Influence of Temperature Chronobiology on Stroke Outcome
Abstract
1. Introduction
2. Results
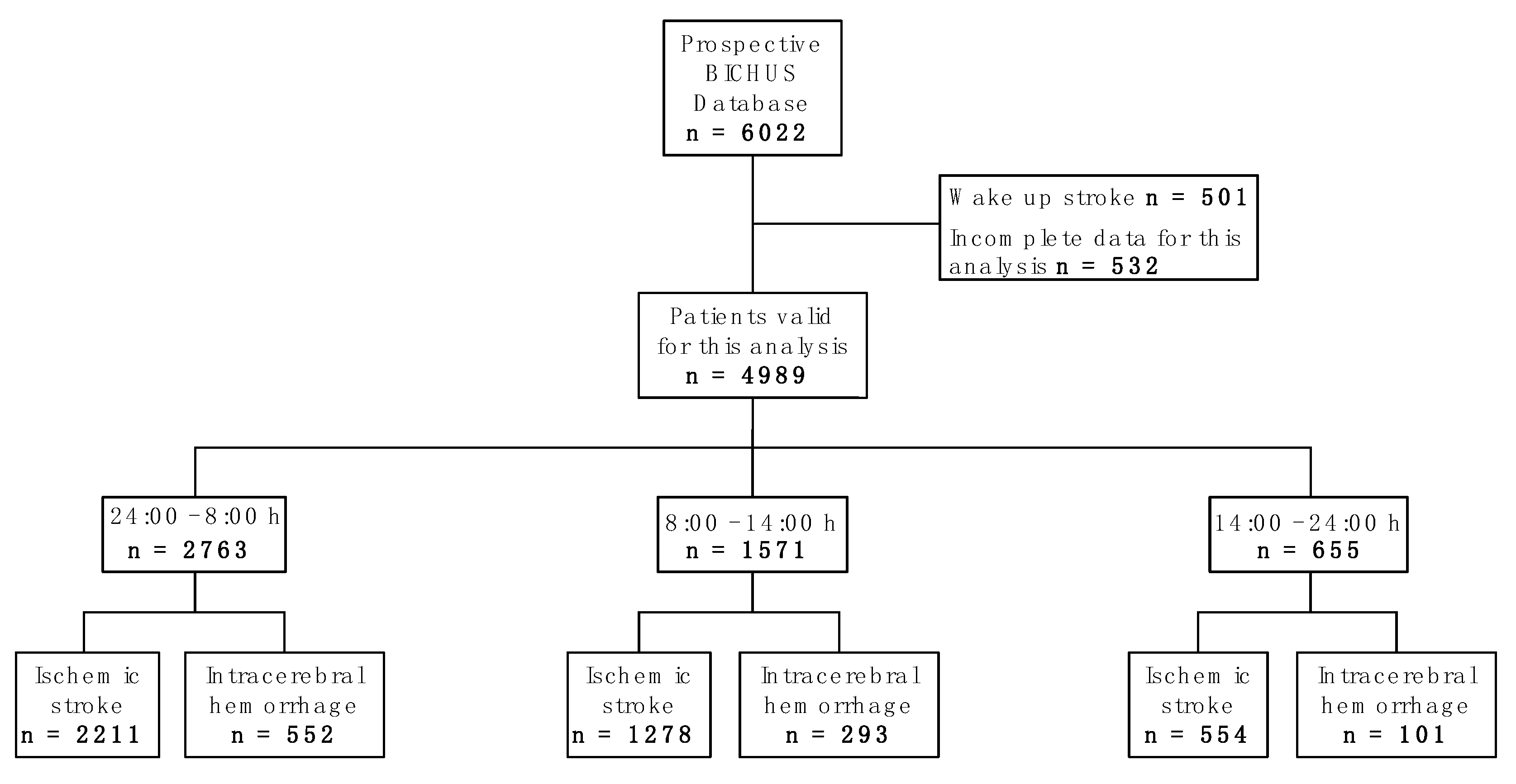
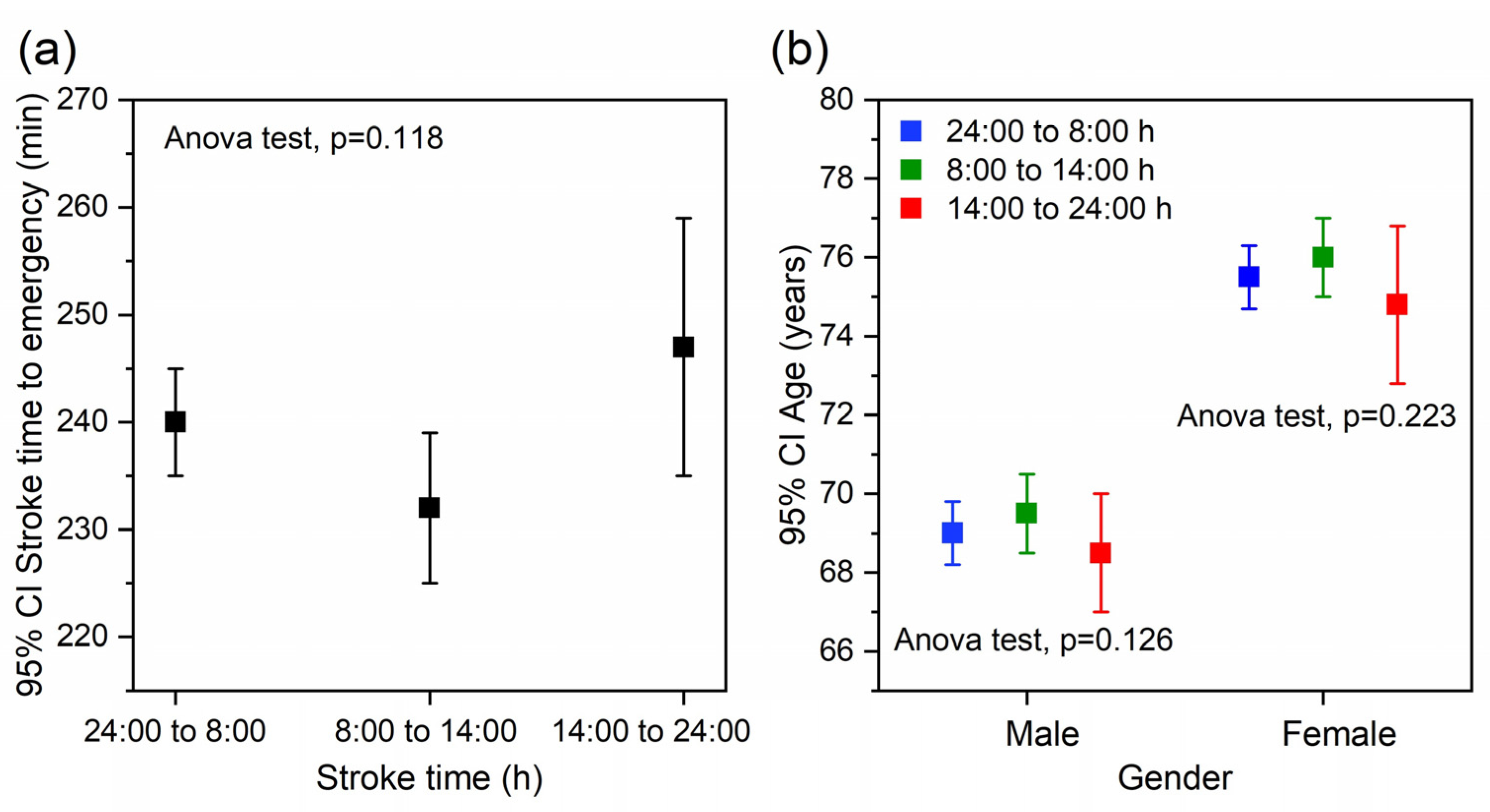
2.1. Sample Description
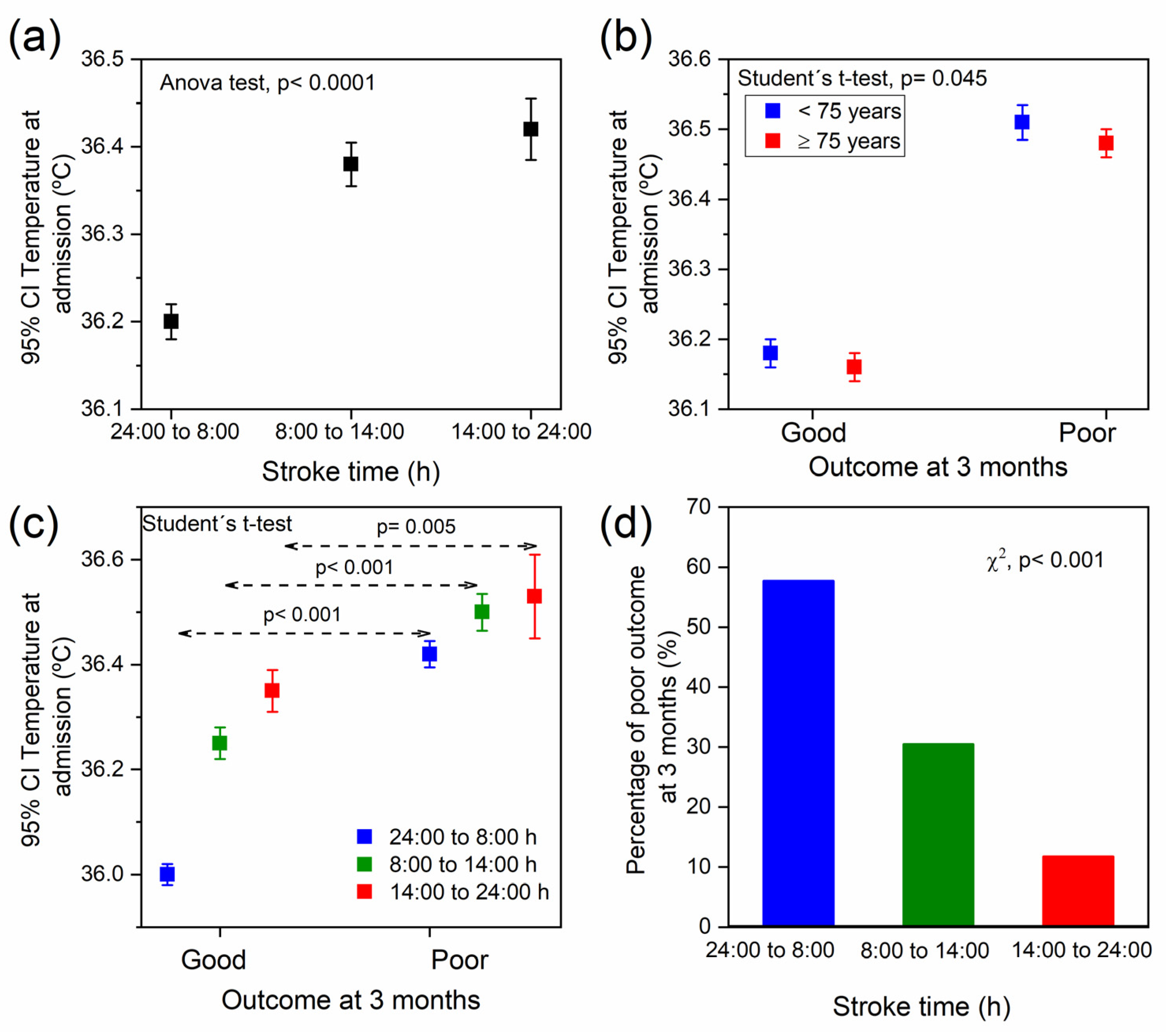
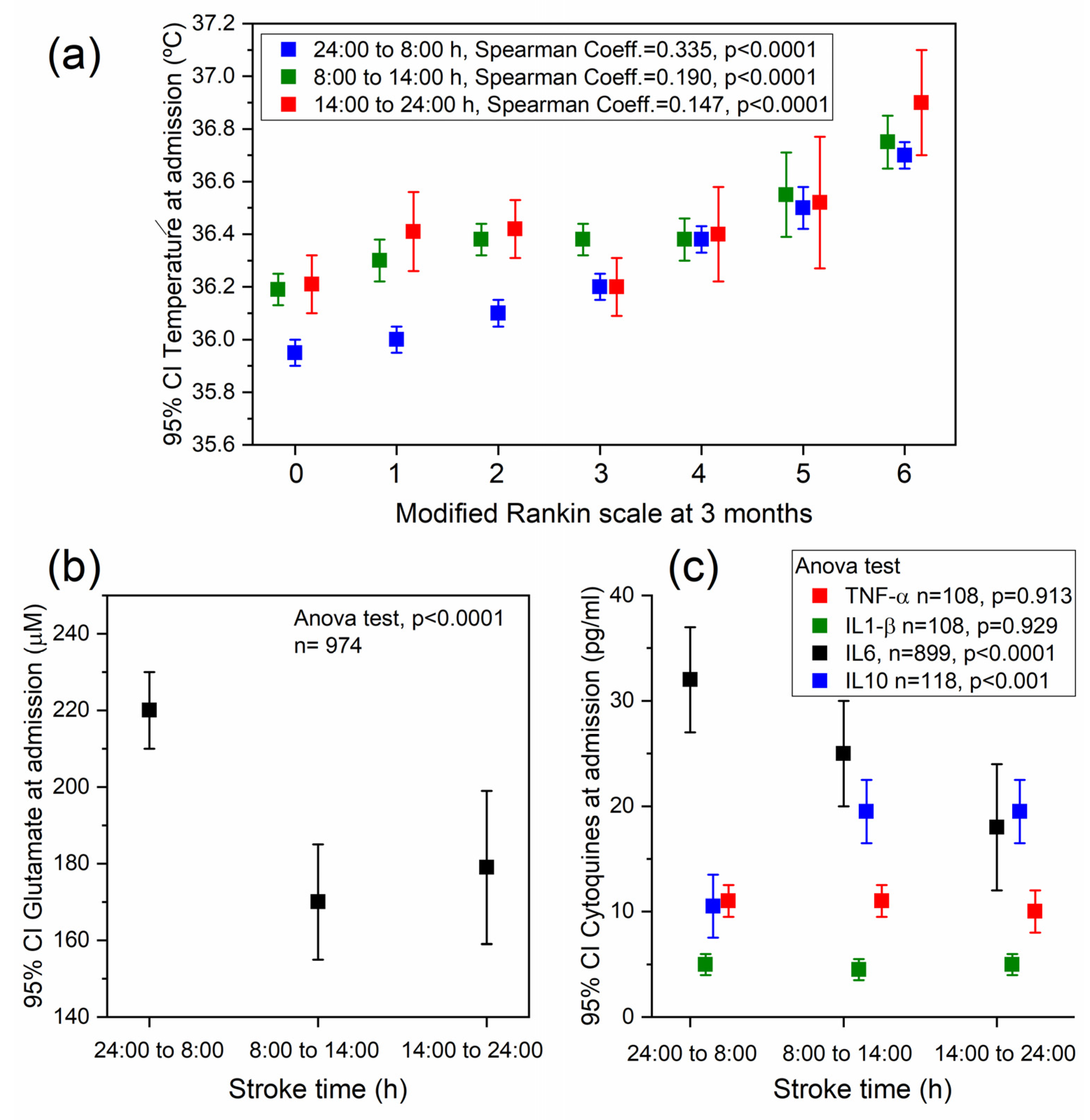
2.2. Association between Temperature, Stroke Time, and the Functional Outcome at 3 Months
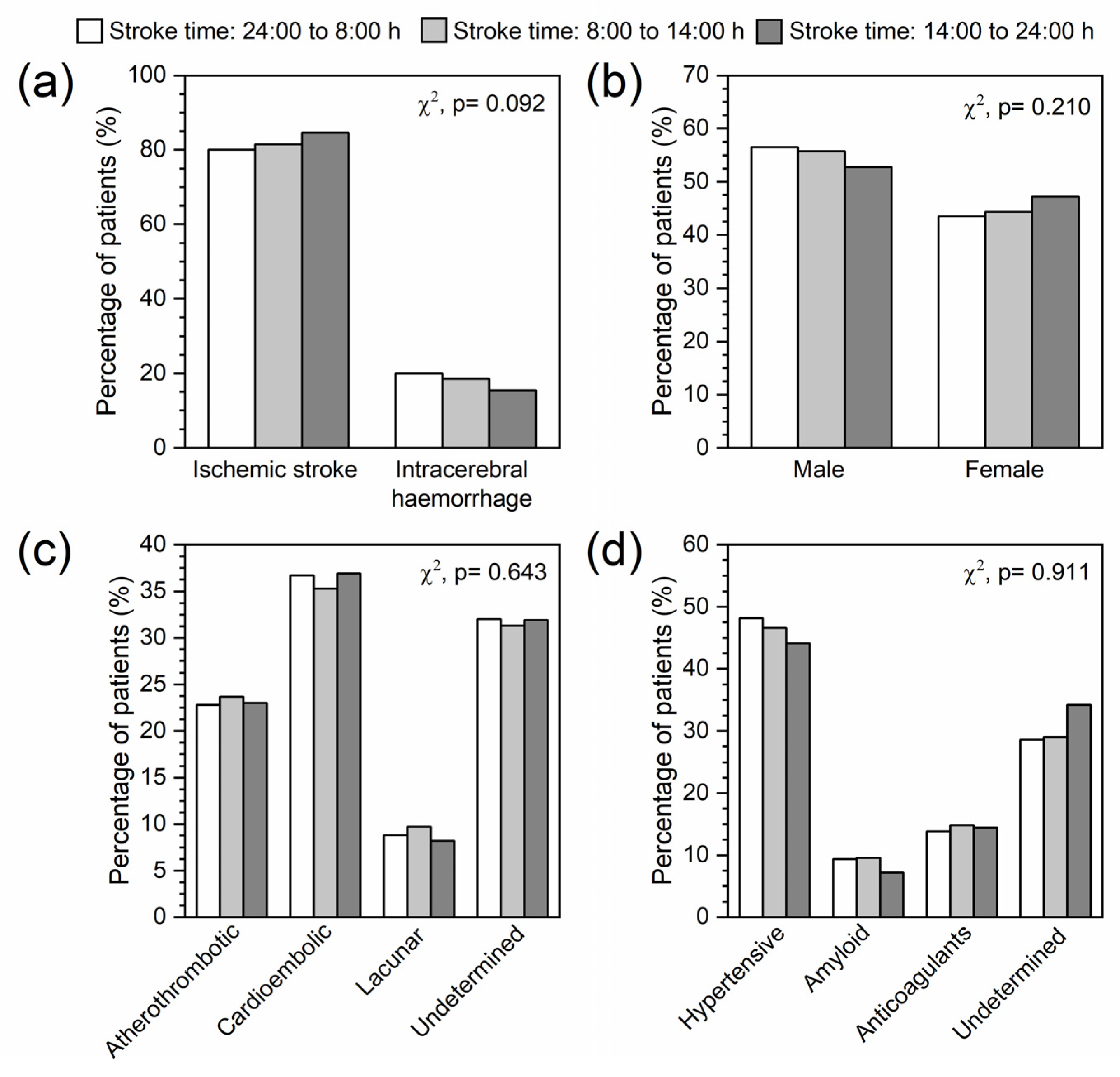
2.3. Association between Stroke Time and Different Biomarkers
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Inclusion and Exclusion Criteria
4.3. Standard Protocol Approvals, Registrations and Patients Consents
4.4. Temperature Control and Biochemical Analysis
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian Control of the Immune System. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. Circadian Rhythmicity of Body Temperature and Metabolism. Temperature 2020, 7, 321–362. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular Regulations of Circadian Rhythm and Implications for Physiology and Diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights Into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the Circadian System in Cardiovascular Disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.W.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, Precision, and Near-24-Hour Period of the Human Circadian Pacemaker. Science 1999, 284, 2177–2181. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Wüst, S.; Hellhammer, D. Consistent Sex Differences in Cortisol Responses to Psychological Stress. Psychosom. Med. 1992, 54, 648–657. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of Life: Circadian Disruption and Brain Disorders across the Lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian Disruption and Human Health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian Rhythm Disruption and Mental Health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Coiffard, B.; Diallo, A.B.; Mezouar, S.; Leone, M.; Mege, J.