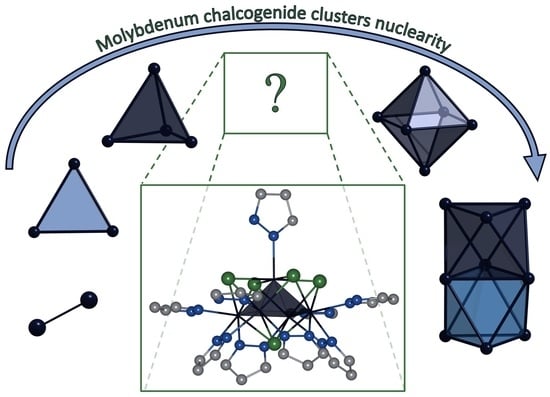
Unusual Square Pyramidal Chalcogenide Mo5 Cluster with Bridging Pyrazolate-Ligands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and General Characterization
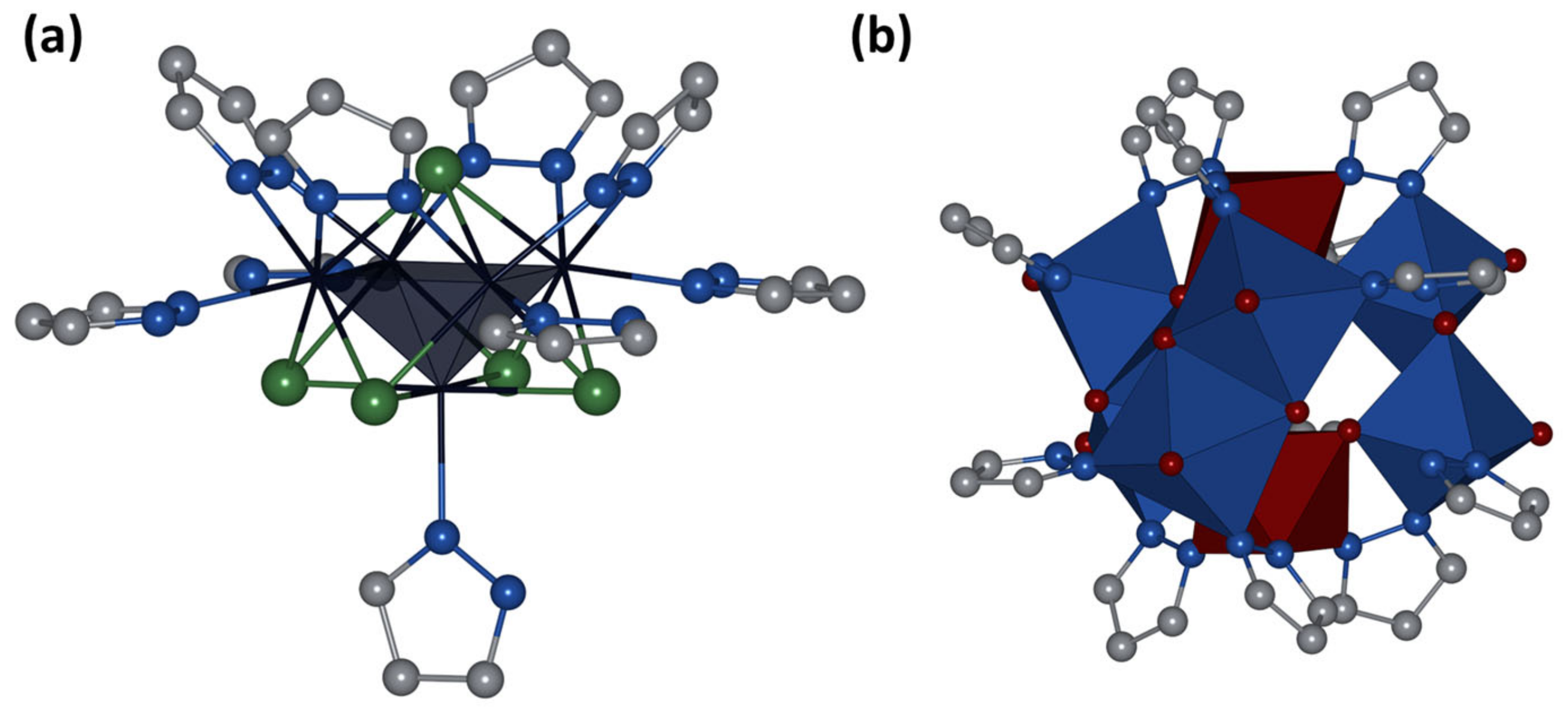
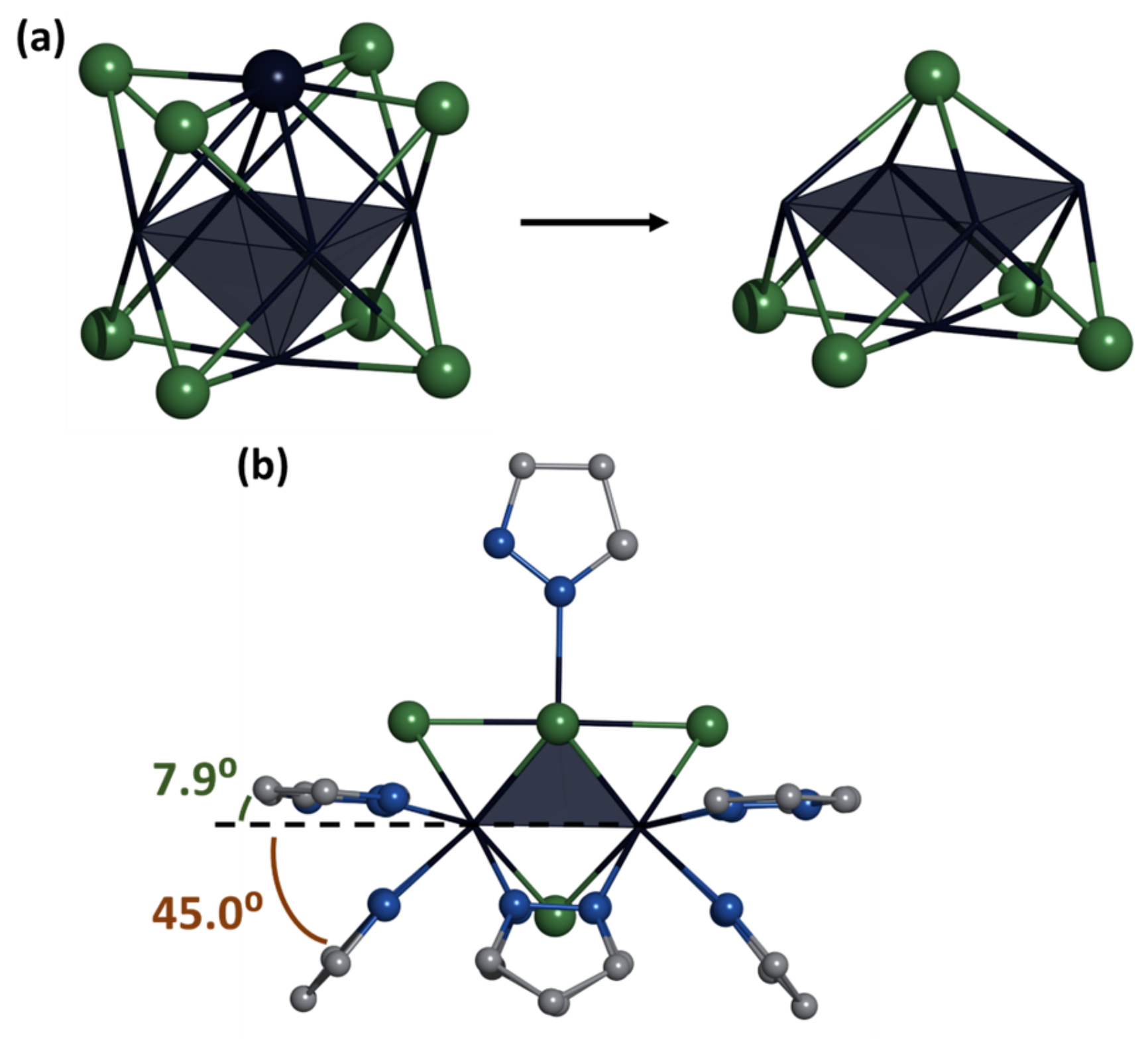
2.2. Crystal Structure of Compounds
| Compound | Mo-Mo (Average), Å | Mo-Q (Average), Å | Mo-N (Average), Å | Formal Mo Oxidation State | VEC | Formal Electrons per Mo-Mo Bond | Ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | Side | μ3-Q | μ4-Q | pzHbs | pzHap | μ-pz | |||||
| Mo5red | 2.8398(2) | 2.6823(3) | 2.5572(2) | 2.5329(3) | 2.217(2) | 2.239(4) | 2.184(2)–2.194(2) (2.189) | 5 × Mo: +3 | 15 | 1.875 | This work |
| Mo5ox | 2.865(1) | 2.660(1)–2.674(1) (2.667) | 2.499(1)–2.5712(8) (2.5326) | 2.547(1)–2.550(1) (2.548) | 2.214(7)–2.230(7) (2.222) | 2.25(1) | 2.164(8)–2.209(7) (2.179) | 4 × Mo: +3 1 × Mo: +4 | 14 | 1.750 | This work |
| (Bu4N)2[Mo5Cl8Cl5] | 2.602 | 2.563 | – | – | – | – | – | 4 × Mo: +2 1 × Mo: +3 | 19 | 2.375 | [35] |
| Ag3.6Mo9Se11 | 2.633–2.748 (2.701) | 2.554–2.642 (2.594) | 2.575–2.666 (2.620) | – | – | – | – | – | – | [9] | |
| [Mo6Se8(PEt3)6] | 2.697–2.705 (2.702) | 2.550–2.569 (2.560) | – | – | – | – | 4 × Mo: +3 2 × Mo: +2 | 20 | 1.667 | [5] | |
| [Mo6Se8(Ph2PC2H4CO2H)6] | 2.695–2.705 (2.700) | 2.531–2.574 (2.557) | – | – | – | – | [7] | ||||
| Mo6Br12 | 2.630–2.640 (2.635) | – | – | – | – | – | 6 × Mo: +2 | 24 | 2 | [36] | |
| (PPh4)2[Mo3Se4(C2O4)3(H2O)3] | 2.811–2.826 (2.819) | 2.445–2.455 (2.450) | – | – | – | – | 3 × Mo: +4 | 6 | 2 | [37] | |
| (Bu4N)2[Mo3Se7Br6] | 2.806–2.820 (2.813) | 2.466–2.473 (2.470) | – | – | – | – | [38] | ||||
| K7[Mo4Se4(CN)12] | 2.900–2.925 (2.910) | 2.493–2.522 (2.507) | – | – | – | – | 3 × Mo: +3 1 × Mo: +4 | 11 | 1.833 | [39] | |
| [Mo4S4(HB(pz)3)4(μ-pz)] | 2.659–2.952 (2.856) | 2.304–2.404 (2.353) | – | 2.19–2.34 (2.24) | 2.20 | [32] | |||||
2.3. DFT Calculations
2.4. X-ray Photoelectron Spectroscopy
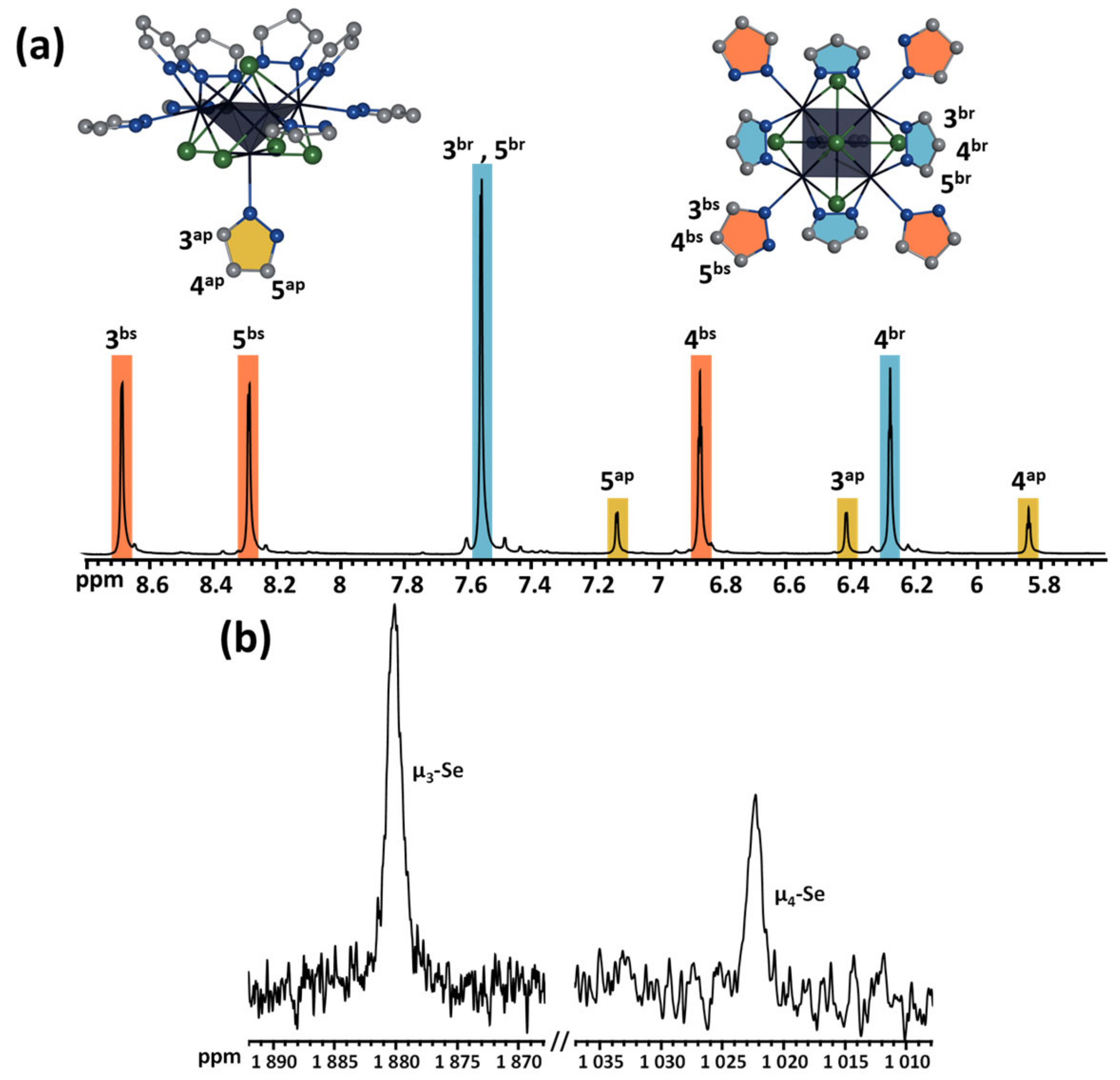
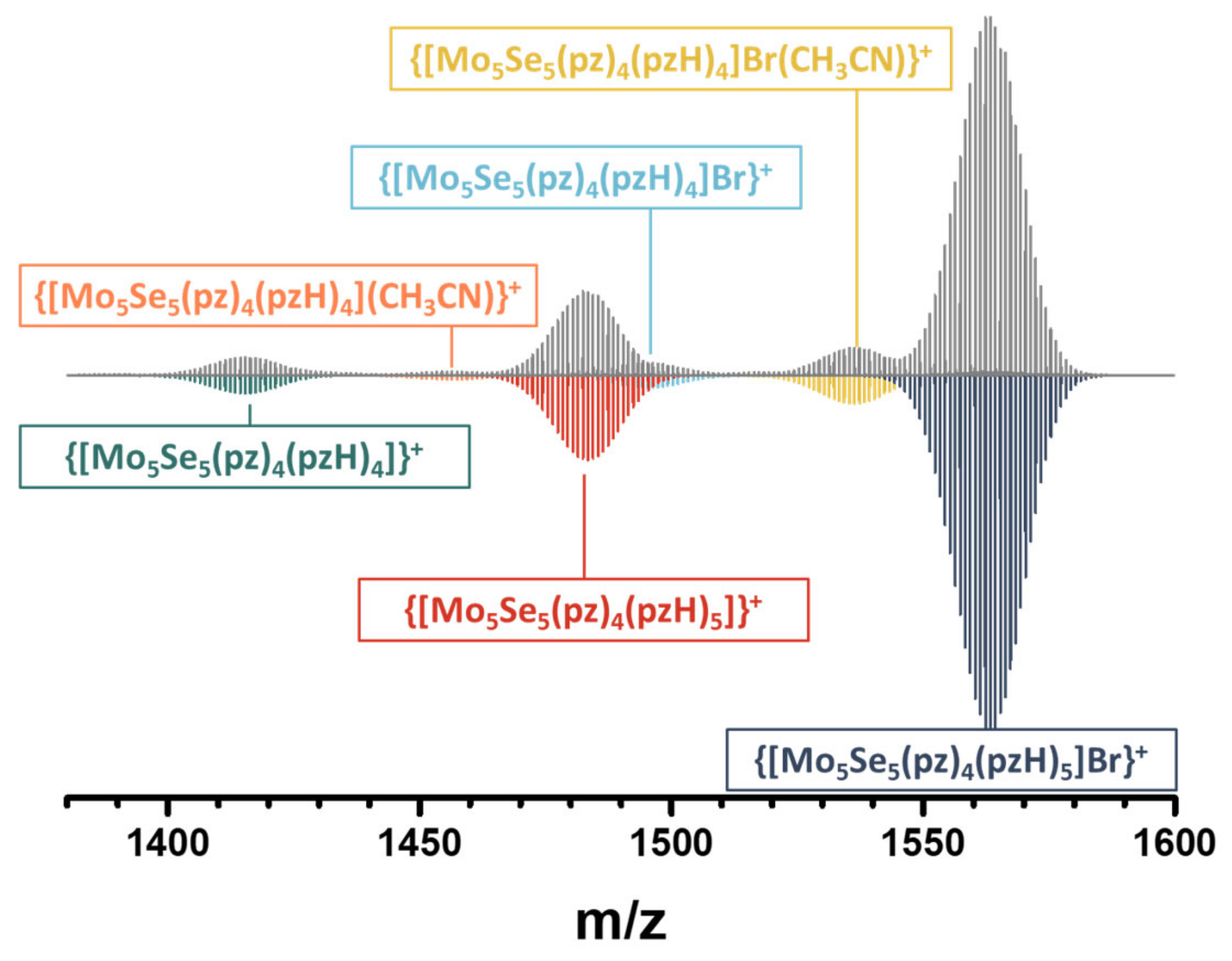
2.5. NMR and HR-ESI-MS Spectroscopy
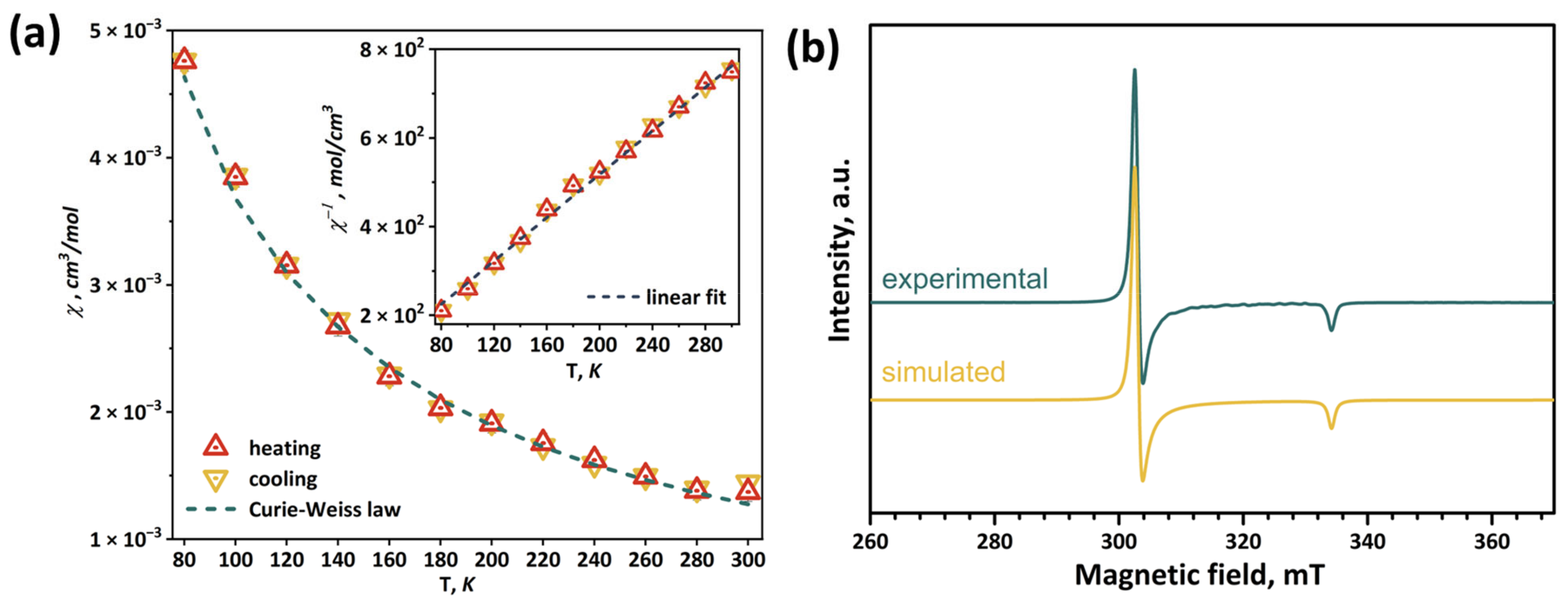
2.6. Magnetic Properties and EPR Spectroscopy
2.7. Redox Properties
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Syntheses
3.2.1. “NaMo6Se8Br4” (Denoted as Mo6)
3.2.2. [{Mo5(μ3-Se)i4(μ4-Se)i(μ-pz)i4}(pzH)t5]Br 4pzH (Denoted as Mo5red)
3.2.3. [{Mo5(μ3-Se)i4(μ4-Se)i(μ-pz)i4}(pzH)t5]Br2 2H2O (Denoted as Mo5ox)
3.3. Physical Methods
3.4. Single-Crystal X-ray Diffraction Analysis (XRD)
