Insight into the Molecule Impact of Critical-Sized UHMWPE-ALN Wear Particles on Cells by the Alginate-Encapsulated Cell Reactor
Abstract
1. Introduction
2. Results
2.1. Biological Evaluation of Critical-Sized UHMWPE-ALN Wear Particles on Macrophages Encapsulated in Cell Reactors
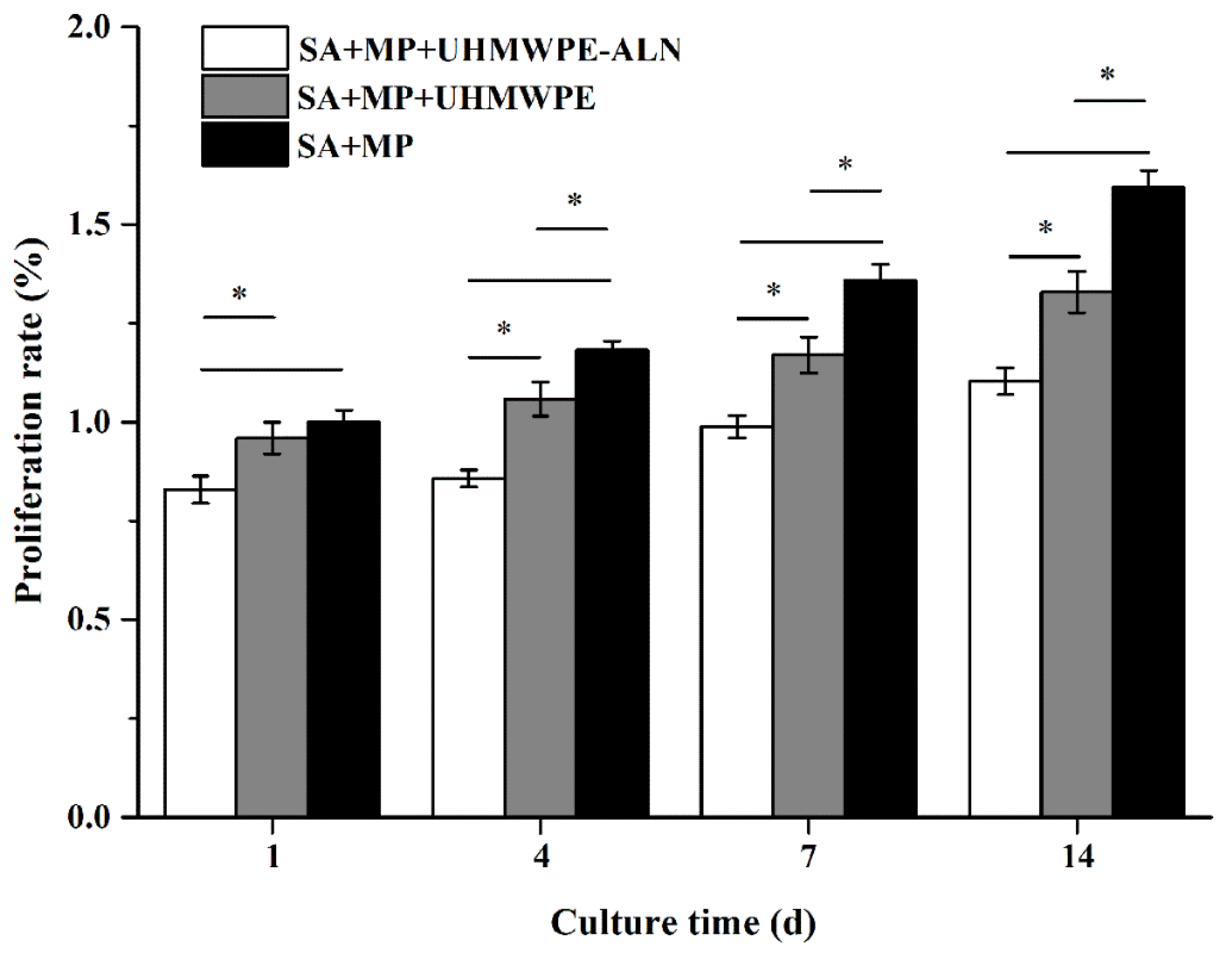
2.1.1. Alamar Blue Assay
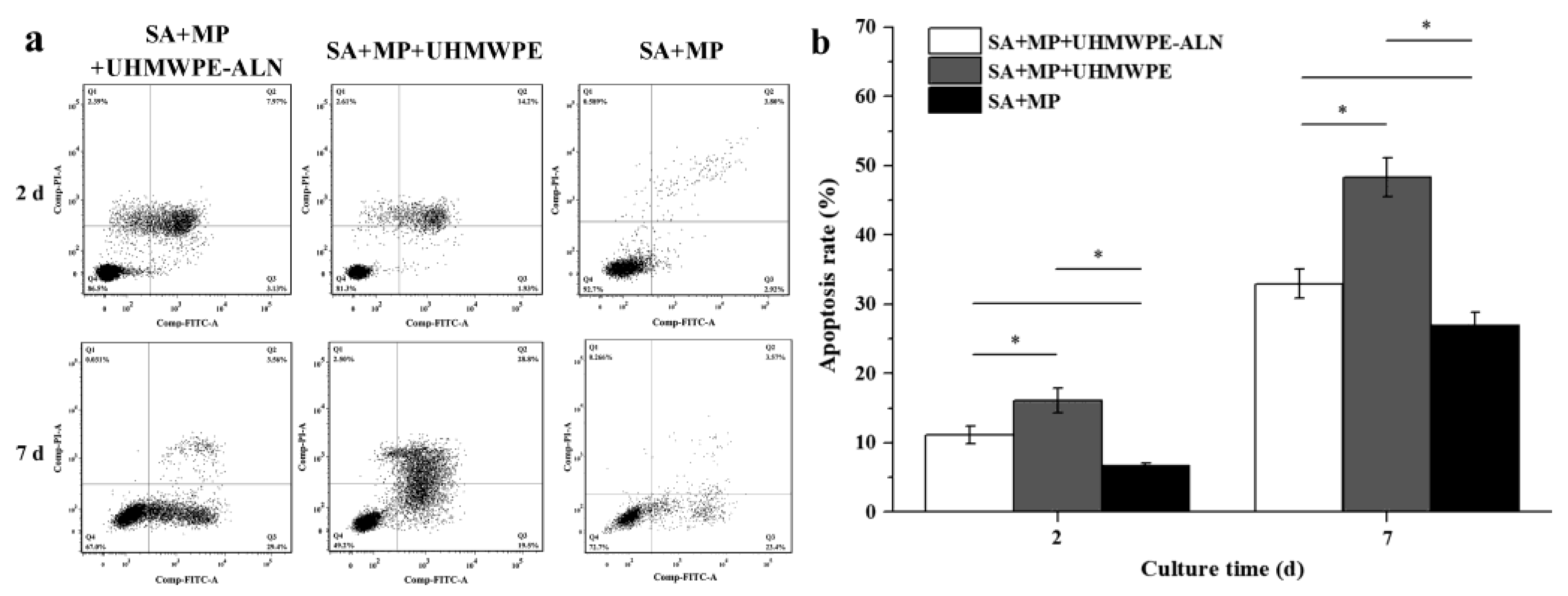
2.1.2. Cell Apoptosis Assay
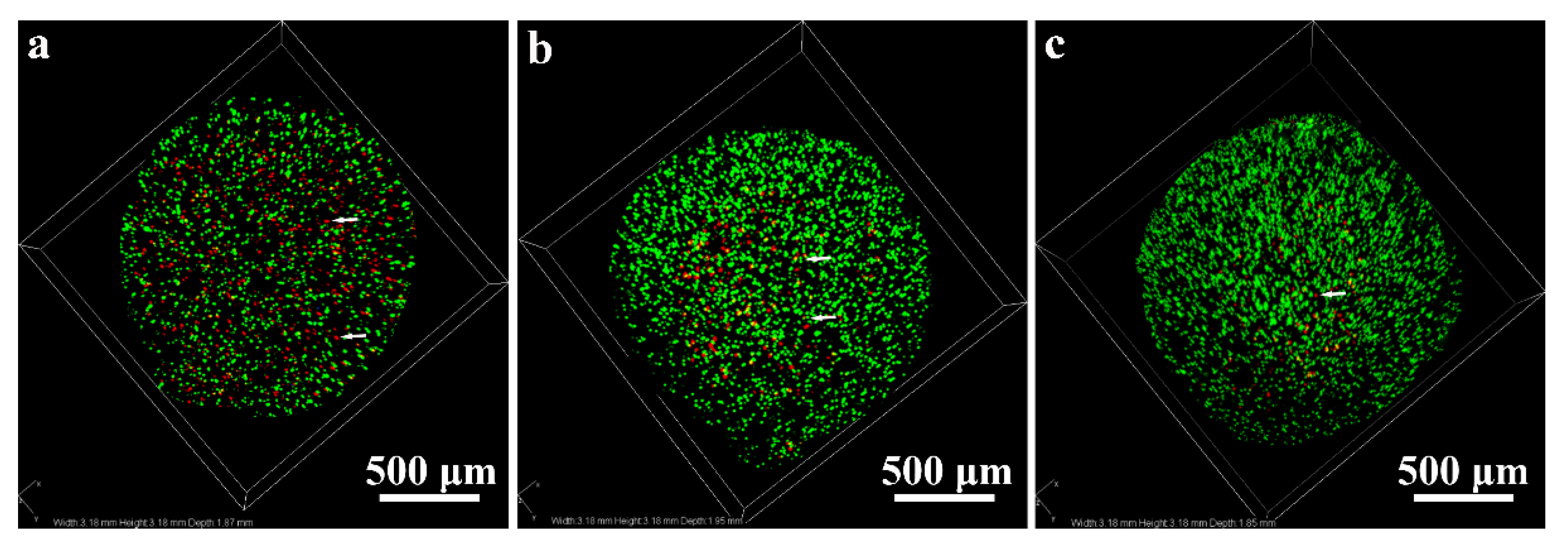
