Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review
Abstract
1. Introduction
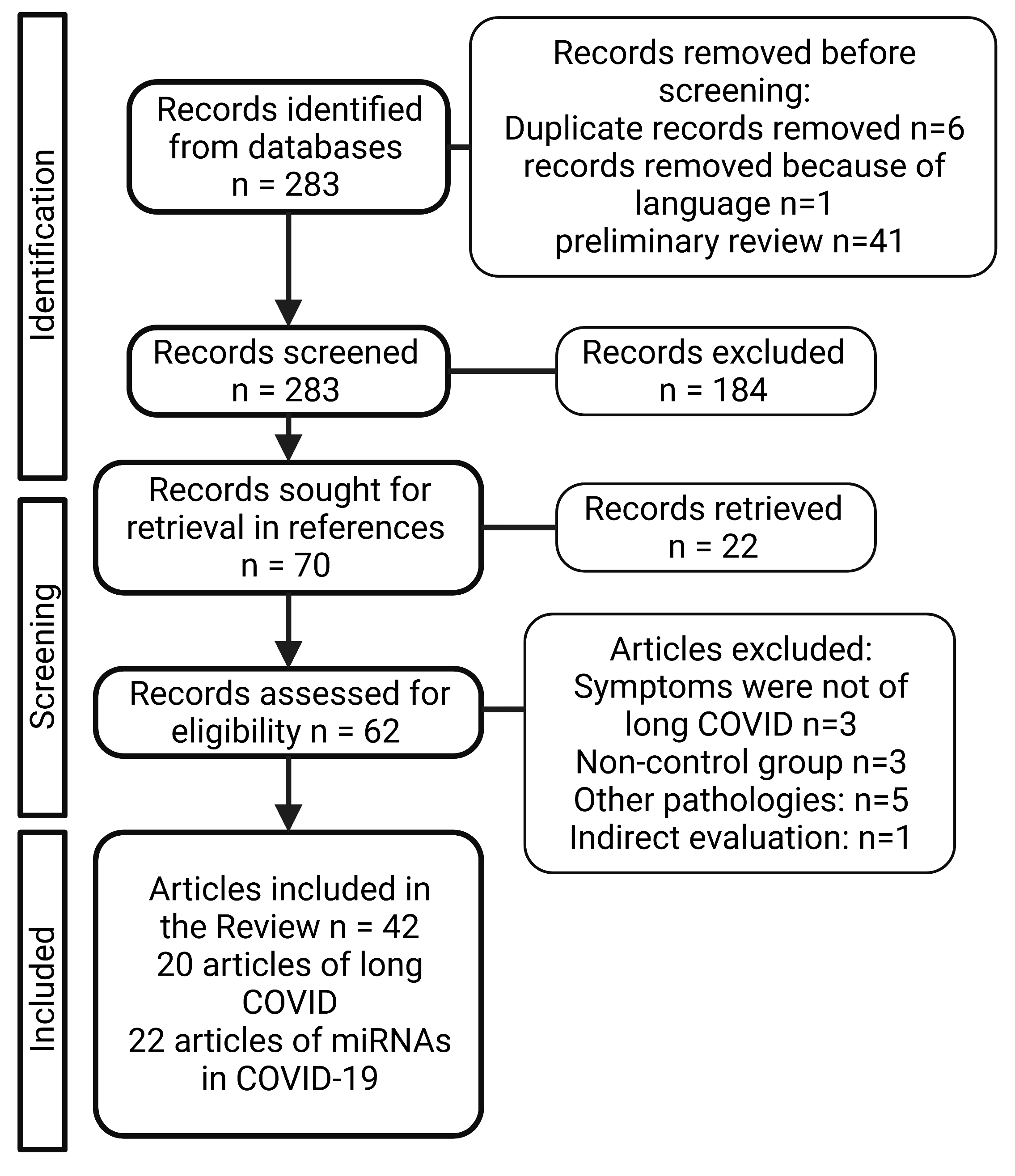
2. Material and Methods
3. Results
3.1. Long COVID Pain-Like Symtpomatology
| Cite | Study Design | COVID-19 Severity (n) | Country/Nationality; Age (Mean) | Long COVID Pain-Related Symptoms | Long COVID Duration |
|---|---|---|---|---|---|
| [29] | Cross sectional | Mild (27), moderate (65), severe (18). | UK; 47, 57,62. | Fatigue 39%, myalgia 22%, Chest pain 13% | 12 weeks |
| [30] | Cross sectional | Noninvasive ventilation (21), invasive ventilation (7) | Italy; 56.5 | Fatigue 53.1%, arthralgia 27.3%, chest pain 21.7% | 60.3 days |
| [31] | Prospective | Moderate (101), severe (29) | France | Arthralgia 21%, chest pain 17% | 60 days |
| [32] | Cross sectional | 488 | USA; 62 | Unable to return to normal activity 38.52%, New or worsening difficulty completing activities of daily living 11.88% | 60 days |
| [33] | Cross sectional | 307 | China | Soreness in the throat 17.9%, Fatigue 11.4% myalgia and arthralgia 8.8% | |
| [34] | Cross sectional | Non-Hospitalized (2133), Visited ER or Urgent Care (1312), Hospitalized (317) | White (85.3%), Hispanic, Latino, Spanish Origin (3.7%), Asian, South Asian, SE Asian (3.3%), Black (2%), Middle Eastern, North African (1.7%), Indigenous Peoples (1.6%), Pacific Islander (0.1%), Other (2.5%) | Headache 77% | 7 months |
| Fatigue 80%, Headache 53.60% | 6 months | ||||
| [35] | Cross sectional | Neurological complications (196), Controls (186) | White (44%, 41%), Black (11%, 14%), Asian (10%, 4%), Native American/Pacific Islander (0.5%, 1%), American Indian (0.5%, 1%); 68 and 69 | Difficulty completing activities of daily living | 6.7 months |
| [36] | Cross sectional | Neurological complications (113), non-neurological complications (129) | White (43%, 34%), Black (8%, 12%), Asian (4%, 4%), American Indian/Alaska Native (0, 1%), Other (19%, 16%), Prefer not to answer (26%, 33%); 64 and 65 | mRS > 0 75%, Barthel index < 100 64%, T-MoCA < 18 50%, Fatigue 9% | 12 months |
| Neurological complications (86), non-neurological complications (88) | A 6 months,88% had at least one abnormal metric. A 12 months, 84% had at least one abnormal metric. | 6 and 12 months | |||
| [37] | NA | Non-Hospitalized (2001), Hospitalized (317) | Netherlands and Belgium; 47 | Fatigue 87%, Chest tightness 44%, Headache 38%, Muscle pain 36%, Pain between shoulder blades 33%, Sore throat 26% | 79 days |
| [38] | Cross sectional | Ward patients (68), UCI patients (32) | White (79.4%, 59.4%), Mixed (1.5%, 0), Asian or Asian British (2.9%, 25%), Black or Black British (7.4%, 9.4%); 70.5 and 58.5 | Worsened pain/discomfort 14.7% and 28.1% | 14 ± 10.3 días |
| [39] | Cross sectional | Severity scale: 3 (439) 4 (1172) 5-6 (122) | China; 57 | Fatigue 81.20%, Joint pain 14.53%, Chest pain 8.55%, Sore throat 4.27%, Myalgia 3.42%, Headache 2.56% | 205 days |
| [24] | Cross sectional | Oxygen alone (217), ICU (54), Intubation (47) | British Caucasian (38.8%), Other Caucasian (17.1%), British Asian (6.5%), Other Asian (10.3%), Black British (6.8%), Other black (7.6%), Other ethnicity (13.9%); 59.9 | Fatigue 67.3%, 73.3%, 76.9% | 54 days |
| [40] | Cross sectional | No pneumonia (20), Mild (15), Severe pneumonia (106) | Spain; 62 | Fatigue 68.1%, Myalgia and arthralgia 38.3%, Headache 34.8%, Severe headache 18.4% | 77 days |
| [41] | Cross sectional | Symptoms at acute phase but not at follow-up (178) | Faroe Islands | Headache 56.7%, Fatigue 48.9% | 81 days |
| [42] | Cross sectional | Mild (57), | Italy, 62.3 | Fatigue 29.8% Myalgia 24.7% Headache12.5% | 6 months |
| Moderate (77) | Italy, 67.3 | Fatigue 31.2% Myalgia 31.6% Headache 6.5% | |||
| Severe (31) | Italy, 63.2 | Fatigue 48.4% Myalgia 38.7% Headache 12.9% | |||
| [43] | Cross sectional | Mild (16), Severe (4) | Germany | Fatigue 55%, Myalgia 15%, Headache 10%, | 225.3 days |
| [44] | Cross sectional | Normal HADS-A/D (70), Pathological HADS-A/D (30) | Italy; 55 and 56 | Pain 7.10%, 20% | 46 days |
| [45] | Cross sectional | 128 | Ireland; 49.3 ± 14.3 and 49.7± 16 | Fatigued 52.3% | 8–12 weeks |
