Somatostatin Containing δ-Cell Number Is Reduced in Type-2 Diabetes
Abstract
1. Introduction
2. Results
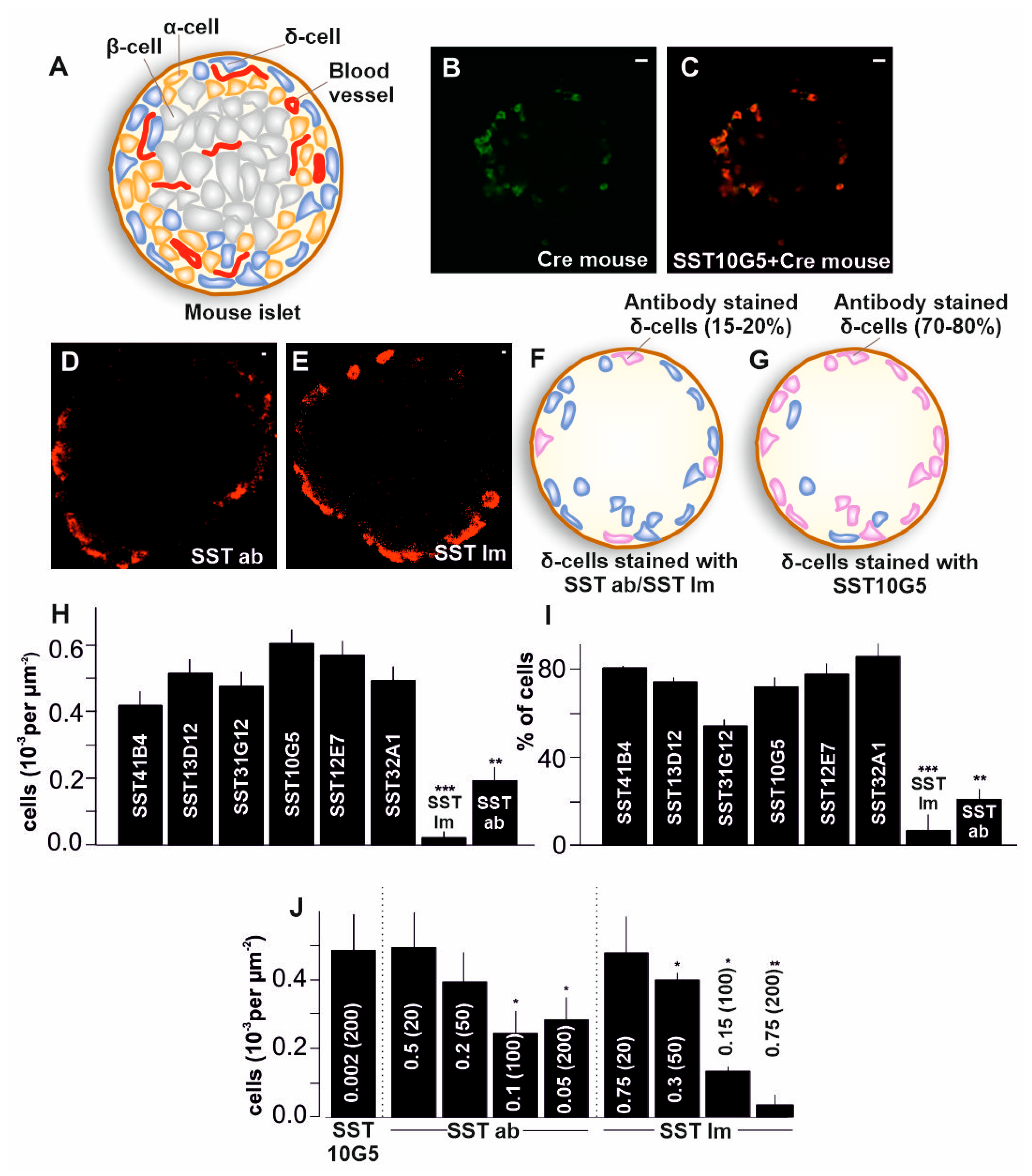
2.1. Co-Localization of Immuno-Stained Cells in Transgenic Islets
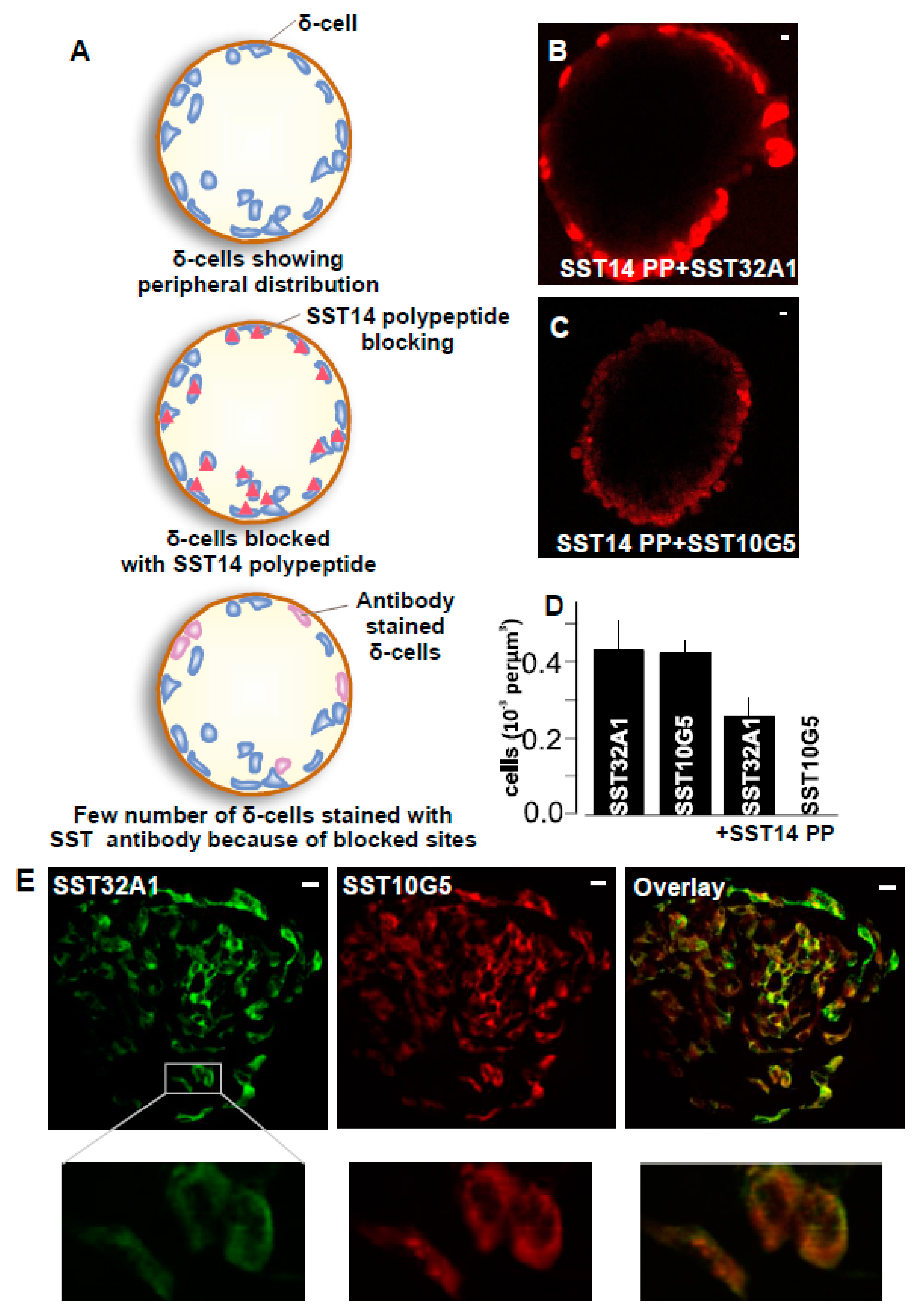
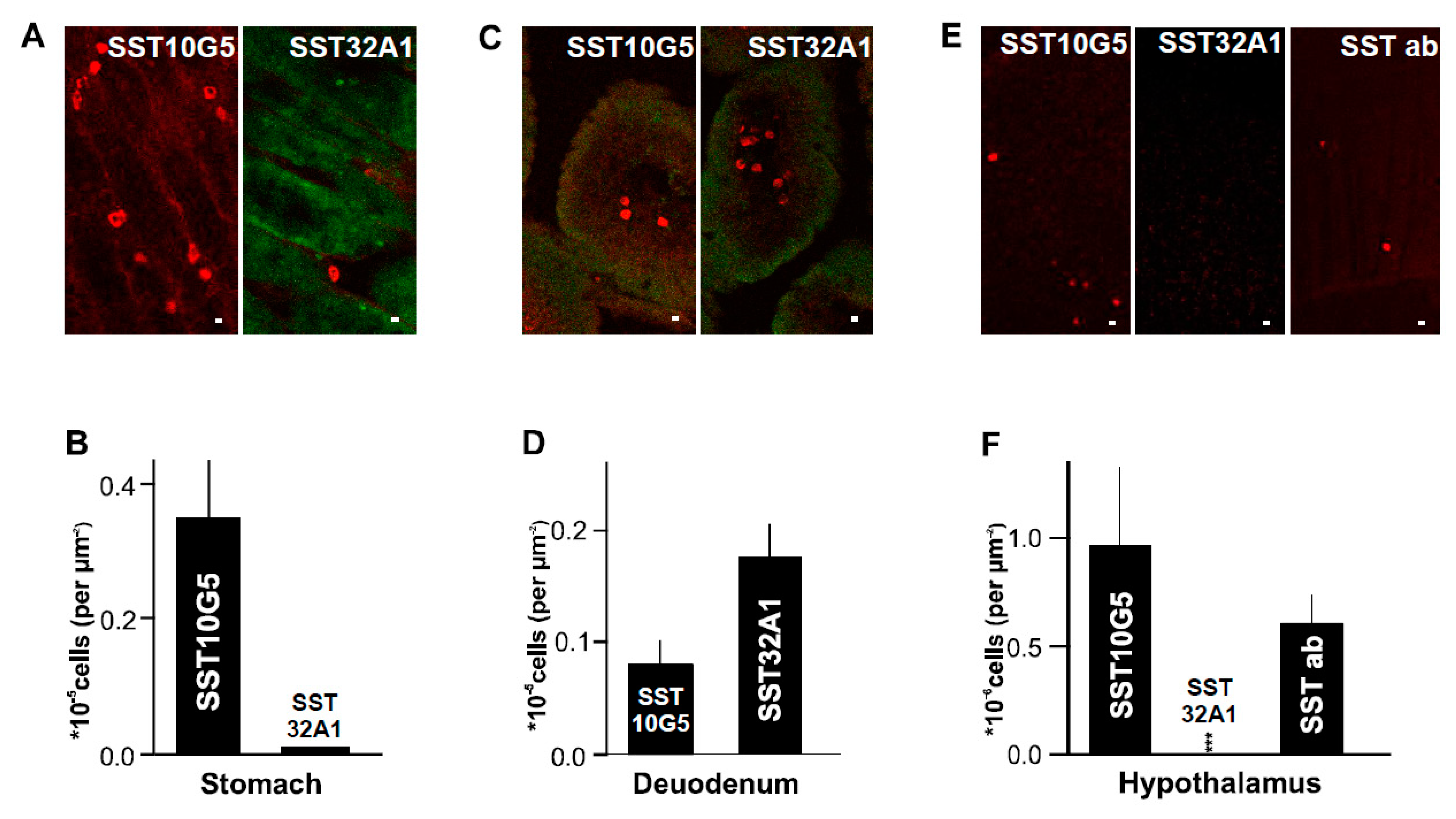
2.2. SST-14 vs. SST-28 Dilemma
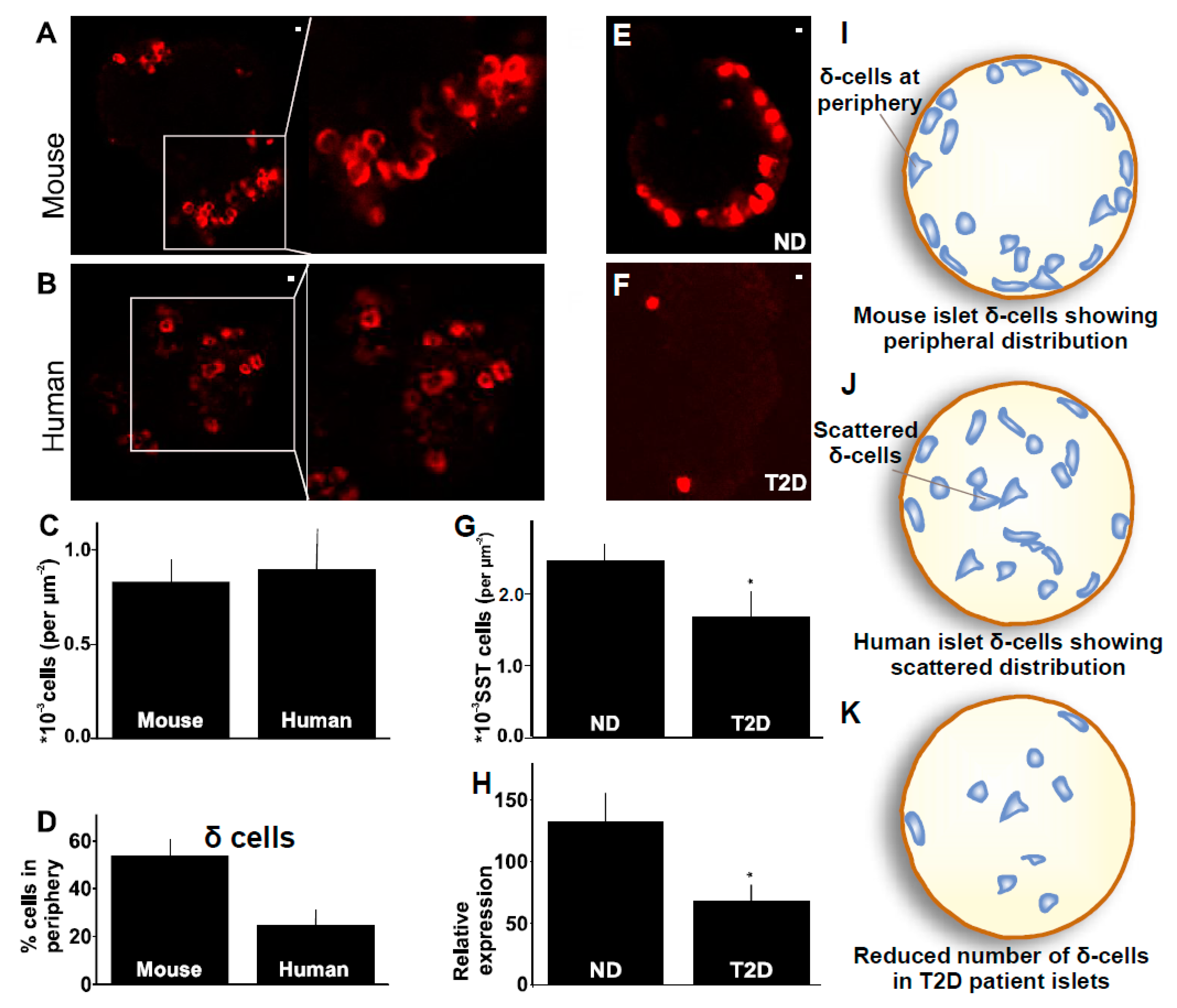
2.3. Distribution of δ-Cells in Mouse Compared to Human Islet
2.4. δ-Cells in Human Type-2 Diabetes
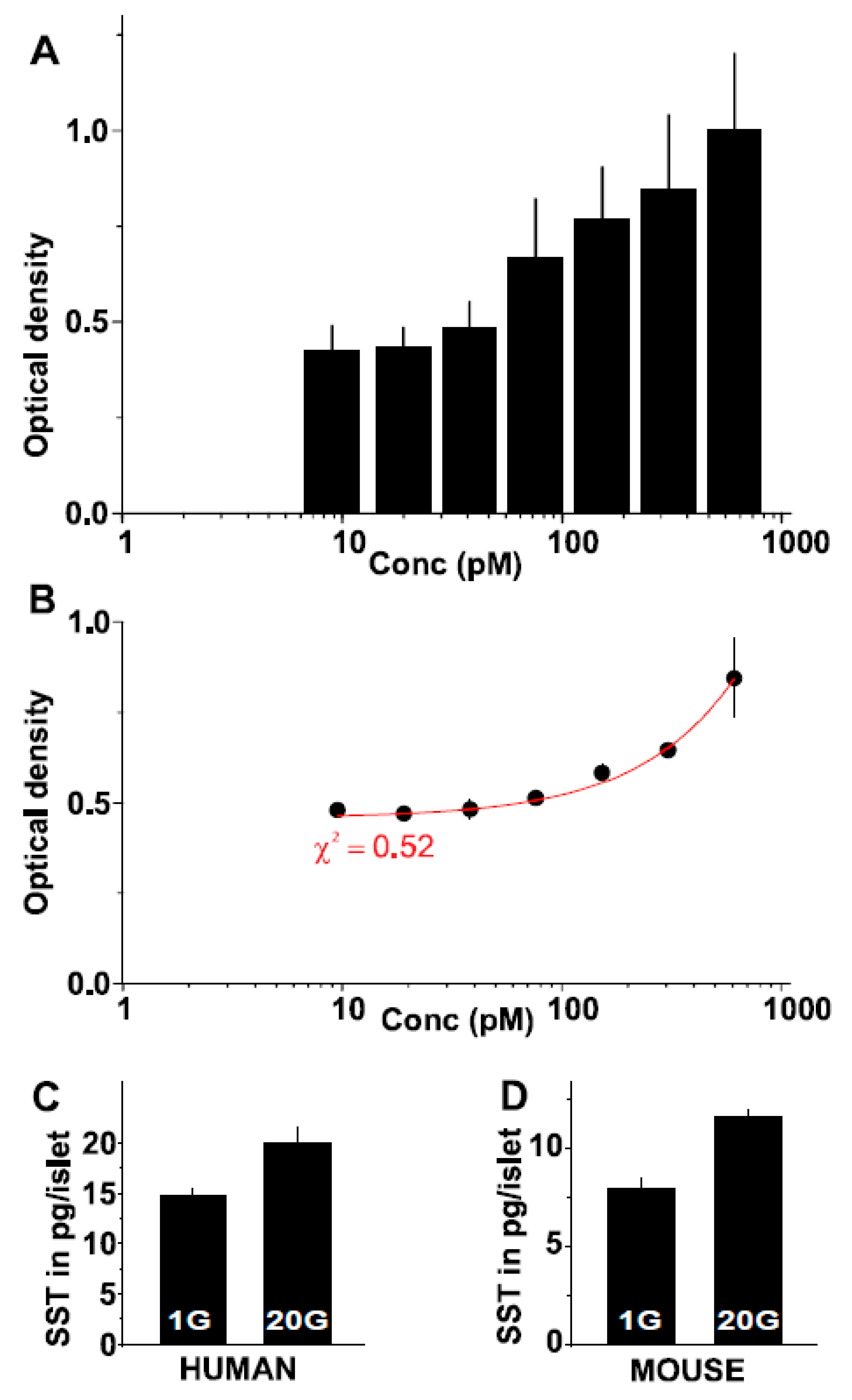
2.5. A Novel Somatostatin Assay
3. Discussion
4. Material and Methods
4.1. Islet Isolation
4.2. Immunostaining
4.3. Cryosectioning
4.4. Confocal Microscopy
4.5. Two-Photon Imaging
4.6. Image Analysis
4.7. Assay Development
4.8. RNA Extraction and Quantitative PCR
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunicardi, F.C.; Kleinman, R.; Moldovan, S.; Nguyen, T.-H.L.; Watt, P.C.; Walsh, J.; Gingerich, R. Immunoneutralization of Somatostatin, Insulin, and Glucagon Causes Alterations in Islet Cell Secretion in the Isolated Perfused Human Pancreas. Pancreas 2001, 23, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gylfe, E.; Gilon, P. Glucose regulation of glucagon secretion. Diabetes Res. Clin. Pract. 