Abstract
As the greatest defense organ of the body, the skin is exposed to endogenous and external stressors that produce reactive oxygen species (ROS). When the antioxidant system of the body fails to eliminate ROS, oxidative stress is initiated, which results in skin cellular senescence, inflammation, and cancer. Two main possible mechanisms underlie oxidative stress-induced skin cellular senescence, inflammation, and cancer. One mechanism is that ROS directly degrade biological macromolecules, including proteins, DNA, and lipids, that are essential for cell metabolism, survival, and genetics. Another one is that ROS mediate signaling pathways, such as MAPK, JAK/STAT, PI3K/AKT/mTOR, NF-κB, Nrf2, and SIRT1/FOXO, affecting cytokine release and enzyme expression. As natural antioxidants, plant polyphenols are safe and exhibit a therapeutic potential. We here discuss in detail the therapeutic potential of selected polyphenolic compounds and outline relevant molecular targets. Polyphenols selected here for study according to their structural classification include curcumin, catechins, resveratrol, quercetin, ellagic acid, and procyanidins. Finally, the latest delivery of plant polyphenols to the skin (taking curcumin as an example) and the current status of clinical research are summarized, providing a theoretical foundation for future clinical research and the generation of new pharmaceuticals and cosmetics.
1. Introduction
The skin is the largest organ in humans, accounting for approximately 10–15% of the body weight [1]. It plays a role in protection, excretion, immunity, body temperature regulation, and perception of external stimuli [2]. Environmental pollution and ozone layer destruction have recently increased the intensity of PM2.5 and ultraviolet (UV) rays in the atmosphere, thereby increasing the risk of skin cancer, dermatitis, and skin cellular senescence. This is because environmental factors stimulate the skin, leading to oxidative stress [3,4,5]. Oxidative stress is caused by ROS overproduction in biological systems, and these ROS cannot be completely removed by the antioxidant system, which is essentially a state of disturbed “redox homeostasis” [6]. On the one hand, excess ROS directly damage lipids, proteins, and DNA, causing oxidative damage to the skin. Binding of ROS to DNA leads to proto-oncogene activation, that to proteins leads to collagen degradation, and that to lipids results in lipid peroxidation and increased cell membrane permeability [6]. UV radiation causes oxidative damage to macromolecules, leading to inflammation, photoaging, and many skin cancer types [7]. On the other hand, excess ROS are involved in cell signaling pathways, altering the expression of numerous genes and leading to skin cellular senescence, inflammatory skin conditions, such as psoriasis and dermatitis, and cancers, such as melanoma and squamous cell carcinoma (SCC).
Several antioxidants are currently available to treat oxidative stress-induced skin cellular senescence, inflammation, and cancer. Biological antioxidants are defined as substances that significantly delay or prevent the oxidation of an oxidizable substrate when the concentration is lower than that of the oxidizable substrate [8]. The human body’s antioxidant system that functions against ROS mainly comprises antioxidant enzymes and non-enzymatic antioxidants capable of preventing oxidative damage in the human skin [9]. Antioxidant enzymes include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GSH-Re), etc. [10]. Non-enzymatic antioxidants mainly are low-molecular-weight substances capable of neutralizing free radicals, including antioxidant vitamins and their derivatives, cofactors, melatonin, minerals, sulfur compounds, non-protein nitrogen compounds, and plant antioxidants [11,12,13,14,15]. Plant polyphenols are types of remarkable antioxidants in a different biological system, including red blood cells, because the ortho-phenolic hydroxyl group in the phenolic hydroxyl structure is easily oxidized into a quinone structure. This structure has considerable potential to scavenge free radicals, such as ROS [16]. Moreover, these polyphenols can activate cell signaling pathways and express antioxidant enzymes. Nonetheless, plant polyphenols are poorly soluble, easily metabolized, and have low bioavailability. Currently, several studies have investigated the oral preparation of plant polyphenols, but studies on the topical application of plant polyphenols to the skin, particularly clinical research, are insufficient. With biomaterial development, plant polyphenols could be stably and efficiently delivered to the skin, eliminating low biocompatibility barriers. This article introduces the targets of plant polyphenols in detail to prevent oxidative stress-induced skin cellular senescence, inflammation, and cancer, as well as the latest plant polyphenol delivery system. These data serve as a theoretical foundation for future clinical research, novel medicine development, and cosmetic product generation.
2. Sources of ROS in the Skin
2.1. Endogenous Factors of ROS
Endogenous ROS production involves intracellular enzymes (Figure 1), including the mitochondrial electron transport chain, xanthine oxidoreductase (XOR), NADPH oxidase, cytochrome P450 family enzymes, several peroxidases, cyclooxygenases, and lipid oxygenase [17]. These enzymes are widely disseminated in the mitochondria, endoplasmic reticulum, cell membrane, and cytosol, with mitochondria and endoplasmic reticulum being major contributors to ROS production. In the mitochondria, oxygen is converted to superoxide anion by mitochondrial complex I or III or xanthine oxidase. This anion is converted to hydrogen peroxide by SOD. Under normal conditions, hydrogen peroxide is decomposed into water and oxygen by antioxidant enzymes (TPx, GPxs, and CAT) in the mitochondria, whereas under oxidative stress, hydrogen peroxide generates ROS through the Fenton reaction [18]. In addition, during the oxidative metabolism of long-chain fatty acids, peroxisomes generate H2O2 radicals [19,20,21]. During protein production, NO• is produced while nitric oxide synthase is being synthesized [19,20,21]. Thus, endogenous ROS are mainly generated as a by-product of oxidative phosphorylation and enzymatic reactions that support aerobic metabolism.
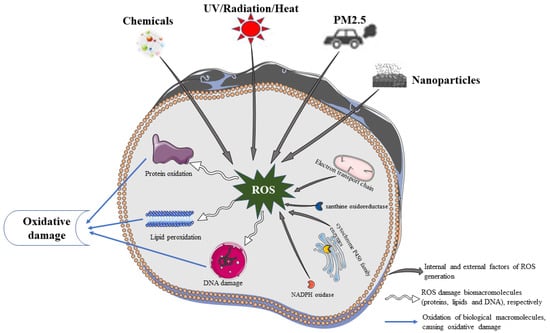
Figure 1.
Oxidative damage of biological macromolecules.
2.2. Exogenous Factors of ROS
Exogenous ROS are mainly induced by environmental factors, with ultraviolet radiation being the main source. UV light is typically divided into three bands based on its biological effects: long-wave UV (UVA), medium-wave UV (UVB), and short-wave UV (UVC). UVA has a strong penetrating power and can reach the dermis, thereby tanning the skin, whereas UVB has a moderate penetrating power and can sunburn the epidermis [22]. Sunlight is the main source of ultraviolet radiation, and ultraviolet radiation reaching the earth’s surface consists of 95% UVA and 5% UVB [23]. Excessive skin exposure to sunlight generates high ROS levels and reduces GSH-Px and GSH-Re activities. ROS production by keratin-forming cells and fibroblasts is associated with UV radiation [24]. In addition, various factors, such as radiation, PM2.5, nanoparticles, chemicals, and thermal stimuli, may contribute to ROS generation. Table 1 summarizes the exogenous factors and adverse consequences of ROS production in some experiments.

Table 1.
Summary of exogenous factors and adverse consequences of ROS production in some experiments.
Both endogenous and exogenous factors stimulate ROS generation, and thus have many adverse consequences on the skin, thereby causing skin cellular senescence, inflammation, and cancer.
3. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer
Oxidative stress causes skin cellular senescence, inflammation, and cancer through two possible mechanisms: (1) ROS directly damage biomolecules, thereby causing oxidative damage; (2) ROS mediate cellular signaling pathways, thus affecting gene expression.
3.1. Oxidative Damage of Biological Macromolecules
Endogenous and exogenous stimuli produce large amounts of ROS that damage biological macromolecules, such as proteins, lipids, and DNA. This leads to skin cellular senescence, inflammation, and cancer (Figure 1).
3.1.1. Biomacromolecule Damage and Skin Cellular Senescence
Oxidative stress is a crucial factor that affects skin cellular senescence. Genetic factors drive intrinsic aging, whereas the external environment drives extrinsic aging. Long-term exposure to UV radiation is the primary cause of extrinsic photoaging [17,43]. Both external and internal stimuli trigger ROS production in the skin, further reducing antioxidant levels in the skin and damaging DNA, proteins, and lipids [44]. Under this condition, the number of dermal fibroblasts and collagen and elastin synthesis in the extracellular matrix (ECM), particularly type I and type III of collagen, are reduced [45]. The skin then exhibits visible signs of aging, including thinning, wrinkles, dehydration, and loss of elasticity.
3.1.2. Biomacromolecule Damage and Skin Inflammation
Exposure of the skin to endogenous or exogenous stimulation triggers excessive oxidative stress, thus inducing ROS production. ROS react with unsaturated fatty acids with acyl double bonds to generate lipid peroxidase. This enzyme forms hydrophilic pores in biological membranes, thereby resulting in increased cellular or mitochondrial membrane permeability, oxidative damage, and inflammatory reactions [46]. Furthermore, several studies have linked oxidative damage to various inflammatory skin conditions, such as psoriasis and dermatitis [47]. Urinary nitrate, malondialdehyde, and 8-hydroxydeoxyguanosine levels were higher in patients with psoriasis and atopic dermatitis (AD) than in healthy controls, which suggested that the development of inflammatory skin diseases is associated with the oxidation of nitric oxide, lipids, and DNA [48,49].
3.1.3. Biomacromolecule Damage and Skin Cancer
Oxidative damage is the primary cause of melanoma and non-melanoma [e.g., SCC and BCC] [50]. Exposure to UV radiation is among the leading causes of oxidative stress-induced skin cancer. UV irradiation of the skin induces extensive ROS generation, causing nuclear DNA damage through the synthesis of pyrimidine (6–4), lobutane pyrimidine dimers (CPDS), 8-OXODG, and pyrimidinone photoproducts. When the genetic damage is not severe, the tumor suppressor gene p53 blocks the cell cycle in the G1 phase by DNA repair enzymes, repairing the mutant gene before replication. When the gene damage is severe and beyond effective repair, p53 controls Bax and Bcl-2 and mediates apoptosis. However, chronic oxidative stress can activate proto-oncogenes (BRAF, N-Ras, RAC1, and PTEN) and deactivate tumor suppressor genes (p53 and PTCH) [46,51]. Thus, the mutated gene-carrying DNA enters the cell division cycle, resulting in the proliferation and metabolic cycle disorder of tumor cells.
3.2. Oxidative Stress-Related Signaling Pathway
ROS produced by cells exposed to oxidative stress mediate mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB), phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) (PI3K/AKT/mTOR), Janus kinase/signal transducers and activators of transcription (JAK/STAT), Kelch-like epichlorohydrin-associated protein-1(Keap1)–nuclear factor(E2)-related factor 2 (Nrf2), and silent information regulator 1 (SIRT1)/forkhead box O (FOXO) (SIRT1/FOXO) signaling pathways. Thus, they regulate the expression of skin cellular senescence-, inflammation-, and cancer-related genes (Figure 2).
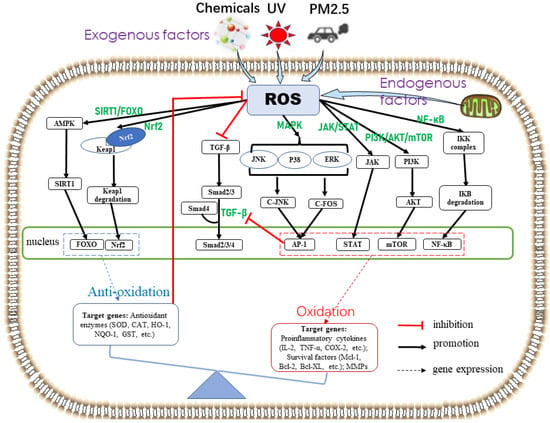
Figure 2.
Skin oxidative stress-related signaling pathways. Exogenous stimuli (e.g., UV, chemicals, and PM2.5) and/or endogenous factors (e.g., mitochondrial oxidase and metabolism) induce ROS overproduction. ROS activate the antioxidant defense system and promote the expression of antioxidant enzymes through Nrf2 and SIRT1/FOXO pathways, which in turn reduce ROS production. When the antioxidant defense system fails to completely scavenge ROS, oxidative stress is induced. Excessive ROS activate MAPK, JAK/STAT, PI3K/AKT/mTOR, and NF-κB signaling pathways, thereby resulting in the expression of cytokines associated with skin cellular senescence, inflammation, and cancer. The transforming growth factor-β signaling pathway, related to collagen synthesis, is inhibited by ROS and the transcription factor AP-1 to accelerate skin cellular senescence.
3.2.1. Skin Cellular Senescence-Related Signaling Pathways
Like aging in other organs, skin cellular senescence is characterized by a gradual loss of function and regenerative potential, whereas cellular senescence is the result of aging when cells stop dividing and reach a state of stasis. Oxidative stress generates ROS, and increased levels of ROS lead to cellular senescence. Senescent cells accumulate in aging tissues and organs, impairing physiological processes, including regeneration, and leading to aging [52]. Compared with young cells, senescent cells exhibit increased beta-galactosidase activity and cell cycle inhibitors P21 and P16, as well as reduced lamin B1, and produce senescence-associated secreted protein (SASP) [53]. Excessive ROS promote the production of matrix metalloproteinases (MMPs) and inflammatory cytokines by activating two major SASP regulatory pathways in the senescent cells: the MAPK and NF-κB pathways [54]. Moreover, the transforming growth factor-β (TGF-β) signaling pathway, which is associated with collagen synthesis, is inhibited in the senescent cells.
More precisely, oxidative stress occurs in cells, and excessive ROS activate the MAPK signaling pathway. This pathway in turn activates the downstream transcription factor c-Jun, which forms a dimer with the transcription factor c-fos, inducing an increase in AP-1 levels. In addition, ROS can activate the NF-κB signaling pathway. AP-1 and NF-κB activation increases the expression of MMPs, which can almost completely degrade various protein components in the ECM, gradually destroying dermal integrity [54]. The long-term effects of MMPs can severely damage skin collagen and produce large amounts of abnormal elastic fibrous substances, which can cause skin cellular senescence. The expression of MMPs is increased in senescent cells, whereas that of tissue inhibitors of metalloproteinases (TIMPs) is decreased [55]. Additionally, inflammatory skin cellular senescence may be triggered when inflammatory factors intervene [56,57]. As ligands, inflammatory factors participate in cell signaling pathways and accelerate cellular senescence. The results of experiments conducted by using UVB-induced immortalized human keratinocytes (HaCaTs) and H2O2-induced human bioactive skin fibroblasts (BJ cells) as models suggested that the antioxidant AYAPE counteracts skin cellular senescence by reducing MMP-1 production in the MAPK/AP-1 signaling pathway and inhibits fibroblast aging [58]. Lee et al. found that cordycepin inhibited UVB-induced MMP expression and reduced skin cellular senescence by inhibiting the NF-κB pathway in human dermal fibroblasts (HDFs) [59].
