High-Efficiency CRISPR/Cas9-Mediated Correction of a Homozygous Mutation in Achromatopsia-Patient-Derived iPSCs
Abstract
1. Introduction
2. Results
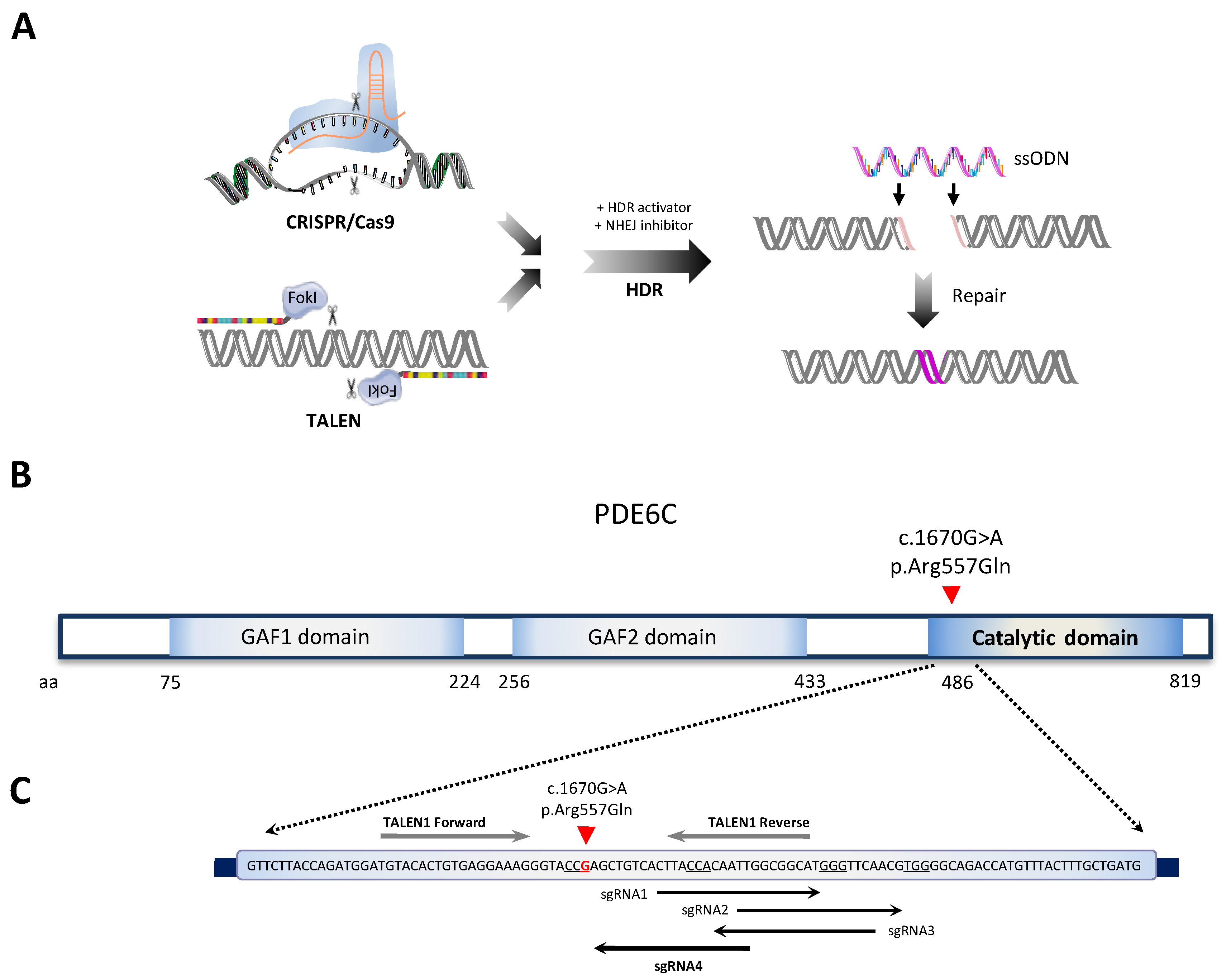
2.1. Single-Nucleotide Gene Editing for Correcting a Homozygous PDE6C Variant Causing Achromatopsia
2.2. Selection of the Optimal sgRNA, TALEN, and ssODN Designs for Targeting the Pathogenic PDE6C Variant
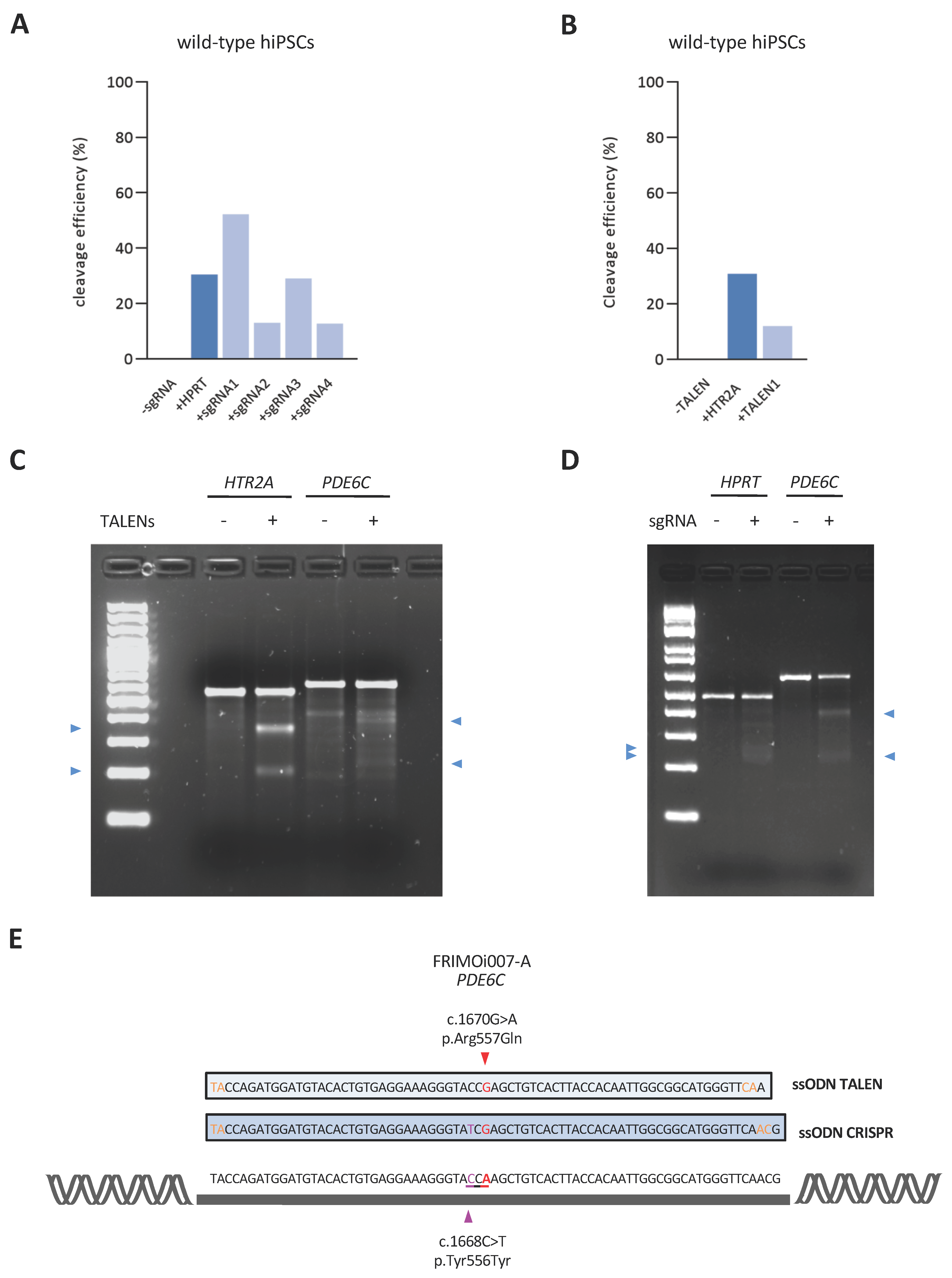
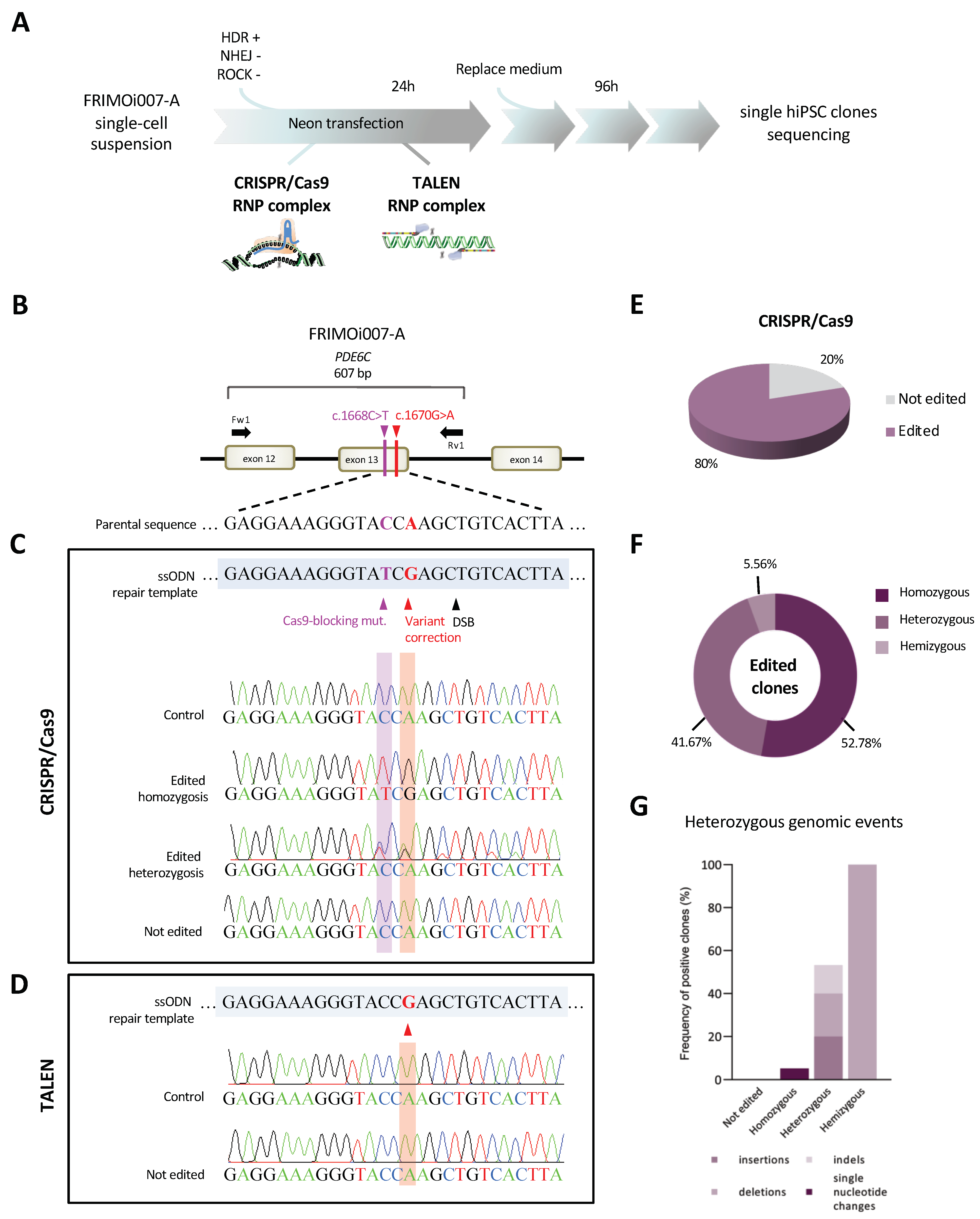
2.3. Highly Efficient Genome Editing of a PDE6C Pathogenic Variant by CRISPR/Cas9 in hiPSCs
2.4. Identification of Heterozygous on-Target Genomic Defects in Some Edited Clones
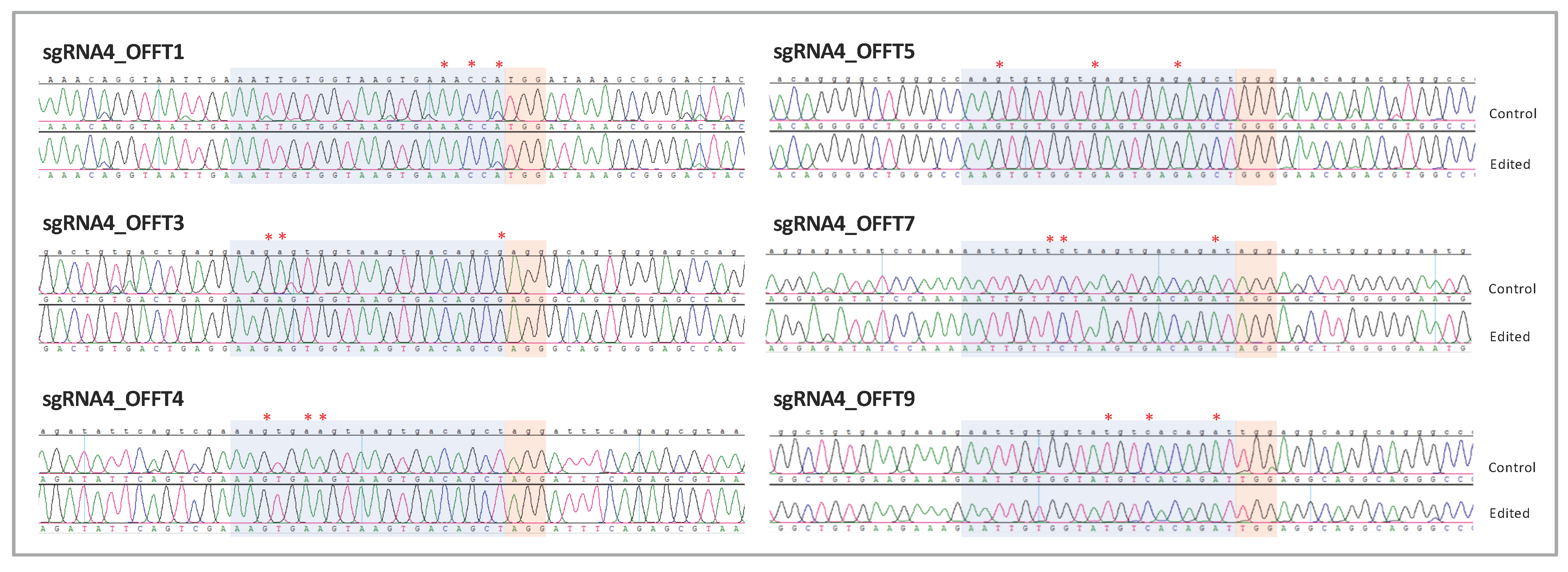
2.5. Single-Nucleotide Gene Editing Preserves hiPSCs Pluripotency and Renders no Genomic Alterations in Potential Off-Targets
3. Discussion
4. Materials and Methods
4.1. hiPSC Culture and Transfection
4.2. sgRNAs, TALENs and ssODNs Design
4.3. Genomic Cleavage Detection Assay
4.4. Human iPSC CRISPR/Cas9 and TALEN-Mediated Genome Editing
4.5. PCR Amplification, Gel Extraction, Sanger Sequencing and Data Analysis
4.6. Off-Target Prediction and Analysis
4.7. Immunofluorescence Staining
4.8. RNA Extraction and Quantitative Real-Time PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grau, T.; Artemyev, N.O.; Rosenberg, T.; Dollfus, H.; Haugen, O.H.; CumhurSener, E.; Jurklies, B.; Andreasson, S.; Kernstock, C.; Larsen, M.; et al. Decreased catalytic activity and altered activation properties of PDE6C mutants associated with autosomal recessive achromatopsia. Hum. Mol. Genet. 2011, 20, 719–730. [Google Scholar] [CrossRef]
- Aboshiha, J.; Dubis, A.M.; Carroll, J.; Hardcastle, A.J.; Michaelides, M. The cone dysfunction syndromes. Br. J. Ophthalmol. 2016, 100, 115–121. [Google Scholar] [CrossRef]
- Hirji, N.; Aboshiha, J.; Georgiou, M.; Bainbridge, J.; Michaelides, M. Achromatopsia: Clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018, 39, 149–157. [Google Scholar] [CrossRef]