3.5. Cyclic Voltammetry
3.6. DFT Calculations
3.7. EPR
3.8. Magnetic Susceptibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughbanks, T.; Hoffmann, R. Molybdenum chalcogenides: Clusters, chains, and extended solids. The approach to bonding in three dimensions. J. Am. Chem. Soc. 1983, 105, 1150–1162. [Google Scholar] [CrossRef]
- Fedorov, V.E.; Mironov, Y.V.; Naumov, N.G.; Sokolov, M.N.; Fedin, V.P. Chalcogenide clusters of group 5–7 metals. Russ. Chem. Rev. 2007, 76, 529–552. [Google Scholar] [CrossRef]
- Chevrel, R.; Sergent, M.; Prigent, J. Sur de nouvelles phases sulfurées ternaires du molybdène. J. Solid State Chem. 1971, 3, 515–519. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Laricheva, Y.A.; Sokolov, M.N.; Llusar, R. Tri- and tetranuclear molybdenum and tungsten chalcogenide clusters: On the way to new materials and catalysts. Russ. Chem. Rev. 2018, 87, 670–706. [Google Scholar] [CrossRef]
- Saito, T.; Yamamoto, N.; Nagase, T.; Tsudoi, T.; Kobayashi, K.; Yamagata, T.; Imoto, H.; Unoura, K. Molecular models of the superconducting chevrel phases: Syntheses and structures of [Mo6X8(PEt3)6] and [PPN][Mo6X8(PEt3)6] (X = S, Se; PPN = (Ph3P)2N). Inorg. Chem. 1990, 29, 764–770. [Google Scholar] [CrossRef]
- Jin, S.; Popp, F.; Boettcher, S.; Yuan, M.; Oertel, C.M.; DiSalvo, F.J. Synthesis, characterization and properties of Mo6S8(4-tert-butylpyridine)6 and related M6S8L6 cluster complexes (M = Mo, W). J. Chem. Soc. Dalton Trans. 2002, 2002, 3096–3100. [Google Scholar] [CrossRef]
- Novikova, E.D.; Gassan, A.D.; Ivanov, A.A.; Vorotnikov, Y.A.; Shestopalov, M.A. Neutral Mo6Q8-clusters with terminal phosphane ligands—A route to water-soluble molecular units of Chevrel phases. N. J. Chem. 2022, 46, 2218–2223. [Google Scholar] [CrossRef]
- Modec, B.; Brenčič, J.V. From small {Mo2O4}2+ aggregates to infinite solids. J. Clust. Sci. 2002, 13, 279–302. [Google Scholar] [CrossRef]
- Gougeon, P.; Padiou, J.; Le Marouille, J.Y.; Potel, M.; Sergent, M. Ag3.6Mo9Se11: Premier compose a clusters Mo9 dans des motifs Mo9Se11. J. Solid State Chem. 1984, 51, 218–226. [Google Scholar] [CrossRef]
- Picard, S.; Gougeon, P.; Potel, M. Synthesis, structural evolution, and electrical properties of the novel Mo12 cluster compounds K1+xMo12S14 (x = 0, 1.1, 1.3, and 1.6) with a Tunnel Structure. Inorg. Chem. 2006, 45, 1611–1616. [Google Scholar] [CrossRef]