2.1.3. Live-Dead Assay
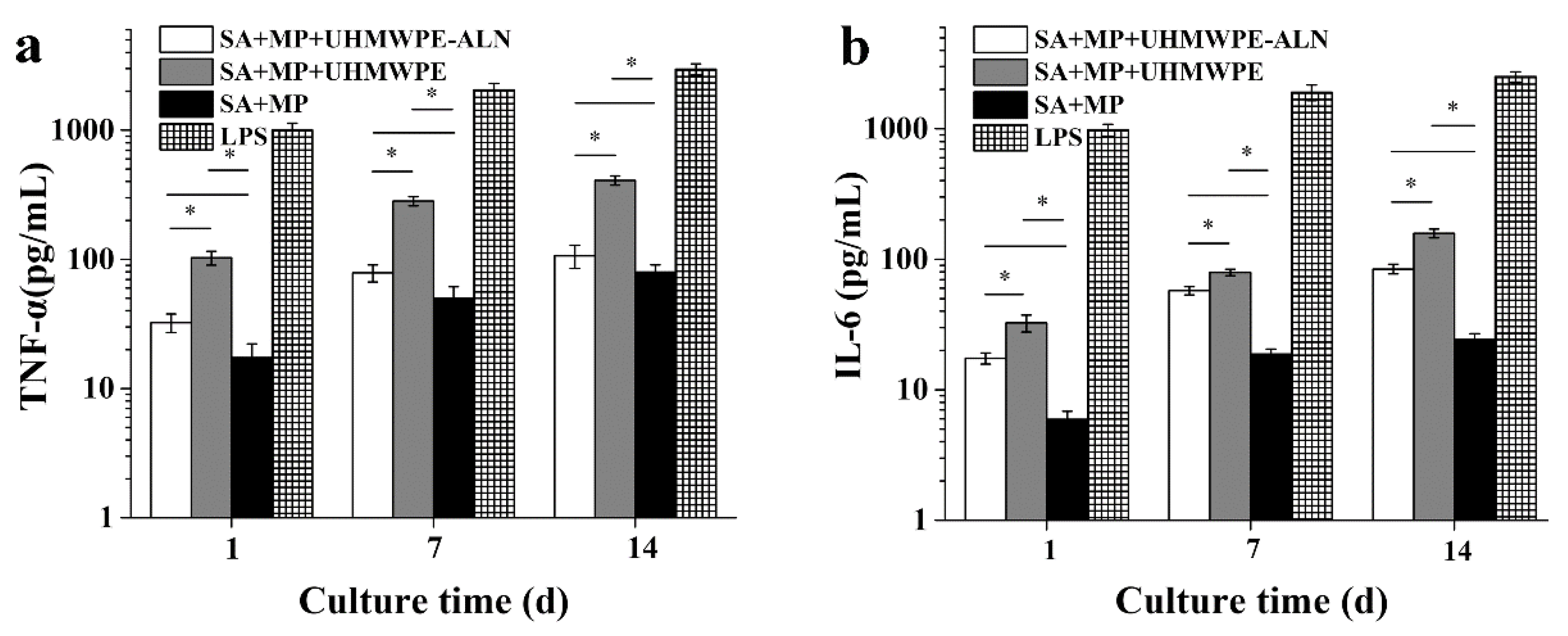
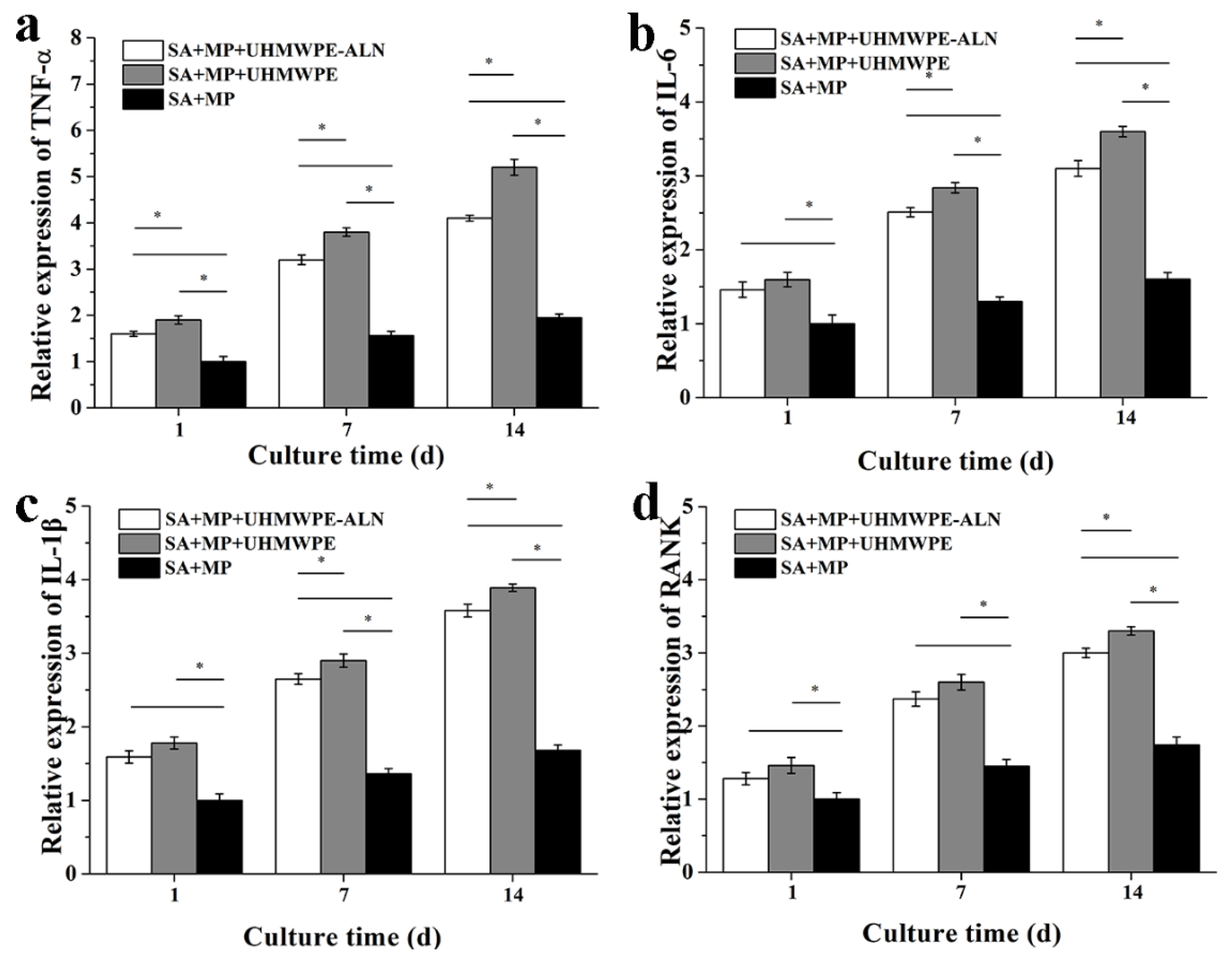
2.1.4. Cytokine Assay
2.1.5. RT-PCR Analysis
2.2. Biological Evaluation of Critical-Sized UHMWPE-ALN Wear Particles with Osteoblasts Encapsulated in Cell Reactors
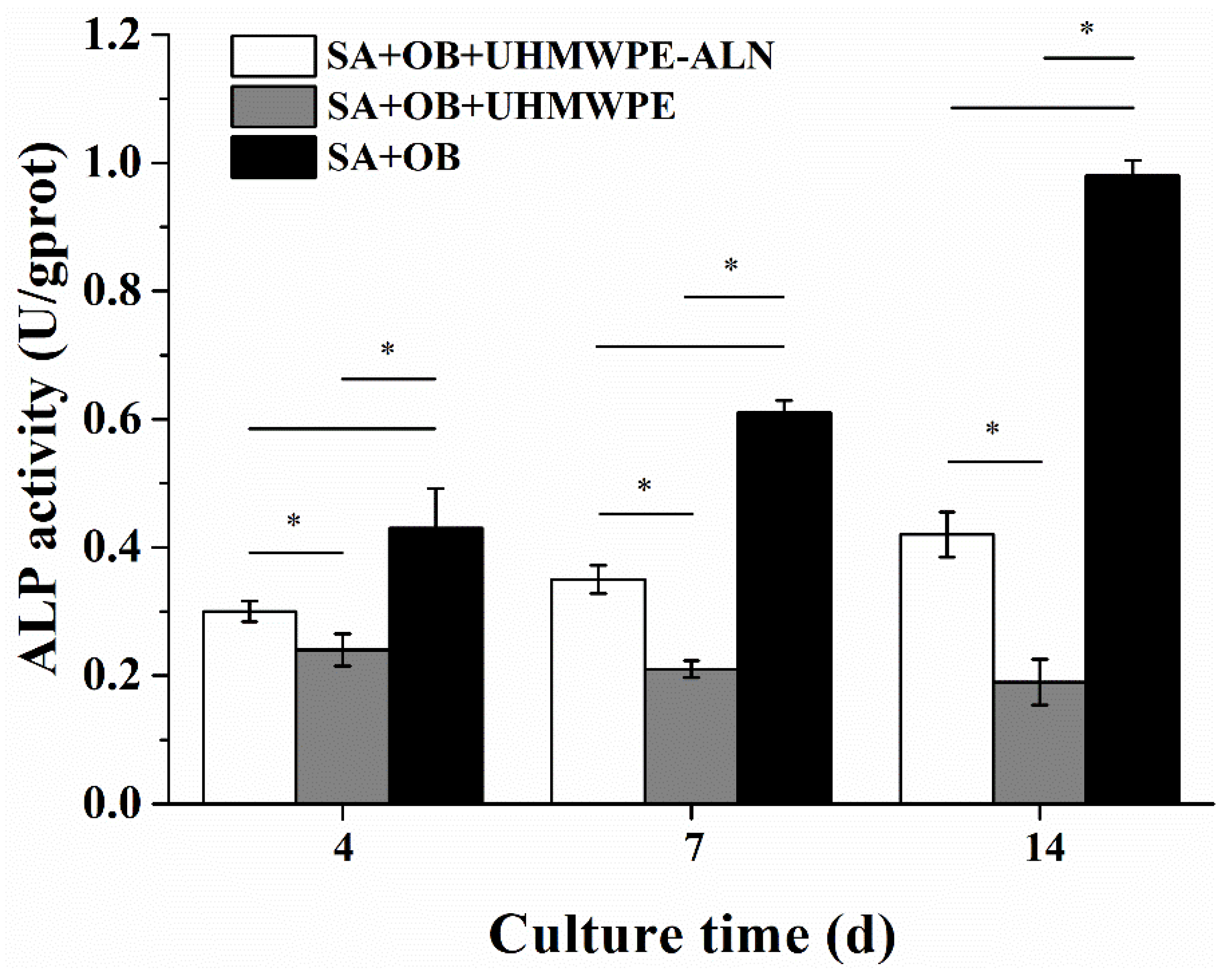
2.2.1. ALP Assay
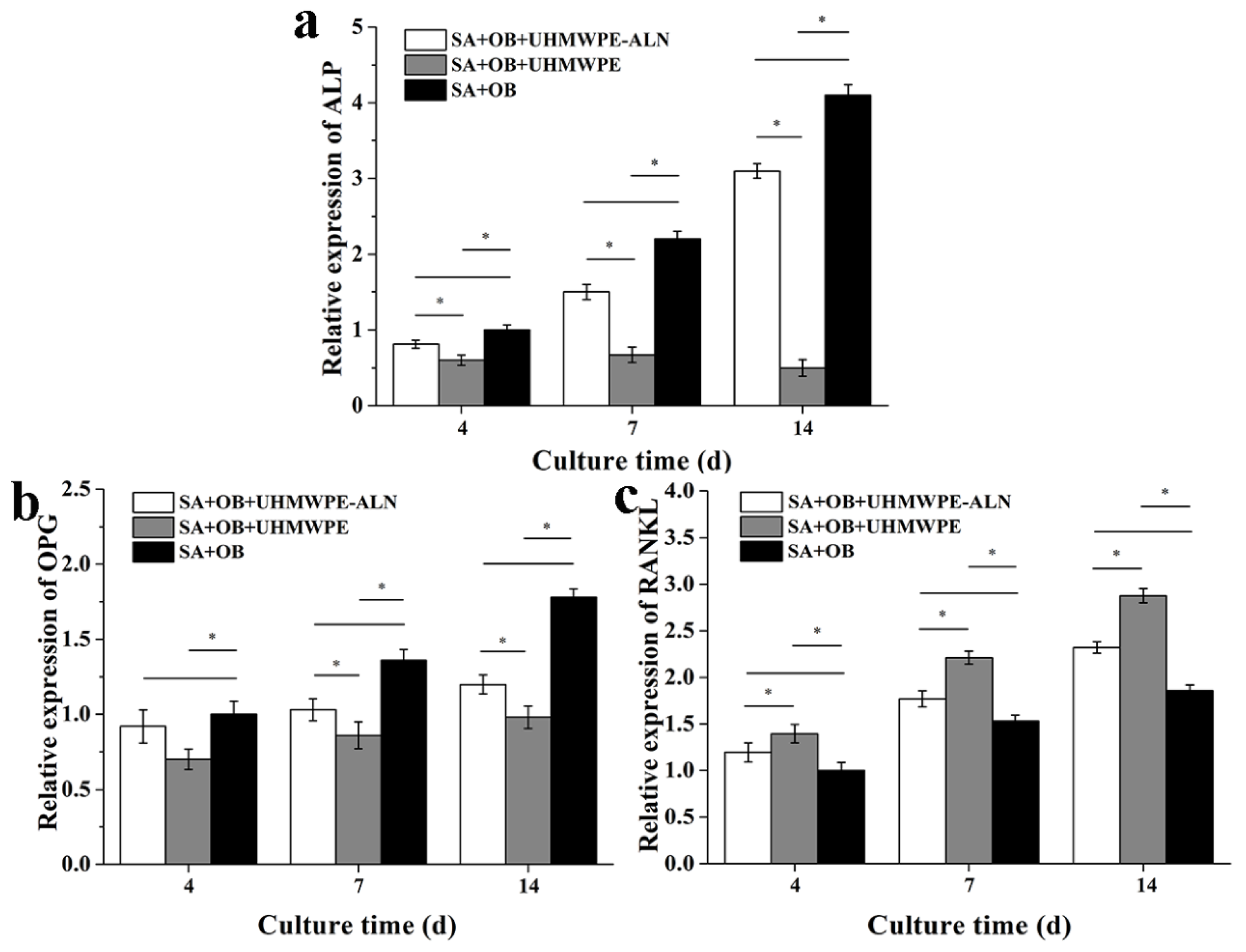
2.2.2. RT-PCR Assay
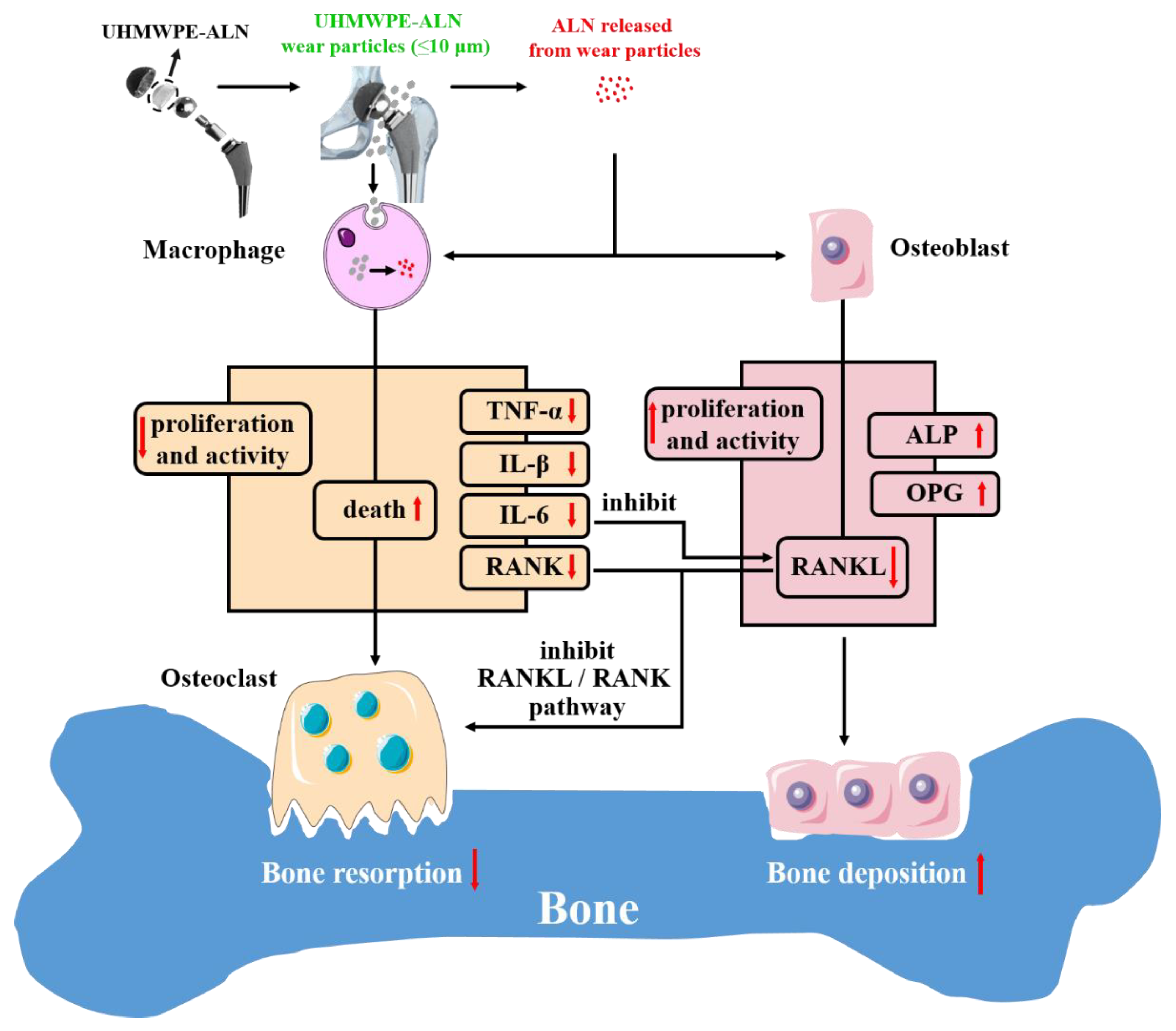
3. Discussion
4. Materials and Methods