- Brunetti-Pierri, R.; Karali, M.; Melillo, P.; Di Iorio, V.; De Benedictis, A.; Iaccarino, G.; Testa, F.; Banfi, S.; Simonelli, F. Clinical and molecular characterization of achromatopsia patients: A longitudinal study. Int. J. Mol. Sci. 2021, 22, 1681. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Coppieters, F.; Meire, F.; Schaich, S.; Roosing, S.; Brennenstuhl, C.; Bolz, S.; van Genderen, M.M.; Riemslag, F.C.; Lukowski, R.; et al. A nonsense mutation in PDE6H causes autosomal-recessive incomplete achromatopsia. Am. J. Hum. Genet. 2012, 91, 527–532. [Google Scholar] [CrossRef]
- Kroeger, H.; Grandjean, J.M.D.; Chiang, W.-C.J.; Bindels, D.D.; Mastey, R.; Okalova, J.; Nguyen, A.; Powers, E.T.; Kelly, J.W.; Grimsey, N.J.; et al. ATF6 is essential for human cone photoreceptor development. Proc. Natl. Acad. Sci. USA 2021, 118, e2103196118. [Google Scholar] [CrossRef]
- Thiadens, A.A.; Hollander, A.I.D.; Roosing, S.; Nabuurs, S.B.; Zekveld-Vroon, R.C.; Collin, R.W.; De Baere, E.; Koenekoop, R.K.; van Schooneveld, M.J.; Strom, T.M.; et al. Homozygosity Mapping Reveals PDE6C Mutations in Patients with Early-Onset Cone Photoreceptor Disorders. Am. J. Hum. Genet. 2009, 85, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Grau, T.; Dangel, S.; Hurd, R.; Jurklies, B.; Sener, E.C.; Andreasson, S.; Dollfus, H.; Baumann, B.; Bolz, S.; et al. A homologous genetic basis of the murine cpfl1 mutant and human achromatopsia linked to mutations in the PDE6C gene. Proc. Natl. Acad. Sci. USA 2009, 106, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Zobor, D.; Chiang, W.-C.; Weisschuh, N.; Staller, J.; Menendez, I.G.; Chang, S.; Beck, S.C.; Garrido, M.G.; Sothilingam, V.; et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet. 2015, 47, 757–765. [Google Scholar] [CrossRef]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’Angelo, R.; Sidoti, A. The impact of modifier genes on cone-rod dystrophy heterogeneity: An explorative familial pilot study and a hypothesis on neurotransmission impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Donato, L.; Alibrandi, S.; Esposito, T.; Alafaci, C.; D’Angelo, R.; Sidoti, A. Transcriptome analysis provides new molecular signatures in sporadic Cerebral Cavernous Malformation endothelial cells. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165956. [Google Scholar] [CrossRef]