In the human skin, TGF-β, a multifunctional cytokine controlling many aspects of cell function [60], stimulates dermal fibroblast proliferation, induces the synthesis of ECM collagen and elastin, and suppresses MMP expression [61]. The process of TGF-β signaling pathway activation is as follows. The extracellular TGF-β ligand binds to receptor type II (TβRII) on the cell membrane, which induces autophosphorylation. TβRII then activates type I receptor, which in turn phosphorylates R-Smad (Smad2/3). The phosphorylated R-Smad binds and phosphorylates co-Smad (Smad4) after activation. Finally, the Smad2/3/4 complex is transported to the nucleus to regulate transcription, upregulate P300 transcriptional activity, and promote histone H4K16 acetylation and collagen transcription. Moreover, TGF-β induces type I collagen production by stimulating the expression of the connective tissue growth factor (CTGF) gene [61,62,63]. Duncan et al., who conducted in vitro and in vivo studies using normal rat kidney fibroblasts, demonstrated that CTGF could induce collagen synthesis and that transfection with the antisense CTGF gene blocked TGF-β-mediated stimulation of collagen synthesis. In a mouse experiment, TGF-β activation of the human CTGF promoter/lacZ reporter gene was inhibited by subcutaneously injecting transgenic mice with collagen carboxy-terminal peptide (CTX), and CTGF was found to be essential for TGF-β-induced collagen synthesis in fibroblasts [64]. However, in cells exposed to UV-stimulated oxidative stress, excessive ROS and AP-1 production inhibits the TGF-β signaling pathway, reducing type I collagen synthesis and leading to skin cellular senescence [55,61].
In conclusion, skin cellular senescence is associated with the degradation of various ECM protein components. MMPs can degrade these components and are regulated by MAPK/AP-1 and NF-κB signaling pathways. The TGF-β signaling pathway can promote protein production in the ECM, but is inhibited by AP-1. In addition, inflammatory cytokine release can accelerate skin cellular senescence.
3.2.2. Skin Inflammation-Related Signaling Pathways
As secondary messengers, ROS mediate pro-inflammatory signaling pathways and regulate the expression of various inflammation-related genes, thereby resulting in oxidative stress. Oxidative stress leads to oxidative damage and causes inflammatory skin diseases, such as psoriasis, solar dermatitis (SD), atopic dermatitis (AD), and contact dermatitis (CD). The development of these diseases is positively associated with the activation of MAPK, JAK/STAT, and NF-κB signaling pathways.
Psoriasis is a chronic inflammatory skin problem with a high global prevalence [65]. Although psoriasis is a multifactorial disease with its exact etiology unknown, its onset and progression are related to the activation of inflammatory signaling pathways [66,67,68]. NF-κB is a multifunctional transcription factor regulating the gene expression of various cytokines and inflammatory mediators. Thus, it regulates immune responses, participates in intracellular signal transduction, and regulates tissue cell pathology and physiology [69]. Various cytokines and adhesion molecules, including interleukin (IL)-1, IL-2, IL-6, IL-8, IL-12, IL-18, TNF-α, and nerve growth factor, are abnormally expressed in the lesions of psoriasis patients, suggesting that psoriasis is associated with the release of NF-κB-regulated inflammatory cytokines [70]. NF-κB is present in the promoters or enhancers of genes encoding these factors. In the absence of exogenous stimulation, IκB exists in the cell cytoplasm in an inactive form and binds to NF-κB, preventing NF-κB pathway activation. Under oxidative stress, ROS mediates TAK1 and RIP1 in cells, and these kinases phosphorylate the downstream NEMO/IKKα/IKKβ complex. The phosphorylated complex then further phosphorylates the downstream IκB, leading to ubiquitination degradation and release of the p50/p65 (NF-κB) complex involved in nuclear transcriptional regulation. Studies have reported that the CpG oligodeoxynucleotide induces psoriasis by expressing IL-17A through the NF-κB signaling pathway [71]. Moreover, psoriasis development has been associated with the MAPK signaling pathway. Keratinocyte hyperproliferation and neutrophil infiltration at the lesions are among the crucial pathological changes in psoriasis [72]. Studies have shown that MAPK can stimulate the expression of inflammatory factors or chemokines by regulating downstream transcription factors, thereby regulating Th cell differentiation and promoting the chemotaxis of T cells, DC cells, and neutrophils [73]. Moreover, MAPK promotes ki-67, PCNA, and keratin expression in keratinocytes, thus promoting keratinocyte proliferation. MAPK was significantly higher in psoriasis patients than in people with normal skin, and phosphorylated ERK was highly expressed in the spines of psoriatic lesions and the basal nucleus [74,75]. Other studies have found that total protein and phosphorylated STAT3 levels are significantly higher in psoriatic lesions than in the normal skin, and high expression of active STAT3 can lead to typical psoriasis-like symptoms in mice [76,77]. Furthermore, psoriasis-related factors, such as IL-17A, IL-17F, IL-22, and IL-23R, can be transcriptionally activated by STAT3 [78], suggesting that STAT3 plays a promoting role in psoriasis development. Thus, STAT3 is an important target for psoriasis treatment.
SD, AD, and CD are also associated with inflammatory signaling pathways. The acute response of the skin to UV rays is inflammation, such as erythema and edema, and ROS production. Oxidative stress-induced ROS can initiate the activation of inflammatory and pro-inflammatory cytokines [56]. Several pro-inflammatory signaling pathways, including NF-kB, MAPK, and JAK/STAT, are activated. Many proinflammatory cytokines and environmental stress can trigger the p38 MAPK cascade. Studies have suggested that the p38 MAPK pathway may be involved in UV-induced inflammation by regulating cyclooxygenase 2 (COX-2) activity [79,80,81], which causes SD. Other studies have observed NF-κB signaling pathway activation, expression of pro-inflammatory mediators, and inflammatory skin lesions in hairless mice chronically exposed to UVB radiation [82]. Genetic studies of UV-irradiated human epidermal cells have revealed that increased inflammatory processes were associated with the upregulation of the JAK/STAT signaling pathway [83]. AD, also known as atopic eczema, is a common chronic inflammatory skin disease characterized by recurrent, pleomorphic skin lesions; dry skin; and intense itching [84]. External application of an oligodeoxynucleotide ointment that induces NF-κB activation could improve dermatitis symptoms in the NC/Nga atopic mouse model, indicating that NF-κB deficiency was related to AD pathogenesis [85]. CD is an immune system-mediated inflammatory skin problem [86]. In the initial stage of allergic CD in mice, haptens activated Langerhans cells, while dendritic cell activation occurred mainly through the activation of MPAK/p38, suggesting that CD pathogenesis is related to the MAPK signaling pathway [87]. Furthermore, the production of IL-22, an immunoregulatory cytokine associated with CD development, was dependent on TLR4, MAPK/p38, NF-κB, and JAK/STAT signaling pathways [88].
In conclusion, oxidative stress-induced skin inflammation is associated with the release of cellular inflammatory factors (e.g., IL-2, TNF-α, and COX-2), and the expression of these factors is regulated by NF-κB, MAPK, and JAK/STAT signaling pathways.
3.2.3. Skin Cancer-Related Signaling Pathways
ROS promotes the abnormal proliferation, metastasis, and penetration of various tumor cells by activating oxidative stress-related pathways, such as the PI3K/AKT/mTOR, MAPK, NF-κB, and JAK/STAT pathways [51]. Two main types of skin cancer are caused by oxidative stress, namely, melanoma and non-melanoma skin cancers (NMSCs).
Both melanoma skin cancers and NMSCs are common and have high metastatic rates. NMSCs mainly include BCC and SCC, accounting for 70% and 25% of NMSCs, respectively [89]. Skin cancer is associated with tumor cell proliferation, metastasis, and angiogenesis [90], and oxidative stress in the skin activates multiple cancer-related signaling pathways. Studies have linked skin cancer development to MAPK/ERK [91,92,93]. The study results revealed that most melanoma patients carry BRAF mutations that may activate the MAPK/ERK signaling pathway [94]. This entire pathway is mainly composed of protein kinases, such as RAS→RAF→MEK→ERK [95], and is sequentially activated by catalytic phosphorylation of superior protein kinases. When constitutively activated, the MAPK/ERK signaling pathway promotes melanoma development by increasing cell proliferation and tumor invasion and metastasis, and inhibiting apoptosis [96,97,98]. Moreover, the MAPK/ERK signaling pathway was activated in both mouse and human cutaneous basal melanomas, suggesting its involvement in BCC development [99].
PI3K is a specific kinase that catalyzes phosphatidylinositol lipids. This heterodimer has a P110 catalytic subunit and a P85 regulatory subunit, with protein and lipid kinase activities [100]. Akt, a serine/threonine protein kinase, is a crucial downstream effector molecule of PI3K. After activation through phosphorylation, PI3K can regulate the cell cycle by inactivating various apoptotic effector molecules, activate telomerase activity, and promote tumor growth and invasion [101]. PI3K/Akt activation occurs as follows. Under normal conditions, lipid phosphatase (PTEN) metabolizes (dephosphorylates) PIP3 back to the metabolite PIP2, thereby terminating the PI3K signaling pathway. Under oxidative stress, PI3K phosphorylates PIP2 to PIP3. The phosphorylated phosphogroup 3 of PIP3 can simultaneously recruit PDK1 and AKT proteins to the plasma membrane, causing PDK1 to phosphorylate threonine (T308) at position 308 of the AKT protein and leading to partial AKT activation. Activated AKT further activates downstream regulatory signaling pathways, including mTOR, MAPK, FOXO, NF-κB, and p53 pathways. Therefore, the tumor suppressor gene PTEN reduces tumor cell adhesion and viability and prevents their invasion and metastasis by regulating the PI3K/Akt pathway. In addition, mTOR is among the key regulatory molecules downstream of AKT, and activated mTOR promotes cancer cell proliferation and angiogenesis.
The JAK/STAT pathway is involved in inflammation and immunity associated with various human pathologies, including cancer [102]. The basic transduction process of this pathway is as follows. Binding of the ligand to the receptor dimerizes the receptor, and the receptor-bound JAK is activated through self-phosphorylation [103,104]. STAT then binds to the receptor and is dimerized, which is then activated by JAK phosphorylation. The phosphorylated STAT dimer is finally translocated to the nucleus, where it binds directly to DNA [105,106]. STAT family proteins act as both cytoplasmic signaling proteins and nuclear transcription factors. As nuclear transcription factors, they activate many different gene types, including some involved in tumorigenesis, such as anti-apoptotic genes (Bcl-2, Bcl-XL) [107,108], cell cycle regulatory genes (c-Myc, CycD) [109], lipid metabolism genes, and cell differentiation genes (AOX, GFAP) [110]. Genes involved in the JAK/STAT signaling pathway are highly expressed in tumors and SCCs at a high metastasis risk [111]. JAK/STAT also endorses the growth and metastasis of carcinogenesis-related skin cancer cells [111,112,113,114].
The NF-κB pathway allows the survival, proliferation, anti-apoptosis, and metastasis of melanoma cells [115]. Growing evidence has shown that NF-κB target genes can significantly promote cell survival. Moreover, ROS can activate NF-κB signaling to promote angiogenesis and melanoma progression [116]. Recent reports have also suggested that the p65 subunit of NF-κB is critical for skin cancer development in mice. p65 loss prevented SCC development and tumor progression [117].
Conclusively, skin cancer is related to tumor cell proliferation, invasion, metastasis, and angiogenesis. The MAPK/ERK signaling pathway enhances cell proliferation, invasion, and metastasis. PI3K/AKT/mTOR and NF-κB signaling pathways promote cell proliferation and angiogenesis. The JAK/STAT signaling pathway expresses the anti-apoptotic genes (Bcl-2 and Bcl-XL) and regulates the cell cycle.
3.2.4. Antioxidant Defense-Related Signaling Pathway
Skin cellular senescence, inflammation, and cancer are associated with ROS generation. Efficient ROS detoxification is the only means to prevent, or at least limit, ROS-induced damage in cells and is achieved through low-molecular-weight antioxidants, the tripeptide glutathione, and ROS detoxification enzymes and antioxidant proteins [118]. Various NADPH-triggered cytoprotective proteins include quinone oxidoreductase-1 (NQO1), heme oxygenase-1 (HO-1), γ-glutamylcysteine synthase (γ-GCS), SOD, GSH-Px, and glutamate sulfur transferase (GST), which are regulated by Nrf2 and SIRT1/FOXO signaling pathways.
The Nrf2 signaling pathway is a key pathway regulating the cellular oxidative stress response. The downstream phase II metabolic enzymes and antioxidant proteins/enzymes regulated by KEAP1 play a crucial role in cellular defense and protection [119]. Nrf2, which belongs to the CNC regulatory protein family, is a transcription factor with a basic leucine zipper structure [120]. It is widely distributed in various human organs and is the main regulator of cellular redox reactions. Under normal physiological conditions, Nrf2 mainly binds to its inhibitor Keap1, which exists in an inactive state in the cytoplasm. Once oxidative stress occurs, Keap1 degrades rapidly through the ubiquitin-proteasome pathway, thus maintaining the low transcriptional activity of Nrf2 [121]. In cells stimulated by ROS or other nucleophiles, Nrf2 is uncoupled from Keap1. The activated Nrf2 enters the nucleus and binds to Maf protein to form a heterodimer. This heterodimer t binds ARE to induce target gene expression and regulate the transcriptional activity of phase II enzymes, antioxidant enzymes, or drug transporters to exert antioxidant effects [122,123]. When activated, the Keap1–Nrf2/ARE signaling pathway can initiate the expression of multiple downstream target proteins. These activated target proteins can regulate the body’s redox balance, allowing the body to recover from an oxidative stress state to a normal physiological state. The Nrf2-regulated downstream target proteins have been confirmed to be categorized into metabolic phase II enzymes, antioxidant proteins/enzymes, proteasome/molecular chaperones, anti-inflammatory factors, and metabolic phase III enzymes (drug transporters). For example, the NADPH-regulated downstream target proteins include NQO1, HO-1, γ-GCS, and so on.