-L. A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System. Biology 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Reith, J.; Jørgensen, H.S.; Pedersen, P.M.; Nakayama, H.; Raaschou, H.O.; Jeppesen, L.L.; Olsen, T.S. Body Temperature in Acute Stroke: Relation to Stroke Severity, Infarct Size, Mortality, and Outcome. Lancet (Lond. Engl.) 1996, 347, 422–425. [Google Scholar] [CrossRef]
- Castillo, J.; Dávalos, A.; Marrugat, J.; Noya, M. Timing for Fever-Related Brain Damage in Acute Ischemic Stroke. Stroke 1998, 29, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Leira, R.; Rodríguez-Yáñez, M.; Castellanos, M.; Blanco, M.; Nombela, F.; Sobrino, T.; Lizasoain, I.; Dávalos, A.; Castillo, J. Hyperthermia Is a Surrogate Marker of Inflammation-Mediated Cause of Brain Damage in Acute Ischaemic Stroke. J. Intern. Med. 2006, 260, 343–349. [Google Scholar] [CrossRef]
- Leira, R.; Sobrino, T.; Blanco, M.; Campos, F.; Rodríguez-Yáñez, M.; Castellanos, M.; Moldes, O.; Millán, M.; Dávalos, A.; Castillo, J. A Higher Body Temperature Is Associated with Haemorrhagic Transformation in Patients with Acute Stroke Untreated with Recombinant Tissue-Type Plasminogen Activator (RtPA). Clin. Sci. 2012, 122, 113–119. [Google Scholar] [CrossRef]
- Blanco, M.; Campos, F.; Rodríguez-Yáñez, M.; Arias, S.; Fernández-Ferro, J.; Gómez-Sánchez, J.C.; Castillo, J. Neuroprotection or Increased Brain Damage Mediated by Temperature in Stroke Is Time Dependent. PLoS ONE 2012, 7, e30700. [Google Scholar] [CrossRef]
- Millán, M.; Grau, L.; Castellanos, M.; Rodríguez-Yáñez, M.; Arenillas, J.F.; Nombela, F.; Pérez de la Ossa, N.; López-Manzanares, L.; Serena, J.; Castillo, J.; et al. Body Temperature and Response to Thrombolytic Therapy in Acute Ischaemic Stroke. Eur. J. Neurol. 2008, 15, 1384–1389. [Google Scholar] [CrossRef]
- Hajat, C.; Hajat, S.; Sharma, P. Effects of Poststroke Pyrexia on Stroke Outcome: A Meta-Analysis of Studies in Patients. Stroke 2000, 31, 410–414. [Google Scholar] [CrossRef]
- Greer, D.M.; Funk, S.E.; Reaven, N.L.; Ouzounelli, M.; Uman, G.C. Impact of Fever on Outcome in Patients with Stroke and Neurologic Injury: A Comprehensive Meta-Analysis. Stroke 2008, 39, 3029–3035. [Google Scholar] [CrossRef]