2014, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Makam, A.A.; Biswas, A.; Kothegala, L.; Gandasi, N.R. Setting the Stage for Insulin Granule Dysfunction during Type-1-Diabetes: Is ER Stress the Culprit? Biomedicines 2022, 10, 2695. [Google Scholar] [CrossRef] [PubMed]
- Orci, L.; Unger, R. Functional subdivision of islets of langerhans and possible role of D cells. Lancet 1975, 306, 1243–1244. [Google Scholar] [CrossRef]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.-O.; Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334–2339. [Google Scholar] [CrossRef]
- Jain, R.; Lammert, E. Cell-cell interactions in the endocrine pancreas. Diabetes Obes. Metab. 2009, 11, 159–167. [Google Scholar] [CrossRef]
- Arrojo Drigo, R.; Jacob, S.; García-Prieto, C.F.; Zheng, X.; Fukuda, M.; Tran Thi Nhu, H.; Stelmashenko, O.; Letícia Martins Peçanha, F.; Rodriguez-Diaz, R.; Bushong, E.; et al. Structural basis for delta cell paracrine regulation in pancreatic islets. Nat. Commun. 2019, 10, 3700. [Google Scholar] [CrossRef]
- Ling, N.; Burgus, R.; Rivier, J.; Vale, W.; Brazeau, P. The use of mass spectrometry in deducing the sequence of somatostatin—A hypothalamic polypeptide that inhibits the secretion of growth hormone. Biochem. Biophys. Res. Commun. 1973, 50, 127–133. [Google Scholar] [CrossRef]