The activity of FOXO, a crucial autophagy regulator, is mainly regulated through SIRT1 deacetylation. The SIRT1/FOXO pathway is activated by cellular oxidative stress and may allow the development of new strategies for the prevention and treatment of skin cellular senescence, inflammation, and cancer. This pathway protects cells in two main ways. On the one hand, SIRT1-regulated FOXO transcription factors can promote autophagy in oxidation-damaged biomacromolecules. Autophagy is a vital basic metabolic mechanism that maintains cell homeostasis through phagocytosis and degradation of unnecessary or dysfunctional cellular components in duplex autosolutes [124]. α-Neoendorphins can reduce UVB-induced skin photoaging by activating autophagy [125]. The expression of lysosomal cathepsins was decreased in the skin of patients with psoriasis and AD. Impaired autophagy may lead to inflammation and impaired keratinocyte differentiation, whereas stimulation of autophagy may have a therapeutic potential for inflammatory skin diseases [126]. Srivastava et al. discovered that the FOXO transcription factor mediated the anti-angiogenic effect of resveratrol and effectively inhibited tumor development and metastases [127]. On the other hand, FOXO can activate antioxidant enzymes, such as SOD and CAT, to eliminate oxidative stress-induced ROS generation [128,129].
In conclusion, both Nrf2 and SIRT1/FOXO signaling pathways can express antioxidant enzymes to reduce oxidative stress-induced ROS production, thereby preventing ROS-mediated oxidative damage. In addition, the SIRT1/FOXO signaling pathway protects cells through autophagy, thereby reducing skin cellular senescence, inflammation, and cancer.
4. Therapeutic Potential of Plant Polyphenols
4.1. Health-Promoting Benefits of Natural Products
In previous studies, it has been found that natural products play an important role in human health. Amin. et al. observed that Chlorella has a protective effect on the pancreas of diabetic animals, which indicates that Chlorella can improve the condition of diabetic patients by regulating insulin secretion [130]. In addition, the plant extract Miswak has been shown to have antioxidant and anti-angiogenic effects, and is a potential antioxidant and antitumor agent [131]. Another study showed that dandelion can prevent oxidative stress, liver fibrosis, and inflammatory response in rats [132]. All these studies showed that natural products are helpful to human health and have great development potential.
4.2. Structure and Classification of Plant Polyphenols
The nutritional and medicinal value of active components of natural plants, such as polyphenols, polysaccharides, proteins, and alkaloids, are commonly known; they are beneficial for the treatment and prevention of skin oxidative damage [133]. Because of their wide variety of sources and high biological action, plant polyphenols are selected as possible phytochemicals for the treatment of oxidative stress-induced skin cellular senescence, inflammation, and cancer. Plants producing fruits, vegetables, and cereals contain polyphenols. Polyphenols are predominantly composed of at least two benzene rings and one or more hydroxyl substituents [134]. Owing to the vast distribution and diversity of plant polyphenols, they are primarily divided into six categories according to their chemical structures, namely, flavonoids, phenolic acids, stilbenes, lignans, tannins, and curcuminoids [135], with flavonoids being the most extensively distributed.
4.3. Antioxidative, Anti-Inflammatory, and Anticancer Activities of Plant Polyphenols
Owing to their unique structures, plant polyphenols possess antioxidant, anti-inflammatory, and anticancer properties. Phloretin, a polyphenol found in apples, exhibit anti-inflammatory and anticancer activities and can prevent cancer cell growth and migration [136]. Cactus produces numerous phenolic compounds, including quercetin, isorhamnetin, and kaempferol derivatives that assist in regulating the infiltration and production of soluble inflammatory mediators in cells and, therefore, play a major role in inflammation [137]. Coumaric acid possesses strong free radical scavenging activity and, hence, can lower the negative consequences of oxidative damage, including arthritis and cardiovascular disease [138]. Flavonoids limit lysozyme, β-glucuronidase, and arachidonic acid production, as well as modulate the expression of genes encoding cytokines and pro-inflammatory molecules [139]. Proanthocyanidins may be used for enhancing body functioning, such as lowering inflammation, alleviating oxidative stress, combating cancer, and influencing the immune system [140]. Phenolic acids, such as chlorogenic acid, positively contribute to improving the intestinal health of animals and enhancing the body’s antioxidant capacity [141].
4.4. Regulatory Mechanism of Plant Polyphenols
Based on the classification and structure of plant polyphenols, seven types of polyphenols were selected for discussion. Among them, flavonoids include catechin and quercetin, phenolic acids include ellagic acid, stilbenes include resveratrol, tannins include proanthocyanidins, lignans include honokiol, and curcuminoids include curcumin. Table 2 summarizes their molecular targets or action mechanisms of preventing oxidative stress-induced skin cellular senescence, inflammation, and cancer.

Table 2.
A summary of molecular targets of several polyphenols in preventing skin cellular senescence, inflammation, and cancer.
4.4.1. Curcumin
Curcumin is a natural plant polyphenol and an orange-yellow diketone pigment found in turmeric rhizomes [220,221]. It exhibits anti-inflammatory, antitumor, and antioxidant properties [222] and can chelate iron ions [143].
Studies have investigated the role of curcumin in oxidative stress-induced skin cellular senescence. UV irradiation and hydrogen peroxide stimulation can induce oxidative stress in human immortalized epidermal keratinocytes (HaCat) and HDFs, as evidenced through elevated levels of ROS and malondialdehyde (MDA), decreased antioxidant enzyme activity, DNA damage, protein carbonylation, and apoptosis [223,224,225,226]. Curcumin not only reduces the aforementioned oxidative damage, but also activates TGF-β and Nrf2 signaling pathways, enhances collagen production and antioxidant enzyme expression, and decreases MMP-1 and MMP-3 levels [146,223,224,227].
Furthermore, curcumin can prevent and reduce oxidative stress-induced skin inflammation. Curcumin inhibited NADPH oxidase and reduced endogenous ROS, thereby inhibiting lipopolysaccharide-induced inflammation [144]. Moreover, curcumin alleviated skin damage in psoriatic mice by decreasing IL-22 and IL-18 expression with a 47% inhibition rate [151]. This study also reported that curcumin protected the skin by neutralizing free radicals and inhibiting MAPK and NF-κB signaling pathways. Thus, AP-1 transcription and production of cytokines (e.g., IL-33, TNF-α, IL-4, IL-5, IL-13, and IL-31) were inhibited by curcumin, thereby reducing skin inflammation in psoriatic and AD mice [150,151,152,228].
Moreover, various studies have demonstrated that curcumin has anticancer properties. Kakar et al. found that curcumin suppressed TPA-induced mRNA expression of proto-oncogenes (C-FOS, C-JUN, and C-MYC) and promoted apoptotic p53 protein expression in mice skin. Furthermore, curcumin inhibited the PI3K/AKT/mTOR pathway in SCC [158]. Because of its poor water solubility and low biological rate, researchers developed hyaluronan, nano-microemulsion, and hydrogel to enhance the biocompatibility and more effectively exert the antiaging, anti-inflammatory, and anticancer properties of curcumin [227,229,230,231].
4.4.2. Catechins
Many types of catechins exist, with the most common being epigallocatechin gallate (EGCG) [161]. Green tea, apples, grapes, and other plants are the primary sources of catechins [232]. In 32 random volunteers who drank 3 cups of green tea per day for 2 weeks, green tea enhanced the body’s ability of free radical elimination [163]. Additionally, epicatechin inhibits UVA-mediated unstable iron release and reduces oxidative damage. In addition, EGCG protected rat skin and keratinocytes against oxidative stress [159,160,161,162].
Several studies have investigated the effect of catechins on oxidative stress-induced skin cellular senescence. According to Wagn et al., when combined with YAG laser, catechin can reduce wrinkles and pigmentation, increase collagen, and enhance SOD activity in the skin of photodamaged mice [233]. Catechins isolated from green tea and elm root bark can inhibit MMP-1 expression and may have antiaging effects on fibroblasts, HaCaT cells, and mice [164,165,166,167]. Green tea catechins were found to prevent skin cellular senescence by regulating NF-κB, AP-1, and MAPK signaling pathways [164].
Some studies have demonstrated that catechins can prevent and reduce oxidative stress-induced skin inflammation. Catechin supplementation prevented UV radiation-induced inflammatory erythema [234]. Catechin derivatives suppressed the cytokines TNF, IL-1, and IL-4 in hapten-induced contact dermatitis models [168]. Psoriasis is a multifactorial, complex disease characterized by an inflammatory skin response. Catechins reduced the inflammatory response in psoriatic mice [169,170].
Moreover, several studies have reported the anticancer role of catechins. By regulating antioxidant and inflammatory biomarkers, the catechin emulsion gel inhibited DMBA/TPA-induced cutaneous SCC in mice [172]. Wu et al. observed that EGCG suppressed Bcl-2 expression to trigger death in melanoma cells [173].
4.4.3. Resveratrol
Resveratrol, a polyphenol belonging to the stilbene family, is found in various plants, including grapes, soybeans, blueberries, plums, and peanuts [235,236,237,238]. When the skin is exposed to certain environments, such as sunlight or pollution, or under pathological conditions that induce oxidative stress, resveratrol at non-toxic doses (20–100 μM) can activate the Nrf2 signaling pathway. Among the genes downstream of Nrf2, glutamylcysteinyl ligase and glutathione peroxidase-2 were induced at mRNA and protein levels, thereby increasing cellular antioxidant levels [239].
Several studies have investigated the function of resveratrol in oxidative stress-induced skin cellular senescence. In a H2O2-induced model of human keratinocyte senescence, resveratrol activated FOXO3 and enhanced the expression of target genes, including catalase. Moreover, the senescent cells lacked SIRT1 compared with the non-senescent keratinocytes. This suggested that resveratrol prevents skin cellular senescence by activating the SIRT1/FOXO pathway [178]. Furthermore, resveratrol inhibited the MAPK signaling pathway, reduced COX-2 and MMP expression, and increased SOD, CAT, GSH-Px, and collagen I expression, and thus exerted its antiaging activity [174,175,176,177,178].
In addition, resveratrol can prevent and reduce oxidative stress-induced skin inflammation. PM (particulate matter) causes inflammation in human keratinocytes and is associated with various oxidative stress-induced skin diseases. Shin et al. discovered that resveratrol lowers inflammation by blocking PM-induced ROS, regulating MAPK activation, and decreasing the expression of proinflammatory cytokines [240]. Dinitrochlorobenzene induced AD in NC/Nga mice. In the experimental group, rice containing resveratrol was applied to the backs of the mice. The experimental group mice scratched less frequently, had less severe dermatitis, and had improved transepidermal water loss and skin hydration [180]. Another study showed that resveratrol downregulated NF-κB, thereby reducing inflammation in mice with imiquimod-induced psoriasis [181].
Moreover, studies have confirmed the anticancer properties of resveratrol. The occurrence of cancer is related to the abnormal proliferation of cancer cells. Resveratrol promotes cancer cell apoptosis by activating p53 [183]. Resveratrol inhibited Bcl-2 expression, promoted Bax expression, and induced programmed cell death [182]. Furthermore, resveratrol regulated apoptosis in human epidermoid carcinoma A431 cells through JAK/STAT and mitochondria-mediated pathways [184].
4.4.4. Quercetin
The natural product quercetin (QC) is a flavonoid widely present in many fruits and vegetables (e.g., onion and apple) [241]. It is an antioxidant; however, maintaining QC in the skin in vivo is difficult owing to its high lipid solubility. On evaluating the biological activity of QC microcapsules (TFcQCMC) in a UVB-irradiated mouse model, Vale et al. found that TFcQCMC inhibited antioxidant degradation, 2,2’-azinobis free radicals, and reactive peroxidation hydrogenase and decreased iron concentration. TFcQCMC also decreased inflammatory cytokine production, MMP-9 activity, skin edema, collagen fiber damage, myeloperoxidase activity/neutrophil recruitment, and mast cell and sunburn cell numbers [242]. QC at 5 μg/mL effectively neutralized free radicals in HaCaT and THP-1 cells.
Several studies have investigated the role of QC in oxidative stress-induced skin cellular senescence. On studying the molecular mechanism underlying QC’s action against UV-induced skin cellular senescence, Shin et al. suggested that QC suppressed UV-induced AP-1 and NF-κB transcription and MMP-1 and COX-2 expression and prevented collagen degradation in human skin tissue. Additional investigation revealed that QC reduced ERK, JNK, AKT, JAK2, and STAT phosphorylation [185]. Evidence indicated that SIRT1 regulates cellular senescence and multiple aging-related cellular processes [243].
Moreover, some studies have demonstrated that QC can prevent and minimize oxidative stress-induced skin inflammation. QC suppressed poly(dA:dT)-induced IL-18 secretion in human keratinocytes by inhibiting the IFN-γ-induced JAK2/STAT1 pathway and downregulating AIM2 and pro-caspase-1 expression [191]. In psoriatic and AD mice, QC quercetin reduced the expression of cytokines, such as COX-2, TNF-α, and IL-1, downregulated inflammation-related NF-κB and MAPK pathways, and upregulated the expression of antioxidant enzymes, such as SOD, CAT, and GPx [192,193,194]. QC has been shown to reduce redness, itching, and inflammation in damaged areas of the human skin [244].
Moreover, several studies have reported the role of QC against cancer. Kim et al. discovered that quercetin enhanced levels of the pro-apoptotic protein Bax, phosphorylated-JNK, and phosphorylated-p38, while drastically decreasing the A375SM tumor volume [195].
4.4.5. Ellagic Acid
Ellagic acid, a polyphenol belonging to the phenolic acid family, is produced by various plants, including pomegranate, strawberry, raspberry, cranberry, and grape [245,246,247,248]. Ellagic acid inhibited UV/hydrogen peroxide/MGO-induced photodamage, photoaging, inflammation, and apoptosis in HaCat cells [197,200,249,250,251].
Ellagic acid and dihydromyricetin synergistically acted against UVB-induced photoaging in mice, presumably by activating the TGF-β pathway [200].