- Daigre, G.; Gougeon, P.; Gall, P.; Merdrignac-Conanec, O.; Al Rahal Al Orabi, R.; Gautier, R.; Dauscher, A.; Candolfi, C.; Lenoir, B. Unravelling the beneficial influence of ag insertion on the thermoelectric properties of the cluster compound K2Mo15Se19. ACS Appl. Energy Mater. 2020, 3, 2846–2855. [Google Scholar] [CrossRef]
- Feliz, M.; Llusar, R.; Uriel, S.; Vicent, C.; Humphrey, M.G.; Lucas, N.T.; Samoc, M.; Luther-Davies, B. Solid state synthesis, structure and optical limiting properties of seleno cuboidal clusters [M3Se4X3(diphosphine)3]+ (M=Mo, W.; X=Cl, Br). Inorg. Chim. Acta 2003, 349, 69–77. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Sokolov, M.N.; Peresypkina, E.V.; Virovets, A.V.; Kozlova, S.G.; Zakharchuk, N.F.; Fedin, V.P. Crystal structure, electronic structure, and solid-state electrochemistry of cluster complexes of M3Se74+ (M = Mo, W) with noninnocent o-phenanthroline and Se22– ligands. Eur. J. Inorg. Chem. 2008, 2008, 3964–3969. [Google Scholar] [CrossRef]
- Herbst, K.; Zanello, P.; Corsini, M.; D’Amelio, N.; Dahlenburg, L.; Brorson, M. A complete family of isostructural cluster compounds with cubane-like M3S4M‘ Cores (M = Mo, W.; M‘ = Ni, Pd, Pt): Comparative crystallography and electrochemistry. Inorg. Chem. 2003, 42, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Llusar, R.; Uriel, S.; Vicent, C.; Clemente-Juan, J.M.; Coronado, E.; Gómez-García, C.J.; Braïda, B.; Canadell, E. Single-component magnetic conductors based on Mo3S7 trinuclear clusters with outer dithiolate ligands. J. Am. Chem. Soc. 2004, 126, 12076–12083. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.A.; Naumov, D.Y.; Llusar, R.; Gómez-García, C.J.; Polo, V.; Konchenko, S.N. Synthesis and structure of a paramagnetic Mo3S4 incomplete cuboidal cluster with seven cluster skeletal electrons. Dalton Trans. 2012, 41, 14031–14034. [Google Scholar] [CrossRef] [PubMed]
- Peña, O. Chevrel phases: Past, present and future. Phys. C Supercond. Appl. 2015, 514, 95–112. [Google Scholar] [CrossRef]
- Feliz, M.; Guillamón, E.; Llusar, R.; Vicent, C.; Stiriba, S.-E.; Pérez-Prieto, J.; Barberis, M. Unprecedented stereoselective synthesis of catalytically active chiral Mo3CuS4 clusters. Chem. Eur. J. 2006, 12, 1486–1492. [Google Scholar] [CrossRef]
- Takei, I.; Wakebe, Y.; Suzuki, K.; Enta, Y.; Suzuki, T.; Mizobe, Y.; Hidai, M. Synthesis of cubane-type Mo3NiS4 clusters and their catalytic activity for the cyclization of alkynoic acids to enol lactones. Organometallics 2003, 22, 4639–4641. [Google Scholar] [CrossRef]