- Francis, B.H.; Baskin, D.G.; Saunders, D.R.; Ensinck, J.W. Distribution of somatostatin-14 and somatostatin-28 gastrointestinal-pancreatic cells of rats and humans. Gastroenterology 1990, 99, 1283–1291. [Google Scholar] [CrossRef]
- Epelbaum, J. Somatostatin in the central nervous system: Physiology and pathological modifications. Prog. Neurobiol. 1986, 27, 63–100. [Google Scholar] [CrossRef]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Laschke, M.W. Molecular Sciences Regulatory Mechanisms of Somatostatin Expression. IJMS 2020, 21, 4170. [Google Scholar] [CrossRef] [PubMed]
- Wendt, A.; Birnir, B.; Buschard, K.; Gromada, J.; Salehi, A.; Sewing, S.; Rorsman, P.; Braun, M. Glucose Inhibition of Glucagon Secretion from Rat-Cells Is Mediated by GABA Released From Neighboring-Cells. Diabetes 2004, 53, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.G.; Thienpont, L.M.; van Uytfanghe, K.; Clark, P.M.; Lindstedt, P.; Nilsson, G.; Steffes, M.W. Toward standardization of insulin immunoassays. Clin. Chem. 2009, 55, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Wewer Albrechtsen, N.J.; Hartmann, B.; Veedfald, S.; Windeløv, J.A.; Plamboeck, A.; Bojsen-Møller, K.N.; Idorn, T.; Feldt-Rasmussen, B.; Knop, F.K.; Vilsbøll, T.; et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: Nonspecific interference or truly elevated levels? Diabetologia 2014, 57, 1919–1926. [Google Scholar] [CrossRef]
- Saponaro, C.; Gmyr, V.; Thévenet, J.; Moerman, E.; Delalleau, N.; Pasquetti, G.; Coddeville, A.; Quenon, A.; Daoudi, M.; Hubert, T.; et al. The GLP1R Agonist Liraglutide Reduces Hyperglucagonemia Induced by the SGLT2 Inhibitor Dapagliflozin via Somatostatin Release. Cell Rep. 2019, 28, 1447–1454.e4. [Google Scholar] [CrossRef]
- Vergari, E.; Denwood, G.; Salehi, A.; Zhang, Q.; Adam, J.; Alrifaiy, A.; Wernstedt Asterholm, I.; Benrick, A.; Chibalina, M.V.; Eliasson, L.; et al. Somatostatin secretion by Na+-dependent Ca2+-induced Ca2+ release in pancreatic delta cells. Nat. Metab. 2020, 2, 32–40. [Google Scholar] [CrossRef]
- Grange, R.D.; Thompson, J.P.; Lambert, D.G.; Mahajan, R.P. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216. [Google Scholar] [CrossRef]
- Brubaker, P.L.; Gronau, K.A.; Asa, S.L.; Greenberg, G.R. Nutrient and Peptide Regulation of Somatostatin-28 Secretion from Intestinal Cultures. Endocrinology 1998, 139, 148–155. [Google Scholar] [CrossRef]