| [46] | Cross sectional | Non-Hospitalized (79), Hospitalized (55), ICU (19) | White (70.9%, 81.8%, 73.7%), Asian (21.5%, 10.9%, 15.8%), Hispanic (2.5%, 0, 0), African (5.1%, 7.3%, 10.5%); 40.2, 56.4 and 54.5 | Fatigue 48% | 75 days |
| [47] | Cross sectional | Anosognosia (26) | Switzerland; 56.58 and 56.49 | Physical pain 85.19%, 69.84% | 227.07 ± 42.69 days |
| Nosognosia (76) | Sore throat 0, 1.3%, Muscle pain 7.7%, 10.5%, Fatigue 23.1%, 53.9%, Chest pain 0, 2.6%, Headache 7.7%, 13.2% | 6–9 months |
3.2. MicroRNAs and COVID-19
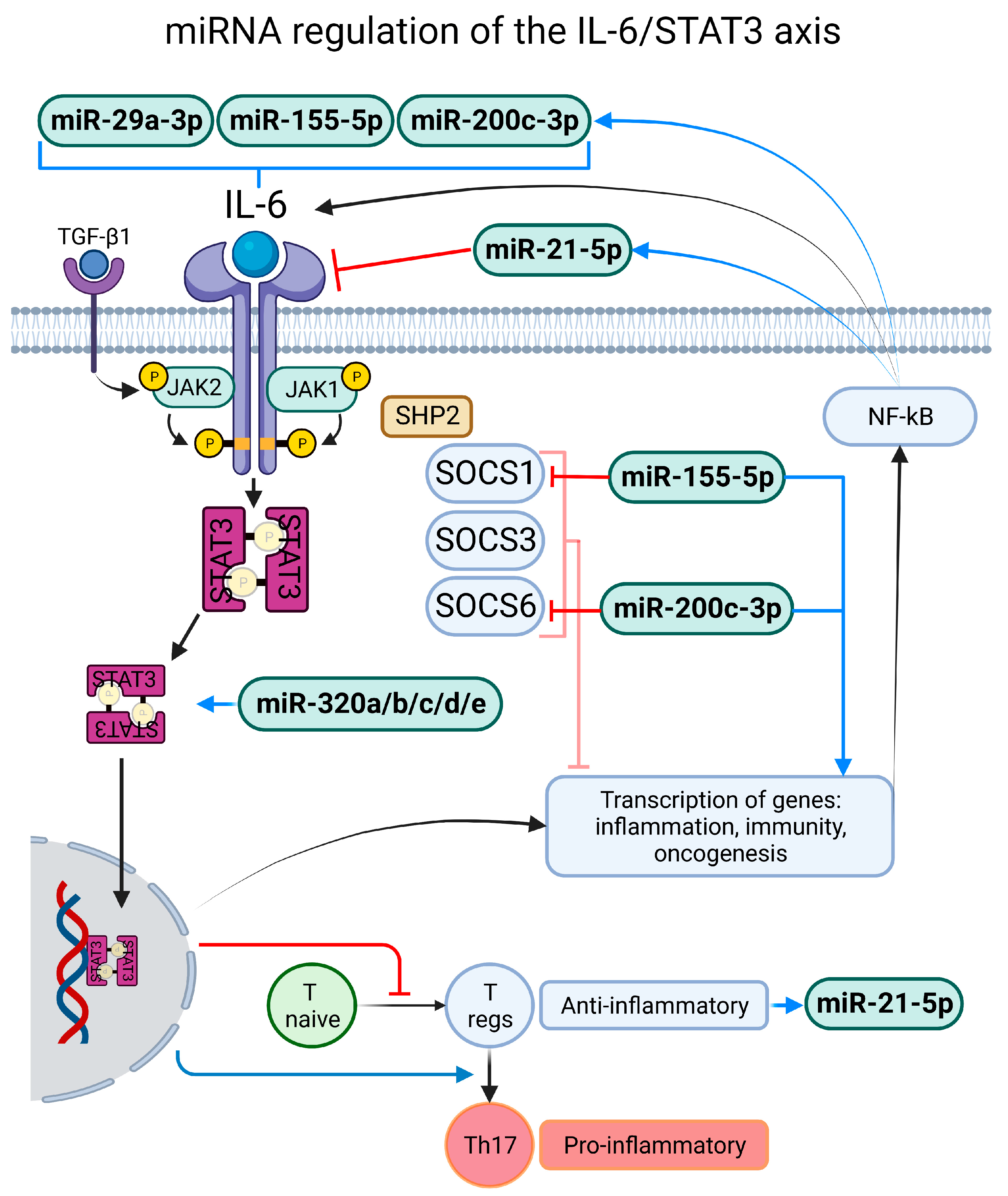
3.2.1. miR-21-5p
3.2.2. miR-29a-3p and miR-29b-3p
3.2.3. miR-92a-3p, -92b-3p and -92b-5p
3.2.4. miR-126-3p
3.2.5. miR-150-5p
3.2.6. miR-155-5p
3.2.7. miR-200a-3p and miR-200c-3p
3.2.8. miR-320a, miR-320b, miR-320c, miR-320d and miR-320e
3.2.9. miR-451a
4. Discussion
4.1. Exacerbated and Persistent Inflammatory Response
4.2. Compromise of the Blood–Nerve Barrier
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lythgoe, M.P.; Middleton, P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol. Sci. 2020, 41, 363–382. [Google Scholar] [CrossRef]
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 Vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Razai, M.S.; Chaudhry, U.A.R.; Doerholt, K.; Bauld, L.; Majeed, A. COVID-19 Vaccination Hesitancy. BMJ 2021, 373, n1138. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Stephenson, T.; Pinto Pereira, S.M.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; Chalder, T.; et al. Physical and Mental Health 3 Months after SARS-CoV-2 Infection (Long COVID) among Adolescents in England (CLoCk): A National Matched Cohort Study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Mehandru, S.; Merad, M. Pathological Sequelae of Long-Haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e1. [Google Scholar] [CrossRef]
- Baig, A.M. Chronic Long-COVID Syndrome: A Protracted COVID-19 Illness with Neurological Dysfunctions. CNS Neurosci. Ther. 2021, 27, 1433–1436. [Google Scholar] [CrossRef]
- Haidar, M.A.; Jourdi, H.; Haj Hassan, Z.; Ashekyan, O.; Fardoun, M.; Wehbe, Z.; Maaliki, D.; Wehbe, M.; Mondello, S.; Abdelhady, S.; et al. Neurological and Neuropsychological Changes Associated with SARS-CoV-2 Infection: New Observations, New Mechanisms. Neuroscientist 2021, 28, 107385842098410. [Google Scholar] [CrossRef]
- Wilson, B.A.; Betteridge, S.; Fish, J. Neuropsychological Consequences of COVID-19. Neuropsychol. Rehabil. 2020, 30, 1625–1628. [Google Scholar] [CrossRef]
- Galván-Tejada, C.E.; Herrera-García, C.F.; Godina-González, S.; Villagrana-Bañuelos, K.E.; Amaro, J.D.D.L.; Herrera-García, K.; Rodríguez-Quiñones, C.; Zanella-Calzada, L.A.; Ramírez-Barranco, J.; de Avila, J.L.R.; et al. Persistence of COVID-19 Symptoms after Recovery in Mexican Population. Int. J. Environ. Res. Public Health 2020, 17, 9367. [Google Scholar] [CrossRef]
- Bandala, C.; Cortes-Altamirano, J.L.; Reyes-Long, S.; Lara-Padilla, E.; Ilizaliturri-Flores, I.; Alfaro-Rodríguez, A. Putative Mechanism of Neurological Damage in COVID-19 Infection. Acta Neurobiol. Exp. 2021, 81, 69–79. [Google Scholar] [CrossRef]
- Bulfamante, G.; Chiumello, D.; Canevini, M.P.; Priori, A.; Mazzanti, M.; Centanni, S.; Felisati, G. First Ultrastructural Autoptic Findings of SARS-CoV-2 in Olfactory Pathways and Brainstem. Minerva Anestesiol. 2020, 86, S0375–S9393. [Google Scholar] [CrossRef]
- Huang, Y.H.; Jiang, D.; Huang, J.T. SARS-CoV-2 Detected in Cerebrospinal Fluid by PCR in a Case of COVID-19 Encephalitis. Brain Behav. Immun. 2020, 87, 149. [Google Scholar] [CrossRef] [PubMed]
- Wilczynska, A.; Bushell, M. The Complexity of MiRNA-Mediated Repression. Cell Death Differ. 2015, 22, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Long, S.; Cortes-Altamirano, J.L.; Clavijio-Cornejo, D.; Gutiérrez, M.; Bertolazzi, C.; Bandala, C.; Pineda, C.; Alfaro-Rodríguez, A. Nociceptive Related MicroRNAs and Their Role in Rheumatoid Arthritis. Mol. Biol. Rep. 2020, 47, 7265–7272. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating MicroRNAs as Potential Cancer Biomarkers: The Advantage and Disadvantage. Clin. Epigenet. 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Corey, D.R. Non-Coding RNAs as Drug Targets. Nat. Rev. Drug Discov. 2017, 16, 167–179. [Google Scholar] [CrossRef]
