Abstract
Despite an uncommon condition, the clinical management of phlegmon appendicitis (retention of the intra-abdominal appendiceal abscess) is still controversial, and probiotics might be partly helpful. Then, the retained ligated cecal appendage (without gut obstruction) with or without oral Lacticaseibacillus rhamnosus dfa1 (started at 4 days prior to the surgery) was used as a representative model. At 5 days post-surgery, the cecal-ligated mice demonstrated weight loss, soft stool, gut barrier defect (leaky gut using FITC-dextran assay), fecal dysbiosis (increased Proteobacteria with reduced bacterial diversity), bacteremia, elevated serum cytokines, and spleen apoptosis without kidney and liver damage. Interestingly, the probiotics attenuated disease severity as indicated by stool consistency index, FITC-dextran assay, serum cytokines, spleen apoptosis, fecal microbiota analysis (reduced Proteobacteria), and mortality. Additionally, impacts of anti-inflammatory substances from culture media of the probiotics were demonstrated by attenuation of starvation injury in the Caco-2 enterocyte cell line as indicated by transepithelial electrical resistance (TEER), inflammatory markers (supernatant IL-8 with gene expression of TLR4 and NF-κB), cell energy status (extracellular flux analysis), and the reactive oxygen species (malondialdehyde). In conclusion, gut dysbiosis and leaky-gut-induced systemic inflammation might be helpful clinical parameters for patients with phlegmon appendicitis. Additionally, the leaky gut might be attenuated by some beneficial molecules from probiotics.
1. Introduction
The intra-abdominal peritonitis using cecal ligation and puncture (CLP) in rodents is an established model of sepsis, a potentially life-threatening condition in response to a severe infection, that represents “ruptured appendicitis” or “perforated diverticulitis” in patients [1]. Indeed, acute appendicitis, resulting from obstruction of the appendiceal lumen commonly by fecaliths and lymphoid follicular hyperplasia, is a common surgical condition in humans that needs an emergency resection [2]. However, in some cases of appendicitis, the inflamed appendix is walled off by the greater omentum inducing an inflammatory tumor-liked lesion referred to as “phlegmon appendicitis” or “abscess appendicitis” which mostly does not develop sepsis and has controversial management (surgical procedures or supportive management). For surgical procedures, antibiotics administration followed by delayed abscess drainage or appendectomy versus an immediate phlegmon removal is under the debate as some cases with immediate surgery end up with an ileocecal resection due to the technical difficulty in an acute inflammatory lesion [3,4]. On the other hand, antibiotics administration followed by delayed surgery might develop sepsis or recurrent symptom with the high cost of hospital stay [4]. Meanwhile, in the cases with successful antibiotic responses, the need for delayed surgical procedures is questioned because of the low risk of recurrent inflammation [4]. Although several interventions (ultrasonogram and computed tomography) were proposed to indicate the necessity of surgical procedures after successful supportive treatment [5], an alteration in gut microbiota or gut barrier damage might be another indicator.
As such, gut dysbiosis is an imbalance of gut microbiota (gut normal flora) correlating with unhealthy outcomes, that is affected by alterations in the intra-intestinal lumen and in the systemic situation [6]. For the intra-intestinal factors, increased gut pathogens, some diets, antibiotics, and the local inflammation (infection or non-infection) [7,8,9,10] induce gut dysbiosis, in part, through the enhanced intestinal immune cells that might have a different impact on different groups of gut organisms causing selective growth in some groups of bacteria (dysbiosis) [11,12,13,14]. For systemic alterations, the selected growth of some organisms over other groups might be due to increased uremic toxin in the gut (the intestine is used as an alternative route for the toxin excretion during renal insufficiency), reduced blood perfusion in sepsis, immune responses defects, deposition of circulating immune complex [15,16,17,18,19,20], and systemic viral infection (COVID-19 and dengue) [21,22]. While gut dysbiosis from both factors damages the gut barrier (a single cell layer separating the host’s circulation and the microbial molecules in the gut contents), gut eubiosis (the balanced microbiota in the healthy regular condition) improves gut integrity [7,23], partly through the increased short-chain fatty acids (SCFAs; the growth factors for gut epithelium) that are altered from the ingested complex carbohydrates by the hosts [24]. Subsequently, the intestinal barrier defect allows the translocation of microbial molecules from the gut into the blood circulation with systemic inflammation (leaky gut or gut leakage) [25,26,27,28]. Hence, gut dysbiosis and leaky gut in phlegmon appendicitis might represent a severe extra-intestinal lumen inflammation that possibly is an indicator of the need for earlier surgical management [12].
Additionally, the use of probiotics (the microbes that are beneficial to the host) for several preventive purposes is currently mentioned and Lacticaseibacillus rhamnosus dfa1, isolated from the Thai healthy volunteers [29] might be beneficial for the protection of the intestine against several issues. Perhaps, phlegmon appendicitis in individuals who regularly have probiotics for the balanced gut microbiota might have some benefits once the phlegmon develops. Then, cecal ligation without puncture model was used to test the correlation between extra-intestinal lumen inflammation and intestinal conditions (leaky gut and gut dysbiosis) and the influences of probiotics. While cecal ligation and puncture (CLP) is a well-established model for sepsis [30,31,32], the information on cecal ligation model is still very scare. Indeed, ligation of the cecal appendix is an easy procedure to induce the retention of necrotic tissue inside the intra-abdominal space of mice which usually surround by the greater omentum (adipose tissue) as a mechanism for the control of infection similar to phlegmon appendicitis in patients [33]. Notably, both the human appendix (from the terminal end of the cecum) and the cecal appendage in rodents are located at the junction of the ileum and cecum; the human appendix is a narrow extension that is an evolutionary vestige, whereas the rodent cecal appendage is very large [34]. In rodents, the cecum appendix resembles a blind sac for the temporary storage of food contents allowing bacteria to break down cellulose in the contents and absorb water for the well-formed feces [35,36,37] and resection of the cecal appendage might reduce fecal SCFAs [37] and decrease energy expenditure from the reduced biomass of gut microbiota [38]. Although there is no available data on the model of cecal ligation without cecal removal in mice, this model might be a model that represents phlegmon appendicitis. We hypothesize that cecal ligation might induce gut dysbiosis and leaky gut that could possibly be attenuated by L. rhamnosus dfa1.
Due to (i) the lack of data on dysbiosis during inflammation at the extra-intestinal lumen without sepsis or any underlying condition, (ii) the possible clinical translation in the patients with phlegmon appendicitis, and (iii) the interest in the cecal ligation model as a model representing a human condition, a model of cecal ligation with or without the probiotics together with several in vitro experiments was performed.
2. Results
2.1. The Clinical Manifestation and Fecal Dysbiosis of Mice with Cecal Ligation
To induce intraperitoneal inflammation and to test the impact of probiotics on the model, cecal ligation was performed with or without probiotic administration. As such, all mice with cecal ligation demonstrated weight loss that was more prominent in cecal-ligated mice without probiotics (Figure 1A). Although there was a low mortality rate in cecal-ligated mice, the operated mice with probiotics showed a higher 7-day survival rate (Figure 1B). There were looser and softer feces (stool consistency index) in the cecal-ligated mice when compared with the sham control (Figure 1C,D), possibly due to the water reabsorption through the mouse cecum. However, the cecal-ligated mice with probiotics demonstrated a better-formed stool than the non-probiotic mice at 2 days (but not at 5 days) post-surgery (Figure 1C,D). Although no puncture was performed in the ligated cecum, bacteremia was detectable in some mice mostly at 5 days post-surgery (Figure 1E). In parallel, the cecal-ligated mice demonstrated gut barrier defect, as evaluated by FITC-dextran assay (Figure 1F), as early as 2 days post-surgery, despite mostly negative hemoculture (Figure 1E), compared with the control mice without the significant difference to the 5 days post-operation. Although the probiotic administration reduced leaky gut (FITC-dextran) at 2 days post-surgery, the values at 5 days were not different between groups (Figure 1F). On the other hand, elevation of serum TNF-α was detected as early as 1 day after cecal ligation, whereas serum IL-6 and IL-10 increased on the 2nd and 5th day post-operation (Figure 1G–I). Probiotics attenuated serum cytokine levels at 2 days post-surgery (all cytokines) and at 5 days post-operation (serum TNF-α and IL-6) (Figure 1G–I). While the increased serum cytokines were not high enough to induce liver damage (liver enzyme) (Figure 1J), the systemic inflammation showed some impacts on the spleen as indicated by spleen apoptosis (Figure 1K). Indeed, the abundance of spleen apoptosis in the probiotic-administered mice was lower than in the cecal-ligated mice without probiotics (Figure 1K and Figure 2), which might be correlated with the attenuation of leaky-gut-induced systemic inflammation by probiotics (Figure 1F–G).