- Patel, Y.C. General Aspects of the Biology and Function of Somatostatin. Somatostatin 1992, 4, 1–16. [Google Scholar]
- Finley JC, W.; Maderdrut, J.L.; Roger, L.J.; Petrusz, P. The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience 1981, 6, 2173–2192. [Google Scholar] [CrossRef]
- Lawlor, N.; George, J.; Bolisetty, M.; Kursawe, R.; Sun, L.; Sivakamasundari, V.; Kycia, I.; Robson, P.; Stitzel, M.L. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 2017, 27, 208–222. [Google Scholar] [CrossRef]
- Rorsman, P.; Huising, M.O. The somatostatin-secreting pancreatic δ-cell in health and disease HHS Public Access. Nat. Rev. Endocrinol. 2018, 14, 404–414. [Google Scholar] [CrossRef]
- Xu, S.; Wang, B.; Liu, W.; Wu, C.; Huang, J. The effects of insulin therapy on mortality in diabetic patients undergoing percutaneous coronary intervention. Ann. Transl. Med. 2021, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Gandasi, N.R.; Yin, P.; Riz, M.; Chibalina, M.V.; Cortese, G.; Lund, P.-E.; Matveev, V.; Rorsman, P.; Sherman, A.; Pedersen, M.G.; et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J. Clin. Investig. 2017, 127, 2353–2364. [Google Scholar] [CrossRef]