- Moszyńska, A.; Gebert, M.; Collawn, J.F.; Bartoszewski, R. SNPs in MicroRNA Target Sites and Their Potential Role in Human Disease. Open Biol. 2017, 7, 170019. [Google Scholar] [CrossRef]
- Baig, A.M. Chronic COVID Syndrome: Need for an Appropriate Medical Terminology for Long-COVID and COVID Long-haulers. J. Med. Virol. 2021, 93, 2555–2556. [Google Scholar] [CrossRef]
- Venkatesan, P. NICE Guideline on Long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A Cross-Sectional Study of Persisting Symptoms, Biomarker and Imaging Abnormalities Following Hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Mahboubi Mehrabani, M.; Karvandi, M.S.; Maafi, P.; Doroudian, M. Neurological Complications Associated with COVID-19; Molecular Mechanisms and Therapeutic Approaches. Rev. Med. Virol. 2022, 32, 21–44. [Google Scholar] [CrossRef]
- Wong, T.L.; Weitzer, D.J. Long COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)—A Systemic Review and Comparison of Clinical Presentation and Symptomatology. Medicina 2021, 57, 418. [Google Scholar] [CrossRef]
- Orrù, G.; Bertelloni, D.; Diolaiuti, F.; Mucci, F.; Di Giuseppe, M.; Biella, M.; Gemignani, A.; Ciacchini, R.; Conversano, C. Long-COVID Syndrome? A Study on the Persistence of Neurological, Psychological and Physiological Symptoms. Healthcare 2021, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Scientists Set out to Connect the Dots on Long COVID. Nat. Methods 2021, 18, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient Outcomes after Hospitalisation with COVID-19 and Implications for Follow-up: Results from a Prospective UK Cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of Adults with Noncritical COVID-19 Two Months after Symptom Onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; O’Malley, M.; Malani, A.N.; Prescott, H.C. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann. Intern. Med. 2021, 174, 576–578. [Google Scholar] [CrossRef]
- Dai, L.-L.; Wang, X.; Jiang, T.-C.; Li, P.-F.; Wang, Y.; Wu, S.-J.; Jia, L.-Q.; Liu, M.; An, L.; Cheng, Z. Anxiety and Depressive Symptoms among COVID-19 Patients in Jianghan Fangcang Shelter Hospital in Wuhan, China. PLoS ONE 2020, 15, e0238416. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. eClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Frontera, J.A.; Yang, D.; Lewis, A.; Patel, P.; Medicherla, C.; Arena, V.; Fang, T.; Andino, A.; Snyder, T.; Madhavan, M.; et al. A Prospective Study of Long-Term Outcomes among Hospitalized COVID-19 Patients with and without Neurological Complications. J. Neurol. Sci. 2021, 426, 117486. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Yang, D.; Medicherla, C.; Baskharoun, S.; Bauman, K.; Bell, L.; Bhagat, D.; Bondi, S.; Chervinsky, A.; Dygert, L.; et al. Trajectories of Neurologic Recovery 12 Months After Hospitalization for COVID-19: A Prospective Longitudinal Study. Neurology 2022, 99, e33–e45. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent Symptoms 3 Months after a SARS-CoV-2 Infection: The Post-COVID-19 Syndrome? ERJ Open Res. 2020, 6, 00542–02020. [Google Scholar] [CrossRef] [PubMed]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge Symptoms and Rehabilitation Needs in Survivors of COVID-19 Infection: A Cross-sectional Evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.-M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-Acute COVID-19 Syndrome. Incidence and Risk Factors: A Mediterranean Cohort Study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; á Steig, B.; Gaini, S.; Strøm, M.; Weihe, P. Long COVID in the Faroe Islands: A Longitudinal Study Among Nonhospitalized Patients. Clin. Infect. Dis. 2021, 73, e4058–e4063. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-Term Neurological Manifestations of COVID-19: Prevalence and Predictive Factors. Neurol. Sci. 2021, 42, 4903–4907. [Google Scholar] [CrossRef]
