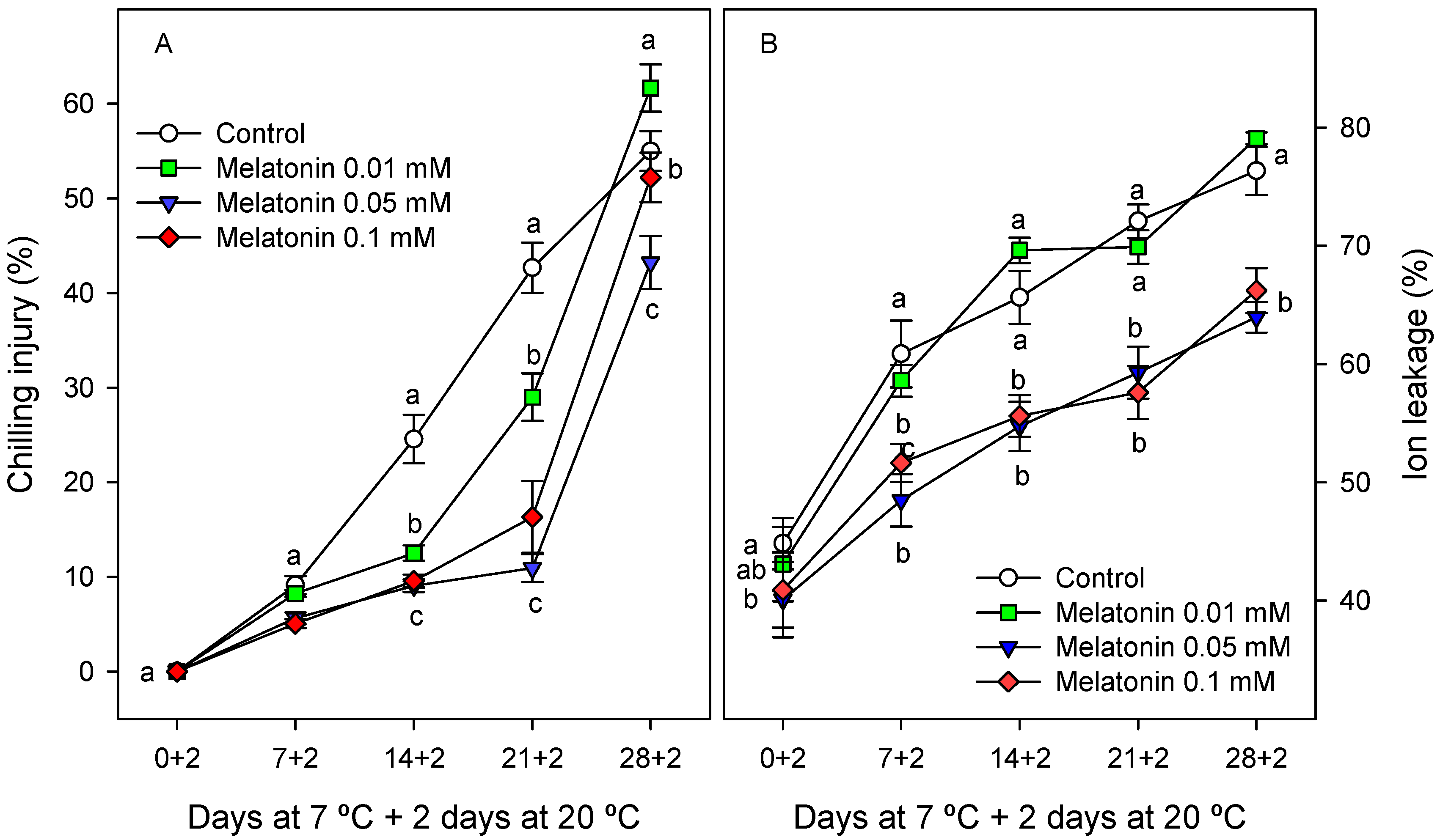
2.2. Effects of Melatonin Treatment on Cherimoya CI, Quality Parameters and Ripening
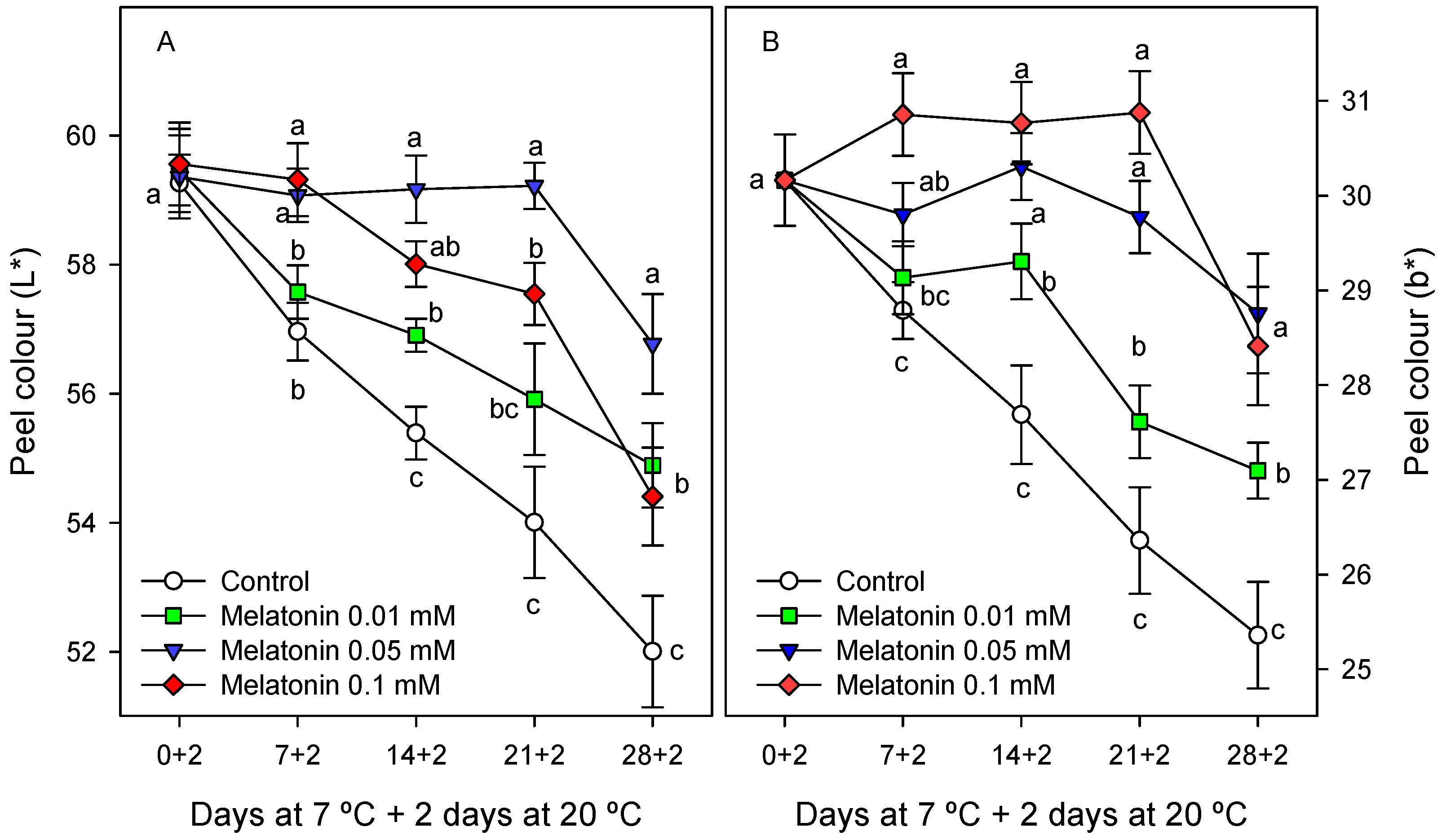
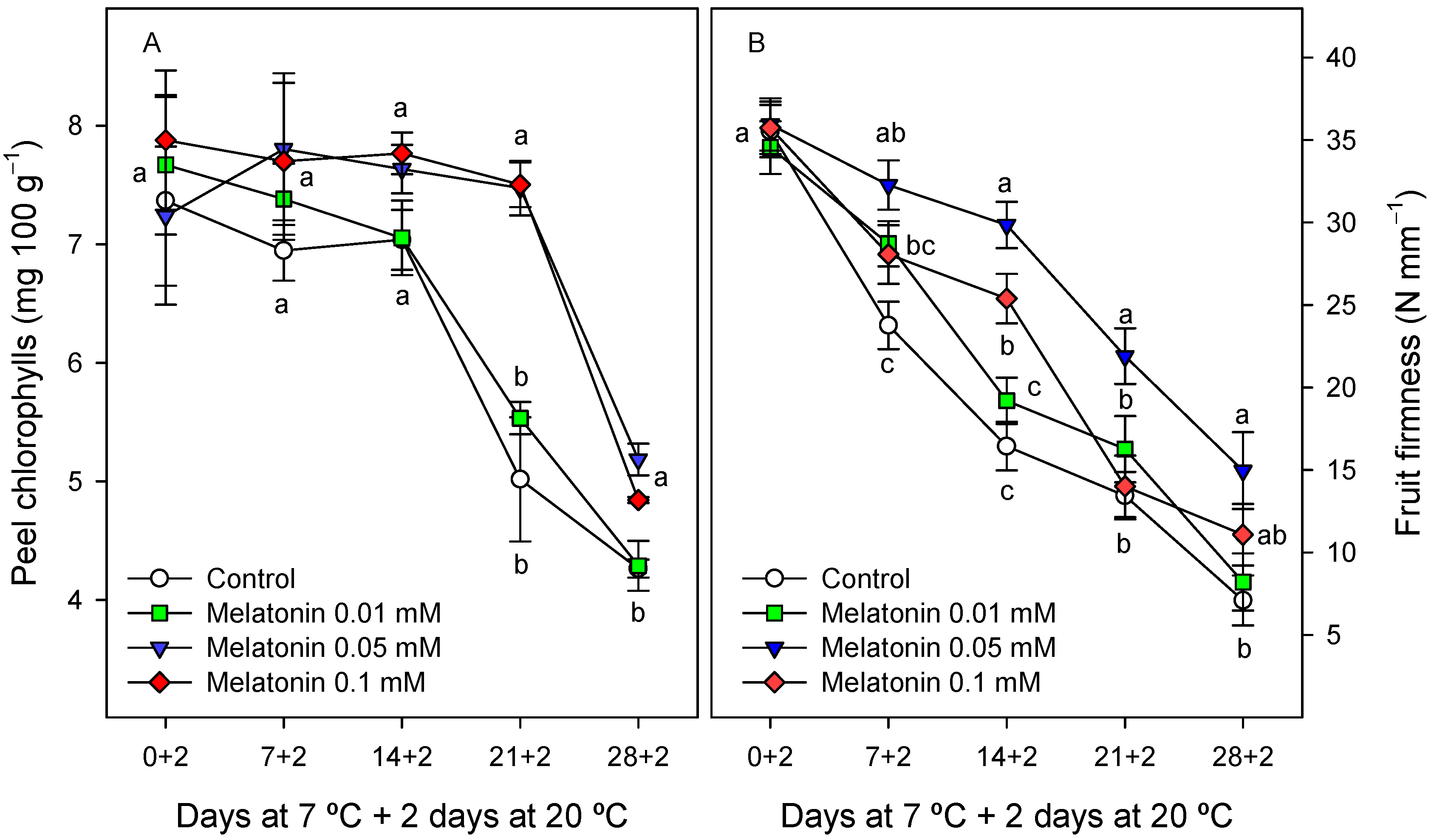
2.2.1. Chilling Injury and External Quality Parameters
2.2.2. Total Phenolic and Antioxidant Activity in Cherimoya Peel
2.2.3. Internal Fruit Quality Parameters
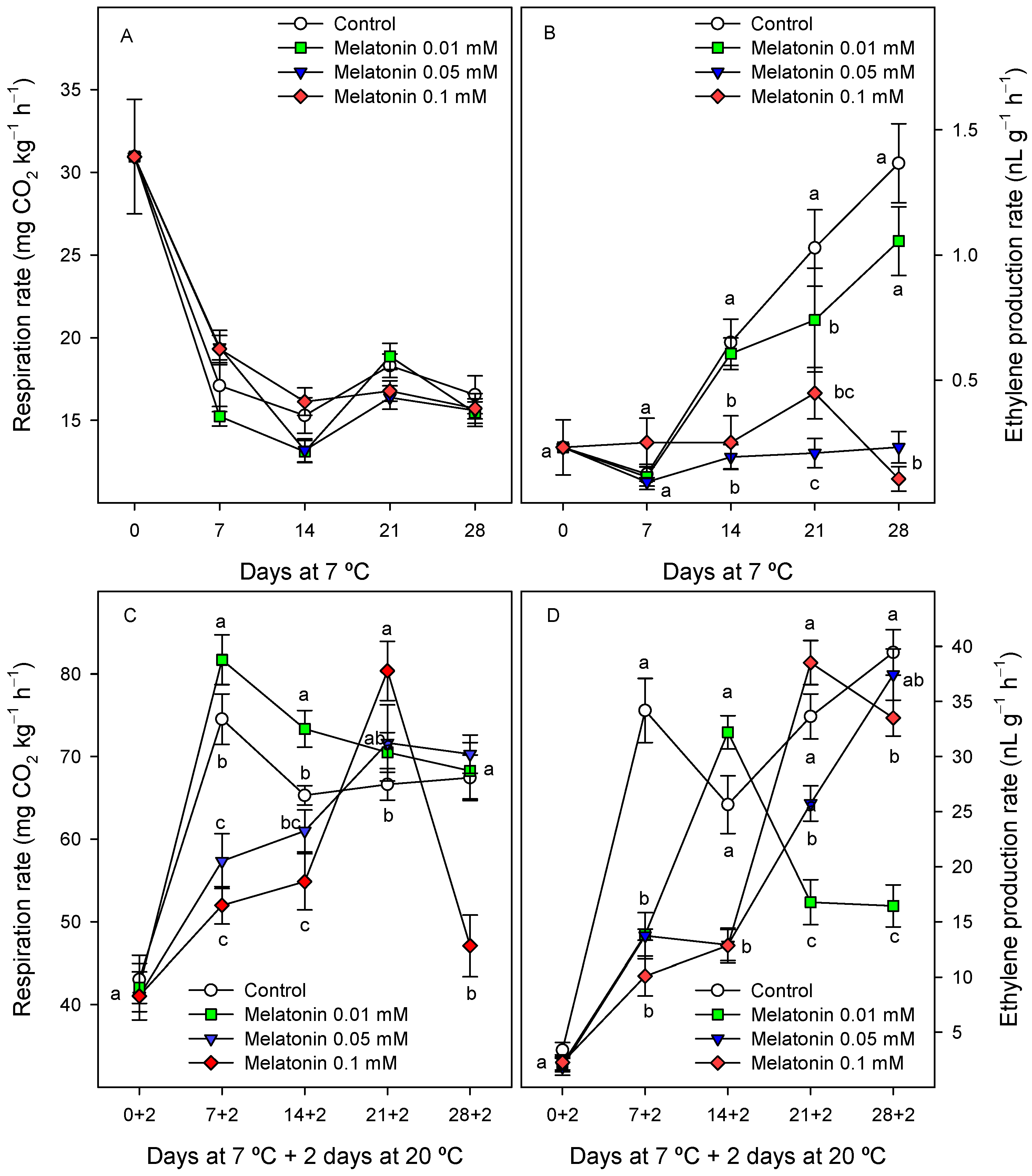
2.2.4. Respiration Rate and Ethylene Production
3. Discussion
4. Materials and Methods
4.1. Plant Material and Melatonin Treatments
4.2. Respiration Rate, Ethylene Production and Quality Parameters
4.3. Total Phenolic Quantification and Total Antioxidant Activity
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larranaga, N.; Albertazzi, F.J.; Hormaza, J.I. Phylogenetics of Annona cherimola (Annonaceae) and some of its closest relatives. J. Syst. Evol. 2019, 57, 211–221. [Google Scholar] [CrossRef]
- Perrone, A.; Yousefi, S.; Salami, A.; Papini, A.; Martinelli, F. Botanical, genetic, phytochemical and pharmaceutical aspects of Annona cherimola Mill. Sci. Hortic. 2022, 296, 110896. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Avallone, L.; Menichini, F. Radical scavenging, antioxidant and metal chelating activities of Annona cherimola Mill. (cherimoya) peel and pulp in relation to their total phenolic and total flavonoid contents. J. Food Compos. Anal. 2012, 25, 179–184. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Camacho, J.F.; Cordeiro, N.; Gouveia, M.; Freire, C.S.R.; Silvestre, A.J.D. Profiling of lipophilic and phenolic phytochemicals of four cultivars from cherimoya (Annona cherimola Mill.). Food Chem. 2016, 211, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Mannino, G.; Palazzolo, E.; Gianguzzi, G.; Perrone, A.; Serio, G.; Farina, V. Pomological, sensorial, nutritional and nutraceutical profile of seven cultivars of cherimoya (Annona cherimola Mill.). Foods 2021, 10, 35. [Google Scholar] [CrossRef]