- Briant, L.J.; Dodd, M.S.; Chibalina, M.V.; Rorsman, N.J.; Johnson, P.R.; Carmeliet, P.; Rorsman, P.; Knudsen, J.G. CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets. Cell Rep. 2018, 23, 3300–3311. [Google Scholar] [CrossRef]
- Omar-Hmeadi, M.; Lund, P.-E.; Gandasi, N.R.; Tengholm, A.; Barg, S. Paracrine control of α-cell glucagon exocytosis is compromised in human type-2 diabetes. Nat. Commun. 2020, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. β-Cell deficit and increased β-cell apoptosis in humans with Type 2 Diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Moss, C.E.; Marsh, W.J.; Parker, H.E.; Ogunnowo-Bada, E.; Riches, C.H.; Habib, A.M.; Evans, M.L.; Gribble, F.M.; Reimann, F. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia 2012, 55, 3094–3103. [Google Scholar] [CrossRef]
- Li, N.; Yang, Z.; Li, Q.; Yu, Z.; Chen, X.; Li, J.-C.; Li, B.; Ning, S.-L.; Cui, M.; Sun, J.-P.; et al. Ablation of somatostatin cells leads to impaired pancreatic islet function and neonatal death in rodents. Cell Death Dis. 2018, 9, 682. [Google Scholar] [CrossRef]
- Kelly, C.; Flatt, P.R.; McClenaghan, N.H. Cell-to-cell communication and cellular environment alter the somatostatin status of delta cells. Biochem. Biophys. Res. Commun. 2010, 399, 162–166. [Google Scholar] [CrossRef]