- Schweitzer, F.; Goereci, Y.; Franke, C.; Silling, S.; Bösl, F.; Maier, F.; Heger, E.; Deiman, B.; Prüss, H.; Onur, O.A.; et al. Cerebrospinal Fluid Analysis Post–COVID-19 Is Not Suggestive of Persistent Central Nervous System Infection. Ann. Neurol. 2022, 91, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Bai, F.; Castoldi, R.; Barbanotti, D.; Falcinella, C.; Mulè, G.; Mondatore, D.; Tavelli, A.; Vegni, E.; Marchetti, G.; et al. Anxiety and Depression Symptoms after Virological Clearance of COVID-19: A Cross-sectional Study in Milan, Italy. J. Med. Virol. 2021, 93, 1175–1179. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent Poor Health after COVID-19 Is Not Associated with Respiratory Complications or Initial Disease Severity. Ann. ATS 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Thomasson, M.; Lalive, P.H.; Lövblad, K.-O.; Braillard, O.; et al. Functional Connectivity Underlying Cognitive and Psychiatric Symptoms in Post-COVID-19 Syndrome: Is Anosognosia a Key Determinant? Brain Commun. 2022, 4, fcac057. [Google Scholar] [CrossRef]
- Abdolahi, S.; Hosseini, M.; Rezaei, R.; Mohebbi, S.R.; Rostami-Nejad, M.; Mojarad, E.N.; Mirjalali, H.; Yadegar, A.; Asadzadeh Aghdaei, H.; Zali, M.R.; et al. Evaluation of MiR-200c-3p and MiR-421-5p Levels during Immune Responses in the Admitted and Recovered COVID-19 Subjects. Infect. Genet. Evol. 2022, 98, 105207. [Google Scholar] [CrossRef]
- Akula, S.M.; Bolin, P.; Cook, P.P. Cellular MiR-150-5p May Have a Crucial Role to Play in the Biology of SARS-CoV-2 Infection by Regulating Nsp10 Gene. RNA Biol. 2022, 19, 1–11. [Google Scholar] [CrossRef]
- Centa, A.; Fonseca, A.S.; da Silva Ferreira, S.G.; Azevedo, M.L.V.; de Paula, C.B.V.; Nagashima, S.; Machado-Souza, C.; dos Santos Miggiolaro, A.F.R.; Pellegrino Baena, C.; de Noronha, L.; et al. Deregulated MiRNA Expression Is Associated with Endothelial Dysfunction in Post-Mortem Lung Biopsies of COVID-19 Patients. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2021, 320, L405–L412. [Google Scholar] [CrossRef]
- Donyavi, T.; Bokharaei-Salim, F.; Baghi, H.B.; Khanaliha, K.; Alaei Janat-Makan, M.; Karimi, B.; Sadri Nahand, J.; Mirzaei, H.; Khatami, A.; Garshasbi, S.; et al. Acute and Post-Acute Phase of COVID-19: Analyzing Expression Patterns of MiRNA-29a-3p, 146a-3p, 155–5p, and Let-7b-3p in PBMC. Int. Immunopharmacol. 2021, 97, 107641. [Google Scholar] [CrossRef]
- Eichmeier, A.; Kiss, T.; Kocanova, M.; Hakalova, E.; Spetik, M.; Cechova, J.; Tichy, B. Conserved MicroRNAs in Human Nasopharynx Tissue Samples from Swabs Are Differentially Expressed in Response to SARS-CoV-2. Genes 2022, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating Cardiovascular microRNAs in Critically Ill COVID-19 Patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Matacchione, G.; Ramini, D.; Di Rosa, M.; Bonfigli, A.R.; Sabbatinelli, J.; Monsurrò, V.; Recchioni, R.; Marcheselli, F.; Marchegiani, F.; et al. Circulating MiR-320b and MiR-483-5p Levels Are Associated with COVID-19 in-Hospital Mortality. Mech. Ageing Dev. 2022, 202, 111636. [Google Scholar] [CrossRef]
- De GONZALO-CALVO, D.; Benítez, I.D.; Pinilla, L.; Carratalá, A.; Moncusí-Moix, A.; Gort-Paniello, C.; Molinero, M.; González, J.; Torres, G.; Bernal, M.; et al. Circulating MicroRNA Profiles Predict the Severity of COVID-19 in Hospitalized Patients. Transl. Res. 2021, 236, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Grehl, C.; Schultheiß, C.; Hoffmann, K.; Binder, M.; Altmann, T.; Grosse, I.; Kuhlmann, M. Detection of SARS-CoV-2 Derived Small RNAs and Changes in Circulating Small RNAs Associated with COVID-19. Viruses 2021, 13, 1593. [Google Scholar] [CrossRef]
- Haroun, R.A.-H.; Osman, W.H.; Amin, R.E.; Hassan, A.K.; Abo-Shanab, W.S.; Eessa, A.M. Circulating Plasma MiR-155 Is a Potential Biomarker for the Detection of SARS-CoV-2 Infection. Pathology 2022, 54, 104–110. [Google Scholar] [CrossRef]
- Fayyad-Kazan, M.; Makki, R.; Skafi, N.; El Homsi, M.; Hamade, A.; El Majzoub, R.; Hamade, E.; Fayyad-Kazan, H.; Badran, B. Circulating MiRNAs: Potential Diagnostic Role for Coronavirus Disease 2019 (COVID-19). Infect. Genet. Evol. 2021, 94, 105020. [Google Scholar] [CrossRef]
- Keikha, R.; Hashemi-Shahri, S.M.; Jebali, A. The MiRNA Neuroinflammatory Biomarkers in COVID-19 Patients with Different Severity of Illness. Neurología 2021. [Google Scholar] [CrossRef]