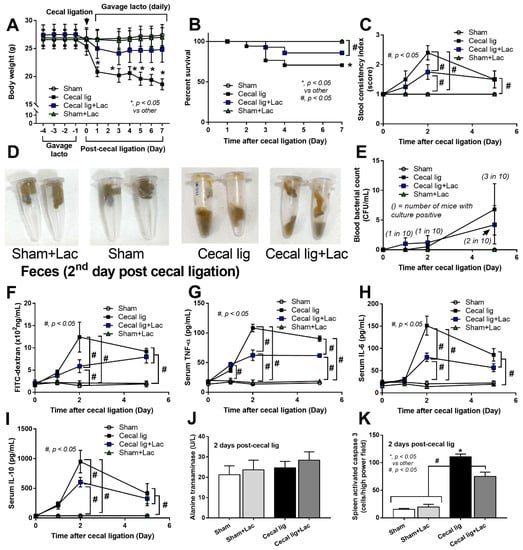
Figure 1.
The characteristics of mice with cecal ligation (without puncture) (Cecal lig) or sham surgery (Sham) with or without Lacticaseibacillus administration (Cecal lig + Lac and Sham + Lac) as indicated by body weight (A), survival analysis (B), stool consistency index (C), representative pictures of the mouse feces (D), blood bacterial count (E), gut barrier determination (FITC-dextran assay) (F), serum cytokines (TNF-α, IL-6, and IL-10) (G–I), liver enzyme (alanine transaminase) (J), and spleen apoptosis (K) are demonstrated (n = 25/group in A and B, n = 8–10/group or time point for others).
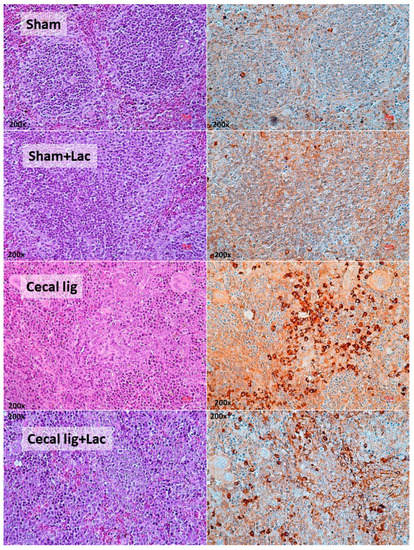
Figure 2.
The representative pictures of spleen histology from mice with cecal ligation (without puncture) (Cecal lig) or sham surgery (Sham) with or without Lacticaseibacillus administration (Cecal lig + Lac and Sham + Lac) at 48 h post-surgery as stained by Hematoxylin and Eosin (H&E) stain (left column) and activated caspase 3 (apoptosis) (right column) are demonstrated (the score of apoptotic cells is presented in Figure 1K).
Because (i) both leaky gut and stool consistency might be partly associated with gut dysbiosis [39], (ii) mouse cecum is an important reservoir of gut organisms [37], and (iii) the possible vulnerability of the balance of gut organisms [40], cecal ligation might interfere with fecal dysbiosis. As such, in comparison with control mice, cecal-ligated mice demonstrated a higher abundance of bacteria in phylum Proteobacteria (the group of several pathogenic Gram-negative bacteria), especially Escherichia-Shigella (Figure 3A–C) with a reduction in the diversity of bacterial species as evaluated by Chao1 richness estimation (the total number of species in the population) and Shannon evenness score (the proportions of species of the microbiota) (Figure 3D).
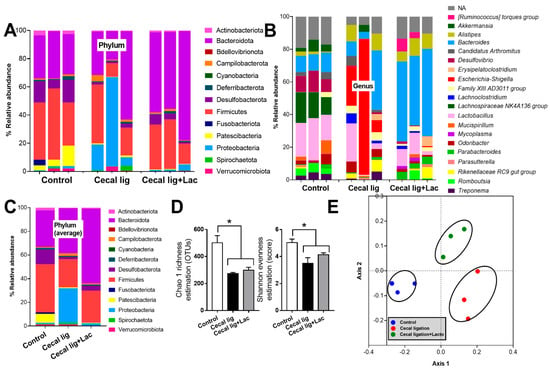
Figure 3.
The characteristics of fecal microbiome analysis from mice with cecal ligation (without puncture) (Cecal lig) or sham surgery (Sham) with or without Lacticaseibacillus administration (Cecal lig + Lac and Sham + Lac) as indicated by the abundance of bacteria in phylum, genus, and the average value on phylum (A–C), the alpha diversity (Chao 1 richness estimation and Shannon evenness evaluation) (D), and the non-metric multidimensional scaling (NMDS) (E) are demonstrated. * p < 0.05.
In comparison with the sham control, the crude characteristics of fecal microbiota from the cecal-ligated mice were demonstrated by the abundance of bacterial phylum (Figure 3C). Indeed, there was lower Firmicutes, the group of potentially beneficial bacteria in the healthy host [41], with the higher Proteobacteria (also referred to as “Pseudomonadota”), the group of several pathogenic Gram-negative bacteria [42], and similar Bacteroidota, the group of mostly Gram-negative anaerobes with possible pathogenicity in some situation [43]. Bacteria in Phylum Desulfobacterota, the thermophilic sulfate-reducing bacteria [44], and Patescibacteria, the uncultivated bacteria from metagenomic analysis [45], also presented in the highest abundance in the sham control mice (Figure 3A–C), but with a very limited amount of data on impacts of these organisms in the host. Nevertheless, the use of probiotics reduced Proteobacteria and increased Bacteroides without the alteration in Firmicutes and bacterial diversity (Figure 3A–D). Additionally, the differences in fecal microbiome among these experimental groups were also indicated by the clear separation in the non-metric multidimensional scaling (NMDS; a statistical analysis allowing the complex multivariate data sets to be visualized in a reduced number of dimensions) (Figure 3E).
2.2. The Protective Effects of the Condition Media from Lacticaseibacillus rhamnosus dfa1 in Enterocytes
Because (i) the excretion of several beneficial substances from Lacticaseibacillus rhamnosus dfa1, especially the exopolysaccharide is well-known [46,47] and (ii) cell starvation might be the most common and most important underlying mechanism of enterocyte damages caused by several different insults (such as hypoxia, overwhelming inflammatory responses, and some toxins) [48,49,50], the in vitro starvation model using an enterocyte cell line (Caco-2 cell) and the Lacticaseibacillus condition media (LCM) might be an interesting experiment to test the general protective effect of probiotics. As such, with 48 h starvation protocol with or without LCM (see method), there was no alteration in the cell viability as evaluated by the MTT assay when compared with the control group (Figure 4A). Surprisingly, the 48 h starvation enhanced the enterocyte integrity, as measured by transepithelial electrical impedance (TEER), when compared with the control (Figure 4B). However, the starvation caused enterocytic pro-inflammatory responses as indicated by increased supernatant cytokine (IL-8) with the up-regulation of Toll-like receptor 4 (TLR-4) and nuclear factor kappa B (NF-κB) (Figure 4C–E). For the cell energy status, the starvation decreased mitochondrial function (oxygen consumption rate; OCR) without an alteration in glycolysis activity (extracellular acidification rate; ECAR) (Figure 4F–I), despite the starvation-induced enterocyte inflammatory responses (Figure 4C–E), supporting a previous publication [51]. Additionally, the cell starvation also induced oxidative stress as indicated by an increase in a reactive oxygen species (ROS) using malondialdehyde (MDA) measurement (Figure 4J), possibly due to mitochondrial injury-induced oxidative stress [52].
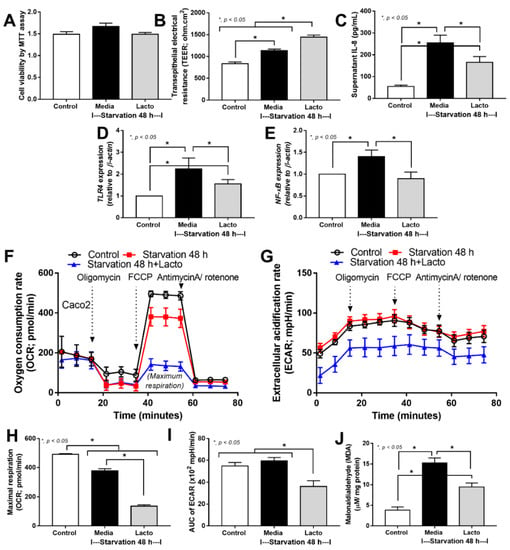
Figure 4.