- Huang, X.-Q. Somatostatin: Likely the most widely effective gastrointestinal hormone in the human body. World J. Gastroenterol. 1997, 3, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Gandasi, N.R.; Yin, P.; Omar-Hmeadi, M.; Ottosson Laakso, E.; Vikman, P.; Barg, S. Glucose-Dependent Granule Docking Limits Insulin Secretion and Is Decreased in Human Type 2 Diabetes. Cell Metab. 2018, 27, 470–478.e4. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.; Manning Fox, J.E.; Spigelman, A.F.; Kim, R.; Smith, N.; O’Gorman, D.; Kin, T.; Shapiro AM, J.; Rajotte, R.V.; MacDonald, P.E. Research-Focused Isolation of Human Islets from Donors with and without Diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 2016, 157, 560–569. [Google Scholar] [CrossRef] [PubMed]

| S. No. | Antibody | Species | Concentration | Monoclonal/Polyclonal | Antibody Against |
|---|---|---|---|---|---|
| 1 | SST14/28 from Immunostar | Rabbit | 15 μg/μL | Polyclonal | SST14/28 |
| 2 | SST14 from Abcam | Rabbit | 10 mg/mL | Polyclonal | SST14 |
| 3 | SST41B4 from Mercodia | Rat | 0.4 mg/mL | Monoclonal | SST28 |
| 4 | SST13D12 from Mercodia | Mouse | 0.8 mg/mL | Monoclonal | SST14 |
| 5 | SST31G12 from Mercodia | Rat | 0.7 mg/mL | Monoclonal | SST28 |