- Beltrán, T.F.; Feliz, M.; Llusar, R.; Mata, J.A.; Safont, V.S. Mechanism of the catalytic hydrodefluorination of pentafluoropyridine by group six triangular cluster hydrides containing phosphines: A combined experimental and theoretical study. Organometallics 2011, 30, 290–297. [Google Scholar] [CrossRef]
- Sorribes, I.; Wienhöfer, G.; Vicent, C.; Junge, K.; Llusar, R.; Beller, M. Chemoselective transfer hydrogenation to nitroarenes mediated by cubane-type Mo3S4 cluster catalysts. Angew. Chem. Int. Ed. 2012, 51, 7794–7798. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides—Efficient and viable materials for electro—And photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Kumar Gangu, K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Mermer, A.; Keles, T.; Sirin, Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review. Bioorg. Chem. 2021, 114, 105076. [Google Scholar] [CrossRef]
- Garnovskii, A.D.; Osipov, O.A.; Kuznetsova, L.I.; Bogdashev, N.N. Advances in the coordination chemistry of azoles. Russ. Chem. Rev. 1973, 42, 89–110. [Google Scholar] [CrossRef]
- Kumar Bhaumika, P.; Ghosh, K.; Chattopadhyay, S. Synthetic strategies, crystal structures and biological activities of metal complexes with the members of azole family: A review. Polyhedron 2021, 200, 115093. [Google Scholar] [CrossRef]
- Castro, I.; Barros, W.P.; Calatayud, M.L.; Lloret, F.; Marino, N.; De Munno, G.; Stumpf, H.O.; Ruiz-García, R.; Julve, M. Dicopper(II) pyrazolenophanes: Ligand effects on their structures and magnetic properties. Coord. Chem. Rev. 2016, 315, 135–152. [Google Scholar] [CrossRef]
- Mironov, Y.V.; Kozhomuratova, Z.S.; Naumov, N.G.; Fedorov, V.E. Synthesis and structure of a new molecular octahedral cluster complex Mo6Se8(Ph3P)6·2H2O. J. Struct. Chem. 2007, 48, 383–387. [Google Scholar] [CrossRef]
- Klayman, D.L.; Griffin, T.S. Reaction of selenium with sodium borohydride in protic solvents. A facile method for the introduction of selenium into organic molecules. J. Am. Chem. Soc. 1973, 95, 197–199. [Google Scholar] [CrossRef]
- Konovalov, D.I.; Ivanov, A.A.; Vorotnikov, Y.A.; Brylev, K.A.; Eltsov, I.V.; Kuratieva, N.V.; Kitamura, N.; Mironov, Y.V.; Shestopalov, M.A. Synthesis and luminescence properties of apically homoleptic octahedral rhenium clusters with pyrazole and 3,5-dimethylpyrazole. Inorg. Chim. Acta 2019, 498, 119128. [Google Scholar] [CrossRef]
- Ehlert, M.K.; Rettig, S.J.; Storr, A.; Thompson, R.C.; Trotter, J. Octamolybdenum oxo-pyrazolate clusters. Syntheses, characterization, and crystal and molecular structures of the Mo(V)/Mo(VI) and Mo(VI) octamolybdenum clusters Mo8(pz)6O18(pzH)6 and Mo8(pz)6O21(pzH)6. Inorg. Chem. 1993, 32, 5176–5182. [Google Scholar] [CrossRef]