- Huang, X.-M.; Liu, Q.; Xu, Z.-Y.; Yang, X.-H.; Xiao, F.; Ouyang, P.-W.; Yi, W.-Z.; Zhao, N.; Meng, J.; Cui, Y.-H.; et al. Down-regulation of HuR inhibits pathological angiogenesis in oxygen-induced retinopathy. Exp. Eye Res. 2023, 227, 109378. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Epitranscriptome Analysis of Oxidative Stressed Retinal Epithelial Cells Depicted a Possible RNA Editing Landscape of Retinal Degeneration. Antioxidants 2022, 11, 1967. [Google Scholar] [CrossRef]
- Chiang, W.-C.; Chan, P.; Wissinger, B.; Vincent, A.; Skorczyk-Werner, A.; Krawczyński, M.R.; Kaufman, R.J.; Tsang, S.H.; Héon, E.; Kohl, S.; et al. Achromatopsia mutations target sequential steps of ATF6 activation. Proc. Natl. Acad. Sci. USA 2017, 114, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Qi, R.; Fang, X.; Wang, X.; Zhou, L.; Sheng, X. Two novel PDE6C gene mutations in Chinese family with achromatopsia. Ophthalmic. Genet. 2020, 41, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Varela, M.D.; Ullah, E.; Yousaf, S.; Brooks, B.P.; Hufnagel, R.B.; Huryn, L.A. PDE6C: Novel mutations, atypical phenotype, and differences among children and adults. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Weisschuh, N.; Stingl, K.; Audo, I.; Biskup, S.; Bocquet, B.; Branham, K.; Burstedt, M.S.; De Baere, E.; De Vries, M.J.; Golovleva, I.; et al. Mutations in the gene PDE6C encoding the catalytic subunit of the cone photoreceptor phosphodiesterase in patients with achromatopsia. Hum. Mutat. 2018, 39, 1366–1371. [Google Scholar] [CrossRef]
- Maxwell, K.L. The Anti-CRISPR Story: A Battle for Survival. Mol. Cell 2017, 68, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.R. Editing the genome of hiPSC with CRISPR/Cas9: Disease models. Mamm. Genome 2017, 28, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Becker, S.; Boch, J. TALE and TALEN genome editing technologies. Gene Genome Ed. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, Y.; Yin, H. Genome Editing with mRNA Encoding ZFN, TALEN, and Cas9. Mol. Ther. 2019, 27, 735–746. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; She, K.; Lu, F.; Yang, Y. CRISPR/Cas systems usher in a new era of disease treatment and diagnosis. Mol. Biomed. 2022, 3, 31. [Google Scholar] [CrossRef]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.W.; Dai, X.Y.; Wang, W.T.; Yang, Z.X.; Zhao, J.J.; Zhang, J.P.; Wen, W.; Zhang, F.; Oberg, K.C.; Zhang, L.; et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing. Nucleic Acids Res. 2021, 49, 969–985. [Google Scholar] [CrossRef]