| 6 | SST10G5 from Mercodia | Mouse | 0.4 mg/mL | Monoclonal | SST14 |
| 7 | SST12E7 from Mercodia | Mouse | 0.7 mg/mL | Monoclonal | SST14 |
| 8 | SST32A1 from Mercodia | Rat | 0.4 mg/mL | Monoclonal | SST28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kothegala, L.; Miranda, C.; Singh, M.; Krieger, J.-P.; Gandasi, N.R. Somatostatin Containing δ-Cell Number Is Reduced in Type-2 Diabetes. Int. J. Mol. Sci. 2023, 24, 3449. https://doi.org/10.3390/ijms24043449
Kothegala L, Miranda C, Singh M, Krieger J-P, Gandasi NR. Somatostatin Containing δ-Cell Number Is Reduced in Type-2 Diabetes. International Journal of Molecular Sciences. 2023; 24(4):3449. https://doi.org/10.3390/ijms24043449
Chicago/Turabian StyleKothegala, Lakshmi, Caroline Miranda, Meetu Singh, Jean-Philippe Krieger, and Nikhil R. Gandasi. 2023. "Somatostatin Containing δ-Cell Number Is Reduced in Type-2 Diabetes" International Journal of Molecular Sciences 24, no. 4: 3449. https://doi.org/10.3390/ijms24043449
APA StyleKothegala, L., Miranda, C., Singh, M., Krieger, J.-P., & Gandasi, N. R. (2023). Somatostatin Containing δ-Cell Number Is Reduced in Type-2 Diabetes. International Journal of Molecular Sciences, 24(4), 3449. https://doi.org/10.3390/ijms24043449