- Keikha, R.; Hashemi-Shahri, S.M.; Jebali, A. The Relative Expression of MiR-31, MiR-29, MiR-126, and MiR-17 and Their MRNA Targets in the Serum of COVID-19 Patients with Different Grades during Hospitalization. Eur. J. Med. Res. 2021, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wen, Y.-Z.; Huang, Z.-L.; Shen, X.; Wang, J.-H.; Luo, Y.-H.; Chen, W.-X.; Lun, Z.-R.; Li, H.-B.; Qu, L.-H.; et al. SARS-CoV-2 Causes a Significant Stress Response Mediated by Small RNAs in the Blood of COVID-19 Patients. Mol. Ther. -Nucleic Acids 2022, 27, 751–762. [Google Scholar] [CrossRef]
- McDonald, J.T.; Enguita, F.J.; Taylor, D.; Griffin, R.J.; Priebe, W.; Emmett, M.R.; Sajadi, M.M.; Harris, A.D.; Clement, J.; Dybas, J.M.; et al. The Great Deceiver: MiR-2392’s Hidden Role in Driving SARS-CoV-2 Infection. bioRxiv 2021. [Google Scholar] [CrossRef]
- De Souza Nicoletti, A.; Visacri, M.B.; da Ronda, C.R.d.S.C.; Vasconcelos, P.E.d.N.S.; Quintanilha, J.C.F.; de Souza, R.N.; Ventura, D.d.S.; Eguti, A.; Silva, L.F.d.S.; Perroud Junior, M.W.; et al. Differentially Expressed Plasmatic MicroRNAs in Brazilian Patients with Coronavirus Disease 2019 (COVID-19): Preliminary Results. Mol. Biol. Rep. 2022, 49, 6931–6943. [Google Scholar] [CrossRef]
- Parray, A.; Mir, F.A.; Doudin, A.; Iskandarani, A.; Danjuma, I.M.M.; Kuni, R.A.T.; Abdelmajid, A.; Abdelhafez, I.; Arif, R.; Mulhim, M.; et al. SnoRNAs and MiRNAs Networks Underlying COVID-19 Disease Severity. Vaccines 2021, 9, 1056. [Google Scholar] [CrossRef]
- Pimenta, R.; Viana, N.; dos Santos, G.; Candido, P.; Guimaraes, V.; Romao, P.; Silva, I.; de Camargo, J.; Hatanaka, D.; Queiroz, P.; et al. MiR-200c-3p Expression May Be Associated with Worsening of the Clinical Course of Patients with COVID-19. Mol. Biol. Res. Commun. 2021, 10, 141. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Giuliani, A.; Matacchione, G.; Latini, S.; Laprovitera, N.; Pomponio, G.; Ferrarini, A.; Svegliati Baroni, S.; Pavani, M.; Moretti, M.; et al. Decreased Serum Levels of the Inflammaging Marker MiR-146a Are Associated with Clinical Non-Response to Tocilizumab in COVID-19 Patients. Mech. Ageing Dev. 2021, 193, 111413. [Google Scholar] [CrossRef]
- Saulle, I.; Garziano, M.; Fenizia, C.; Cappelletti, G.; Parisi, F.; Clerici, M.; Cetin, I.; Savasi, V.; Biasin, M. MiRNA Profiling in Plasma and Placenta of SARS-CoV-2-Infected Pregnant Women. Cells 2021, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.C.; Kealy, D.; James, S.R.; Plowman, T.; Newling, K.; Jagger, C.; Filbey, K.; Mann, E.R.; Konkel, J.E.; Menon, M.; et al. Integrated MiRNA/Cytokine/Chemokine Profiling Reveals Severity-Associated Step Changes and Principal Correlates of Fatality in COVID-19. iScience 2022, 25, 103672. [Google Scholar] [CrossRef]
- Wu, W.; Choi, E.-J.; Wang, B.; Zhang, K.; Adam, A.; Huang, G.; Tunkle, L.; Huang, P.; Goru, R.; Imirowicz, I.; et al. Changes of Small Non-Coding RNAs by Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Front. Mol. Biosci. 2022, 9, 821137. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. MiR-21 and MiR-146a: The MicroRNAs of Inflammaging and Age-Related Diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The Noncoding and Coding Transcriptional Landscape of the Peripheral Immune Response in Patients with COVID-19. Clin. Transl. Med. 2020, 10, e200. [Google Scholar] [CrossRef]
- Li, Y.; Wu, R.; Liu, Z.; Fan, J.; Yang, H. Enforced Expression of MicroRNA-21 Influences the Replication of Varicella-Zoster Virus by Triggering Signal Transducer and Activator of Transcription 3. Exp. Ther. Med. 2014, 7, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- De Melo, P.; Pineros Alvarez, A.R.; Ye, X.; Blackman, A.; Alves-Filho, J.C.; Medeiros, A.I.; Rathmell, J.; Pua, H.; Serezani, C.H. Macrophage-Derived MicroRNA-21 Drives Overwhelming Glycolytic and Inflammatory Response during Sepsis via Repression of the PGE2/IL-10 Axis. J. Immunol. 2021, 207, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Narita, M.; Yamashita, A.; Horiuchi, H.; Hamada, Y.; Kondo, T.; Watanabe, M.; Igarashi, K.; Kawata, M.; Shibasaki, M.; et al. Changes in the Expression of IL-6-Mediated MicroRNAs in the Dorsal Root Ganglion under Neuropathic Pain in Mice: Changes in Micrornas Under Neuropathic Pain. Synapse 2016, 70, 317–324. [Google Scholar] [CrossRef]