4.1. Preparation of Alg-Encapsulated Bead with Cells and Critical-Sized UHMWPE-ALN Wear Particles
4.2. Biological Evaluation of Critical-Sized UHMWPE-ALN Wear Particles on Macrophages Encapsulated in Cell Reactors
4.2.1. Alamar Blue Assay
4.2.2. Cell Apoptosis Assay
4.2.3. Live-Dead Assay
4.2.4. Cytokine Assay
4.2.5. Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
4.3. Biological Evaluation of Critical-Sized UHMWPE-ALN Wear Particles on Osteoblasts Encapsulated in Cell Reactors
4.3.1. Alkaline Phosphatase (ALP) Activity Assay
4.3.2. RT-PCR Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurtz, S.M. The Clinical Performance of UHMWPE in Knee Replacements. In UHMWPE Biomaterials Handbook, 3rd ed.; Kurtz, S.M., Ed.; William Andrew Publishing: Oxford, UK, 2016; pp. 123–144. [Google Scholar] [CrossRef]
- Kandahari, A.M.; Yang, X.; Laroche, K.A.; Dighe, A.S.; Pan, D.; Cui, Q. A review of UHMWPE wear-induced osteolysis: The role for early detection of the immune response. Bone Res. 2016, 4, 16014. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mukherjee, K.; Pal, B. Polyethylene in Orthopedic Implants: Recent Trends and Limitations. In Encyclopedia of Materials: Plastics and Polymers; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2022; pp. 777–794. [Google Scholar] [CrossRef]
- Liu, G.; Guo, T.; Zhang, Y.; Liu, N.; Chen, J.; Chen, J.; Zhang, J.; Zhao, J. Apoptotic pathways