In addition, the effects of ellagic acid on TNF-α/IFN-γ-stimulated HaCaT keratinocytes and dust mite-induced AD skin damage have been investigated in NC/Nga mice. By regulating critical inflammatory signaling pathways, such as MAPK and STAT signaling, and transcriptional activators, ellagic acid suppressed inflammatory responses [252].
Khwairakpam et al. discovered that pomegranate has potential anticancer properties. It downregulates multiple signaling pathways, such as NF-κB and PI3K/AKT/mTOR, and decreases the expression of downstream cancer genes, including antiapoptotic genes, VEGF, c-met, cyclins, Cdks, and proinflammatory cytokines [207].
4.4.6. Honokiol
Magnolia officinalis is a physiologically active lignan utilized for centuries in traditional Chinese medicine. It exerts various pharmacological properties, including anticancer, anti-inflammatory, and anxiolytic effects [253].
In UV-induced mouse and keratinocyte aging models, honokiol reduced MMP expression by inhibiting MAPK nuclear translocation and activated NF-κB [216,217].
Honokiol also inhibits inflammatory factors in AD mice, and honokiol acetylation or methylation can improve skin transmission and anti-inflammatory effects [254,255,256].
Honokiol is also very effective in reducing skin cancer incidence. Studies have shown that honokiol reduced skin cancer diversity by 49–58% and tumor volume by 70–89% compared with the control. The 30 μg dose exhibited chemopreventive effects, while the 60 μg dose significantly reduced cancer incidence by 40% compared with the control [257]. Honokiol downregulated the expression of inflammatory factors and cyclin D1, cyclin D2, cdk2, cdk4, and cdk6 proteins, whereas it upregulated the expression of cdk inhibitory proteins p21 and p27, thus inducing apoptosis and DNA fragmentation [258,259,260,261].
Cumulatively, the aforementioned research results demonstrate that plant polyphenols can be used to treat oxidative stress-induced skin cellular senescence, inflammation, and cancer, primarily through the five routes outlined below (Figure 3). Route 1: Plant polyphenols reduce endogenous ROS generation, including chelation of iron ions, to inhibit the Fenton reaction on the electron transport chain and inhibit intracellular oxidase activity. Route 2: The structural peculiarities of polyphenols allow them to directly neutralize excess ROS. Route 3: Polyphenols can directly reduce the oxidative damage caused to macromolecular substances. Route 4: Polyphenols can block ROS-mediated signaling pathways associated with skin cellular senescence, inflammation, and cancer. Route 5: Polyphenols can activate the cellular antioxidant system signaling pathway to produce antioxidant enzymes.
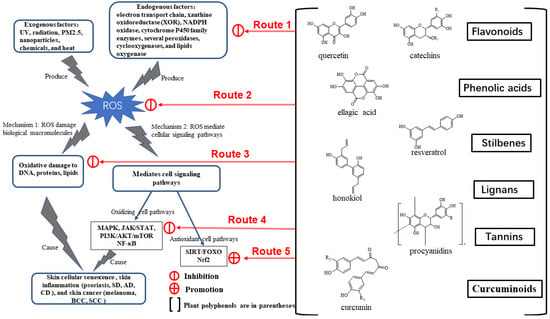
Figure 3.
The regulatory mechanism of plant polyphenols.
4.4.7. Proanthocyanidins
Proanthocyanidins, also known as condensed tannins, are oligomers or polymers of the flavan-3-ol moiety. They are commonly found in fruits, vegetables, grains, nuts, and leaves [262,263]. Proanthocyanidins may be used for enhancing body functions, such as anti-inflammation and anticancer activities, decreasing oxidative stress, and regulating immunity [140].
Proanthocyanidin is a crucial player in fighting skin cellular senescence. Oligomeric proanthocyanidin nanoliposomes reduced UV radiation-induced damage to epidermal epithelial cells (HFF-1) and had strong protective effects on collagen degradation and/or synthesis following UVA exposure. In addition, proanthocyanidin cream reduced UV-induced UPE and protected the skin from UV ray-induced harm [264].
By inhibiting the release of inflammatory substances through the MAPK and NF-κB signaling pathways, proanthocyanidins can alleviate inflammatory skin damage, such as dermatitis and psoriasis [209,210,211].
Proanthocyanidins may inhibit MAPK/ERK to limit skin cancer cell proliferation, reduce the release of NF-κB pathway inflammatory factors, and increase cancer cell autophagy [212,213].
4.5. Delivery Systems for Topical Use of Plant Polyphenols
Although plant polyphenols have considerable bioactivity, their bioavailability is minimal, as most polyphenols are poorly soluble and unstable [265]. At present, cutaneous delivery has emerged as among the most attractive routes in the cosmetic and pharmaceutical industries. This delivery route can overcome some disadvantages of oral drug delivery, such as low bioavailability, metabolic interactions, and cytotoxicity, while ensuring that the polyphenol is continuously and stably released at the site of action. Nevertheless, normal skin possesses a formidable barrier to drug absorption, mostly because of its particular lipid composition and stratum corneum tissue. To improve polyphenol solubility and bioavailability and to offer site-specific drug delivery with enhanced pharmacokinetic features, nanoengineered polyphenol delivery systems must be created urgently. Gels, particularly hydrogels, are the most prevalent type of topical application to date. However, development of additional delivery strategies, including lipid and polymer nanoparticles, microparticles, and transsomes, is underway (Table 3).

Table 3.
Plant polyphenol delivery systems (example of curcumin).
4.6. Clinical Evidence That Plant Polyphenols Prevent Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer
At present, preclinical research—that is, research on skin cells and animal skin tis-sues—has revealed that plant polyphenols prevent skin cellular senescence, inflammation, and cancer caused by oxidative stress. However, human clinical trials are relatively insufficient. Table 4 summarizes some human clinical trials. The findings of these studies revealed the following: (1) Plant polyphenols are mostly used as topical or ingested products in clinical research. (2) To ensure the therapeutic effect, plant polyphenols are tested in clinical trials at high concentrations and for longer periods, which may cause irritation to the skin, intestines, and stomach. (3) In the clinic, plant polyphenols are often combined with other compounds to improve their bioavailability. (4) More diversified and objective evaluation methods of clinical results should be established.

Table 4.
Human clinical trials of plant polyphenols for oxidative stress-induced skin cellular senescence and inflammation.
5. Conclusions
To summarize, oxidative stress is extremely harmful to the skin and is associated with the onset and progression of skin cellular senescence, inflammation, and cancer. Plant polyphenols can prevent this skin damage through their molecular targets and have attracted great research owing to their low toxicity. The most recently discovered skin drug delivery technology for plant polyphenols is more durable and effective. This system will surely encourage clinical research on plant polyphenol-based products. In the near future, the limitations associated with the use of plant polyphenols in skin applications will be overcome, and new skin products will be brought to the market and society.
Author Contributions
Conceptualization, H.-M.L. and M.-Y.C.; methodology, H.-M.L., M.-H.X. and M.-Y.C.; formal analysis, Z.-W.Z., Y.Z. and W.T.; data curation, M.-Y.C., J.C. and J.N.; writing—original draft preparation, M.-Y.C.; writing—review and editing, M.-Y.C. and H.-M.L.; supervision, W.W.; project administration, H.-M.L. and W.W.; funding acquisition, H.-M.L. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shanghai Alliance Program, grant number LM201941.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ROS | reactive oxygen species |
| B16 | mouse melanoma cells |
| A375 and C8161 | melanoma cells |
| A431 | human epidermoid carcinoma cells |
| HST | human skin tissues |
| JB6 P+ | mouse epithelial cells |
| HFF-1 | skin epithelial cells |
| HaCaT | human immortalized epidermal cells |
| HDF | human dermal fibroblasts |
| BCC | basal cell carcinoma |
| SCC | squamous cell carcinoma |
| AD | atopic dermatitis |
| CD | contact dermatitis |
| SD | solar dermatitis |
| AP-1 | activator protein 1 |
| Bcl-2 | B-cell lymphoma 2 |
| p53 | tumor suppressor proteins and transcription factors |
| BOX | tumor suppressor proteins and transcription factors |
| caspase-3 | terminal shearing enzyme during apoptosis |
| Bax | proapoptotic gene |
| Mcl-1 | proapoptotic gene |
| ARE | antioxidant reaction element |
| ERK | extracellular receptor kinase |
| PTEN | phosphatase and tensin homolog |
| PCNA | proliferating cell nuclear antigen |
| PDCD4 | programmed cell death 4 |
| cdk2 | cyclin-dependent kinases |
| MDA | malondialdehyde |
| MMPs | matrix metalloproteinases |
| SOD | superoxide dismutase |
| GSH | glutathione |
| GSH-Px | glutathione peroxidase |
| GST | glutamate sulfur transferase |
| GR | glutathione reductase |
| TIMP | tissue inhibitor of metalloproteinase |
| TNF-a | tumor necrosis factor alpha |
| EGCG | epigallocatechin gallate |
| CAT | catalase |
| COX-2 | cyclooxygenase-2 |
| ECM | extracellular matrix |
| HO-1 | heme oxygenase |
| IκB | NF-κB inhibitory protein |
| IKK | nuclear factor kappa-B inhibitory protein kinase |
| IL- | interleukin- |
| IFN-γ | gamma interferon |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun amino-terminal kinases |
| Keap1 | kelch-like ECH-associated protein 1 |
| NADPH | nicotinamide adenine dinucleotide phosphate |
References
- Jabłońska-Trypuć, A.; Krętowski, R.; Kalinowska, M.; Świderski, G.; Cechowska-Pasko, M.; Lewandowski, W. Possible Mechanisms of the Prevention of Doxorubicin Toxicity by Cichoric Acid—Antioxidant Nutrient. Nutrients 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, S230–S242. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.A.; Karimi, J.; Talebi, S.S.; Piri, H. The Association of COVID-19 and Reactive Oxygen Species Modulator 1 (ROMO1) with Oxidative Stress. Chonnam Med. J. 2022, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zarbafian, M.; Dayan, S.; Fabi, S.G. Teachings from COVID-19 and aging-An oxidative process. J. Cosmet. Dermatol. 2020, 19, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Krutmann, J. Air pollution (particulatematter and nitrogen dioxide) and skin aging. Hautarzt 2019, 70, 158–162. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef]
- Godic, A.; Poljsak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Yu, X.M.; Shoaib, M.; Cheng, X.R.; Cui, Y.L.; Hussain, S.; Yan, J.; Zhou, J.; Chen, Q.; Gu, Y.F.; Zou, L.K.; et al. Role of rhizobia in promoting non-enzymatic antioxidants to mitigate nitrogen-deficiency and nickel stresses in Pongamia pinnata. Ecotoxicol. Environ. Saf. 2022, 241, 113789. [Google Scholar] [CrossRef]
- Tolmacheva, A.S.; Nevinsky, G.A. Essential Protective Role of Catalytically Active Antibodies (Abzymes) with Redox Antioxidant Functions in Animals and Humans. Int. J. Mol. Sci. 2022, 23, 3898. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kleszczynski, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.-K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local Melatoninergic System as the Protector of Skin Integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Boehm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczynski, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Izykowska, I.; Piotrowska, A.; Podhorska-Okolow, M.; Cegielski, M.; Zabel, M.; Dziegiel, P. The protective role of melatonin in the course of UV exposure. Postep. Hig. I Med. Dosw. 2008, 62, 23–27. [Google Scholar]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation Stress as a Mechanism of Aging in Human Erythrocytes: Protective Effect of Quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Perez, L.S.; Yee, N.G.; Peralta-Hernandez, J.M.; Bandala, E.R. Comparison between Fenton and Fenton-like reactions for l-proline degradation. Int. J. Environ. Sci. Technol. 2019, 16, 1515–1526. [Google Scholar] [CrossRef]
- Canas, P.E. The role of xanthine oxidase and the effects of antioxidants in ischemia reperfusion cell injury. Acta Physiol. Pharmacol. Et Ther. Latinoam. Organo Asoc. Latinoam. Cienc. Fisiol. Asoc. Latinoam. Farmacol. 1999, 49, 13–20. [Google Scholar]
- Lewen, A.; Matz, P.; Chan, P.H. Free radical pathways in CNS injury. J. Neurotrauma 2000, 17, 871–890. [Google Scholar] [CrossRef]