The characteristics of enterocytes (Caco-2 cell line) in control media versus the 48 h starvation with or without Lacticaseibacillus condition media (LCM) (Lacto) as indicated by cell viability test using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT assay) (A), enterocyte integrity (transepithelial electrical resistance; TEER) (B), supernatant IL-8 (C), gene expression of inflammatory molecules, including Toll-like receptor 4 (TLR4), nuclear factor kappa B (NF-κB) (D,E), cell energy status as evaluated by the oxygen consumption rate (OCR; mitochondrial function), extracellular acidification rate (ECAR; glycolysis activity), maximal respiration (calculated from OCR), and the area under the curve (AUC) of ECAR (F–I), with malondialdialdehyde (MDA; a reactive oxygen species) (J) are demonstrated (independent triplicated experiments were performed).
In starvation with LCM, the cell viability was not altered but enterocyte integrity (TEER) was enhanced with a reduction in inflammatory responses (supernatant IL-8 and gene expression of TLR4 and NF-κB) (Figure 4A–E), implying some beneficial impacts of probiotics. Notably, in Caco-2 cells with starvation and LCM, the expression of NF-κB was reduced to the level of the control group (Figure 4E); however, the anti-inflammatory property of LCM was not high enough to normalize supernatant IL-8 and TLR4 expression (Figure 4C,D). In the cell energy status, LCM reduced both mitochondrial function (OCR) and glycolysis activity (ECAR) along with decreased oxidative stress (MDA) (Figure 4F–J). Perhaps, the reduced cell energy status might be correlated with the anti-inflammatory cell activity that reduced mitochondrial injury and ROS production. Although exploration of the mechanistic details of probiotics is out of the scope of the current interest, our data suggest that L. rhamnosus dfa1 attenuates disease severity of the mice with cecal ligation partly through the improved gut dysbiosis and excretion of some anti-inflammatory substances that strengthen gut barrier integrity partly via cell energy interference (Figure 5).
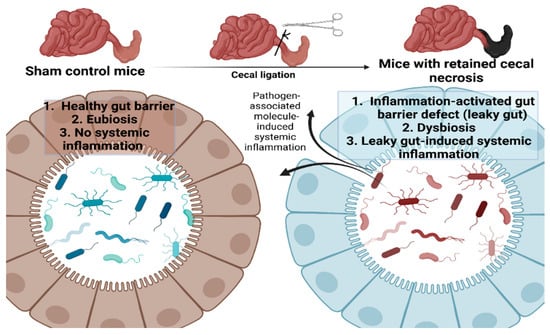
Figure 5.
The proposed working hypothesis of the gut barrier defect (leaky gut) from the retained necrotic lesion in the abdomen possibly through the active intraperitoneal inflammation that induces fecal dysbiosis (prominence in Proteobacteria; the phylum of several Gram-negative pathogenic bacteria) leading to the translocation of microbial molecules from the gut into the blood circulation (leaky gut) that facilitating systemic inflammation is demonstrated. Picture created by BioRender.com.
3. Discussion
For the characteristics of the model, necrosis of the mouse cecal appendage resulted in a significant weight loss, possibly due to reduced intake and poor water reabsorption (more fecal fluid loss). Indeed, the rodent cecal appendage is similar to appendix in human that possibly be used to solidify the rodent feces [35,36,37]. Some cecal-ligated mice died as early 2 days post-operation possibly due to bacteremia; however, bacteremia was detectable only in a few mice per group (especially at 5 days post-operation) resulting in the non-statistically significant value of bacteremia among the experimental groups. Despite a low rate of bacteremia in cecal-ligated mice, the intestinal barrier defect in these mice as determined by the translocation of FITC-dextran (molecular weight 4 kDa), approximately 4 × 10−3 µm in diameter (smaller than the size of the whole viable bacteria at 1–2 µm in diameter) [53,54], from the gut into the blood circulation (leaky gut) was demonstrated. Likewise, systemic inflammation (low levels of the elevated serum cytokines) in cecal-ligated mice was also demonstrated which might be due to the immune responses against the intra-abdominal necrotic tissue [55,56] together with the leaky gut-induced systemic inflammation [57]. Different from the cecal ligation and puncture (CLP) sepsis model which gut perforation and fecal peritonitis are initiated by a puncture of the ligated cecum, mice with cecal ligation without puncture, here, show only a subtle systemic inflammation with relatively low mortality compared with the high mortality rate in CLP sepsis model [58,59,60]. The limited mortality in cecal-ligated mice supported the role of greater omentum in the control of intra-abdominal sources of infection (abscess or necrotic tissue) as reported in phlegmon appendicitis [5]. Although the systemic inflammation after cecal ligation was not severe enough to induce kidney or liver injury, different from the inflammation of the gut–kidney–liver axis in sepsis [7], the significant inflammatory responses in cecal-ligated mice were demonstrated by an increase in apoptosis of spleen. Indeed, the overwhelming inflammatory activation can cause apoptosis in several cell types is well-known [27,61].
While serum cytokines in cecal-ligated mice might be a result of both leaky gut and other factors, fecal dysbiosis in these mice supported a possible dysbiosis-induced leaky gut after cecal ligation surgery. As such, an increased Proteobacteria (mostly pathogenic Gram-negative bacteria) and reduced bacterial diversity in the feces of cecal-ligated mice support gut dysbiosis in this model. With a presence of intra-abdominal necrotic tissue in this model, the inflammation in the peritoneal cavity affects the balance of gut microbiota, despite the inflammatory lesions being walled off. Although these data are similar to the dysbiosis in several peritonitis models, such as spontaneous bacterial peritonitis, fecal peritonitis, and peritonitis in peritoneal dialysis [62,63,64], the dysbiosis in these situations might not be only from peritonitis alone but also from the underlying overt systemic responses, including cirrhosis, sepsis, and uremia, in these situations that might affect the gut dysbiosis. Here, despite the subtle systemic responses in the cecal ligation model, the alteration in fecal dysbiosis was prominent, supporting a vulnerability of the balance in microbiota in the gut to the microenvironment [65]. Indeed, the alteration in psychological conditions, environmental changes, and physical stresses can also affect gut microbiota [66,67] and the detection of dysbiosis might be one of the sensitive biomarkers to recognize some important clinical impacts in patients. Indeed, the interplay between gut dysbiosis and inflammation is well-known [68]; however, data on the correlation between a subtle systemic inflammation and gut dysbiosis without the additional impacts from the underlying disease are still very limited. Hence, the cecal ligation (without puncture) model might be one of the interesting models for the exploration of dysbiosis with an inactive intra-abdominal lesion (abscess or necrotic tissue).
For the probiotic impacts, the stool bulk-forming property [34] and the enhanced intestinal integrity by probiotics are well-known through several mechanisms, including direct impacts on enterocytes (SCFAs; the energy sources of intestinal cells) [69], immune modulation (such as exopolysaccharides) [70,71], and reduced gut pathogens (nutrient competition) [72]. Here, probiotics attenuated dysbiosis after cecal ligation as indicated by reduced Proteobacteria, but not good enough to enhance bacterial diversity. Probiotics also improved gut barrier defect (FITC-dextran assay) and decreased serum cytokines in cecal-ligated mice, supporting that elevated serum cytokines in these mice might be, at least in part, correlated with leaky gut. In the in vitro experiments, starvation protocol on enterocytes was used as a model for inducing cell injury because cell starvation might be a common factor of cell injury in several stimulations. Although there was a good adaptation of enterocytes toward several insults (such as hypoxia and lipopolysaccharide) [73,74,75], the overwhelming inflammation induces cell injury, partly through the use-up of cell energy and mitochondrial injury [76]. Likewise, hypoxia, cellular acidosis, and poor blood perfusion (from too much vasodilation or cardiac dysfunction) are also similar to cell starvation as these factors limit energy production [77]. As expected, cell starvation reduced the mitochondrial function of enterocytes (extracellular flux analysis) which might be correlated with an increase in oxidative stress (MDA). Notably, mitochondria are important sources of oxidants in the cells, partly because of components in the electron transport chain [78], that can transform lipids in cell membrane into MDA [79]. However, the starvation had a low impact on glycolysis activity, perhaps due to the lower cell energy production (the synthesis of adenosine triphosphate; ATP) by glycolysis compared with mitochondrial oxidative phosphorylation [80].