- Cotton, F.A.; Dori, Z.; Llusar, R.; Schwotzer, W. Derivatization of the cuboidal Mo4S4-aquo ion: Preparation and structure of Mo4S4(HB(pz)3)4(pz) (pz = pyrazolyl, C3N2H3−). Inorg. Chem. 1986, 25, 3529–3532. [Google Scholar] [CrossRef]
- Potel, M.; Chevrel, R.; Sergent, M. In2Mo15Se19: Nouvel exemple de structure à motifs Mo6Se8 et Mo9Se11. Acta Cryst. B 1981, 37, 1007–1010. [Google Scholar] [CrossRef]
- Chevrel, R.; Potel, M.; Sergent, M.; Decroux, M.; Fischer, O. New ternary molybdenum chalcogenides containing Mo6 and Mo9 clusters: M2Mo15Se19 (M = K, Ba, In, Tl) and K2Mo15S19. Mater. Res. Bull. 1980, 15, 867–874. [Google Scholar] [CrossRef]
- Jödden, K.; von Schnering, H.G.; Schäfer, H. [(n-C4H9)4N]2Mo5Cl13— a compound with the cluster group [Mo5Cli8]. Angew. Chem. 1975, 14, 570–571. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Grin, Y.; von Schnering, H.G. Crystal structure of molybdenum(II) bromide, Mo6Br12. Z. Krist. N. Cryst. Struct. 1998, 213, 469–470. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Gushchin, A.L.; Naumov, D.Y.; Gerasko, O.A.; Fedin, V.P. Cluster oxalate complexes [M3(μ3-Q)(μ2-Q2)3(C2O4)3]2- and [Mo3(μ3-Q)(μ2-Q)3(C2O4)3(H2O)3]2− (M = Mo, W.; Q = S, Se): Mechanochemical synthesis and crystal structure. Inorg. Chem. 2005, 44, 2431–2436. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Ooi, B.-L.; Harris, P.; Vicent, C.; Sokolov, M.N. Synthesis and characterization of mixed chalcogen triangular complexes with new Mo3(μ3-S)(μ2-Se2)34+ and M3(μ3-S)(μ2-Se)34+(M = Mo, W) cluster cores. Inorg. Chem. 2009, 48, 3832–3839. [Google Scholar] [CrossRef]
- Magliocchi, C.; Xie, X.; Hughbanks, T. Cyanide-melt synthesis of reduced molybdenum selenide clusters. Inorg. Chem. 2004, 43, 1902–1911. [Google Scholar] [CrossRef]
- Mironov, Y.V.; Virovets, A.V.; Naumov, N.G.; Ikorskii, V.N.; Federov, V.E. Excision of the {Mo6Se8} cluster core from a chevrel phase: Synthesis and properties of the first molybdenum octahedral cluster selenocyanide anions [Mo6Se8(CN)6]7− and [Mo6Se8(CN)6]6−. Chem. Eur. J. 2000, 6, 1361–1365. [Google Scholar] [CrossRef]
- Levi, E.; Aurbach, D.; Gatti, C. Metal–metal bond in the light of Pauling’s rules. Molecules 2021, 26, 304. [Google Scholar] [CrossRef] [PubMed]
- Baranovski, V.I.; Korolkov, D.V. Electron density distribution, polarizability and bonding in the octahedral clusters [Mo6S8(CN)6]6−, [Re6S8(CN)6]4− and Rh6(CO)16. Polyhedron 2004, 23, 1519–1526. [Google Scholar] [CrossRef]