- Shaul, P.W. Regulation of endothelial nitric oxide synthase: Location, location, location. Annu. Rev. Physiol. 2002, 64, 749–774. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y.Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.F.; Masih, A.P.; Alqasmi, I.; Alsulimani, A.; Khan, F.H.; Haque, S. Oxidative-Stress-Induced Cellular Toxicity and Glycoxidation of Biomolecules by Cosmetic Products under Sunlight Exposure. Antioxidants 2021, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, Y.; Yu, S.-H.; Lee, S.-E.; Park, J.H.; Cho, G.; Choi, C.; Han, K.; Kim, C.-H.; Kang, Y.C. Platelet-derived mitochondria transfer facilitates wound-closure by modulating ROS levels in dermal fibroblasts. Platelets 2023, 34, 2151996. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Lee, C.-L.; Korivi, M.; Liao, J.-W.; Rajendran, P.; Wu, J.-J.; Hseu, Y.-C. Zerumbone protects human skin keratinocytes against UVA-irradiated damages through Nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef]
- Huang, K.-F.; Ma, K.-H.; Chang, Y.-J.; Lo, L.-C.; Jhap, T.-Y.; Su, Y.-H.; Liu, P.-S.; Chueh, S.-H. Baicalein inhibits matrix metalloproteinase 1 expression via activation of TRPV1-Ca-ERK pathway in ultraviolet B-irradiated human dermal fibroblasts. Exp. Dermatol. 2019, 28, 568–575. [Google Scholar] [CrossRef]
- Lee, E.H.; Faulhaber, D.; Hanson, K.M.; Ding, W.H.; Peters, S.; Kodali, S.; Granstein, R.D. Dietary lutein reduces ultraviolet radiation-induced inflammation and immunosuppression. J. Investig. Dermatol. 2004, 122, 510–517. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Q.; Zhang, Y.; Shi, W.; Wang, H.; Zheng, Z.; Meng, L.; Xin, Y.; Jiang, X. Sulforaphane-Mediated Nrf2 Activation Prevents Radiation-Induced Skin Injury through Inhibiting the Oxidative-Stress-Activated DNA Damage and NLRP3 Inflammasome. Antioxidants 2021, 10, 1850. [Google Scholar] [CrossRef]
- Lin, K.-T.; Chang, T.-C.; Lai, F.-Y.; Lin, C.-S.; Chao, H.-L.; Lee, S.-Y. Rhodiola crenulata Attenuates gamma-Ray Induced Cellular Injury via Modulation of Oxidative Stress in Human Skin Cells. Am. J. Chin. Med. 2018, 46, 175–190. [Google Scholar] [CrossRef]
- Dong, L.; Hu, R.; Yang, D.; Zhao, J.; Kan, H.; Tan, J.; Guan, M.; Kang, Z.; Xu, F. Fine Particulate Matter (PM2.5) upregulates expression of Inflammasome NLRP1 via ROS/NF-kappa B signaling in HaCaT Cells. Int. J. Med. Sci. 2020, 17, 2200–2206. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.-M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, N.; Mao, M.; Zhou, Y.; Wu, Y.; Li, J.; Zhang, W.; Peng, C.; Chen, X.; Li, J. Fine particulate matter (PM2.5) promotes IgE-mediated mast cell activation through ROS/Gadd45b/JNK axis. J. Dermatol. Sci. 2021, 102, 47–57. [Google Scholar] [CrossRef]
- Liang, P.; Xing, X.; Wu, J.; Song, J.; Liu, Q. PM2.5 promotes apoptosis of human epidermal melanocytes through promoting oxidative damage and autophagy. Gen. Physiol. Biophys. 2020, 39, 569–577. [Google Scholar] [CrossRef]
- Balke, J.; Volz, P.; Neumann, F.; Brodwolf, R.; Wolf, A.; Pischon, H.; Radbruch, M.; Mundhenk, L.; Gruber, A.D.; Ma, N.; et al. Visualizing Oxidative Cellular Stress Induced by Nanoparticles in the Subcytotoxic Range Using Fluorescence Lifetime Imaging. Small 2018, 14, e1800310. [Google Scholar] [CrossRef]
- Ali, D.; Alarifi, S.; Alkahtani, S.; AlKahtane, A.A.; Almalik, A. Cerium Oxide Nanoparticles Induce Oxidative Stress and Genotoxicity in Human Skin Melanoma Cells. Cell Biochem. Biophys. 2015, 71, 1643–1651. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alakhtani, S.; Al Suhaibani, E.S.; Al-Qahtani, A.A. Reactive Oxygen Species-Mediated DNA Damage and Apoptosis in Human Skin Epidermal Cells After Exposure to Nickel Nanoparticles. Biol. Trace Elem. Res. 2014, 157, 84–93. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Chen, W.; Li, N.; Zhai, C.; Liu, Z.; Xiao, Z.; Li, P.; Zhao, J. The expression and roles of chemokine CCL20 in the skin lesions of psoriasis. Immunol. J. 2017, 33, 505–511. [Google Scholar]
- Yoon, Y.S.; Sajo, M.; Ignacio, R.M.C.; Kim, S.K.; Kim, C.S.; Lee, K.J. Positive Effects of Hydrogen Water on 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in NC/Nga Mice. Biol. Pharm. Bull. 2014, 37, 1480–1485. [Google Scholar] [CrossRef]
- Huang, J.K.; Feng, X.Y.; Zeng, J.; Zhang, S.C.; Zhang, J.; Guo, P.; Yu, H.Y.; Sun, M.K.; Wu, J.M.; Li, M.Y.; et al. Aberrant HO-1/NQO1-Reactive Oxygen Species-ERK Signaling Pathway Contributes to Aggravation of TPA-Induced Irritant Contact Dermatitis in Nrf2-Deficient Mice. J. Immunol. 2022, 208, 1424–1433. [Google Scholar] [CrossRef]
- Lou, Q.; Zhang, M.C.; Zhang, K.Y.; Liu, X.A.; Zhang, Z.H.; Zhang, X.; Yang, Y.M.; Gao, Y.H. Arsenic exposure elevated ROS promotes energy metabolic reprogramming with enhanced AKT-dependent HK2 expression. Sci. Total Environ. 2022, 836. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-W.; Liu, F.-C.; Wang, Y.-R.; Tsai, H.-I.; Yu, H.-P. Aloin Protects Skin Fibroblasts from Heat Stress-Induced Oxidative Stress Damage by Regulating the Oxidative Defense System. PLoS ONE 2015, 10, e0143528. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Dahmane, R.G.; Godic, A. Intrinsic skin aging: The role of oxidative stress. Acta Dermatovenerol. Alp. Pannonica Et Adriat. 2012, 21, 33–36. [Google Scholar]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.S.; Jiang, G. Research progress on skin photoaging and oxidative stress. Postep. Dermatol. I Alergol. 2021, 38, 931–936. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the Redox Balance in Inflammatory Skin Conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Nakai, K.; Yoneda, K.; Maeda, R.; Munehiro, A.; Fujita, N.; Yokoi, I.; Moriue, J.; Moriue, T.; Kosaka, H.; Kubota, Y. Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1405–1408. [Google Scholar] [CrossRef]
- Omata, N.; Tsukahara, H.; Ito, S.; Ohshima, Y.; Yasutomi, M.; Yamada, A.; Jiang, M.; Hiraoka, M.; Nambu, M.; Deguchi, Y.; et al. Increased oxidative stress in childhood atopic dermatitis. Life Sci. 2001, 69, 223–228. [Google Scholar] [CrossRef]
- Sander, C.S.; Hamm, F.; Elsner, P.; Thiele, J.J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef]
- Xian, D.; Lai, R.; Song, J.; Xiong, X.; Zhong, J. Emerging Perspective: Role of Increased ROS and Redox Imbalance in Skin Carcinogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Csekes, E.; Rackova, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free. Radic. Res. 2017, 51, 555–567. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef] [PubMed]
- Thornfeldt, C.R. Chronic inflammation is etiology of extrinsic aging. J. Cosmet. Dermatol. 2008, 7, 78–82. [Google Scholar] [CrossRef]
- He, Y.-L.; Lin, L.; Zheng, H.; Mo, Y.; Zhou, C.; Sun, S.; Hong, P.; Qian, Z.-J. Potential anti-skin aging effect of a peptide AYAPE isolated from Isochrysis zhanjiangensis on UVB-induced HaCaT cells and H2O2-induced BJ cells. J. Photochem. Photobiol. B-Biol. 2022, 233, 112481. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Noh, E.-M.; Jeong, E.-Y.; Yun, S.-K.; Jeong, Y.-J.; Kim, J.-H.; Kwon, K.-B.; Kim, B.-S.; Lee, S.-H.; Park, C.-S.; et al. Cordycepin inhibits UVB-induced matrix metalloproteinase expression by suppressing the NF-kappa B pathway in human dermal fibroblasts. Exp. Mol. Med. 2009, 41, 548–554. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.J. TGF-β Signal as Common Targets for Cancer Prevention and Therapy. TGF-β Prevention and Treatment of Cancer through signal Modulators. J. Cancer Prev. 2009, 14, 188–200. [Google Scholar]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Sternlicht, M.; Gerritsen, K.; Goldschmeding, R. Could Aging Human Skin Use a Connective Tissue Growth Factor Boost to Increase Collagen Content? J. Investig. Dermatol. 2010, 130, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Gojniczek, K.; Jurzak, M.; Garncarczyk, A. The role of connective tissue growth factor (CTGF) in fibroproliferative processes and tissues fibrosis. Postep. Biol. Komorki 2008, 35, 113–131. [Google Scholar] [CrossRef]
- Duncan, M.R.; Frazier, K.S.; Abramson, S.; Williams, S.; Klapper, H.; Huang, X.F.; Grotendorst, G.R. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: Downregulation by cAMP. FASEB J. 1999, 13, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xie, K.; Liu, X.; Chen, H. Identification of key pathways and genes in psoriasis via gene microarray analysis. Mol. Med. Rep. 2016, 13, 2327–2337. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, X.; Wang, X.; Lv, H.; Zhao, J.; Guo, X.; Xian, F.; Ji, Y.; Zhang, G. Plasma MicroRNA Expression Profiles in Psoriasis. J. Immunol. Res. 2020, 2020, 1561278. [Google Scholar] [CrossRef]
- Woo, Y.R.; Cho, D.H.; Park, H.J. Molecular Mechanisms and Management of a Cutaneous Inflammatory Disorder: Psoriasis. Int. J. Mol. Sci. 2017, 18, 2684. [Google Scholar] [CrossRef]
- Garcia-Melendo, C.; Cubiro, X.; Puig, L. Janus Kinase Inhibitors in Dermatology: Part 2: Applications in Psoriasis, Atopic Dermatitis, and Other Dermatoses. Actas Dermo-Sifiliogr. 2021, 112, 586–600. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, Y.S.; Jeong, M.H. The Role of Nuclear Factor Kappa B Activation in Atherosclerosis and Ischemic Cardiac Injury. Korean Circ. J. 2006, 36, 245–251. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, Z.; Zhang, L.; Wang, Y.; Li, P. 4′-O-beta-D-glucosyl-5-O-methylvisamminol ameliorates imiquimod-induced psoriasis-like dermatitis and inhibits inflammatory cytokines production by suppressing the NF-kappa B and MAPK signaling pathways. Braz. J. Med. Biol. Res. 2020, 53. [Google Scholar] [CrossRef]
- Li, Z.J.; Choi, D.-K.; Sohn, K.-C.; Lim, S.K.; Im, M.; Lee, Y.; Seo, Y.-J.; Kim, C.D.; Lee, J.-H. Induction of Interleukin-22 (IL-22) production in CD4(+) T Cells by IL-17A Secreted from CpG-Stimulated Keratinocytes. Ann. Dermatol. 2016, 28, 579–585. [Google Scholar] [CrossRef]
- Martinez-Torres, I.; Tepale-Segura, A.; Castro-Escamilla, O.; Cancino-Diaz, J.C.; Rodriguez-Martinez, S.; Perez-Tapia, S.M.; Bonifaz, L.C.; Cancino-Diaz, M.E. The Protective Role of pVHL in Imiquimod-Induced Psoriasis-like Skin Inflammation. Int. J. Mol. Sci. 2022, 23, 5226. [Google Scholar] [CrossRef]
- Jendrysik, M.A.; Vasilevsky, S.; Yi, L.; Wood, A.; Zhu, N.; Zhao, Y.; Koontz, S.M.; Jackson, S.H. NADPH Oxidase-2 Derived ROS Dictates Murine DC Cytokine-Mediated Cell Fate Decisions during CD4 T Helper-Cell Commitment. PLoS ONE 2011, 6, e28198. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.-X.; Li, W.; Wu, Y.; Chen, S.-J.; Yue, Q.; Xiao, M.; Li, J.-W. IL-36 Cytokine Expression and Its Relationship with p38 MAPK and NF-kappa B Pathways in Psoriasis Vulgaris Skin Lesions. J. Huazhong Univ. Sci. Technol.-Med. Sci. 2013, 33, 594–599. [Google Scholar] [CrossRef]
- Xiao-hong, M.A.N.; Xiao-yan, Z.; Hang-yu, Y.; Juan, T.; Li-xin, Z.; Li-ping, Y.O.U. High expression of phosphorylated MEK/ERK/NF-kappaB in lesions of psoriasis vulgaris. Chin. J. Dermatol. 2010, 43, 160–163. [Google Scholar]
- Man, X.-Y.; Zheng, M. Advances in pathogenesis of psoriasis. Zhejiang Da Xue Xue Bao. Yi Xue Ban J. Zhejiang Univ. Med. Sci. 2006, 35, 673–677. [Google Scholar]
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H.; et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.; Dang, M.; Chen, X.; Yan, X. Rehmannia radix Extract Ameliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in a Mouse Model via the Janus-Kinase Signal Transducer and Activator of Transcription Pathway. Pharmacogn. Mag. 2020, 16, 613–619. [Google Scholar] [CrossRef]
- Hoozemans, J.J.M.; Veerhuis, R.; Rozemuller, A.J.M.; Arendt, T.; Eikelenboom, P. Neuronal COX-2 expression and phosphorylation of pRb precede p38 MAPK activation and neurofibrillary changes in AD temporal cortex. Neurobiol. Dis. 2004, 15, 492–499. [Google Scholar] [CrossRef]
- Sun, H.; Xu, B.; Inoue, H.; Chen, Q.M. p38 MAPK mediates COX-2 gene expression by corticosterone in cardiomyocytes. Cell. Signal. 2008, 20, 1952–1959. [Google Scholar] [CrossRef]
- Lin, C.H.; Kuan, I.H.; Wang, C.H.; Lee, H.M.; Lee, W.S.; Sheu, J.R.; Hsiao, G.; Wu, C.H.; Kuo, H.P. Lipoteichoic acid-induced cyclooxygenase-2 expression requires activations of p44/42 and p38 mitogen-activated protein kinase signal pathways. Eur. J. Pharmacol. 2002, 450, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Choi, M.S.; Lee, H.G.; Lee, S.-H.; Noh, K.H.; Kwon, S.; Jeong, A.J.; Lee, H.; Yi, E.H.; Park, J.Y.; et al. Photoprotective Potential of Penta-O-Galloyl-beta-D-Glucose by Targeting NF-kappa B and MAPK Signaling in UVB Radiation-Induced Human Dermal Fibroblasts and Mouse Skin. Mol. Cells 2015, 38, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, N.; Adamski, H.; Bouvet, R.; Corre, S.; Courbebaisse, Y.; Watier, E.; Mosser, J.; Chesne, C.; Galibert, M.-D. In Vivo Identification of Solar Radiation-Responsive Gene Network: Role of the p38 Stress-Dependent Kinase. PLoS ONE 2010, 5, e10776. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Shao, H.; Deng, J.; Liu, Y. Network pharmacology-based analysis to explore the therapeutic mechanism of Cortex Dictamni on atopic dermatitis. J. Ethnopharmacol. 2023, 304, 116023. [Google Scholar] [CrossRef]