With the condition media of Lacticaseibacillus rhamnosus dfa1 (LCM), there were improved enterocyte integrity (TEER), reduced inflammatory responses, decreased cell energy status (both mitochondrial function and glycolysis activity), and decreased oxidative stress in starvation-induced enterocyte injury. While the starvation injury reduced cell energy with elevated cytokine production and enhanced oxidative stress implying the starvation-induced mitochondrial injury [81], the prominently lower energy status from probiotics induced lower inflammatory responses with less oxidative stress suggesting enterocyte protection [82]. Potentially, the necessity of energy production from mitochondria and glycolysis is reduced by the short-chain fatty acids (SCFAs) in LCM that can be metabolized mostly by enterocytes (and hepatocytes) [83]. It is well-known that the presence of SCFAs inhibits the glycolysis pathway, reduces equivalents of the mitochondrial respiratory chain, decreases the efficacy of oxidative ATP synthesis, and enhances lipogenesis and gluconeogenesis [84,85]. More mechanistic studies will be of interest.
Although our current study is only a proof of concept on gut dysbiosis induction by the inflammation in the intraperitoneal cavity, the cecal ligation model might be a good representative model of phlegmon appendicitis [4]. Because leaky gut, fecal dysbiosis, and systemic inflammation are the main characteristics that correlate with mortality rate, we propose using these factors (or one of these factors) as the indicator for the removal of intra-abdominal phlegmon lesions. For example, patients with phlegmon appendicitis who develop endotoxemia (an indicator of leaky gut) might need an urgent phlegmon removal, whereas the patients without endotoxemia might benefit more from conservative antibiotic treatment. More studies on this model and in patients are needed for testing this hypothesis. For the use of probiotics, our current results implied that the probiotics might prevent the severity of dysbiosis caused by the retained intra-abdominal abscess; however, the use of probiotics in the patients might be inappropriate as the avoidance of oral intake will be routinely performed. Nevertheless, regular intake of probiotics for other health benefits might be also protective against intra-peritoneal abscess-induced dysbiosis and systemic inflammation. Additionally, isolation of the active anti-inflammatory substances from probiotics might be interesting.
Finally, there were several limitations in our study. First, the animal model study with a limited number of mice in each group is only a proof of concept study that need further experiments for a solid conclusion. Second, the gender and age of the mice might affect the model as our current study only uses young male 8-week-old mice that represent young humans (approximately 20 years old) [86]. Studies on female mice of different ages might have different results. Third, more details in mechanistic parts are needed for understanding the role of ligated cecal appendage on gut dysbiosis and the working mechanisms of probiotics. Despite these limitations, the cecal ligation (without puncture) model might be another model representing some conditions of the patients that might be useful for translation research.
4. Materials and Methods
4.1. Animal Model and Mouse Sample Analyses
The animal study protocol (CU-ACUP No. 011/2564) was endorsed by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University following the U.S. National Institutes of Health (NIH) animal care and use protocol. As such, C57BL/6J mice were purchased from Nomura Siam (Pathumwan, Bangkok, Thailand) and 8-week-old male mice were used in the experiments. All mice were housed in an animal facility under a light/dark cycle of 12:12 h at 22 ± 2 °C with 50 ± 10% relative humidity with free access to water and food (SmartHeart Rodent; Perfect companion pet care, Bangkok, Thailand). Then, Lacticaseibacillus rhamnosus dfa1 (Chulalongkorn University, Bangkok, Thailand) that isolated from the Thai healthy volunteers from a previous project [29] at 1 × 108 CFU in 0.3 mL phosphate-buffered solution (PBS), or PBS alone, were daily orally administered for 4 days prior to cecal ligation or sham operation. The cecal ligation protocol was modified from previous publications [87,88,89,90,91,92]. Briefly, the whole length of the cecum was ligated by silk 2-0 before closing the abdominal wall layer by layer, whereas sham surgery was only the identification of the cecum before suturing the abdominal wall with nylon 6–0. After that, 1 mL of prewarmed normal saline solution (NSS) with tramadol at 25 mg/kg/dose was subcutaneously administered after surgery, at 6 and 18 h post-CLP. The oral gavage by L. rhamnosus dfa1 or PBS was omitted on the day of surgery and continued after that. All mice were observed and monitored daily with body weight measurement. For blood and organ sample collection, the mice were euthanized by cardiac puncture under isoflurane anesthesia. The serum was stored at −80 °C until use, the spleen was placed in 10% neutral formalin for histological analysis, and feces were collected for microbiome analysis. The stool consistency index was graded into four scores as follows; 0: normal, 1: soft, 2: loose, and 3: diarrhea followed previous publications [93,94]. Blood bacterial abundance (bacteremia) was evaluated using the direct spread of mouse blood onto blood agar plates (Oxoid, Hampshire, UK) in serial dilutions and incubating at 37 °C for 24 h before colony enumeration.
For leaky gut evaluation, 0.5 mL of 25 mg/mL fluorescein isothiocyanate (FITC)-dextran 4 kDa (Sigma-Aldrich, St. Louis, MO, USA) in sterile water was orally administered at 3 h prior to sacrifice and FITC-dextran in blood was measured by fluorospectrometer (Varioskan, Thermo Fisher Scientific, Foster City, CA, USA) relative to FITC-dextran standards as previously described [10,15,16]. Notably, the results of FITC-dextran from different time points were determined from the different mice due to the retention of FITC-dextran in the intestinal lumens for a few days after administration. Serum cytokines (TNF-α, IL-6, and IL-10) and alanine transaminase (the liver enzyme) were measured by the enzyme-linked immunosorbent assay (ELISA) (Invitrogen, Waltham, MA, USA) and EnzyChrom Alanine Transaminase assay (EALT-100) (BioAssay, Hayward, CA, USA). Serum creatine (kidney function) was evaluated by QuantiChrom (DICT-500, BioAssay). The spleen samples on 4 mm paraffin sections were stained by hematoxylin and eosin (H&E) color and by an immunohistochemistry stain using an anti-activated caspase-3 antibody (Cell Signaling Technology, Beverly, MA, USA). Apoptotic cells in the spleen were evaluated in 10 randomly selected ×200 magnified fields per slide and expressed as positive cells per high-power field following previous publications [27,95,96,97].
4.2. Fecal Microbiome Analysis
Mouse feces were collected by placing mice in metabolic cages (Hatteras Instruments, Cary, NC, USA) for a few hours. Then, feces (0.25 g) from each mouse in different cages were collected for microbiome analysis to avoid the influence of allocoprophagy (a habit of mice that ingest feces from other mice) on fecal microbiota analysis following previous publications [93,98,99]. Briefly, the metagenomic DNA was extracted from the prepared samples using a DNAeasy Kit (Qiagen, Redwood City, CA, USA). The quality and concentration of the extracted DNA were measured by nanodrop spectrophotometry. Universal prokaryotic primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) with appended 50 Illumina adapter and 30 Golay barcode sequences were used for 16S rRNA gene V4 library construction in Miseq300 platform (Illumina, San Diego, CA, USA). The raw sequences and operational taxonomic unit (OTU) were classified following Mothur’s Standard Operating Procedures (SOP) [100].
4.3. The In Vitro Experiments
Caco-2 (HTB-37), human colorectal adenocarcinoma cells from the American Type Culture Collection (ATCC, Manassas, VA, USA), were cultured with Dulbecco’s modified Eagle medium (DMEM) supplemented with 20% fetal bovine serum (FBS), 1% penicillin/streptomycin, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) with sodium pyruvate (Thermo Fisher Scientific) in a humidified 5% CO2 incubator at 37 °C. Then, the cells were sub-cultured before using for the starvation protocol by transferring the cells into the serum-free culture medium (DMEM supplemented with 1% penicillin/streptomycin, 1% HEPES, and 1% sodium pyruvate) for 48 h following a previous protocol [73]. The regular culture media were used as a control group. Meanwhile, the condition media (modified DMEM as mentioned above) after 48 h culture with L. rhamnosus dfa1, referred to as “Lacticaseibacillus condition media (LCM)”, was prepared by the incubation of L. rhamnosus dfa1 at an OD600 of 0.1 in anaerobic condition for 48 h before supernatant collection by centrifugation and filtration with a 0.22 μm membrane filter (Minisart; Sartorius Stedim Biotech GmbH, Göttingen, Germany). After that, this cell-free supernatant (0.5 mL) was concentrated by speed vacuum drying at 40 °C for 3 h (Savant Instruments, Farmingdale, NY, USA) and stored at −20 °C until use [101]. Then, the LCM pellets, resuspended in DMEM (5% LCM) or DMEM alone (Media), in an equal volume were incubated in the Caco-2 cells with starvation before the sample collection for supernatant cytokines by ELISA (Invitrogen) or gene expression with quantitative real-time polymerase chain reaction (PCR) following previous publications [102,103,104].