- Best, S.A.; Walton, R.A. X-ray photoelectron spectra of inorganic molecules. 22. Halogen core electron binding energies of low oxidation state molybdenum bromide and molybdenum iodide clusters and niobium and tantalum chlorides containing the [M6Cl12]n+ cores. Inorg. Chem. 1979, 18, 484–488. [Google Scholar] [CrossRef]
- Richard, J.; Benayad, A.; Colin, J.-F.; Martinet, S. Charge transfer mechanism into the Chevrel phase Mo6S8 during Mg intercalation. J. Phys. Chem. C 2017, 121, 17096–17103. [Google Scholar] [CrossRef]
- Akashi, H.; Shibahara, T. Molybdenum–copper–sulfur Mo3CuS4 cubes. Inorg. Chim. Acta 2000, 300–302, 572–580. [Google Scholar] [CrossRef]
- Fedorenko, A.D.; Semushkina, G.I.; Peregudova, N.N.; Lavrukhina, S.A.; Gushchin, A.L.; Fomenko, Y.S.; Sokolov, M.N.; Gusel′nikov, A.V.; Kalinkin, A.V.; Nikolenko, A.D.; et al. Studying the electronic structure of trinuclear molybdenum cluster sulfides with {Mo3S4} and {Mo3S7} cores by X-ray spectroscopy. J. Struct. Chem. 2021, 62, 853–864. [Google Scholar] [CrossRef]
- Wang, X.-L.; Xue, C.; Kong, N.; Wu, Z.; Zhang, J.; Wang, X.; Zhou, R.; Lin, H.; Li, Y.; Li, D.-S.; et al. Molecular modulation of a molybdenum–selenium cluster by sulfur substitution to enhance the hydrogen evolution reaction. Inorg. Chem. 2019, 58, 12415–12421. [Google Scholar] [CrossRef]
- Abramov, P.A.; Sokolov, M.N.; Peresypkina, E.V.; Mirzaeva, I.V.; Moroz, N.K.; Vicent, C.; Hernandez-Molina, R.; Goya, M.C.; Arévalo, M.C.; Fedin, V.P. Oxoselenide triangular molybdenum clusters: Synthesis and characterization of [Mo3SeO3(acac)3(py)3]PF6. Inorg. Chim. Acta 2011, 375, 314–319. [Google Scholar] [CrossRef]
- Gassan, A.D.; Ivanov, A.A.; Pozmogova, T.N.; Eltsov, I.V.; Kuratieva, N.V.; Mironov, Y.V.; Shestopalov, M.A. Water-soluble chalcogenide W6-clusters: On the way to biomedical applications. Int. J. Mol. Sci. 2022, 23, 8734. [Google Scholar] [CrossRef]
- Zietlow, T.C.; Gray, H.B. Preparation and characterization of pentanuclear molybdenum halide clusters. Inorg. Chem. 1986, 25, 631–634. [Google Scholar] [CrossRef]
- Tariq, M. Electrochemistry of Br−/Br2 redox couple in acetonitrile, methanol and mix media of acetonitrile–methanol: An insight into redox behavior of bromide on platinum (Pt) and gold (Au) electrode. Z. Phys. Chem. 2020, 234, 295–312. [Google Scholar] [CrossRef]
- Nasreldin, M.; Henkel, G.; Kampmann, G.; Krebs, B.; Lamprecht, G.J.; Routledge, C.A.; Geoffrey, A. Preparation, structure and properties of dinuclear, trinuclear incomplete cuboidal and cuboidal molybdenum-selenium cluster complexes. J. Chem. Soc. Dalton Trans. 1993, 1993, 737–746. [Google Scholar] [CrossRef]