- Martínez, G.; Serrano, M.; Pretel, M.T.; Riquelme, F.; Romojaro, F. Ethylene biosynthesis and physico-chemical changes during fruit ripening of cherimoya (Annona cherimola Mill.). J. Hortic. Sci. 1993, 68, 477–483. [Google Scholar] [CrossRef]
- Pareek, S.; Yahia, E.M.; Pareek, O.P.; Kaushik, R.A. Postharvest physiology and technology of Annona fruits. Food Res. Int. 2011, 44, 1741–1751. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Lahoz, J.M.; Sola, M.M.; Pascual, L.; Vargas, A.M. Postharvest changes in total soluble solids and tissue pH of cherimoya fruits stored at chilling and non-chilling temperatures. J. Hortic. Sci. 1994, 69, 459–463. [Google Scholar] [CrossRef]
- Montero, L.M.; Escribano, M.I.; De La Plaza, J.L.; Merodio, C. Chilling temperature storage induces changes in protein patterns and protease activity in cherimoya fruit. Postharvest Biol. Technol. 1995, 5, 251–260. [Google Scholar] [CrossRef]
- Palma, T.; Aguilera, J.M.; Stanley, D.W. A review of postharvest events in cherimoya. Postharvest Biol. Technol. 1993, 2, 187–208. [Google Scholar] [CrossRef]
- Alique, R.; Oliveira, G.S. Changes in sugars and organic acids in cherimoya (Annona cherimola Mill.) fruit under controlled atmosphere storage. J. Agric. Food Chem. 1994, 42, 799–803. [Google Scholar] [CrossRef]
- Maldonado, R.; Sanchez-Ballesta, M.T.; Alique, R.; Escribano, M.I.; Merodio, C. Malate metabolism and adaptation to chilling temperature storage by pretreatment with high CO2 levels in Annona cherimola fruit. J. Agric. Food Chem. 2004, 52, 4758–4763. [Google Scholar] [CrossRef] [PubMed]