Briefly, the total RNA was prepared by Trizol, quantified by a Nanodrop ND-1000 (Thermo Fisher Scientific, Rockford, IL, USA), converted to cDNA with High-Capacity cDNA Reverse Transcription (Thermo Scientific), and examined gene expression with the SYBR Green PCR Master Mix (Applied biosystem, Foster City, CA, USA). The results were demonstrated in relative quantification of the comparative threshold method (2−ΔΔCt) and normalized by a housekeeping gene (β-actin). The following primers (for human cells) were used to amplify cDNA fragments: TLR4, forward 5′-CACAGACTTGCGGGTTCTAC-3′, reverse 5′-AGGACCGACACACCAATGATG-3′; NF-κB, forward 5′-CAGAGCTGCGCTTGCAGAG-3′, reverse 5′-GTCAGCAGCCGGTTACCAAG-3′; β-actin forward 5′-CCTGGCACCCAGCACAAT-3′, reverse5′-GCCGATCCACACGGAGTACT-3′. In parallel, the cell viability was determined by tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) solution (Thermo Fisher Scientific) [13] with the incubation by 0.5 mg/mL of MTT solution at 37 °C in the dark for 2 h and diluted by dimethyl sulfoxide (DMSO; Thermo Fisher Scientific) before measurement with a Varioskan Flash microplate reader at absorbance OD570 nm. Moreover, the impact of starvation on intestinal integrity was determined by transepithelial electrical resistance (TEER) as previously described [19]. Briefly, Caco-2 cells at 5 × 104 cells per well in modified DMEM were seeded onto the upper compartment of the 24-well Boyden chamber trans-well for 15 days to establish the confluent cell monolayer. Then, the starvation protocol was performed and TEER in ohm (Ω) × cm2 was measured with an epithelial volt-ohm meter (EVOM2TM, World precision instruments, Sarasota, FL, USA) by placing electrodes in the supernatant at basolateral and in apical chambers. Notably, TEER values in media culture without Caco-2 cells were used as a blank and were subtracted from the values for the measurements. For measurement of malondialdehyde (MDA; an indicator of reactive oxygen species of lipid peroxidation) [105], the activated Caco-2 cells were homogenized by the Ultra-Turrax homogenizer (IKA, Staufen, Germany) and centrifuged at 12,000× g for 15 min at 4 °C to separate the supernatant. Then, malondialdehyde (MDA) in the supernatant was measured by an MDA assay kit (colorimetric) (Abcam, Cambridge, UK) according to the manufacturer’s protocol and representing the intracellular reactive oxygen species (ROS).
4.4. Extracellular Flux Analysis
Seahorse XFp Analyzers (Agilent, Santa Clara, CA, USA) was used to determine the energy status of the cells (extracellular flux analysis), with oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) representing mitochondrial function (respiration) and glycolysis activity, respectively, follow previous publications [17,88,91,106,107]. Briefly, Caco-2 cells (1 × 104 cells/well) were grown in Seahorse culture plates with DMEM for 48 h before transferring to the serum-free culture medium (starvation protocol) or control DMEM for another 48 h with or without 5%LCM. Then, the cell media were replaced by Seahorse media (DMEM complemented with glucose, pyruvate, and L-glutamine) (Agilent, 103575–100) at 37 °C for 1 h and subsequently activated by different metabolic interference compounds, including 1.5 μM oligomycin, 1 μM carbonyl cyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP), and 0.5 μM rotenone/antimycin A for the oxygen consumption rate (OCR) evaluation. The maximal respiration was calculated by the Seahorse Wave 2.6 software based on the following equation, maximal respiration = OCR between FCCP and rotenone/antimycin A—OCR after rotenone/antimycin A. Additionally, glycolysis stress tests were calculated from the mitochondrial stress test using the wave program of Seahorse XF Analyzers (Agilent) and demonstrated by the area under the curve of the ECAR graph as calculated by the trapezoidal rule [91].
4.5. Statistical Analysis
All data in mean ± standard error (SE) were analyzed by Graph Pad Prism version 7.0 software (La Jolla, CA, USA) and Statistical Package for Social Sciences software (SPSS 22.0, SPSS Inc., Chicago, IL, USA). The differences between multiple groups were examined by one-way analysis of variance (ANOVA) with Tukey’s analysis, whereas the survival analysis was determined by the log-rank test. A p-value < 0.05 was considered statistically significant.
5. Conclusions
The vulnerability of the balance of fecal organisms was demonstrated by gut dysbiosis after cecal ligation. Gut dysbiosis and leaky-gut-induced systemic inflammation could be demonstrated in some mice which might be possibly used as biomarkers for patients with an intra-abdominal abscess as an indication of the severe disease. In addition, the reduced severity of cecal-ligated mice with probiotic prevention also supports the possible benefit of balanced eubiosis on the reduction of systemic inflammation and disease severity. More studies are warranted.
Author Contributions
Conceptualization, T.T., W.K. and A.L.; methodology, W.K. and A.L.; formal analysis, W.K., P.P. and A.L.; investigation, W.K., T.T. and A.L.; resources, A.L.; data curation, W.K., T.T., W.C. and A.L.; writing—original draft preparation, W.K. and A.L.; writing—review and editing, A.L.; supervision, A.L.; funding acquisition, A.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovation Fund Chulalongkorn University (Fundamental fund 66) (HEA663000017), and National Research Council of Thailand (grant number NRCT5-RGJ63001) and (NRCT-N41A640076) with NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation. T.T. was supported by Pink diamond program, Faculty of Medicine, Chulalongkorn University.