- Saini, M.; Saqqur, M.; Kamruzzaman, A.; Lees, K.R.; Shuaib, A. VISTA Investigators Effect of Hyperthermia on Prognosis after Acute Ischemic Stroke. Stroke 2009, 40, 3051–3059. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; Sampedro-Viana, A.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Mosqueira, A.J.; Ouro, A.; Ávila-Gómez, P.; Sobrino, T.; Campos, F.; et al. Antihyperthermic Treatment in the Management of Malignant Infarction of the Middle Cerebral Artery. J. Clin. Med. 2022, 11, 2874. [Google Scholar] [CrossRef]
- Elliott, W.J. Circadian Variation in the Timing of Stroke Onset: A Meta-Analysis. Stroke 1998, 29, 992–996. [Google Scholar] [CrossRef]
- Fabbian, F.; Manfredini, R.; De Giorgi, A.; Gallerani, M.; Cavazza, M.; Grifoni, S.; Fabbri, A.; Cervellin, G.; Ferrari, A.M.; Imberti, D. “Timing” of Arrival and in-Hospital Mortality in a Cohort of Patients under Anticoagulant Therapy Presenting to the Emergency Departments with Cerebral Hemorrhage: A Multicenter Chronobiological Study in Italy. Chronobiol. Int. 2016, 33, 245–256. [Google Scholar] [CrossRef]
- Ripamonti, L.; Riva, R.; Maioli, F.; Zenesini, C.; Procaccianti, G. Daily Variation in the Occurrence of Different Subtypes of Stroke. Stroke Res. Treat. 2017, 2017, 9091250. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.A.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Disruptions of Circadian Rhythms and Thrombolytic Therapy During Ischemic Stroke Intervention. Front. Neurosci. 2021, 15, 675732. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Conde, J.; Ois, A.; Rodríguez-Campello, A.; Gomis, M.; Roquer, J. Does Sleep Protect against Ischemic Stroke? Less Frequent Ischemic Strokes but More Severe Ones. J. Neurol. 2007, 254, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Stubblefield, J.J.; Lechleiter, J.D. Time to Target Stroke: Examining the Circadian System in Stroke. Yale J. Biol. Med. 2019, 92, 349–357. [Google Scholar]