- Tinebra, I.; Scuderi, D.; Busetta, G.; Palazzolo, E.; Settanni, L.; Farina, V. Modified atmosphere packaging and low temperature storage extend marketability of cherimoya (Annona cherimola Mill.). Italus Hortus 2022, 29, 115–137. [Google Scholar] [CrossRef]
- Sevillano, L.; Sola, M.; Vargas, A. Induction of small heat-shock proteins in mesocarp of cherimoya fruit (Annona cherimola Mill.) produces chilling tolerance. J. Food Biochem. 2010, 34, 625–638. [Google Scholar] [CrossRef]
- Wu, X.; Ren, J.; Huang, X.; Zheng, X.; Tian, Y.; Shi, L.; Dong, P.; Li, Z. Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Sci. Hortic. 2021, 288, 110392. [Google Scholar] [CrossRef]
- Acosta, M.G.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a possible natural safener in crops. Plants 2022, 11, 890. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Sun, Y.; Zhang, L.; Zheng, S. Versatile roles of melatonin in growth and stress tolerance in plants. J. Plant Growth Regul. 2022, 41, 507–523. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef]
- Jayarajan, S.; Sharma, R.R. Melatonin: A blooming biomolecule for postharvest management of perishable fruits and vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Madebo, M.P.; Zheng, Y.; Jin, P. Melatonin-mediated postharvest quality and antioxidant properties of fresh fruits: A comprehensive meta-analysis. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3205–3226. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, L.; Li, K.; Cao, J.; Zhao, Z. Integrative transcriptomic and metabolomic alterations unravel the effect of melatonin on mitigating postharvest chilling injury upon plum (cv. Friar) fruit. Postharvest Biol. Technol. 2022, 186, 111819. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Pareek, S.