- Nami, F.; Basiri, M.; Satarian, L.; Curtiss, C.; Baharvand, H.; Verfaillie, C. Strategies for In Vivo Genome Editing in Nondividing Cells. Trends Biotechnol. 2018, 36, 770–786. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S. The road less traveled: Strategies to enhance the frequency of homology-directed repair (HDR) for increased efficiency of CRISPR/Cas-mediated transgenesis. BMB Rep. 2018, 51, 437–443. [Google Scholar] [CrossRef]
- Song, B.; Yang, S.; Hwang, G.H.; Yu, J.; Bae, S. Analysis of nhej-based dna repair after crispr-mediated dna cleavage. Int. J. Mol. Sci. 2021, 22, 6397. [Google Scholar] [CrossRef]
- Moon, S.B.; Lee, J.M.; Kang, J.G.; Lee, N.-E.; Ha, D.-I.; Kim, D.Y.; Kim, S.H.; Yoo, K.; Kim, D.; Ko, J.-H.; et al. Highly efficient genome editing by CRISPR-Cpf1 using CRISPR RNA with a uridinylate-rich 3′-overhang. Nat. Commun. 2018, 9, 3651. [Google Scholar] [CrossRef]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef]
- Maguire, A.M.; High, K.A.; Auricchio, A.; Wright, J.F.; Pierce, E.A.; Testa, F.; Mingozzi, F.; Bennicelli, J.L.; Ying, G.-S.; Rossi, S.; et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet 2009, 374, 1597–1605. [Google Scholar] [CrossRef]
- Li, P.; Kleinstiver, B.P.; Leon, M.Y.; Prew, M.S.; Navarro-Gomez, D.; Greenwald, S.H.; Pierce, E.A.; Joung, J.K.; Liu, Q. Allele-Specific CRISPR-Cas9 Genome Editing of the Single-Base P23H Mutation for Rhodopsin-Associated Dominant Retinitis Pigmentosa. Cris. J. 2018, 1, 55–64. [Google Scholar] [CrossRef]
- Courtney, D.G.; Moore, J.E.; Atkinson, S.D.; Maurizi, E.; Allen, E.H.A.; Pedrioli, D.M.L.; McLean, W.H.I.; Nesbit, M.A.; Moore, C.B.T. CRISPR/Cas9 DNA cleavage at SNP-derived PAM enables both in vitro and in vivo KRT12 mutation-specific targeting. Gene Ther. 2016, 23, 108–112. [Google Scholar] [CrossRef]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef]
- Thompson, D.A.; Iannaccone, A.; Ali, R.R.; Arshavsky, V.Y.; Audo, I.; Bainbridge, J.W.B.; Besirli, C.G.; Birch, D.G.; Branham, K.E.; Cideciyan, A.V.; et al. Advancing clinical trials for inherited retinal diseases: Recommendations from the second monaciano symposium. Transl. Vis. Sci. Technol. 2020, 9, 2. [Google Scholar] [CrossRef]