Institutional Review Board Statement
The study was conducted in accordance with the National Institutes of Health (NIH) criteria, and the animal study protocol was approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (CU-ACUP No. 011/2564).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wallin, D.; Bock, D.; Angenete, E.; Öhman, L.; Magnusson, M.M.; Haglind, E. Model of Cecal Ligation and Puncture Mimicking Perforated Diverticulitis with Purulent Peritonitis. Clin. Surg. 2021, 6, 3225. [Google Scholar]
- Chen, Y.G.; Chang, H.M.; Chen, Y.L.; Cheng, Y.C.; Hsu, C.H. Perforated acute appendicitis resulting from appendiceal villous adenoma presenting with small bowel obstruction: A case report. BMC Gastroenterol. 2011, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Elkbuli, A.; Diaz, B.; Polcz, V.; Hai, S.; McKenney, M.; Boneva, D. Operative versus non-operative therapy for acute phlegmon of the appendix: Is it safer? A case report and review of the literature. Int. J. Surg. Case Rep. 2018, 50, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Tannoury, J.; Abboud, B. Treatment options of inflammatory appendiceal masses in adults. World J. Gastroenterol. 2013, 19, 3942–3950. [Google Scholar] [CrossRef]
- Ahmed, A.; Feroz, S.H.; Dominic, J.L.; Muralidharan, A.; Thirunavukarasu, P. Is Emergency Appendicectomy Better Than Elective Appendicectomy for the Treatment of Appendiceal Phlegmon: A Review. Cureus 2020, 12, e12045. [Google Scholar] [CrossRef]
- Charoensappakit, A.; Sae-Khow, K.; Leelahavanichkul, A. Gut Barrier Damage and Gut Translocation of Pathogen Molecules in Lupus, an Impact of Innate Immunity (Macrophages and Neutrophils) in Autoimmune Disease. Int. J. Mol. Sci. 2022, 23, 8223. [Google Scholar] [CrossRef]
- Amornphimoltham, P.; Yuen, P.S.T.; Star, R.A.; Leelahavanichkul, A. Gut Leakage of Fungal-Derived Inflammatory Mediators: Part of a Gut-Liver-Kidney Axis in Bacterial Sepsis. Dig. Dis. Sci. 2019, 64, 2416–2428. [Google Scholar] [CrossRef]
- Hiengrach, P.; Panpetch, W.; Worasilchai, N.; Chindamporn, A.; Tumwasorn, S.; Jaroonwitchawan, T.; Wilantho, A.; Chatthanathon, P.; Somboonna, N.; Leelahavanichkul, A. Administration of Candida Albicans to Dextran Sulfate Solution Treated Mice Causes Intestinal Dysbiosis, Emergence and Dissemination of Intestinal Pseudomonas Aeruginosa and Lethal Sepsis. Shock 2020, 53, 189–198. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef]
- Panpetch, W.; Phuengmaung, P.; Hiengrach, P.; Issara-Amphorn, J.; Cheibchalard, T.; Somboonna, N.; Tumwasorn, S.; Leelahavanichkul, A. Candida Worsens Klebsiella pneumoniae Induced-Sepsis in a Mouse Model with Low Dose Dextran Sulfate Solution through Gut Dysbiosis and Enhanced Inflammation. Int. J. Mol. Sci. 2022, 23, 7050. [Google Scholar] [CrossRef]
- Panpetch, W.; Hiengrach, P.; Nilgate, S.; Tumwasorn, S.; Somboonna, N.; Wilantho, A.; Chatthanathon, P.; Prueksapanich, P.; Leelahavanichkul, A. Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut Microbes 2020, 11, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Panpetch, W.; Phuengmaung, P.; Cheibchalard, T.; Somboonna, N.; Leelahavanichkul, A.; Tumwasorn, S. Lacticaseibacillus casei Strain T21 Attenuates Clostridioides difficile Infection in a Murine Model Through Reduction of Inflammation and Gut Dysbiosis With Decreased Toxin Lethality and Enhanced Mucin Production. Front. Microbiol. 2021, 12, 745299. [Google Scholar] [CrossRef]
- Panpetch, W.; Visitchanakun, P.; Saisorn, W.; Sawatpanich, A.; Chatthanathon, P.; Somboonna, N.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin. PLoS ONE 2021, 16, e0261189. [Google Scholar] [CrossRef] [PubMed]
- Visitchanakun, P.; Panpetch, W.; Saisorn, W.; Chatthanathon, P.; Wannigama, D.L.; Thim-Uam, A.; Svasti, S.; Fucharoen, S.; Somboonna, N.; Leelahavanichkul, A. Increased susceptibility to dextran sulfate-induced mucositis of iron-overload β-thalassemia mice, another endogenous cause of septicemia in thalassemia. Clin. Sci. 2021, 135, 1467–1486. [Google Scholar] [CrossRef]
- Ataide, M.A.; Knöpper, K.; Cruz de Casas, P.; Ugur, M.; Eickhoff, S.; Zou, M.; Shaikh, H.; Trivedi, A.; Grafen, A.; Yang, T.; et al. Lymphatic migration of unconventional T cells promotes site-specific immunity in distinct lymph nodes. Immunity 2022, 55, 1813–1828.e9. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Panpetch, W.; Chindamporn, A.; Leelahavanichkul, A. Macrophage depletion alters bacterial gut microbiota partly through fungal overgrowth in feces that worsens cecal ligation and puncture sepsis mice. Sci. Rep. 2022, 12, 9345. [Google Scholar] [CrossRef]
- Issara-Amphorn, J.; Dang, C.P.; Saisorn, W.; Limbutara, K.; Leelahavanichkul, A. Candida Administration in Bilateral Nephrectomy Mice Elevates Serum (1→3)-β-D-glucan That Enhances Systemic Inflammation through Energy Augmentation in Macrophages. Int. J. Mol. Sci. 2021, 22, 5031. [Google Scholar] [CrossRef] [PubMed]
- Panpetch, W.; Sawaswong, V.; Chanchaem, P.; Ondee, T.; Dang, C.P.; Payungporn, S.; Leelahavanichkul, A. Candida Administration Worsens Cecal Ligation and Puncture-Induced Sepsis in Obese Mice Through Gut Dysbiosis Enhanced Systemic Inflammation, Impact of Pathogen-Associated Molecules from Gut Translocation and Saturated Fatty Acid. Front. Immunol. 2020, 11, 561652. [Google Scholar] [CrossRef]
- Tungsanga, S.; Katavetin, P.; Panpetch, W.; Udompornpitak, K.; Saisorn, W.; Praditpornsilpa, K.; Eiam-Ong, S.; Tungsanga, K.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus L34 attenuates chronic kidney disease progression in a 5/6 nephrectomy mouse model through the excretion of anti-inflammatory molecules. Nephrol. Dial. Transpl. 2022, 37, 1429–1442. [Google Scholar] [CrossRef]
- Tungsanga, S.; Panpetch, W.; Bhunyakarnjanarat, T.; Udompornpitak, K.; Katavetin, P.; Chancharoenthana, W.; Chatthanathon, P.; Somboonna, N.; Tungsanga, K.; Tumwasorn, S.; et al. Uremia-Induced Gut Barrier Defect in 5/6 Nephrectomized Mice Is Worsened by Candida Administration through a Synergy of Uremic Toxin, Lipopolysaccharide, and (1→3)-β-D-Glucan, but Is Attenuated by Lacticaseibacillus rhamnosus L34. Int. J. Mol. Sci. 2022, 23, 2511. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Kamolratanakul, S.; Ariyanon, W.; Thanachartwet, V.; Phumratanaprapin, W.; Wilairatana, P.; Leelahavanichkul, A. Abnormal Blood Bacteriome, Gut Dysbiosis, and Progression to Severe Dengue Disease. Front. Cell. Infect. Microbiol. 2022, 12, 890817. [Google Scholar] [CrossRef] [PubMed]
- Sirivongrangson, P.; Kulvichit, W.; Payungporn, S.; Pisitkun, T.; Chindamporn, A.; Peerapornratana, S.; Pisitkun, P.; Chitcharoen, S.; Sawaswong, V.; Worasilchai, N.; et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensive Care Med. Exp. 2020, 8, 72. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F.; et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar] [PubMed]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, A.; Panpetch, W.; Worasilchai, N.; Somparn, P.; Chancharoenthana, W.; Nilgate, S.; Finkelman, M.; Chindamporn, A.; Tumwasorn, S. Evaluation of gastrointestinal leakage using serum (1→3)-β-D-glucan in a Clostridium difficile murine model. FEMS Microbiol. Lett. 2016, 363, fnw204. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Leelahavanichkul, A.; Ariyanon, W.; Vadcharavivad, S.; Phatcharophaswattanakul, S.; Kamolratanakul, S.; Leaungwutiwong, P.; Phumratanaprapin, W.; Wilairatana, P. Leaky Gut Syndrome Is Associated with Endotoxemia and Serum (1→3)-β-D-Glucan in Severe Dengue Infection. Microorganisms 2021, 9, 2390. [Google Scholar] [CrossRef]
- Thim-Uam, A.; Surawut, S.; Issara-Amphorn, J.; Jaroonwitchawan, T.; Hiengrach, P.; Chatthanathon, P.; Wilantho, A.; Somboonna, N.; Palaga, T.; Pisitkun, P.; et al. Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus. Sci. Rep. 2020, 10, 777. [Google Scholar] [CrossRef] [PubMed]