- Ávila-Gómez, P.; Hervella, P.; Da Silva-Candal, A.; Pérez-Mato, M.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Castillo, J.; Sobrino, T.; Iglesias-Rey, R.; et al. Temperature-Induced Changes in Reperfused Stroke: Inflammatory and Thrombolytic Biomarkers. J. Clin. Med. 2020, 9, 2108. [Google Scholar] [CrossRef]
- Geneva, I.I.; Cuzzo, B.; Fazili, T.; Javaid, W. Normal Body Temperature: A Systematic Review. Open Forum Infect. Dis. 2019, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Zitting, K.-M.; Chinoy, E.D. Aging and Circadian Rhythms. Sleep Med. Clin. 2015, 10, 423–434. [Google Scholar] [CrossRef]
- Weinert, D.; Waterhouse, J. The Circadian Rhythm of Core Temperature: Effects of Physical Activity and Aging. Physiol. Behav. 2007, 90, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Rzechorzek, N.M.; Thrippleton, M.J.; Chappell, F.M.; Mair, G.; Ercole, A.; Cabeleira, M.; Rhodes, J.; Marshall, I.; O’Neill, J.S. A Daily Temperature Rhythm in the Human Brain Predicts Survival after Brain Injury. Brain 2022, 145, 2031–2048. [Google Scholar] [CrossRef] [PubMed]
- Lushington, K.; Dawson, D.; Lack, L. Core Body Temperature Is Elevated during Constant Wakefulness in Elderly Poor Sleepers. Sleep 2000, 23, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, M.; Bollu, P.C.; French, B.; Sahota, P. Sleep Medicine: Stroke and Sleep. Mo. Med. 2018, 115, 527–532. [Google Scholar]
- Rodríguez-Castro, E.; López-Dequit, I.; Santamaría-Cadavid, M.; Arias-Rivas, S.; Rodríguez-Yáñez, M.; Pumar, J.M.; Hervella, P.; López-Arias, E.; da Silva-Candal, A.; Estany, A.; et al. Trends in Stroke Outcomes in the Last Ten Years in a European Tertiary Hospital. BMC Neurol. 2018, 18, 164. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, S.J.; Bae, J.; Lee, E.J.; Kwon, O.D.; Jeong, H.-Y.; Kim, Y.; Jeong, H.-B. Impact of Onset-to-Door Time on Outcomes and Factors Associated with Late Hospital Arrival in Patients with Acute Ischemic Stroke. PLoS ONE 2021, 16, e0247829. [Google Scholar] [CrossRef]