- Khutornoi, V.A.; Naumov, N.G.; Mironov, Y.V.; Oeckler, O.; Simon, A.; Fedorov, V.E. Novel complexes [M(DMF)6][Mo6Br8(NCS)6] (M = Mn2+, Co2+, Ni2+, Cu2+, and Cd2+): Synthesis, structure determination, and properties. Russ. J. Coord. Chem. 2002, 28, 183–190. [Google Scholar] [CrossRef]
- Bruker. APEX2, version 1.08; SAINT, version 07.03; SADABS, version 02.11; SHELXTL, version 06.12; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Swart, M. A new family of hybrid density functionals. Chem. Phys. Lett. 2013, 580, 166–171. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Van der Avoird, A.; Wormer, P.E.S. Density functional calculations of molecular hyperfine interactions in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1998, 108, 4783–4796. [Google Scholar] [CrossRef]
- Van Lenthe, E.; Wormer, P.E.S.; van der Avoird, A. Density functional calculations of molecular g-tensors in the zero-order regular approximation for relativistic effects. J. Chem. Phys. 1997, 107, 2488–2498. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Nalewajski, R.F.; Mrozek, J. Modified valence indices from the two-particle density matrix. Int. J. Quantum Chem. 1994, 51, 187–200. [Google Scholar] [CrossRef]
- Nalewajski, R.F.; Mrozek, J.; Mazur, G. Quantum chemical valence indices from the one-determinantal difference approach. Can. J. Chem. 1996, 74, 1121–1130. [Google Scholar] [CrossRef]
- Nalewajski, R.F.; Mrozek, J.; Michalak, A. Two-electron valence indices from the Kohn-Sham orbitals. Int. J. Quantum Chem. 1997, 61, 589–601. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]




| Atom | Number | Mo-Mo | Mo-L | All | |
|---|---|---|---|---|---|
| Mo5red | Moap | 1 | 2.62 | 3.41 | 6.03 |
| Mobs | 4 | 1.73 | 3.95 | 5.68 | |
| Total | 9.55 | 19.20 | 28.75 | ||
| Mo5ox | Moap | 1 | 2.62 | 3.27 | 5.89 |
| Mobs | 4 | 1.62 | 4.17 | 5.79 | |
| Total | 9.11 | 19.95 | 29.06 |
| Compound | Mo 3d5/2–3/2 (%(a)), Assignment | Ref |
|---|---|---|
| Mo6 | 229.2–232.4 (54%), Mo2+/3+ | This work |
| 232.8–236.0 (46%), Mo6+ | ||
| Mo5red | 228.9–232.1 (95%), Mo3+ | This work |
| 231.7–234.8 (4%), Mo4+ | ||
| 233.3–236.4 (1%), Mo6+ | ||
| Mo5ox | 229.3–232.4 (78%), Mo3+ | This work |
| 230.7–233.9 (20%), Mo4+ | ||
| 232.1–235.2 (2%), Mo6+ | ||
| Mo6Br12 | 229.5–232.5 (100%), Mo2+ | [43] |
| Mo6Br12 | 229.8–232.9 (100%), Mo2+ | This work |
| Mo6S8 | 228.5–231.7 (71%), Mo2+ | [44] |
| 229.2–234.4 (24%), Mo3+ | ||