- Sae-Khow, K.; Charoensappakit, A.; Visitchanakun, P.; Saisorn, W.; Svasti, S.; Fucharoen, S.; Leelahavanichkul, A. Pathogen-Associated Molecules from Gut Translocation Enhance Severity of Cecal Ligation and Puncture Sepsis in Iron-Overload β-Thalassemia Mice. J. Inflamm. Res. 2020, 13, 719–735. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Janchot, K.; Kanacharoen, S.; Lertmongkolaksorn, T.; Wongsaroj, L.; Somboonna, N.; Ngamwongsatit, N.; Leelahavanichkul, A. Lactiplantibacillus plantarum dfa1 Outperforms Enterococcus faecium dfa1 on Anti-Obesity in High Fat-Induced Obesity Mice Possibly through the Differences in Gut Dysbiosis Attenuation, despite the Similar Anti-Inflammatory Properties. Nutrients 2022, 14, 80. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Leelahavanichkul, A.; Tsunoda, S.; Dear, J.W.; Takahashi, Y.; Ito, S.; Hu, X.; Zhou, H.; Doi, K.; Childs, R.; et al. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2008, 294, F1050–F1058. [Google Scholar] [CrossRef]
- Panahi, P.; Ibrahim, R.; Veeralakshmanan, P.; Ackah, J.; Coleman, M. Appendiceal phlegmon in adults: Do we know how to manage it yet? Ann. Med. Surg. 2020, 59, 274–277. [Google Scholar] [CrossRef]
- Smith, H.F.; Fisher, R.E.; Everett, M.L.; Thomas, A.D.; Bollinger, R.R.; Parker, W. Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix. J. Evol. Biol. 2009, 22, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Voravuthikunchai, S.P.; Lee, A. Cecectomy causes long-term reduction of colonization resistance in the mouse gastrointestinal tract. Infect. Immun. 1987, 55, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.E.; Argenzio, R.A.; Roberts, M.C. Comparative physiology of the mammalian colon and suggestions for animal models of human disorders. Clin. Gastroenterol. 1986, 15, 763–785. [Google Scholar] [PubMed]
- Brown, K.; Abbott, D.W.; Uwiera, R.R.E.; Inglis, G.D. Removal of the cecum affects intestinal fermentation, enteric bacterial community structure, and acute colitis in mice. Gut Microbes 2018, 9, 218–235. [Google Scholar] [CrossRef]
- Pearson, N.A.; Riedl, R.A.; Atkinson, S.N.; Ollinger, T.L.; Burnett, C.M.; Edwards, R.A.; Mokadem, M.; Kirby, J.R.; Grobe, J.L. Surgical Removal of Gut Bacteria Biomass Promotes Weight Gain via Suppression of Energy Expenditure. FASEB J. 2017, 31, 890–891. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Herrmann, M.; Wegner, C.E.; Taubert, M.; Geesink, P.; Lehmann, K.; Yan, L.; Lehmann, R.; Totsche, K.U.; Küsel, K. Predominance of Cand. Patescibacteria in Groundwater Is Caused by Their Preferential Mobilization from Soils and Flourishing Under Oligotrophic Conditions. Front. Microbiol. 2019, 10, 1407. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, T.T.; Bui, D.C.; Hong, P.T.; Hoang, Q.K.; Nguyen, H.T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. [Google Scholar] [CrossRef]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dziubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef]
- Lahiani, A.; Yavin, E.; Lazarovici, P. The Molecular Basis of Toxins’ Interactions with Intracellular Signaling via Discrete Portals. Toxins 2017, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, J. Regulation of energy balance by inflammation: Common theme in physiology and pathology. Rev. Endocr. Metab. Disord. 2015, 16, 47–54. [Google Scholar] [CrossRef]
- Raffan, E.; Dennis, R.J.; O’Donovan, C.J.; Becker, J.M.; Scott, R.A.; Smith, S.P.; Withers, D.J.; Wood, C.J.; Conci, E.; Clements, D.N.; et al. A Deletion in the Canine POMC Gene Is Associated with Weight and Appetite in Obesity-Prone Labrador Retriever Dogs. Cell Metab. 2016, 23, 893–900. [Google Scholar] [CrossRef]
- Bayir, H.; Kagan, V.E. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis—There is nothing more practical than a good theory. Crit. Care 2008, 12, 206. [Google Scholar] [CrossRef]
- Weiss, V.; Golesne, M.; Friedbacher, G.; Alban, S.; Szymanski, W.; MarchettiDeschmann, M.; Allmaier, G. Size and molecular weight determination of poly-saccharides by means of nano electrospray gas-phase electrophoretic mobility molecular analysis (nES GEMMA). Electrophoresis 2018, 39, 1142–1150. [Google Scholar] [CrossRef]
- Levin, P.A.; Angert, E.R. Small but Mighty: Cell Size and Bacteria. Cold Spring Harb. Perspect. Biol. 2015, 7, a019216. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Reijnen, M.M.; Bleichrodt, R.P.; van Goor, H. Pathophysiology of intra-abdominal adhesion and abscess formation, and the effect of hyaluronan. Br. J. Surg. 2003, 90, 533–541. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, A.; Bocharov, A.V.; Kurlander, R.; Baranova, I.N.; Vishnyakova, T.G.; Souza, A.C.; Hu, X.; Doi, K.; Vaisman, B.; Amar, M.; et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J. Immunol. 2012, 188, 2749–2758. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Worasilchai, N.; Wannalerdsakun, S.; Jutivorakool, K.; Somparn, P.; Issara-Amphorn, J.; Tachaboon, S.; Srisawat, N.; Finkelman, M.; Chindamporn, A. Gastrointestinal Leakage Detected by Serum (1→3)-β-D-Glucan in Mouse Models and a Pilot Study in Patients with Sepsis. Shock 2016, 46, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, A.; Somparn, P.; Panich, T.; Chancharoenthana, W.; Wongphom, J.; Pisitkun, T.; Hirankarn, N.; Eiam-Ong, S. Serum miRNA-122 in acute liver injury induced by kidney injury and sepsis in CD-1 mouse models. Hepatol. Res. 2015, 45, 1341–1352. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Liu, Z.; Yu, R.; Wang, D. LPS-induced inflammatory response and apoptosis are mediated by Fra-1 upregulation and binding to YKL-40 in A549 cells. Exp. Ther. Med. 2021, 22, 1474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lv, H.; Lv, J.; Shi, Y.; Huang, H.; Chen, L.; Shi, D. Alterations of gut microbiota in cirrhotic patients with spontaneous bacterial peritonitis: A distinctive diagnostic feature. Front. Cell. Infect. Microbiol. 2022, 12, 999418. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Lee, M.S.; Park, I.; Ko, H.S.; Yun, S.; Jang, D.H.; Kim, S.; Kim, H.; Kang, J.H.; Lee, J.H.; et al. Analysis of Porcine Model of Fecal-Induced Peritonitis Reveals the Tropism of Blood Microbiome. Front. Cell. Infect. Microbiol. 2021, 11, 676650. [Google Scholar] [CrossRef] [PubMed]
- Simões-Silva, L.; Araujo, R.; Pestana, M.; Soares-Silva, I.; Sampaio-Maia, B. Peritoneal Microbiome in End-Stage Renal Disease Patients and the Impact of Peritoneal Dialysis Therapy. Microorganisms 2020, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018, 9, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Sanchez-Labraca, N.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Lobo, L.A.; Benjamim, C.F.; Oliveira, A.C. The interplay between microbiota and inflammation: Lessons from peritonitis and sepsis. Clin. Transl. Immunol. 2016, 5, e90. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Zorriehzahra, M.J.; Delshad, S.T.; Adel, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Lazado, C.C. Probiotics as beneficial microbes in aquaculture: An update on their multiple modes of action: A review. Vet. Q. 2016, 36, 228–241. [Google Scholar] [CrossRef]
- Ross, A.M.; Walsh, D.R.; Cahalane, R.M.; Marcar, L.; Mulvihill, J.J.E. The effect of serum starvation on tight junctional proteins and barrier formation in Caco-2 cells. Biochem. Biophys. Rep. 2021, 27, 101096. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lacourt, T.E.; Vichaya, E.G.; Chiu, G.S.; Dantzer, R.; Heijnen, C.J. The High Costs of Low-Grade Inflammation: Persistent Fatigue as a Consequence of Reduced Cellular-Energy Availability and Non-adaptive Energy Expenditure. Front. Behav. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef]