- Dávalos, A.; Castillo, J.; Martinez-Vila, E. Delay in Neurological Attention and Stroke Outcome. Stroke 1995, 26, 2233–2237. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, X.X.; Ma, J.; Jia, M.; Wu, L.; Li, W.; Li, C.; Wu, C.; Ren, C.; Chen, X.; et al. Association between the Time of Day at Stroke Onset and Functional Outcome of Acute Ischemic Stroke Patients Treated with Endovascular Therapy. J. Cereb. Blood Flow Metab. 2022, 42, 2191–2200. [Google Scholar] [CrossRef]
- Ryu, W.-S.; Hong, K.-S.; Jeong, S.-W.; Park, J.E.; Kim, B.J.; Kim, J.-T.; Lee, K.B.; Park, T.H.; Park, S.-S.; Park, J.-M.; et al. Association of Ischemic Stroke Onset Time with Presenting Severity, Acute Progression, and Long-Term Outcome: A Cohort Study. PLoS Med. 2022, 19, e1003910. [Google Scholar] [CrossRef]
- Reidler, P.; Brehm, A.; Sporns, P.B.; Burbano, V.G.; Stueckelschweiger, L.; Broocks, G.; Liebig, T.; Psychogios, M.-N.; Ricke, J.; Dimitriadis, K.; et al. Circadian Rhythm of Ischaemic Core Progression in Human Stroke. J. Neurol. Neurosurg. Psychiatry 2023, 94, 70–73. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, G.J.; Bang, O.Y. Biomarkers for Stroke. J. Stroke 2013, 15, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kamtchum-Tatuene, J.; Jickling, G.C. Blood Biomarkers for Stroke Diagnosis and Management. NeuroMol. Med. 2019, 21, 344–368. [Google Scholar] [CrossRef]
- Bsat, S.; Halaoui, A.; Kobeissy, F.; Moussalem, C.; El Houshiemy, M.N.; Kawtharani, S.; Omeis, I. Acute Ischemic Stroke Biomarkers: A New Era with Diagnostic Promise? Acute Med. Surg. 2021, 8, e696. [Google Scholar] [CrossRef] [PubMed]
- Dagonnier, M.; Donnan, G.A.; Davis, S.M.; Dewey, H.M.; Howells, D.W. Acute Stroke Biomarkers: Are We There Yet? Front. Neurol. 2021, 12, 619721. [Google Scholar] [CrossRef] [PubMed]
- Waje-Andreassen, U.; Krakenes, J.; Ulvestad, E.; Thomassen, L.; Myhr, K.-M.; Aarseth, J.; Vedeler, C.A. IL-6: An Early Marker for Outcome in Acute Ischemic Stroke. Acta Neurol. Scand. 2005, 111, 360–365. [Google Scholar] [CrossRef]