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Valero, D.; Serrano, M. An exogenous pre-storage melatonin alleviates chilling injury in some mango fruit cultivars, by acting on the enzymatic and non-enzymatic antioxidant system. Antioxidants 2022, 11, 384. [Google Scholar] [CrossRef]
- Guillén, F.; Medina-Santamarina, J.; García-Pastor, M.E.; Chen, N.J.; Uruu, G.; Paull, R.E. Postharvest melatonin treatment delays senescence and increases chilling tolerance in pineapple. LWT-Food Sci. Technol. 2022, 169, 113989. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Ruiz-Aracil, M.C.; Ilea, M.J.M.; Martínez-Romero, D.; Guillén, F. A synergistic effect based on the combination of melatonin with 1-methylcyclopropene as a new strategy to increase chilling tolerance and general quality in zucchini fruit. Foods 2022, 11, 2784. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.-X.; et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Dattola, A.; Sortino, G.; Vonella, V.; Zappia, R.; Gullo, G. Effect of fruit-set time on the quality performance of Anona cherimola Mill. fruit in south Italy. Sci. Hortic. 2019, 246, 272–278. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Kong, X.-M.; Ge, W.-Y.; Wei, B.-D.; Zhou, Q.; Zhou, X.; Zhao, Y.-B.; Ji, S.-J. Melatonin ameliorates chilling injury in green bell peppers during storage by regulating membrane lipid metabolism and antioxidant capacity. Postharvest Biol. Technol. 2020, 170, 111315. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Jiang, X.; Lin, M.; Fan, Z. Amelioration of chilling injury and enhancement of quality maintenance in cold-stored guava fruit by melatonin treatment. Food Chem. X 2022, 14, 100297. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Luo, T.; Yin, F.; Liao, L.; Liu, Y.; Guan, B.; Wang, M.; Lai, T.; Wu, Z.; Shuai, L. Postharvest melatonin treatment inhibited longan (Dimocarpus longan Lour.) pericarp browning by increasing ROS scavenging ability and protecting cytomembrane integrity. Food Sci. Nutr. 2021, 9, 4963–4973. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Duan, W.; Li, W.; Wang, Q.; Li, J.; Song, H.; Xu, X. Melatonin maintained higher contents of unsaturated fatty acid and cell membrane structure integrity in banana peel and alleviated postharvest chilling injury. Food Chem. 2022, 397, 133836. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Plaza, M.; Marina, M.L. High-performance thin-layer chromatography and direct analysis in real time-high resolution mass spectrometry of non-extractable polyphenols from tropical fruit peels. Food Res. Int. 2021, 147, 110455. [Google Scholar] [CrossRef]
- Rojas-García, A.; Rodríguez, L.; Cádiz-Gurrea, M.D.L.L.; García-Villegas, A.; Fuentes, E.; Villegas-Aguilar, M.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Determination of the bioactive effect of custard apple by-products by in vitro assays. Int. J. Mol. Sci. 2022, 23, 9238. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B. Decreasing postharvest chilling injury of guava fruit by using melatonin treatment. Adv. Hortic. Sci. 2022, 36, 37–42. [Google Scholar] [CrossRef]
- Alique, R.; Zamorano, J.P.; Calvo, M.L.; Merodio, C.; De la Plaza, J.L. Tolerance of cherimoya (Annona cherimola Mill.) to cold storage. J. Am. Soc. Hortic. Sci. 1994, 119, 524–528. [Google Scholar] [CrossRef]
- Li, C.; Shen, W.; Lu, W.; Jiang, Y.; Xie, J.; Chen, J. 1-MCP delayed softening and affected expression of XET and EXP genes in harvested cherimoya fruit. Postharvest Biol. Technol. 2009, 52, 254–259. [Google Scholar] [CrossRef]