- Tanaka, A.; Muto, S.; Jung, K.; Itai, A.; Matsuda, H. Topical application with a new NF-kappa B inhibitor improves atopic dermatitis in NC/NgaTnd mice. J. Investig. Dermatol. 2007, 127, 855–863. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, M.; Ren, J.; Wu, X.; Long, M.; Chen, L.; Chen, Z. Electroacupuncture inhibits mast cell degranulation via cannabinoid CB2 receptors in a rat model of allergic contact dermatitis. Acupunct. Med. 2019, 37, 348–355. [Google Scholar] [CrossRef]
- Sasaki, Y.; Aiba, S. Dendritic cells and contact dermatitis. Clin. Rev. Allergy Immunol. 2007, 33, 27–34. [Google Scholar] [CrossRef]
- Bechara, R.; Nabhan, M.; Antonios, D.; Azouri, H.; Pallardy, M. IL-27 Production and Regulation in Human Dendritic Cells Treated with the Chemical Sensitizer NiSO4. Chem. Res. Toxicol. 2018, 31, 1323–1331. [Google Scholar] [CrossRef]
- Ciuciulete, A.-R.; Stepan, A.E.; Andreiana, B.C.; Simionescu, C.E. Non-Melanoma Skin Cancer: Statistical Associations between Clinical Parameters. Curr. Health Sci. J. 2022, 48, 110–115. [Google Scholar] [CrossRef]
- Durante, G.; Comito, F.; Lambertini, M.; Broseghini, E.; Dika, E.; Ferracin, M. Non-coding RNA dysregulation in skin cancers. Essays Biochem. 2021, 65, 641–655. [Google Scholar]
- Dhanwada, K.R.; Dickens, M.; Neades, R.; Davis, R.; Pelling, J.C. Differential-effects of UV-B and UV-C components of solar-radiation on map kinase signal-transduction pathways in epidermal-keratinocytes. Oncogene 1995, 11, 1947–1953. [Google Scholar]
- Liu, Z.; Zhu, P.; Tao, Y.; Shen, C.; Wang, S.; Zhao, L.; Wu, H.; Fan, F.; Lin, C.; Chen, C.; et al. Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem. Toxicol. 2015, 81, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Malakhova, M.; Mottamal, M.; Reddy, K.; Kurinov, I.; Carper, A.; Langfald, A.; Oi, N.; Kim, M.O.; Zhu, F.; et al. Norathyriol Suppresses Skin Cancers Induced by Solar Ultraviolet Radiation by Targeting ERK Kinases. Cancer Res. 2012, 72, 260–270. [Google Scholar] [CrossRef]
- Zhao, K.; Dai, Q.; Wu, J.; Wei, Z.; Duan, Y.; Chen, B. Morusin enhances the antitumor activity of MAPK pathway inhibitors in BRAF-mutant melanoma by inhibiting the feedback activation of STAT3. Eur. J. Cancer 2022, 165, 58–70. [Google Scholar] [CrossRef]
- Lopez-Bergami, P.; Fitchman, B.; Ronai, Z.e. Understanding signaling cascades in melanoma. Photochem. Photobiol. 2008, 84, 289–306. [Google Scholar] [CrossRef]
- Cohen, C.; Zavala-Pompa, A.; Sequeira, J.H.; Shoji, M.; Sexton, D.G.; Cotsonis, G.; Cerimele, F.; Govindarajan, B.; Macaron, N.; Arbiser, J.L. Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin. Cancer Res. 2002, 8, 3728–3733. [Google Scholar]
- Zipser, M.C.; Eichhoff, O.M.; Widmer, D.S.; Schlegel, N.C.; Schoenewolf, N.L.; Stuart, D.; Liu, W.; Gardner, H.; Smith, P.D.; Nuciforo, P.; et al. A proliferative melanoma cell phenotype is responsive to RAF/MEK inhibition independent of BRAF mutation status. Pigment. Cell Melanoma Res. 2011, 24, 326–333. [Google Scholar] [CrossRef]
- Pathria, G.; Verma, S.; Yin, J.; Scott, D.A.; Ronai, Z.E.A. MAPK signaling regulates c-MYC for melanoma cell adaptation to asparagine restriction. Embo Rep. 2021, 22, e51436. [Google Scholar] [CrossRef]
- Xie, J.W.; Aszterbaum, M.; Zhang, X.L.; Bonifas, J.M.; Zachary, C.; Epstein, E.; McCormick, F. A role of PDGFR alpha in basal cell carcinoma proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 9255–9259. [Google Scholar] [CrossRef]
- Geering, B.; Cutillas, P.R.; Vanhaesebroeck, B. Regulation of class IA PI3Ks: Is there a role for monomeric PI3K subunits? Biochem. Soc. Trans. 2007, 35, 199–203. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Long, X.; Jiao, L.; Zhou, M.; Wu, K. MRPS16 facilitates tumor progression via the PI3K/AKT/Snail signaling axis. J. Cancer 2020, 11, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Lai, A.G. An immunoevasive strategy through clinically-relevant pan-cancer genomic and transcriptomic alterations of JAK-STAT signaling components. Mol. Med. 2019, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, F.; Zhang, G.-L. Natural products and their derivatives regulating the janus kinase/signal transducer and activator of transcription pathway. J. Asian Nat. Prod. Res. 2014, 16, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 1–33. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, W.; Yan, S.; Zhang, J.; Wang, D.; Shen, J. JAK/STAT signaling regulates the Harmonia axyridis leg regeneration by coordinating cell proliferation. Dev. Biol. 2022, 483, 98–106. [Google Scholar] [CrossRef]
- Bach, E.A.; Ekas, L.A.; Ayala-Camargo, A.; Flaherty, M.S.; Lee, H.; Perrimon, N.; Baeg, G.-H. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 2007, 7, 323–331. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Sun, Y.-X.; Yu, M.; Shen, B.-F. IL-6 inhibits apoptosis of human myeloma cell line XG-7 through activation of JAK/STAT pathway and up-regulation of Mcl-1. Ai Zheng. Chin. J. Cancer 2002, 21, 113–116. [Google Scholar]
- Zhang, M.; Griner, L.A.M.; Ju, W.; Duveau, D.Y.; Guha, R.; Petrus, M.N.; Wen, B.; Maeda, M.; Shinn, P.; Ferrer, M.; et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 2015, 112, 12480–12485. [Google Scholar] [CrossRef]
- Migone, T.S.; Humbert, M.; Rascle, A.; Sanden, D.; D’Andrea, A.; Johnston, J.A. The deubiquitinating enzyme DUB-2 prolongs cytokine-induced signal transducers and activators of transcription activation and suppresses apoptosis following cytokine withdrawal. Blood 2001, 98, 1935–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, S.B.; Ogilvie, J.M.; McCool, D.J.; Sarthy, V. Ciliary neurotrophic factor induces glial fibrillary acidic protein in retinal Muller cells through the JAK/STAT signal transduction pathway. Curr. Eye Res. 2002, 24, 305–312. [Google Scholar] [CrossRef]
- Burgo, M.A.; Roudiani, N.; Chen, J.; Santana, A.L.; Doudican, N.; Proby, C.; Felsen, D.; Carucci, J.A. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight 2018, 3, 120750. [Google Scholar] [CrossRef]
- Pan, F.; Wang, Q.; Li, S.; Huang, R.; Wang, X.; Liao, X.; Mo, H.; Zhang, L.; Zhou, X. Prognostic value of key genes of the JAK-STAT signaling pathway in patients with cutaneous melanoma. Oncol. Lett. 2020, 19, 1928–1946. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Ma, T. Mechanisms of JAK-STAT signaling pathway mediated by CXCL8 gene silencing on epithelial-mesenchymal transition of human cutaneous melanoma cells. Oncol. Lett. 2020, 20, 1973–1981. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Chaudhuri, A.; Iqubal, A.; Saleem, S.; Gupta, M.M.; Ahuja, A.; Ali, J.; Baboota, S. Targeted Delivery of Natural Bioactives and Lipid-nanocargos against Signaling Pathways Involved in Skin Cancer. Curr. Med. Chem. 2021, 28, 8003–8035. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Jing, G.; Dong, D. LncRNA HOTAIR Promotes Proliferation of Malignant Melanoma Cells through NF-kappa B Pathway. Iran. J. Public Health 2020, 49, 1931–1939. [Google Scholar]
- Usman, H.A.; Hernowo, B.S.; Tobing, M.D.L.; Hindritiani, R. The Major Role of NF-kappa B in the Depth of Invasion on Acral Melanoma by Decreasing CD8(+) T Cells. J. Pathol. Transl. Med. 2018, 52, 164–170. [Google Scholar] [CrossRef]
- Feehan, R.P.; Shantz, L.M. Molecular signaling cascades involved in nonmelanoma skin carcinogenesis. Biochem. J. 2016, 473, 2973–2994. [Google Scholar] [CrossRef]
- Schaefer, M.; Werner, S. Nrf2-A regulator of keratinocyte redox signaling. Free. Radic. Biol. Med. 2015, 88, 243–252. [Google Scholar] [CrossRef]
- De la Vega, M.R.; Krajisnik, A.; Zhang, D.D.; Wondrak, G.T. Targeting NRF2 for Improved Skin Barrier Function and Photoprotection: Focus on the Achiote-Derived Apocarotenoid Bixin. Nutrients 2017, 9, 1371. [Google Scholar] [CrossRef]
- Motohashi, H.; O’Connor, T.; Katsuoka, F.; Engel, J.D.; Yamamoto, M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 2002, 294, 1–12. [Google Scholar] [CrossRef]
- Wong, D.P.W.; Wells, G.; Hagen, T. Heteroaromatic 4-arylquinols are novel inducers of Nuclear factor-erythroid 2-related factor 2 (Nrf2). Eur. J. Pharmacol. 2010, 643, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Angelova, P.R.; Holmstroem, K.M.; Zhang, Y.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Et Biophys. Acta-Gen. Subj. 2015, 1850, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Sajadimajd, S.; Khazaei, M. Oxidative Stress and Cancer: The Role of Nrf2. Curr. Cancer Drug Targets 2018, 18, 538–557. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Hu, Y.; Sheng, R. Autophagy Regulators as Potential Cancer Therapeutic agents: A Review. Curr. Top. Med. Chem. 2015, 15, 720–744. [Google Scholar] [CrossRef]
- Lim, G.-E.; Park, J.E.; Cho, Y.H.; Lim, D.S.; Kim, A.J.; Moh, S.H.; Lee, J.H.; Lee, J.S. Alpha-neoendorphin can reduce UVB-induced skin photoaging by activating cellular autophagy. Arch. Biochem. Biophys. 2020, 689, 108437. [Google Scholar] [CrossRef]
- Hailfinger, S.; Schulze-Osthoff, K. Impaired Autophagy in Psoriasis and Atopic Dermatitis: A New Therapeutic Target. J. Investig. Dermatol. 2021, 141, 2775–2777. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Unterman, T.G.; Shankar, S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol. Cell. Biochem. 2010, 337, 201–212. [Google Scholar] [CrossRef]
- Dansen, T.B. Forkhead Box O Transcription Factors: Key Players in Redox Signaling. Antioxid. Redox Signal. 2011, 14, 559–561. [Google Scholar] [CrossRef]
- Kim, S.; Koh, H. Role of FOXO transcription factors in crosstalk between mitochondria and the nucleus. J. Bioenerg. Biomembr. 2017, 49, 335–341. [Google Scholar] [CrossRef]
- Amin, A.; Adeghate, E.; Lotfy, M. Pancreas-Protective Effects of Chlorella in STZ-Induced Diabetic Animal Model: Insights into the Mechanism. Diabetes 2011, 60, A703. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Murali, C.; Madhoon, A.A.; Amin, A. Salvadora persica (Miswak): Antioxidant and Promising Antiangiogenic Insights. Am. J. Plant Sci. 2018, 9, 1228–1244. [Google Scholar] [CrossRef]
- Hamza, A.A.; Mohamed, M.G.; Lashin, F.M.; Amin, A. Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. J. Basic Appl. Zool. 2020, 81, 1–13. [Google Scholar] [CrossRef]
- Mohd Nor, N.A.; Budin, S.B.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.F.; Mohamad Anuar, N.N. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Choi, B.Y. Biochemical Basis of Anti-Cancer-Effects of PhloretinA Natural Dihydrochalcone. Molecules 2019, 24, 278. [Google Scholar] [CrossRef]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Liu, M.; Wu, S.; Fang, L.; Guo, D.; He, J. Research and Application of Proanthocyanidins in Livestock Production. Chin. J. Anim. Nutr. 2018, 30, 2902–2910. [Google Scholar]
- Wan, F.; Hou, F.; Yi, B.; Zhang, H. Physiological Functions of Chlorogenic Acid and Its Application in Livestock and Poultry Production. Chin. J. Anim. Nutr. 2021, 33, 2416–2427. [Google Scholar]
- Djawad, K.; Patellongi, I.J.; Miskad, U.A.; Massi, M.N.; Yusuf, I.; Faruk, M. Single or Daily Application of Topical Curcumin Prevents Ultraviolet B-Induced Apoptosis in Mice. Molecules 2023, 28, 371. [Google Scholar] [CrossRef] [PubMed]
- Minear, S.; O’Donnell, A.F.; Ballew, A.; Giaever, G.; Nislow, C.; Stearns, T.; Cyert, M.S. Curcumin Inhibits Growth of Saccharomyces cerevisiae through Iron Chelation. Eukaryot. Cell 2011, 10, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Meng, Z.; Tao, H.; Bai, Z.; Yan, C.; Li, L. Curcumin inhibits LPS-induced inflammation in VSMCs via Toll-like receptor 4/NADPH oxidase/reactive oxygen species signaling pathway. J. Xi’an Jiaotong Univ. Med. Sci. 2015, 36, 543–548. [Google Scholar]
- Hur, G.-H.; Ryu, A.R.; Kim, Y.-W.; Lee, M.-Y. The Potential Anti-Photoaging Effect of Photodynamic Therapy Using Chlorin e6-Curcumin Conjugate in UVB-Irradiated Fibroblasts and Hairless Mice. Pharmaceutics 2022, 14, 968. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Lima, C.F.; Pereira-Wilson, C.; Rattan, S.I.S. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol. Nutr. Food Res. 2011, 55, 430–442. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Zheng, X.; Zhang, K.; Du, Z. Chemoprevention effects of a sulindac-based compound on TPA-induced skin inflammation in mice. Medchemcomm 2015, 6, 1605–1611. [Google Scholar] [CrossRef]
- Li, H.; Gao, A.; Jiang, N.; Liu, Q.; Liang, B.; Li, R.; Zhang, E.; Li, Z.; Zhu, H. Protective Effect of Curcumin Against Acute Ultraviolet B Irradiation-induced Photo-damage. Photochem. Photobiol. 2016, 92, 808–815. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.Y.; Dong, B.H.; Jiang, Y.X.; Yu, L.Y.; Chen, Z.M.; Hu, C.J.; Xu, R.C. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Zhang, J.C.; Ma, Y.; Li, W. Curcumin reduces inflammation in mice with the psoriasis model by inhibiting NLRP3 inflammatory bodies. Cell. Mol. Biol. 2021, 67, 48–54. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef]