- Leasure, A.C.; Kuohn, L.R.; Vanent, K.N.; Bevers, M.B.; Kimberly, W.T.; Steiner, T.; Mayer, S.A.; Matouk, C.C.; Sansing, L.H.; Falcone, G.J.; et al. Association of Serum IL-6 (Interleukin 6) With Functional Outcome After Intracerebral Hemorrhage. Stroke 2021, 52, 1733–1740. [Google Scholar] [CrossRef]
- Basic Kes, V.; Simundic, A.-M.; Nikolac, N.; Topic, E.; Demarin, V. Pro-Inflammatory and Anti-Inflammatory Cytokines in Acute Ischemic Stroke and Their Relation to Early Neurological Deficit and Stroke Outcome. Clin. Biochem. 2008, 41, 1330–1334. [Google Scholar] [CrossRef]
- Vila, N.; Castillo, J.; Dávalos, A.; Esteve, A.; Planas, A.M.; Chamorro, A. Levels of Anti-Inflammatory Cytokines and Neurological Worsening in Acute Ischemic Stroke. Stroke 2003, 34, 671–675. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Nan, S. The Prognostic Determinant of Interleukin-10 in Patients with Acute Ischemic Stroke: An Analysis from the Perspective of Disease Management. Dis. Markers 2021, 2021, 6423244. [Google Scholar] [CrossRef]
- Castellanos, M.; Sobrino, T.; Pedraza, S.; Moldes, O.; Pumar, J.M.; Silva, Y.; Serena, J.; Garcia-Gil, M.; Castillo, J.; Davalos, A. High Plasma Glutamate Concentrations Are Associated with Infarct Growth in Acute Ischemic Stroke. Neurology 2008, 71, 1862–1868. [Google Scholar] [CrossRef]
- Meng, X.; Li, N.; Guo, D.-Z.; Pan, S.-Y.; Li, H.; Yang, C. High Plasma Glutamate Levels Are Associated with Poor Functional Outcome in Acute Ischemic Stroke. Cell. Mol. Neurobiol. 2015, 35, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.-M.; Prolo, P.; Trakada, G.; Chrousos, G.P. IL-6 and Its Circadian Secretion in Humans. Neuroimmunomodulation 2005, 12, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Dimitrov, S.; Born, J. Effects of Sleep and Circadian Rhythm on the Human Immune System. Ann. N. Y. Acad. Sci. 2010, 1193, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Gudewill, S.; Pollmächer, T.; Vedder, H.; Schreiber, W.; Fassbender, K.; Holsboer, F. Nocturnal Plasma Levels of Cytokines in Healthy Men. Eur. Arch. Psychiatry Clin. Neurosci. 1992, 242, 53–56. [Google Scholar] [CrossRef]