- Leinders, M.; Üçeyler, N.; Thomann, A.; Sommer, C. Aberrant MicroRNA Expression in Patients with Painful Peripheral Neuropathies. J. Neurol. Sci. 2017, 380, 242–249. [Google Scholar] [CrossRef]
- Reinhold, A.K.; Krug, S.M.; Salvador, E.; Sauer, R.S.; Karl-Schöller, F.; Malcangio, M.; Sommer, C.; Rittner, H.L. MicroRNA-21-5p Functions via RECK/MMP9 as a Proalgesic Regulator of the Blood Nerve Barrier in Nerve Injury. Ann. NY Acad. Sci. 2022, 1515, 184–195. [Google Scholar] [CrossRef]
- Qiu, M.; Mo, L.; Li, J.; Liang, H.; Zhu, W.; Zheng, X.; Duan, X.; Xu, W. Effects of MiR-150-5p on the Growth and SOCS1 Expression of Rheumatoid Arthritis Synovial Fibroblasts. Clin. Rheumatol. 2020, 39, 909–917. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.T.; Enguita, F.J.; Taylor, D.; Griffin, R.J.; Priebe, W.; Emmett, M.R.; Sajadi, M.M.; Harris, A.D.; Clement, J.; Dybas, J.M.; et al. Role of MiR-2392 in Driving SARS-CoV-2 Infection. Cell Rep. 2021, 37, 109839. [Google Scholar] [CrossRef]
- Maucher, D.; Schmidt, B.; Schumann, J. Loss of Endothelial Barrier Function in the Inflammatory Setting: Indication for a Cytokine-Mediated Post-Transcriptional Mechanism by Virtue of Upregulation of MiRNAs MiR-29a-3p, MiR-29b-3p, and MiR-155-5p. Cells 2021, 10, 2843. [Google Scholar] [CrossRef]
- Magenta, A.; D’Agostino, M.; Sileno, S.; Di Vito, L.; Uras, C.; Abeni, D.; Martino, F.; Barillà, F.; Madonna, S.; Albanesi, C.; et al. The Oxidative Stress-Induced MiR-200c Is Upregulated in Psoriasis and Correlates with Disease Severity and Determinants of Cardiovascular Risk. Oxidat. Med. Cell. Longev. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Nersisyan, S.; Shkurnikov, M.; Turchinovich, A.; Knyazev, E.; Tonevitsky, A. Integrative Analysis of MiRNA and MRNA Sequencing Data Reveals Potential Regulatory Mechanisms of ACE2 and TMPRSS2. PLoS ONE 2020, 15, e0235987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-Y.; Xu, M.; Yang, C.-X.; Tian, R.-R.; Zhang, M.; Li, J.-J.; Wang, X.-C.; Ding, Z.-L.; Li, G.-M.; Li, X.-L.; et al. Longitudinal Transcriptome Analyses Show Robust T Cell Immunity during Recovery from COVID-19. Sig Transduct Target 2020, 5, 294. [Google Scholar] [CrossRef]
- Shakola, F.; Suri, P.; Ruggiu, M. Splicing Regulation of Pro-Inflammatory Cytokines and Chemokines: At the Interface of the Neuroendocrine and Immune Systems. Biomolecules 2015, 5, 2073–2100. [Google Scholar] [CrossRef]
- Abaurrea, A.; Araujo, A.M.; Caffarel, M.M. The Role of the IL-6 Cytokine Family in Epithelial–Mesenchymal Plasticity in Cancer Progression. Int. J. Mol. Sci. 2021, 22, 8334. [Google Scholar] [CrossRef]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS Development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, V.; Kuner, R. Pain Hypersensitivity Mechanisms at a Glance. Dis. Model. Mech. 2013, 6, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, R.; Naime, M. The Deregulated Immune Reaction and Cytokines Release Storm (CRS) in COVID-19 Disease. Int. Immunopharmacol. 2021, 90, 107225. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kubli, S.P.; Yoshinaga, S.K.; Pfeffer, K.; Mak, T.W. An Aberrant STAT Pathway Is Central to COVID-19. Cell Death Differ. 2020, 27, 3209–3225. [Google Scholar] [CrossRef]
- Murray, P.J. The JAK-STAT Signaling Pathway: Input and Output Integration. J. Immunol. 2007, 178, 2623–2629. [Google Scholar] [CrossRef]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The IL-6/Gp130/STAT3 Signaling Axis: Recent Advances towards Specific Inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S. Contribution of STAT3 to the Pathogenesis of COVID-19. Microb. Pathog. 2021, 154, 104836. [Google Scholar] [CrossRef] [PubMed]
- Horita, M.; Farquharson, C.; Stephen, L.A. The Role of MiR-29 Family in Disease. J. Cell Biochem. 2021, 122, 696–715. [Google Scholar] [CrossRef]