- Limtrakul, P.N.; Anuchapreeda, S.; Lipigorngoson, S.; Dunn, F.W. Inhibition of carcinogen induced c-Ha-ras and c-fos proto-oncogenes expression by dietary curcumin. BMC Cancer 2001, 1. [Google Scholar] [CrossRef]
- Kakar, S.S.; Roy, D. Curcumin inhibits TPA induced expression of c-fos, c-jun and c-myc protooncogenes messenger-RNAs in mouse skin. Cancer Lett. 1994, 87, 85–89. [Google Scholar] [CrossRef]
- Kumar, G.; Tajpara, P.; Maru, G.B. Dietary Turmeric Post-Treatment Decreases DMBA-Induced Hamster Buccal Pouch Tumor Growth by Altering Cell Proliferation and Apoptosis-Related Markers. J. Environ. Pathol. Toxicol. Oncol. 2012, 31, 295–312. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L.M. The Curcumin Analog EF24 Targets NF-kappa B and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.-A.O. Curcumin Inhibits Skin Squamous Cell Carcinoma Tumor Growth In Vivo. Otolaryngol. Head Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef]
- Salaheldin, T.A.; Adhami, V.M.; Fujioka, K.; Mukhtar, H.; Mousa, S.A. Photochemoprevention of ultraviolet Beam Radiation-induced DNA damage in keratinocytes by topical delivery of nanoformulated Epigallocatechin-3-gallate. Nanomed.-Nanotechnol. Biol. Med. 2022, 44, 102580. [Google Scholar] [CrossRef]
- Ding, J.X.; Gao, B.B.; Chen, Z.H.; Mei, X.F. An NIR-Triggered Au Nanocage Used for Photo-Thermo Therapy of Chronic Wound in Diabetic Rats Through Bacterial Membrane Destruction and Skin Cell Mitochondrial Protection. Front. Pharmacol. 2021, 12, 3235. [Google Scholar] [CrossRef]
- Li, D.H.; Martini, N.; Wu, Z.M.; Chen, S.; Falconer, J.R.; Locke, M.; Zhang, Z.W.; Wen, J.Y. Niosomal Nanocarriers for Enhanced Dermal Delivery of Epigallocatechin Gallate for Protection against Oxidative Stress of the Skin. Pharmaceutics 2022, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Wang, L.X.; Zheng, X.Q.; Xiang, L.P.; Liang, Y.R. Protective Effect of (-)-Epigallocatechin Gallate against Photo-Damage Induced by Ultraviolet A in Human Skin Fibroblasts. Trop. J. Pharm. Res. 2014, 13, 1079–1084. [Google Scholar] [CrossRef]
- Megow, I.; Darvin, M.E.; Meinke, M.C.; Lademann, J. A Randomized Controlled Trial of Green Tea Beverages on the in vivo Radical Scavenging Activity in Human Skin. Ski. Pharmacol. Physiol. 2017, 30, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Cui, Y.R.; Ahn, G.; Jeon, Y.-J. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-kappa B, AP-1, and MAPKs signaling pathways. Environ. Pollut. 2019, 252, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Won, H.-R.; Lee, P.; Oh, S.-r.; Kim, Y.-M. Epigallocatechin-3-Gallate Suppresses the Expression of TNF-alpha-Induced MMP-1 via MAPK/ERK Signaling Pathways in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2021, 44, 18–24. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-alpha-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar] [CrossRef]
- Yang, W.K.; Park, Y.; Kim, B.K.; Choi, J.J.; Ryu, G.S.; Kim, S.H. Effects of Catechin-rich Green Tea Extract on the MMP-1 Activity of HaCaT Keratinocyte Cells and on UVB-induced Skin Damage in Hairless Mice. Korean J. Med. Crop Sci. 2019, 27, 143–150. [Google Scholar] [CrossRef]
- Nakano, E.; Kamei, D.; Murase, R.; Taki, I.; Karasawa, K.; Fukuhara, K.; Iwai, S. Anti-inflammatory effects of new catechin derivatives in a hapten-induced mouse contact dermatitis model. Eur. J. Pharmacol. 2019, 845, 40–47. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Mei, L.; Wang, H.; Fang, F. Epigallocatechin-3-gallate (EGCG) inhibits imiquimod-induced psoriasis-like inflammation of BALB/c mice. BMC Complement. Altern. Med. 2016, 16, 334. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Siddiqui, I.A.; Adhami, V.M.; Esnault, S.; Bharali, D.J.; Babatunde, A.S.; Adame, S.; Massey, R.J.; Wood, G.S.; Longley, B.J.; et al. Chitosan-based nanoformulated (-)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int. J. Nanomed. 2018, 13, 4189–4206. [Google Scholar] [CrossRef]
- Bito, T.; Roy, S.; Sen, C.K.; Shirakawa, T.; Gotoh, A.; Ueda, M.; Ichihashi, M.; Packer, L. Flavonoids differentially regulate IFN gamma-induced ICAM-1 expression in human keratinocytes: Molecular mechanisms of action. Febs Lett. 2002, 520, 145–152. [Google Scholar] [CrossRef]
- Monga, J.; Aggarwal, V.; Suthar, S.K.; Monika; Nongalleima, K.; Sharma, M. Topical (+)-catechin emulsified gel prevents DMBA/TPA-induced squamous cell carcinoma of the skin by modulating antioxidants and inflammatory biomarkers in BALB/c mice. Food Funct. 2014, 5, 3197–3207. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Jin, P.; Xu, Y.; Yin, J.; Qin, D.; Wang, K.; Du, Q. Epigallocatechin gallate-beta-lactoglobulin nanoparticles improve the antitumor activity of EGCG for inducing cancer cell apoptosis. J. Funct. Foods 2017, 39, 257–263. [Google Scholar] [CrossRef]
- Leis, K.; Pisanko, K.; Jundzill, A.; Mazur, E.; Mecinska-Jundzill, K.; Witmanowski, H. Resveratrol as a factor preventing skin aging and affecting its regeneration. Postep. Dermatol. I Alergol. 2022, 39, 439–445. [Google Scholar] [CrossRef]
- Lephart, E.D.; Sommerfeldt, J.M.; Andrus, M.B. Resveratrol: Influences on gene expression in human skin. J. Funct. Foods 2014, 10, 377–384. [Google Scholar] [CrossRef]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.-P.; Kotzbeck, P. The impact of resveratrol on skin wound healing, scarring, and aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef]
- Cao, C.; Lu, S.; Kivlin, R.; Wallin, B.; Card, E.; Bagdasarian, A.; Tamakloe, T.; Wang, W.-J.; Song, X.; Chu, W.-M.; et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J. Cell. Mol. Med. 2009, 13, 3632–3643. [Google Scholar] [CrossRef]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol Prevents Oxidative Stress-Induced Senescence and Proliferative Dysfunction by Activating the AMPK-FOXO3 Cascade in Cultured Primary Human Keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Y.; Cao, X.; Xiao, L.; Ding, Q.; Luo, F.; Huang, P.; Gao, Y.; Liu, M.; Zhao, H. Effects of Natural Polyphenols on Skin and Hair Health: A Review. Molecules 2022, 27, 7832. [Google Scholar] [CrossRef]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef]
- Kjaer, T.N.; Thorsen, K.; Jessen, N.; Stenderup, K.; Pedersen, S.B. Resveratrol Ameliorates Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice. PLoS ONE 2015, 10, e0126599. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Kalra, N.; Prasad, S.; George, J.; Shukla, Y. Chemopreventive Potential of Resveratrol in Mouse Skin Tumors Through Regulation of Mitochondrial and PI3K/AKT Signaling Pathways. Pharm. Res. 2009, 26, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Afaq, F.; Aziz, M.H.; Ahmad, N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004, 23, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Madan, E.; Prasad, S.; Roy, P.; George, J.; Shukla, Y. Regulation of apoptosis by resveratrol through JAK/STAT and mitochondria mediated pathway in human epidermoid carcinoma A431 cells. Biochem. Biophys. Res. Commun. 2008, 377, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKC delta and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Sohn, E.-J.; Kim, J.M.; Kang, S.-H.; Kwon, J.; An, H.J.; Sung, J.-S.; Cho, K.A.; Jang, I.-S.; Choi, J.-S. Restoring Effects of Natural Anti-Oxidant Quercetin on Cellular Senescent Human Dermal Fibroblasts. Am. J. Chin. Med. 2018, 46, 853–873. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Lee, E.; Shin, A.; Park, Y.G.; Kim, Y. Effect of Quercetin in the UV-Irradiated Human Keratinocyte HaCaT Cells and A Model of Its Binding To p38 MAPK. Bull. Korean Chem. Soc. 2014, 35, 2787–2790. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J.V. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B-Biol. 2006, 84, 21–27. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Pauff, J.M.; Hille, R. Inhibition Studies of Bovine Xanthine Oxidase by Luteolin, Silibinin, Quercetin, and Curcumin. J. Nat. Prod. 2009, 72, 725–731. [Google Scholar] [CrossRef]
- Lee, K.-M.; Kang, J.H.; Yun, M.; Lee, S.-B. Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-gamma-primed human keratinocytes. Biochem. Biophys. Res. Commun. 2018, 503, 116–122. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Modulation of HMGB1 translocation and RAGE/NFB cascade by quercetin treatment mitigates atopic dermatitis in NC/Nga transgenic mice. Exp. Dermatol. 2015, 24, 418–423. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Chen, H.M.; Lu, C.J.; Liu, H.Z.; Wang, M.J.; Zhao, H.; Yan, Y.H.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-kappa B pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, S.H.; Kim, H.J.; Jung, J.Y. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur. J. Pharmacol. 2019, 860, 172568. [Google Scholar] [CrossRef]
- Hundsberger, H.; Stierschneider, A.; Sarne, V.; Ripper, D.; Schimon, J.; Weitzenbock, H.P.; Schild, D.; Jacobi, N.; Eger, A.; Atzler, J.; et al. Concentration-Dependent Pro- and Antitumor Activities of Quercetin in Human Melanoma Spheroids: Comparative Analysis of 2D and 3D Cell Culture Models. Molecules 2021, 26, 717. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Choi, J.-S.; Kang, S.-W.; Lee, Y.-J.; Park, J.; Kang, Y.-H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, E182–E190. [Google Scholar] [CrossRef]
- Jimenez, F.; Mitts, T.F.; Liu, K.; Wang, Y.; Hinek, A. Ellagic and tannic acids protect newly synthesized elastic fibers from premature enzymatic degradation in dermal fibroblast cultures. J. Investig. Dermatol. 2006, 126, 1272–1280. [Google Scholar] [CrossRef]
- Kasai, K.; Yoshimura, M.; Koga, T.; Arii, M.; Kawasaki, S. Effects of oral administration of ellagic acid-rich pomegranate extract on ultraviolet-induced pigmentation in the human skin. J. Nutr. Sci. Vitaminol. 2006, 52, 383–388. [Google Scholar] [CrossRef]
- Moon, N.R.; Kang, S.; Park, S. Consumption of ellagic acid and dihydromyricetin synergistically protects against UV-B induced photoaging, possibly by activating both TGF-beta 1 and wnt signaling pathways. J. Photochem. Photobiol. B-Biol. 2018, 178, 92–100. [Google Scholar] [CrossRef]
- Raudone, L.; Bobinaite, R.; Janulis, V.; Viskelis, P.; Trumbeckaite, S. Effects of raspberry fruit extracts and ellagic acid on respiratory burst in murine macrophages. Food Funct. 2014, 5, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Gil, T.-Y.; Hong, C.-H.; An, H.-J. Anti-Inflammatory Effects of Ellagic Acid on Keratinocytes via MAPK and STAT Pathways. Int. J. Mol. Sci. 2021, 22, 1277. [Google Scholar] [CrossRef] [PubMed]
- Lembo, S.; Balato, A.; Di Caprio, R.; Cirillo, T.; Giannini, V.; Gasparri, F.; Monfrecola, G. The Modulatory Effect of Ellagic Acid and Rosmarinic Acid on Ultraviolet-B-Induced Cytokine/Chemokine Gene Expression in Skin Keratinocyte (HaCaT) Cells. Biomed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Songkro, S.; Reanmongkol, W. Topical Anti-inflammatory Potential of Standardized Pomegranate Rind Extract and Ellagic Acid in Contact Dermatitis. Phytother. Res. 2014, 28, 629–632. [Google Scholar] [CrossRef]
- Jensen, J.D.; Dunn, J.H.; Luo, Y.; Liu, W.; Fujita, M.; Dellavalle, R.P. Ellagic acid inhibits melanoma growth in vitro. Dermatol. Rep. 2011, 3, e36. [Google Scholar] [CrossRef]
- Huang, Q.; Chai, W.-M.; Ma, Z.-Y.; Deng, W.-L.; Wei, Q.-M.; Song, S.; Zou, Z.-R.; Peng, Y.-Y. Antityrosinase mechanism of ellagic acid in vitro and its effect on mouse melanoma cells. J. Food Biochem. 2019, 43, e12996. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, F.; McClements, D.J.; Xie, B.; Sun, Z.; Deng, Q. Oligomeric Procyanidin Nanoliposomes Prevent Melanogenesis and UV Radiation-Induced Skin Epithelial Cell (HFF-1) Damage. Molecules 2020, 25, 1458. [Google Scholar] [CrossRef]
- Lai, R.; Xian, D.; Xiong, X.; Yang, L.; Song, J.; Zhong, J. Proanthocyanidins: Novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018, 23, 130–135. [Google Scholar] [CrossRef]
- Chen, F.; Ye, X.; Yang, Y.; Teng, T.; Li, X.; Xu, S.; Ye, Y. Proanthocyanidins from the bark of Metasequoia glyptostroboides ameliorate allergic contact dermatitis through directly inhibiting T cells activation and Thl/Th17 responses. Phytomedicine 2015, 22, 510–515. [Google Scholar] [CrossRef]
- Katiyar, S.K. Grape seed proanthocyanidines and skin cancer prevention: Inhibition of oxidative stress and protection of immune system. Mol. Nutr. Food Res. 2008, 52, S71–S76. [Google Scholar] [CrossRef]