- Shen, W.B.; Li, C.R.; Chen, J.Y.; Xie, J.H.; Lu, W.J. Expansin gene expression in cherimoya fruit is correlated with flesh firmness during fruit ripening and softening. J. Hortic. Sci. Biotechnol. 2009, 84, 333–339. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment on ripening attributes and expression of cell all related genes in cherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–11. [Google Scholar] [CrossRef]
- Alique, R.; Zamorano, J.P. Influence of harvest date within the season and cold storage on cherimoya fruit ripening. J. Agric. Food Chem. 2000, 48, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Yonemoto, Y.; Hagiwara, S.; Tomita, E. Fruit morphological characteristics and the fruit qualities of cherimoya (Annona cherimola Mill.) cultivars. J. Trop. Agric. 1997, 42, 69–70. [Google Scholar]
- Manríquez, D.A.; Muñoz-Robredo, P.; Gudenschwager, O.; Robledo, P.; Defilippi, B.G. Development of flavor-related metabolites in cherimoya (Annona cherimola Mill.) fruit and their relationship with ripening physiology. Postharvest Biol. Technol. 2014, 94, 58–65. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef]
- Goñi, I.; Escribano, M.I.; Merodio, C. Gelatinization and retrogradation of native starch from cherimoya fruit during ripening, using differential scanning calorimetry. LWT-Food Sci. Technol. 2008, 41, 303–310. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Higuchi, H.; Kitano, Y. Effects of storage temperature and wax coating on ethylene production, respiration and shelf life in cherimoya fruit. J. Jpn. Soc. Hortic. Sci. 2002, 71, 643–650. [Google Scholar] [CrossRef]