- Aloi, M.S.; Prater, K.E.; Sopher, B.; Davidson, S.; Jayadev, S.; Garden, G.A. The Pro-inflammatory microRNA miR-155 Influences Fibrillar β-Amyloid 1-42 Catabolism by Microglia. Glia 2021, 69, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yang, J.; Xiang, K.; Tan, Q.; Guo, Q. Suppression of MicroRNA-155 Attenuates Neuropathic Pain by Regulating SOCS1 Signalling Pathway. Neurochem. Res. 2015, 40, 550–560. [Google Scholar] [CrossRef]
- Alexander, W.S.; Hilton, D.J. The Role of Suppressors of Cytokine Signaling (SOCS) Proteins in Regulation of the Immune Response. Annu. Rev. Immunol. 2004, 22, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Machado, D.; Terrier, O.; Pouzol, S.; Messaoudi, M.; Basualdo, W.; Espínola, E.E.; Guillen, R.M.; Rosa-Calatrava, M.; Picot, V.; et al. Viral and Bacterial Co-Infection in Severe Pneumonia Triggers Innate Immune Responses and Specifically Enhances IP-10: A Translational Study. Sci. Rep. 2016, 6, 38532. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Sileno, S.; D’Agostino, M.; Persiani, F.; Beji, S.; Paolini, A.; Camilli, D.; Platone, A.; Capogrossi, M.C.; Furgiuele, S. Atherosclerotic Plaque Instability in Carotid Arteries: MiR-200c as a Promising Biomarker. Clin. Sci. 2018, 132, 2423–2436. [Google Scholar] [CrossRef]
- Li, F.; Li, S.-S.; Chen, H.; Zhao, J.-Z.; Hao, J.; Liu, J.-M.; Zu, X.-G.; Cui, W. MiR-320 Accelerates Chronic Heart Failure with Cardiac Fibrosis through Activation of the IL6/STAT3 Axis. Aging 2021, 13, 22516–22527. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, A.; Xiang, J.; Lv, Y.; Zhang, X. MiR-451 Acts as a Suppressor of Angiogenesis in Hepatocellular Carcinoma by Targeting the IL-6R-STAT3 Pathway. Oncol. Rep. 2016, 36, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, A.; Rittner, H. Barrier Function in the Peripheral and Central Nervous System—A Review. Pflugers Arch. -Eur. J. Physiol. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- Richner, M.; Ferreira, N.; Dudele, A.; Jensen, T.S.; Vaegter, C.B.; Gonçalves, N.P. Functional and Structural Changes of the Blood-Nerve-Barrier in Diabetic Neuropathy. Front. Neurosci. 2019, 12, 1038. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in Translation: From Molecule to Brain Physiology, Pathology, and Therapy. J. Neurochem. 2016, 139, 91–114. [Google Scholar] [CrossRef] [PubMed]
- Demestre, M.; Wells, G.M.; Miller, K.M.; Smith, K.J.; Hughes, R.A.C.; Gearing, A.J.; Gregson, N.A. Characterisation of Matrix Metalloproteinases and the Effects of a Broad-Spectrum Inhibitor (BB-1101) in Peripheral Nerve Regeneration. Neuroscience 2004, 124, 767–779. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Zheng, H.; Xiao, H.-L.; She, Z.-J.; Zhao, S.-M.; Chen, Z.-L.; Zhou, G.-M. Comparison of Blood–Nerve Barrier Disruption and Matrix Metalloprotease-9 Expression in Injured Central and Peripheral Nerves in Mice. Neurosci. Lett. 2008, 434, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Gabriely, G.; Wurdinger, T.; Kesari, S.; Esau, C.C.; Burchard, J.; Linsley, P.S.; Krichevsky, A.M. MicroRNA 21 Promotes Glioma Invasion by Targeting Matrix Metalloproteinase Regulators. Mol. Cell Biol. 2008, 28, 5369–5380. [Google Scholar] [CrossRef]
- Nan, Y.; Guo, L.; Zhen, Y.; Wang, L.; Ren, B.; Chen, X.; Lu, Y.; Yu, K.; Zhong, Y.; Huang, Q. MiRNA-451 Regulates the NF-ΚB Signaling Pathway by Targeting IKKβ to Inhibit Glioma Cell Growth. Cell Cycle 2021, 20, 1967–1977. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The Neuropathic Pain Triad: Neurons, Immune Cells and Glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Xu, Z.-Z.; Wang, X.; Park, J.Y.; Zhuang, Z.-Y.; Tan, P.-H.; Gao, Y.-J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct Roles of Matrix Metalloproteases in the Early- and Late-Phase Development of Neuropathic Pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An Interactive Web Tool for MicroRNA-Target Enrichment and Network-Based Analysis. BMC Bioinform. 2019, 20, 545. [Google Scholar] [CrossRef] [PubMed]

| Author | miRNAs | Level Changes | Country/ Nationality | N (Exp—Control) | Sample |
|---|---|---|---|---|---|
| Abdolahi et al. [48] | miR-200c-3p | ↓ | Iran | 30—18 | Peripheral blood |
| Akula et al. [49] | miR-150-5p | ↓ | USA | 12—8 | Plasma |
| Centa et al. [50] | miR-29b-3p | ↓ | Brazil | 9—10 | Lung tissue |