- Michalakis, S.; Gerhardt, M.; Rudolph, G.; Priglinger, S.; Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 2022, 26, 51–59. [Google Scholar] [CrossRef]
- Reichel, F.F.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Sothilingam, V.; Kuehlewein, L.; Kahle, N.; et al. Three-year results of phase i retinal gene therapy trial for CNGA3-mutated achromatopsia: Results of a non randomised controlled trial. Br. J. Ophthalmol. 2021, 106, 1567–1572. [Google Scholar] [CrossRef]
- Zein, W.M.; Jeffrey, B.G.; Wiley, H.E.; Turriff, A.E.; Tumminia, S.J.; Tao, W.; Bush, R.A.; Marangoni, D.; Wen, R.; Wei, L.L.; et al. CNGB3-achromatopsia clinical trial with CNTF: Diminished rod pathway responses with no evidence of improvement in cone function. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6301–6308. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef]
- Li, B.; Niu, Y.; Ji, W.; Dong, Y. Strategies for the CRISPR-Based Therapeutics. Trends Pharmacol. Sci. 2020, 41, 55–65. [Google Scholar] [CrossRef]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Chapman, M.; Evans, K.; Azevedo, L.; Hayden, M.; Heywood, S.; Millar, D.S.; Phillips, A.D.; et al. The Human Gene Mutation Database (HGMD(®)): Optimizing its use in a clinical diagnostic or research setting. Hum. Genet. 2020, 139, 1197–1207. [Google Scholar] [CrossRef]
- Sothilingam, V.; Garcia Garrido, M.; Jiao, K.; Buena-Atienza, E.; Sahaboglu, A.; Trifunović, D.; Balendran, S.; Koepfli, T.; Mühlfriedel, R.; Schön, C.; et al. Retinitis pigmentosa: Impact of different Pde6a point mutations on the disease phenotype. Hum. Mol. Genet. 2015, 24, 5486–5499. [Google Scholar] [CrossRef]
- Simkin, D.; Papakis, V.; Bustos, B.I.; Ambrosi, C.M.; Ryan, S.J.; Baru, V.; Williams, L.A.; Dempsey, G.T.; McManus, O.B.; Landers, J.E.; et al. Homozygous might be hemizygous: CRISPR/Cas9 editing in iPSCs results in detrimental on-target defects that escape standard quality controls. Stem Cell Rep. 2022, 17, 993–1008. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Shaw, K.; Phillips, A.D.; Cooper, D.N. The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum. Genet. 2014, 133, 1–9. [Google Scholar] [CrossRef]