| 233.3–236.5 (5%), Mo6+ | ||
| [Mo6Se8(PEt3)6] | 227.8–230.8, Mo2+ and Mo3+ | [5] |
| K2[Mo3S4(Hnta)3] | 230.1–233.3 (100%), Mo4+ | [45] |
| (NH4)2[Mo3S13] | 229.0–232.5 (100%), Mo4+ | [46] |
| Mo3Se13 | 228.7–231.4, Mo4+ | [47] |
| 232.1–235.0, Mo6+ |
| Compound | Process (a) | Ea | Ec | E1/2 | Ref |
|---|---|---|---|---|---|
| Mo5red | MoIII5 to MoIII4MoII, qrev | −0.54 | −0.60 | −0.57 | This work |
| MoIII5 to MoIII4MoIV, rev | 0.14 | 0.07 | 0.11 | ||
| [Mo3Se4Br3(dmpe)3]PF6 (b) | MoIV3 to MoIIIMoIV2, rev | – | – | −0.54 | [12] |
| MoIIIMoIV2 to MoIII2MoIV, rev | – | – | −0.87 | ||
| MoIV3 to MoIVMoV2, rev | – | – | 1.10 | ||
| (NMe4)3[Mo4Se4(edta)2] (c) | MoIII3MoIV to MoIII4, rev | −0.20 | −0.28 | −0.24 | [52] |
| MoIII3MoIV to MoIII2MoIV2, rev | 0.48 | 0.42 | 0.45 | ||
| [Mo6Se8(PEt3)6] (d) | MoII2MoIII4 to MoII3MoIII3, rev | – | – | −0.96 | [5] |
| MoII2MoIII4 to MoIIMoIII5, rev | – | – | 0.31 | ||
| (Bu4N)2[Mo5Br13] (e) | MoII4MoIII to MoII5, rev | – | – | −0.33 | [50] |
| MoII4MoIII to MoII3MoIII2, rev | – | – | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savina, I.V.; Ivanov, A.A.; Evtushok, D.V.; Gayfulin, Y.M.; Komarovskikh, A.Y.; Syrokvashin, M.M.; Ivanova, M.N.; Asanov, I.P.; Eltsov, I.V.; Kuratieva, N.V.; et al. Unusual Square Pyramidal Chalcogenide Mo5 Cluster with Bridging Pyrazolate-Ligands. Int. J. Mol. Sci. 2023, 24, 3440. https://doi.org/10.3390/ijms24043440
Savina IV, Ivanov AA, Evtushok DV, Gayfulin YM, Komarovskikh AY, Syrokvashin MM, Ivanova MN, Asanov IP, Eltsov IV, Kuratieva NV, et al. Unusual Square Pyramidal Chalcogenide Mo5 Cluster with Bridging Pyrazolate-Ligands. International Journal of Molecular Sciences. 2023; 24(4):3440. https://doi.org/10.3390/ijms24043440
Chicago/Turabian StyleSavina, Iulia V., Anton A. Ivanov, Darya V. Evtushok, Yakov M. Gayfulin, Andrey Y. Komarovskikh, Mikhail M. Syrokvashin, Mariia N. Ivanova, Igor P. Asanov, Ilia V. Eltsov, Natalia V. Kuratieva, and et al. 2023. "Unusual Square Pyramidal Chalcogenide Mo5 Cluster with Bridging Pyrazolate-Ligands" International Journal of Molecular Sciences 24, no. 4: 3440. https://doi.org/10.3390/ijms24043440
APA StyleSavina, I. V., Ivanov, A. A., Evtushok, D. V., Gayfulin, Y. M., Komarovskikh, A. Y., Syrokvashin, M. M., Ivanova, M. N., Asanov, I. P., Eltsov, I. V., Kuratieva, N. V., Mironov, Y. V., & Shestopalov, M. A. (2023). Unusual Square Pyramidal Chalcogenide Mo5 Cluster with Bridging Pyrazolate-Ligands. International Journal of Molecular Sciences, 24(4), 3440. https://doi.org/10.3390/ijms24043440