- Wei, D.; Yang, J.; Xiang, Y.; Meng, L.; Pan, Y.; Zhang, Z. Attenuation of postharvest browning in rambutan fruit by melatonin is associated with inhibition of phenolics oxidation and reinforcement of antioxidative process. Front. Nutr. 2022, 9, 905006. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Kim, H.K.; Yang, K.I. Melatonin and melatonergic drugs in sleep disorders. Transl. Clin. Pharmacol. 2022, 30, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, F.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Mao, L.C.; Wang, G.Z.; Zhu, C.G.; Pang, H.Q. Involvement of phospholipase D and lipoxygenase in response to chilling stress in postharvest cucumber fruits. Plant Sci. 2007, 172, 400–405. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Santamarina, J.; Guillén, F.; Ilea, M.I.M.; Ruiz-Aracil, M.C.; Valero, D.; Castillo, S.; Serrano, M. Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit. Int. J. Mol. Sci. 2023, 24, 3787. https://doi.org/10.3390/ijms24043787
Medina-Santamarina J, Guillén F, Ilea MIM, Ruiz-Aracil MC, Valero D, Castillo S, Serrano M. Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit. International Journal of Molecular Sciences. 2023; 24(4):3787. https://doi.org/10.3390/ijms24043787
Chicago/Turabian StyleMedina-Santamarina, Jorge, Fabián Guillén, Mihaela Iasmina Madalina Ilea, María Celeste Ruiz-Aracil, Daniel Valero, Salvador Castillo, and María Serrano. 2023. "Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit" International Journal of Molecular Sciences 24, no. 4: 3787. https://doi.org/10.3390/ijms24043787
APA StyleMedina-Santamarina, J., Guillén, F., Ilea, M. I. M., Ruiz-Aracil, M. C., Valero, D., Castillo, S., & Serrano, M. (2023). Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit. International Journal of Molecular Sciences, 24(4), 3787. https://doi.org/10.3390/ijms24043787