| Donyavi et al. [51] | miR-29-3p, 155-5p | ↑,↑ | Iran | 18—5 | PBMC |
| Eichmeier et al. [52] | miR-21-5p, 29a-3p, 200a | ↑,↑,↑ | NA | 10—10 | Nasopharyngeal tissue |
| Garg et al. [53] | miR-21-5p, 126-3p, 155-5p, | ↑,↓,↑ | Germany | 18—15 | Serum |
| Giuliani et al. [54] | miR-320b | ↑ | Italy | 6—6 | Serum |
| Gonzalo-Calvo et al. [55] | miR-92a-3p, 150-5p, 451a | ↓,↓,↓ | Spain | 84—79 | Plasma |
| Grehl et al. [56] | miR-29a-3p, 126a-3p, 320a-3p, 320b, 320c, 320d, 451a | ↓,↓,↑,↑,↑,↑,↓ | Caucasian | 8—2 | Plasma |
| Haroun et al. [57] | miR-155-5p | ↑ | Egypt | 150—50 | Peripheral blood |
| Fayyad-Kazan et al. [58] | miR-92a-3p, 320a | ↑,↑ | Lebanon | 33—10 | Plasma |
| Keikha et al. [59] | miR-21-5p, 155-5p | ↓,↑ | Iran | 103—20 | Serum |
| Keikha et al. [60] | miR-29a-3p, 126-3p | ↓,↓ | Iran | 103—20 | Serum |
| Liu et al. [61] | miR-29b-3p | ↓ | China | 10—4 | Peripheral blood |
| McDonald et al. [62] | miR-29b-3p, 155-5p | ↓,↓ | USA | 10* | Serum |
| Nicoletti et al. [63] | miR-126-3p, 150-5p, 320b, 320c, 320d | ↓,↓,↑,↑,↑ | Brazil | 8—4 | Plasma |
| Parray et al. [64] | miR-92b-5p | ↓ | Qatar | 29 * | Peripheral blood |
| Pimenta et al. [65] | miR-200c-3p | ↑ | Brazil | 72—39 | Saliva |
| Sabbattinelli et al. [66] | miR-21-5p, 126-3p | ↓,↓ | Italy | 29—29 | Plasma |
| Saulle et al. [67] | miR-21-5p, 29a, 29c, 92a-3p, 150-5p, 155-5p | ↑,↑,↑,↑,↑,↑ | Italy | 15—6 | Plasma |
| Wilson et al. [68] | miR-29b-3p, 150-5p, 320e, 451a | ↑,↑,↑,⇅ | England | 58 * | Plasma |
| Wu et al. [69] | miR-92b-3p, 92b-5p, 320b, 320c | ↓,↑,↑,↑ | USA | 6—7 | Nasopharyngeal Swab |
| Gene Symbol | p-Value | FDR | Odd Ratio | miRNAs |
|---|---|---|---|---|
| MCL1 | 2.22 × 10−9 | 8.54 × 10−6 | 0.071951 | miR-29a-3p; miR-29b-3p; miR-29c-3p; miR-200a-3p; miR-92b-3p; miR-92a-3p; miR-320b; miR-320c; miR-320d |
| COL4A2 | 4.85 × 10−9 | 9.32 × 10−6 | 0.015698 | miR-29b-3p; miR-29c-3p; miR-29a-3p; miR-155-5p; miR-92a-3p |
| MMP2 | 9.66 × 10−7 | 0.000161 | 0.040554 | miR-29b-3p; miR-451a; miR-21-5p; miR-29c-3p; miR-29a-3p |
| SIRT1 | 2.42 × 10−6 | 0.0003 | 0.048403 | miR-29c-3p; miR-126-3p; miR-155-5p; miR-200c-3p; miR-92a-3p |
| VEGFA | 6.92 × 10−6 | 0.000682 | 0.117738 | miR-126-3p; miR-29b-3p; miR-150-5p; miR-200c-3p; miR-29c-3p; miR-21-5p; miR-29a-3p |
| STAT3 | 0.000113 | 0.003962 | 0.104656 | miR-155-5p; miR-21-5p; miR-92a-3p; miR-200a-3p; miR-29b-3p |
| MMP16 | 0.000542 | 0.369273 | 0.292207 | miR-150-5p; miR-155-5p; miR-200a-3p; -200c-3p; miR-29a-3p, -29b-3p, -29c-3p; miR-320b, -320c, -320d; miR-92a-3p, -92b-3p |
| DICER1 | 0.001145 | 0.014206 | 0.122643 | miR-29a-3p; miR-29c-3p; miR-21-5p; miR-200a-3p |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Long, S.; Cortés-Altamirano, J.L.; Bandala, C.; Avendaño-Ortiz, K.; Bonilla-Jaime, H.; Bueno-Nava, A.; Ávila-Luna, A.; Sánchez-Aparicio, P.; Clavijo-Cornejo, D.; Dotor-LLerena, A.L.; et al. Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review. Int. J. Mol. Sci. 2023, 24, 3574. https://doi.org/10.3390/ijms24043574
Reyes-Long S, Cortés-Altamirano JL, Bandala C, Avendaño-Ortiz K, Bonilla-Jaime H, Bueno-Nava A, Ávila-Luna A, Sánchez-Aparicio P, Clavijo-Cornejo D, Dotor-LLerena AL, et al. Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review. International Journal of Molecular Sciences. 2023; 24(4):3574. https://doi.org/10.3390/ijms24043574
Chicago/Turabian StyleReyes-Long, Samuel, Jose Luis Cortés-Altamirano, Cindy Bandala, Karina Avendaño-Ortiz, Herlinda Bonilla-Jaime, Antonio Bueno-Nava, Alberto Ávila-Luna, Pedro Sánchez-Aparicio, Denise Clavijo-Cornejo, Ana Lilia Dotor-LLerena, and et al. 2023. "Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review" International Journal of Molecular Sciences 24, no. 4: 3574. https://doi.org/10.3390/ijms24043574
APA StyleReyes-Long, S., Cortés-Altamirano, J. L., Bandala, C., Avendaño-Ortiz, K., Bonilla-Jaime, H., Bueno-Nava, A., Ávila-Luna, A., Sánchez-Aparicio, P., Clavijo-Cornejo, D., Dotor-LLerena, A. L., Cabrera-Ruiz, E., & Alfaro-Rodríguez, A. (2023). Role of the MicroRNAs in the Pathogenic Mechanism of Painful Symptoms in Long COVID: Systematic Review. International Journal of Molecular Sciences, 24(4), 3574. https://doi.org/10.3390/ijms24043574