- Eid, A.; Alshareef, S.; Mahfouz, M.M. CRISPR base editors: Genome editing without double-stranded breaks. Biochem. J. 2018, 475, 1955–1964. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; Maclaren, R.E. Crispr-cas9 dna base-editing and prime-editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- McGrath, E.; Shin, H.; Zhang, L.; Phue, J.-N.; Wu, W.W.; Shen, R.-F.; Jang, Y.-Y.; Revollo, J.; Ye, Z. Targeting specificity of APOBEC-based cytosine base editor in human iPSCs determined by whole genome sequencing. Nat. Commun. 2019, 10, 5353. [Google Scholar] [CrossRef]
- Zuo, E.; Sun, Y.; Wei, W.; Yuan, T.; Ying, W.; Sun, H.; Yuan, L.; Steinmetz, L.M.; Li, Y.; Yang, H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 2019, 364, 289–292. [Google Scholar] [CrossRef]
- Xu, P.; Tong, Y.; Liu, X.Z.; Wang, T.T.; Cheng, L.; Wang, B.Y.; Lv, X.; Huang, Y.; Liu, D.P. Both TALENs and CRISPR/Cas9 directly target the HBB IVS2-654 (C > T) mutation in β-thalassemiaderived iPSCs. Sci. Rep. 2015, 5, 12065. [Google Scholar] [CrossRef]
- Houghton, B.C.; Panchal, N.; Haas, S.A.; Chmielewski, K.O.; Hildenbeutel, M.; Whittaker, T.; Mussolino, C.; Cathomen, T.; Thrasher, A.J.; Booth, C. Genome Editing With TALEN, CRISPR-Cas9 and CRISPR-Cas12a in Combination With AAV6 Homology Donor Restores T Cell Function for XLP. Front. Genome Ed. 2022, 4, 828489. [Google Scholar] [CrossRef]
- Khiabani, A.; Kohansal, M.H.; Keshavarzi, A.; Shahraki, H.; Kooshesh, M.; Karimzade, M.; GholizadehNavashenaq, J. CRISPR/Cas9, a promising approach for the treatment of β-thalassemia: A systematic review. Mol. Genet. Genom. 2023, 298, 1–11. [Google Scholar] [CrossRef]
- Shamshirgaran, Y.; Liu, J.; Sumer, H.; Verma, P.J.; Taheri-Ghahfarokhi, A. Tools for Efficient Genome Editing; ZFN, TALEN, and CRISPR. In Applications of Genome Modulation and Editing; Springer: New York, NY, USA, 2022; pp. 29–46. [Google Scholar] [CrossRef]
- Li, C.; Chu, W.; Gill, R.A.; Sang, S.; Shi, Y.; Hu, X.; Yang, Y.; Zaman, Q.U.; Zhang, B. Computational tools and resources for CRISPR/Cas genome editing. Genomics. Proteom. Bioinform. 2022. [CrossRef]
- Richardson, C.D.; Ray, G.J.; DeWitt, M.A.; Curie, G.L.; Corn, J.E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 2016, 34, 339–344. [Google Scholar] [CrossRef]