- Jiménez-Zárate, B.S.; Piña-Leyva, C.; Rodríguez-Sánchez, M.; Florán-Garduño, B.; Jiménez-Zamudio, L.A.; Jiménez-Estrada, I. Day-Night Variations in the Concentration of Neurotransmitters in the Rat Lumbar Spinal Cord. J. Circadian Rhythms 2021, 19, 9. [Google Scholar] [CrossRef]
- He, S.; Zhang, X.; Qu, S. Glutamate, Glutamate Transporters, and Circadian Rhythm Sleep Disorders in Neurodegenerative Diseases. ACS Chem. Neurosci. 2019, 10, 175–181. [Google Scholar] [CrossRef]
- Campos, F.; Blanco, M.; Barral, D.; Agulla, J.; Ramos-Cabrer, P.; Castillo, J. Influence of Temperature on Ischemic Brain: Basic and Clinical Principles. Neurochem. Int. 2012, 60, 495–505. [Google Scholar] [CrossRef]
- Ávila-Gómez, P.; Pérez-Mato, M.; Hervella, P.; Dopico-López, A.; da Silva-Candal, A.; Bugallo-Casal, A.; López-Amoedo, S.; Candamo-Lourido, M.; Sobrino, T.; Iglesias-Rey, R.; et al. Associations between RNA-Binding Motif Protein 3, Fibroblast Growth Factor 21, and Clinical Outcome in Patients with Stroke. J. Clin. Med. 2022, 11, 949. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver Agreement for the Assessment of Handicap in Stroke Patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- White, J.A.; Hart, R.J.; Fry, J.C. An Evaluation of the Waters Pico-Tag System for the Amino-Acid Analysis of Food Materials. J. Automat. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef]
| Stroke time from 24:00 to 8:00 h | ||||||
| Not Adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Temperature at admission | 2.76 | 2.45–3.12 | <0.0001 | 2.79 | 2.36–3.28 | <0.0001 |
| Age | 1.04 | 1.03–1.05 | <0.0001 | 1.03 | 1.02–1.04 | <0.0001 |
| Reperfusion treatment | 0.47 | 0.39–0.56 | <0.0001 | 0.19 | 0.15–0.24 | <0.0001 |
| NIHSS at admission | 1.13 | 1.12–1.15 | <0.0001 | 1.14 | 1.12–1.16 | <0.0001 |
| Stroke time from 8:00 to 14:00 h | ||||||
| Not adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Temperature at admission | 1.66 | 1.43–1.91 | <0.0001 | 1.52 | 1.26–1.85 | <0.0001 |
| Age | 1.04 | 1.03–1.05 | <0.0001 | 1.03 | 1.02–1.04 | <0.0001 |