- Vaid, M.; Singh, T.; Katiyar, S.K. Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS ONE 2011, 6, e21539. [Google Scholar] [CrossRef]
- Hah, Y.-S.; Kim, J.G.; Cho, H.Y.; Park, J.S.; Heo, E.P.; Yoon, T.-J. Procyanidins from Vitis vinifera seeds induce apoptotic and autophagic cell death via generation of reactive oxygen species in squamous cell carcinoma cells. Oncol. Lett. 2017, 14, 1925–1932. [Google Scholar] [CrossRef]
- Choi, Y.H. Honokiol attenuates oxidative stress-induced cytotoxicity in human keratinocytes via activating AMPK signaling. Asian Pac. J. Trop. Biomed. 2021, 11, 222–230. [Google Scholar] [CrossRef]
- Costa, A.; Facchini, G.; Pinheiro, A.L.T.A.; da Silva, M.S.; Bonner, M.Y.; Arbiser, J.; Eberlin, S. Honokiol protects skin cells against inflammation, collagenolysis, apoptosis, and senescence caused by cigarette smoke damage. Int. J. Dermatol. 2017, 56, 754–761. [Google Scholar] [CrossRef]
- Tanaka, K.; Hasegawa, J.; Asamitsu, K.; Okamoto, T. Magnolia ovovata extract and its active component magnolol prevent skin photoaging via inhibition of nuclear factor kappaB. Eur. J. Pharmacol. 2007, 565, 212–219. [Google Scholar] [CrossRef]
- Im, A.R.; Song, J.H.; Lee, M.Y.; Chae, S. Magnolol reduces UVB-induced photodamage by regulating matrix metalloproteinase activity. Environ. Toxicol. Pharmacol. 2015, 39, 417–423. [Google Scholar] [CrossRef]
- Lee, J.-H.; Im, D.-S. Honokiol suppresses 2,6-dinitrochlorobenzene-induced atopic dermatitis in mice. J. Ethnopharmacol. 2022, 289, 115023. [Google Scholar] [CrossRef]
- Chilampalli, C.; Guillermo, R.; Kaushik, R.S.; Young, A.; Chandrasekher, G.; Fahmy, H.; Dwivedi, C. Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells. Exp. Biol. Med. 2011, 236, 1351–1359. [Google Scholar] [CrossRef]
- Stanic, Z. Curcumin, a Compound from Natural Sources, a True Scientific Challenge—A Review. Plant Foods Hum. Nutr. 2017, 72, 1–12. [Google Scholar] [CrossRef]
- Zielinska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Med. Lith. 2020, 56, 336. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Y.; Wan, M.J.; Li, H.P.; Chen, Q.; Li, R.X.; Liang, B.H.; Zhu, H.L. Curcumin protection against ultraviolet-induced photo-damage in Hacat cells by regulating nuclear factor erythroid 2-related factor 2. Bioengineered 2021, 12, 9993–10006. [Google Scholar] [CrossRef] [PubMed]
- Semita, I.N.; Utomo, D.N.; Suroto, H.; Sudiana, I.K.; Gandi, P. The mechanism of human neural stem cell secretomes improves neuropathic pain and locomotor function in spinal cord injury rat models: Through antioxidant, anti-inflammatory, anti-matrix degradation, and neurotrophic activities. Korean J. Pain 2023, 36, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.G.; Fang, F.; Kang, S.N.; Wang, Z.C.; Yang, Y.M. Curcumin from Jianghuang (Rhizoma Curcumae Longae) protects against exposure to ultraviolet B by antioxidation and attenuating mitochondrion-dependent apoptosis. J. Tradit. Chin. Med. 2020, 40, 782–791. [Google Scholar]
- Liu, Y.H.; Lin, Y.S.; Huang, Y.W.; Fang, S.U.; Lin, S.Y.; Hou, W.C. Protective Effects of Minor Components of Curcuminoids on Hydrogen Peroxide-Treated Human HaCaT Keratinocytes. J. Agric. Food Chem. 2016, 64, 3598–3608. [Google Scholar] [CrossRef]
- Greenwald, M.B.Y.; Frusic-Zlotkin, M.; Soroka, Y.; Ben Sasson, S.; Bitton, R.; Bianco-Peled, H.; Kohen, R. Curcumin Protects Skin against UVB-Induced Cytotoxicity via the Keap1-Nrf2 Pathway: The Use of a Microemulsion Delivery System. Oxidative Med. Cell. Longev. 2017, 2017, 5205471. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Skin regenerative potentials of curcumin. Biofactors 2013, 39, 141–149. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nacher, A.; Valenti, D.; Fernandez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Alyoussef, A.; El-Gogary, R.I.; Ahmed, R.F.; Farid, O.A.A.; Bakeer, R.M.; Nasr, M. The beneficial activity of curcumin and resveratrol loaded in nanoemulgel for healing of burn-induced wounds. J. Drug Deliv. Sci. Technol. 2021, 62, 102360. [Google Scholar] [CrossRef]
- Hu, B.; Gao, M.Z.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.Y.; Zhang, X.Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, extraction and encapsulation: A review. Food Bioprod. Process. 2015, 93, 122–138. [Google Scholar] [CrossRef]
- Wagn, F.; Li, W. Experimental study of effect of 2 940 nm Er: YAG laser combined with catechin on photo damage in mice skin. J. Clin. Dermatol. 2015, 44, 351–355. [Google Scholar]
- Kapoor, M.P.; Sugita, M.; Fukuzawa, Y.; Timm, D.; Ozeki, M.; Okubo, T. Green Tea Catechin Association with Ultraviolet Radiation-Induced Erythema: A Systematic Review and Meta-Analysis. Molecules 2021, 26, 3702. [Google Scholar] [CrossRef]
- Tian, B.R.; Liu, J.Y. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Arora, D.; Jaglan, S. Therapeutic applications of resveratrol nanoformulations. Environ. Chem. Lett. 2018, 16, 35–41. [Google Scholar] [CrossRef]
- Hou, C.Y.; Tain, Y.L.; Yu, H.R.; Huang, L.T. The Effects of Resveratrol in the Treatment of Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 535. [Google Scholar] [CrossRef]
- Irnidayanti, Y.; Sutiono, D.R. Tempeh & Soybean Seed Coat: The Alternative Sources of Trans-Resveratrol as Neuroprotective Agents. Int. J. Morphol. 2019, 37, 1164–1171. [Google Scholar] [CrossRef]
- Soeur, J.; Eilstein, J.; Lereaux, G.; Jones, C.; Marrot, L. Skin resistance to oxidative stress induced by resveratrol: From Nrf2 activation to GSH biosynthesis. Free. Radic. Biol. Med. 2015, 78, 213–223. [Google Scholar] [CrossRef]
- Shin, J.W.; Lee, H.S.; Na, J.I.; Huh, C.H.; Park, K.C.; Choi, H.R. Resveratrol Inhibits Particulate Matter-Induced Inflammatory Responses in Human Keratinocytes. Int. J. Mol. Sci. 2020, 21, 3446. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Vale, D.L.; Martinez, R.M.; Medeiros, D.C.; da Rocha, C.; Sfeir, N.; Lopez, R.F.V.; Vicentini, F.; Verri, W.A.; Georgetti, S.R.; Baracat, M.M.; et al. A topical formulation containing quercetin-loaded microcapsules protects against oxidative and inflammatory skin alterations triggered by UVB irradiation: Enhancement of activity by microencapsulation. J. Drug Target. 2021, 29, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zi, C.; Chen, D.; Li, J.; He, R.; Hu, J.-M. Target acquisition of anti-aging manno-oligosaccharide that triggers ECM process via TGF-beta/Smads-SIRT1 signalling pathway. Carbohydr. Polym. 2023, 302, 120380. [Google Scholar] [CrossRef] [PubMed]
- Maramaldi, G.; Togni, S.; Pagin, I.; Giacomelli, L.; Cattaneo, R.; Eggenhoffner, R.; Burastero, S.E. Soothing and anti-itch effect of quercetin phytosome in human subjects: A single-blind study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Girme, A.; Saste, G.; Pawar, S.; Ghule, C.; Mirgal, A.; Patel, N.; Singh, R.; Hingorani, L. Development and Validation of a Sensitive Method for Analysis of Ellagic Acid in Dietary Supplements from Punica granatum. Curr. Top. Nutraceutical Res. 2021, 19, 90–105. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Velez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxidative Med. Cell. Longev. 2022, 2022, 1–24. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chou, C.-W.; Kumar, K.J.S.; Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef]
- Guo, H.; Liu, C.; Tang, Q.; Li, D.; Wan, Y.; Li, J.-H.; Gao, X.-H.; Seeram, N.P.; Ma, H.; Chen, H.-D. Pomegranate (Punica granatum) extract and its polyphenols reduce the formation of methylglyoxal-DNA adducts and protect human keratinocytes against methylglyoxal-induced oxidative stress. J. Funct. Foods 2021, 83, 104564. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: A comprehensive review. Phytomedicine Int. J. Phytother. Phytopharm. 2023, 109, 154581. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Wang, L.; Zu, Y.; Wang, S.; Liu, P.; Zhao, X. Preparation of honokiol nanoparticles by liquid antisolvent precipitation technique, characterization, pharmacokinetics, and evaluation of inhibitory effect on HepG2 cells. Int. J. Nanomed. 2018, 13, 5469–5483. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Huang, T.-H.; Hung, C.-F.; Huang, Y.-L.; Aljuffali, I.A.; Liao, W.-C.; Lin, C.-F. Derivatization of honokiol by integrated acetylation and methylation for improved cutaneous delivery and anti-inflammatory potency. Eur. J. Pharm. Sci. 2018, 114, 189–198. [Google Scholar] [CrossRef]
- Lin, C.-F.; Hwang, T.-L.; Al-Suwayeh, S.A.; Huang, Y.-L.; Hung, Y.-Y.; Fang, J.-Y. Maximizing dermal targeting and minimizing transdermal penetration by magnolol/honokiol methoxylation. Int. J. Pharm. 2013, 445, 153–162. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, T.; Katiyar, S.K. Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Guillermo, R.F.; Chilampalli, C.; Zhang, X.; Zeman, D.; Fahmy, H.; Dwivedi, C. Time and dose-response effects of honokiol on UVB-induced skin cancer development. Drug Discov. Ther. 2012, 6, 140–146. [Google Scholar] [CrossRef]
- Chen, J.; Tao, C.; Huang, X.; Chen, Z.; Wang, L.; Li, X.; Ma, M.; Wu, Z. CT2-3, a novel magnolol analogue suppresses NSCLC cells through triggering cell cycle arrest and apoptosis. Bioorganic Med. Chem. 2020, 28, 115352. [Google Scholar] [CrossRef]
- Vaid, M.; Sharma, S.D.; Katiyar, S.K. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: Development of topical formulation. Carcinogenesis 2010, 31, 2004–2011. [Google Scholar] [CrossRef]
- Chilampalli, S.; Zhang, X.; Fahmy, H.; Kaushik, R.S.; Zeman, D.; Hildreth, M.B.; Dwivedi, C. Chemopreventive Effects of Honokiol on UVB-induced Skin Cancer Development. Anticancer. Res. 2010, 30, 777–783. [Google Scholar]
- Guillermo-Lagae, R.; Santha, S.; Thomas, M.; Zoelle, E.; Stevens, J.; Kaushik, R.S.; Dwivedi, C. Antineoplastic Effects of Honokiol on Melanoma. Biomed. Res. Int. 2017, 2017, 5496398. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, E.P.A.; Van Wijk, R.; Bosman, S. Using ultra-weak photon emission to determine the effect of oligomeric proanthocyanidins on oxidative stress of human skin. J. Photochem. Photobiol. B Biol. 2010, 98, 199–206. [Google Scholar] [CrossRef]
- Rao, Z.; Chen, Y.; Wang, Q.; Lei, X.; Zhao, J.; Zeng, K.; Ming, J. Construction of food hydrogel-polyphenol delivery system and their enhancement of polyphenol bioavailability: A review. Food Ferment. Ind. 2022, 48, 304–311. [Google Scholar]
- Gupta, N.K.; Dixit, V.K. Development and evaluation of vesicular system for curcumin delivery. Arch. Dermatol. Res. 2011, 303, 89–101. [Google Scholar] [CrossRef]
- Agrawal, R.; Sandhu, S.K.; Sharma, I.; Kaur, I.P. Development and Evaluation of Curcumin-loaded Elastic Vesicles as an Effective Topical Anti-inflammatory Formulation. AAPS PharmSciTech 2015, 16, 364–374. [Google Scholar] [CrossRef]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanic, Z.; Skalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Song, Z.; Wen, Y.; Teng, F.; Wang, M.; Liu, N.; Feng, R. Carbopol 940 hydrogel containing curcumin-loaded micelles for skin delivery and application in inflammation treatment and wound healing. New J. Chem. 2022, 46, 3674–3686. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Yang, C.; Li, F.; Qiu, B.; Ding, W. Enhanced transdermal efficiency of curcumin-loaded peptide-modified liposomes for highly effective antipsoriatic therapy. J. Mater. Chem. B 2021, 9, 4846–4856. [Google Scholar] [CrossRef]
- Thuy, L.T.; Kang, N.; Choi, M.; Lee, M.; Choi, J.S. Dendrimeric micelles composed of polyamidoamine dendrimer-peptide-cholesterol conjugates as drug carriers for the treatment of melanoma and bacterial infection. J. Ind. Eng. Chem. 2022, 114, 361–376. [Google Scholar] [CrossRef]
- Wolf, J.R.; Gewandter, J.S.; Bautista, J.; Heckler, C.E.; Strasser, J.; Dyk, P.; Anderson, T.; Gross, H.; Speer, T.; Dolohanty, L.; et al. Utility of topical agents for radiation dermatitis and pain: A randomized clinical trial. Support. Care Cancer 2020, 28, 3303–3311. [Google Scholar] [CrossRef]
- Bahraini, P.; Rajabi, M.; Mansouri, P.; Sarafian, G.; Chalangari, R.; Azizian, Z. Turmeric tonic as a treatment in scalp psoriasis: A randomized placebo-control clinical trial. J. Cosmet. Dermatol. 2018, 17, 461–466. [Google Scholar] [CrossRef]
- Chatterjee, S.; Datta, R.N.; Bhattacharyya, B.; Bandopadhyay, S.K. Emollient and antipruritic effect of Itch cream in dermatological disorders: A randomized controlled trial. Indian J. Pharmacol. 2005, 37, 253–254. [Google Scholar] [CrossRef]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-blinded, placebo-controllecl trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005, 31, 855–859. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).