- Weisheit, I.; Kroeger, J.A.; Malik, R.; Klimmt, J.; Crusius, D.; Dannert, A.; Dichgans, M.; Paquet, D. Detection of Deleterious On-Target Effects after HDR-Mediated CRISPR Editing. Cell Rep. 2020, 31, 107689. [Google Scholar] [CrossRef]
- Park, S.H.; Cao, M.; Pan, Y.; Davis, T.H.; Saxena, L.; Deshmukh, H.; Fu, Y.; Treangen, T.; Sheehan, V.A.; Bao, G. Comprehensive analysis and accurate quantification of unintended large gene modifications induced by CRISPR-Cas9 gene editing. Sci. Adv. 2022, 8, eabo7676. [Google Scholar] [CrossRef]
- Bloh, K.; Kanchana, R.; Bialk, P.; Banas, K.; Zhang, Z.; Yoo, B.C.; Kmiec, E.B. Deconvolution of Complex DNA Repair (DECODR): Establishing a Novel Deconvolution Algorithm for Comprehensive Analysis of CRISPR-Edited Sanger Sequencing Data. Cris. J. 2021, 4, 120–131. [Google Scholar] [CrossRef]
- Liang, X.; Potter, J.; Kumar, S.; Ravinder, N.; Chesnut, J.D. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. J. Biotechnol. 2017, 241, 136–146. [Google Scholar] [CrossRef]
- Lander, E.S. The Heroes of CRISPR. Cell 2016, 164, 18–28. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Hassall, M.M.; Barnard, A.R.; MacLaren, R.E. Gene Therapy for Color Blindness. Yale J. Biol. Med. 2017, 90, 543–551. [Google Scholar] [PubMed]
- Jayakody, S.A.; Gonzalez-Cordero, A.; Ali, R.R.; Pearson, R.A. Cellular strategies for retinal repair by photoreceptor replacement. Prog. Retin. Eye Res. 2015, 46, 31–66. [Google Scholar] [CrossRef]
- Pierce, E.A.; Pennesi, M.E.; Lam, B.L.; Jayasundera, K.T.; Myers, R.; Mukherjee, S.; Chen, E.; Roy, M.; Kleinman, D.M.; Michaels, L.A. BRILLIANCE: A Phase 1/2 Single Ascending Dose Study of EDIT-101, an in vivo CRISPR Gene Editing Therapy, in CEP290-Related Retinal Degeneration. Hum. GENE Ther. 2021, 32, A13. [Google Scholar]
- Domingo-Prim, J.; Abad-Morales, V.; Riera, M.; Navarro, R.; Corcostegui, B.; Pomares, E. Generation of an induced pluripotent stem cell line (FRIMOi007-A) derived from an incomplete achromatopsia patient carrying a novel homozygous mutation in PDE6C gene. Stem Cell Res. 2019, 40, 101569. [Google Scholar] [CrossRef]