| Reperfusion treatment | 0.40 | 0.31–0.52 | <0.0001 | 0.16 | 0.12–0.22 | <0.0001 |
| NIHSS at admission | 1.13 | 1.11–1.15 | <0.0001 | 1.15 | 1.13–1.17 | <0.0001 |
| Stroke time from 14:00 to 24:00 h | ||||||
| Not adjusted | Adjusted | |||||
| OR | CI 95% | p | OR | CI 95% | p | |
| Temperature at admission | 1.31 | 1.08–1.59 | 0.006 | 1.23 | 0.94–1.60 | 0.132 |
| Age | 1.05 | 1.04–1.07 | <0.0001 | 1.05 | 1.03–1.06 | <0.0001 |
| Reperfusion treatment | 0.40 | 0.22–0.50 | <0.0001 | 0.17 | 0.10–0.28 | <0.0001 |
| NIHSS at admission | 1.10 | 1.07–1.12 | <0.0001 | 0.10 | 1.06–1.13 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Alonso, M.L.; Sampedro-Viana, A.; Rodríguez-Yáñez, M.; López-Dequidt, I.; Pumar, J.M.; Mosqueira, A.J.; Fernández-Rodicio, S.; Bazarra-Barreiros, M.; Sobrino, T.; Campos, F.; et al. Influence of Temperature Chronobiology on Stroke Outcome. Int. J. Mol. Sci. 2023, 24, 3746. https://doi.org/10.3390/ijms24043746
Alonso-Alonso ML, Sampedro-Viana A, Rodríguez-Yáñez M, López-Dequidt I, Pumar JM, Mosqueira AJ, Fernández-Rodicio S, Bazarra-Barreiros M, Sobrino T, Campos F, et al. Influence of Temperature Chronobiology on Stroke Outcome. International Journal of Molecular Sciences. 2023; 24(4):3746. https://doi.org/10.3390/ijms24043746
Chicago/Turabian StyleAlonso-Alonso, Maria Luz, Ana Sampedro-Viana, Manuel Rodríguez-Yáñez, Iria López-Dequidt, José M. Pumar, Antonio J. Mosqueira, Sabela Fernández-Rodicio, Marcos Bazarra-Barreiros, Tomás Sobrino, Francisco Campos, and et al. 2023. "Influence of Temperature Chronobiology on Stroke Outcome" International Journal of Molecular Sciences 24, no. 4: 3746. https://doi.org/10.3390/ijms24043746
APA StyleAlonso-Alonso, M. L., Sampedro-Viana, A., Rodríguez-Yáñez, M., López-Dequidt, I., Pumar, J. M., Mosqueira, A. J., Fernández-Rodicio, S., Bazarra-Barreiros, M., Sobrino, T., Campos, F., Castillo, J., Hervella, P., & Iglesias-Rey, R. (2023). Influence of Temperature Chronobiology on Stroke Outcome. International Journal of Molecular Sciences, 24(4), 3746. https://doi.org/10.3390/ijms24043746