- Cuvier, C.; Jang, A.; Hill, R.P. Exposure to hypoxia, glucose starvation and acidosis: Effect on invasive capacity of murine tumor cells and correlation with cathepsin (L+B) secretion. Clin. Exp. Metastasis 1997, 15, 19–25. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Awad, S.; Constantin-Teodosiu, D.; Macdonald, I.A.; Lobo, D.N. Short-term starvation and mitochondrial dysfunction—A possible mechanism leading to postoperative insulin resistance. Clin. Nutr. 2009, 28, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Khodaii, Z.; Ghaderian, S.M.H.; Natanzi, M.M. Probiotic Bacteria and their Supernatants Protect Enterocyte Cell Lines from Enteroinvasive Escherichia coli (EIEC) Invasion. Int. J. Mol. Cell. Med. 2017, 6, 183–189. [Google Scholar] [CrossRef]
- Carey, R.A.; Montag, D. Exploring the relationship between gut microbiota and exercise: Short-chain fatty acids and their role in metabolism. BMJ Open Sport Exerc. Med. 2021, 7, e000930. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Sutnu, N.; Visitchanakun, P.; Sawaswong, V.; Chitcharoen, S.; Payungporn, S.; Schuetz, A.; Schultz, M.J.; Leelahavanichkul, A. Critical roles of sepsis-reshaped fecal virota in attenuating sepsis severity. Front. Immunol. 2022, 13, 940935. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Kaewduangduen, W.; Chareonsappakit, A.; Susantitaphong, P.; Pisitkun, P.; Ritprajak, P.; Townamchai, N.; Leelahavanichkul, A. Interference on Cytosolic DNA Activation Attenuates Sepsis Severity: Experiments on Cyclic GMP–AMP Synthase (cGAS) Deficient Mice. Int. J. Mol. Sci. 2021, 22, 11450. [Google Scholar] [CrossRef]
- Visitchanakun, P.; Tangtanatakul, P.; Trithiphen, O.; Soonthornchai, W.; Wongphoom, J.; Tachaboon, S.; Srisawat, N.; Leelahavanichkul, A. Plasma miR-370-3P as a Biomarker of Sepsis-Associated Encephalopathy, the Transcriptomic Profiling Analysis of Microrna-Arrays From Mouse Brains. Shock 2020, 54, 347–357. [Google Scholar] [CrossRef]
- Chancharoenthana, W.; Udompronpitak, K.; Manochantr, Y.; Kantagowit, P.; Kaewkanha, P.; Issara-Amphorn, J.; Leelahavanichkul, A. Repurposing of High-Dose Erythropoietin as a Potential Drug Attenuates Sepsis in Preconditioning Renal Injury. Cells 2021, 10, 3133. [Google Scholar] [CrossRef]
- Hiengrach, P.; Visitchanakun, P.; Tongchairawewat, P.; Tangsirisatian, P.; Jungteerapanich, T.; Ritprajak, P.; Wannigama, D.L.; Tangtanatakul, P.; Leelahavanichkul, A. Sepsis Encephalopathy Is Partly Mediated by miR370-3p-Induced Mitochondrial Injury but Attenuated by BAM15 in Cecal Ligation and Puncture Sepsis Male Mice. Int. J. Mol. Sci. 2022, 23, 5445. [Google Scholar] [CrossRef] [PubMed]
- Issara-Amphorn, J.; Chancharoenthana, W.; Visitchanakun, P.; Leelahavanichkul, A. Syk Inhibitor Attenuates Polymicrobial Sepsis in FcgRIIb-Deficient Lupus Mouse Model, the Impact of Lupus Characteristics in Sepsis. J. Innate Immun. 2020, 12, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Hiengrach, P.; Visitchanakun, P.; Finkelman, M.A.; Chancharoenthana, W.; Leelahavanichkul, A. More Prominent Inflammatory Response to Pachyman than to Whole-Glucan Particle and Oat-β-Glucans in Dextran Sulfate-Induced Mucositis Mice and Mouse Injection through Proinflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4026. [Google Scholar] [CrossRef]
- Saithong, S.; Saisorn, W.; Visitchanakun, P.; Sae-Khow, K.; Chiewchengchol, D.; Leelahavanichkul, A. A Synergy Between Endotoxin and (1→3)-Beta-D-Glucan Enhanced Neutrophil Extracellular Traps in Candida Administered Dextran Sulfate Solution Induced Colitis in FcGRIIB-/- Lupus Mice, an Impact of Intestinal Fungi in Lupus. J. Inflamm. Res. 2021, 14, 2333–2352. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Huang, Y.; Hu, X.; Zhou, H.; Tsuji, T.; Chen, R.; Kopp, J.B.; Schnermann, J.; Yuen, P.S.; Star, R.A. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int. 2011, 80, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Taratummarat, S.; Sangphech, N.; Vu, C.; Palaga, T.; Ondee, T.; Surawut, S.; Sereemaspun, A.; Ritprajak, P.; Leelahavanichkul, A. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization. BMC Microbiol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Leelahavanichkul, A.; Yasuda, H.; Doi, K.; Hu, X.; Zhou, H.; Yuen, P.S.; Star, R.A. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am. J. Physiol. Ren. Physiol. 2008, 295, F1825–F1835. [Google Scholar] [CrossRef]
- Binmama, S.; Dang, C.P.; Visitchanakun, P.; Hiengrach, P.; Somboonna, N.; Cheibchalard, T.; Pisitkun, P.; Chindamporn, A.; Leelahavanichkul, A. Beta-Glucan from S. cerevisiae Protected AOM-Induced Colon Cancer in cGAS-Deficient Mice Partly through Dectin-1-Manipulated Macrophage Cell Energy. Int. J. Mol. Sci. 2022, 23, 951. [Google Scholar] [CrossRef]
- Kaewduangduen, W.; Visitchanakun, P.; Saisorn, W.; Phawadee, A.; Manonitnantawat, C.; Chutimaskul, C.; Susantitaphong, P.; Ritprajak, P.; Somboonna, N.; Cheibchalard, T.; et al. Blood Bacteria-Free DNA in Septic Mice Enhances LPS-Induced Inflammation in Mice through Macrophage Response. Int. J. Mol. Sci. 2022, 23, 1907. [Google Scholar] [CrossRef]
- Panpetch, W.; Somboonna, N.; Bulan, D.E.; Issara-Amphorn, J.; Finkelman, M.; Worasilchai, N.; Chindamporn, A.; Palaga, T.; Tumwasorn, S.; Leelahavanichkul, A. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1→3)-β-D-glucan. PLoS ONE 2017, 12, e0181439. [Google Scholar] [CrossRef]
- Panpetch, W.; Chancharoenthana, W.; Bootdee, K.; Nilgate, S.; Finkelman, M.; Tumwasorn, S.; Leelahavanichkul, A. Lactobacillus rhamnosus L34 Attenuates Gut Translocation-Induced Bacterial Sepsis in Murine Models of Leaky Gut. Infect. Immun. 2018, 86, e00700-17. [Google Scholar] [CrossRef] [PubMed]
- Singkham-In, U.; Phuengmaung, P.; Makjaroen, J.; Saisorn, W.; Bhunyakarnjanarat, T.; Chatsuwan, T.; Chirathaworn, C.; Chancharoenthana, W.; Leelahavanichkul, A. Chlorhexidine Promotes Psl Expression in Pseudomonas aeruginosa That Enhances Cell Aggregation with Preserved Pathogenicity Demonstrates an Adaptation against Antiseptic. Int. J. Mol. Sci. 2022, 23, 8308. [Google Scholar] [CrossRef]
- Hiengrach, P.; Panpetch, W.; Chindamporn, A.; Leelahavanichkul, A. Helicobacter pylori, Protected from Antibiotics and Stresses Inside Candida albicans Vacuoles, Cause Gastritis in Mice. Int. J. Mol. Sci. 2022, 23, 8568. [Google Scholar] [CrossRef] [PubMed]
- Phuengmaung, P.; Mekjaroen, J.; Saisorn, W.; Chatsuwan, T.; Somparn, P.; Leelahavanichkul, A. Rapid Synergistic Biofilm Production of Pseudomonas and Candida on the Pulmonary Cell Surface and in Mice, a Possible Cause of Chronic Mixed Organismal Lung Lesions. Int. J. Mol. Sci. 2022, 23, 9202. [Google Scholar] [CrossRef] [PubMed]
- Selvam, A.K.; Jawad, R.; Gramignoli, R.; Achour, A.; Salter, H.; Björnstedt, M. A Novel mRNA-Mediated and MicroRNA-Guided Approach to Specifically Eradicate Drug-Resistant Hepatocellular Carcinoma Cell Lines by Se-Methylselenocysteine. Antioxidants 2021, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Saisorn, W.; Saithong, S.; Phuengmaung, P.; Udompornpitak, K.; Bhunyakarnjanarat, T.; Visitchanakun, P.; Chareonsappakit, A.; Pisitkun, P.; Chiewchengchol, D.; Leelahavanichkul, A. Acute Kidney Injury Induced Lupus Exacerbation Through the Enhanced Neutrophil Extracellular Traps (and Apoptosis) in Fcgr2b Deficient Lupus Mice with Renal Ischemia Reperfusion Injury. Front. Immunol. 2021, 12, 669162. [Google Scholar] [CrossRef] [PubMed]
- Makjaroen, J.; Thim-Uam, A.; Dang, C.P.; Pisitkun, T.; Somparn, P.; Leelahavanichkul, A. A Comparison Between 1 Day versus 7 Days of Sepsis in Mice with the Experiments on LPS-Activated Macrophages Support the Use of Intravenous Immunoglobulin for Sepsis Attenuation. J. Inflamm. Res. 2021, 14, 7243–7263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).