- Okamoto, S.; Amaishi, Y.; Maki, I.; Enoki, T.; Mineno, J. Highly efficient genome editing for single-base substitutions using optimized ssODNs with Cas9-RNPs. Sci. Rep. 2019, 9, 4811. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Liu, Y.; Ma, T.; Liu, K.; Xu, S.; Zhang, Y.; Liu, H.; La Russa, M.; Xie, M.; Ding, S.; et al. Small molecules enhance crispr genome editing in pluripotent stem cells. Cell Stem Cell 2015, 16, 142–147. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Park, J.; Kim, J.S. Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef] [PubMed]

| Sequence ID | Technology | Sequence | PAM | Strand | Distance | Off-Targets (3 Mismatches) | Exonic Off-Targets |
|---|---|---|---|---|---|---|---|
| sgRNA1 | CRISPR | CTTACCACAATTGGCGGCAT | GGG | + | +25 | 1 | 0 |
| sgRNA2 | CRISPR | TTGGCGGCATGGGTTCAACG | TGG | + | +35 | 2 | 1 |
| sgRNA3 | CRISPR | CAATTGGCGGCATGGGTTCA | TGG | - | +18 | 3 | 1 |
| sgRNA4 | CRISPR | AGCTGTCACTTACCACAATT | CGG | - | +3 | 10 | 0 |
| TALEN1 | TALEN | TGTACACTGTGAGGAAAG, TGCCGCCAATTGTGGTAA | -- | +/− | -- | -- | -- |
| Genomic Events in Heterozygosis | |||||||
|---|---|---|---|---|---|---|---|
| Technology | Clones Screened | Variant Correction | Insertions | Deletions | Indels | Single-Base Changes | |
| CRISPR/Cas9 | 45 | unedited | 9 | 0 | 0 | 0 | 0 |
| homozygosis | 19 | 0 | 0 | 0 | 1 | ||
| heterozygosis | 15 | 3 | 3 | 2 | 0 | ||
| hemizygosis | 2 | 0 | 2 | 0 | 0 | ||
| TALEN | 22 | 0 | 0 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siles, L.; Gaudó, P.; Pomares, E. High-Efficiency CRISPR/Cas9-Mediated Correction of a Homozygous Mutation in Achromatopsia-Patient-Derived iPSCs. Int. J. Mol. Sci. 2023, 24, 3655. https://doi.org/10.3390/ijms24043655
Siles L, Gaudó P, Pomares E. High-Efficiency CRISPR/Cas9-Mediated Correction of a Homozygous Mutation in Achromatopsia-Patient-Derived iPSCs. International Journal of Molecular Sciences. 2023; 24(4):3655. https://doi.org/10.3390/ijms24043655
Chicago/Turabian StyleSiles, Laura, Paula Gaudó, and Esther Pomares. 2023. "High-Efficiency CRISPR/Cas9-Mediated Correction of a Homozygous Mutation in Achromatopsia-Patient-Derived iPSCs" International Journal of Molecular Sciences 24, no. 4: 3655. https://doi.org/10.3390/ijms24043655
APA StyleSiles, L., Gaudó, P., & Pomares, E. (2023). High-Efficiency CRISPR/Cas9-Mediated Correction of a Homozygous Mutation in Achromatopsia-Patient-Derived iPSCs. International Journal of Molecular Sciences, 24(4), 3655. https://doi.org/10.3390/ijms24043655