of macrophages within osteolytic interface membrane in periprosthestic osteolysis after total hip replacement. APMIS 2017, 125, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Anindyajati, A.; Boughton, P.; Ruys, A.J. Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant. Materials 2019, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhu, W.; Liang, S.; Li, H.; Li, S. Cross-Linked Versus Conventional Polyethylene for Long-Term Clinical Outcomes After Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Investig. Surg. 2021, 34, 307–317. [Google Scholar] [CrossRef]
- Gong, K.; Qu, S.; Liu, Y.; Wang, J.; Zhang, Y.; Jiang, C.; Shen, R. The mechanical and tribological properties of UHMWPE loaded ALN after mechanical activation for joint replacements. J. Mech. Behav. Biomed. Mater. 2016, 61, 334–344. [Google Scholar] [CrossRef]
- Pietrzak, W.S. Ultra-High Molecular Weight Polyethylene for Total Hip Acetabular Liners: A Brief Review of Current Status. J. Investig. Surg. 2021, 34, 321–323. [Google Scholar] [CrossRef]
- Rogers, M.J.; Mönkkönen, J.; Munoz, M.A. Molecular mechanisms of action of bisphosphonates and new insights into their effects outside the skeleton. Bone 2020, 139, 115493. [Google Scholar] [CrossRef]
- Thavornyutikarn, B.; Wright, P.F.A.; Feltis, B.; Kosorn, W.; Turney, T.W. Bisphosphonate activation of crystallized bioglass scaffolds for enhanced bone formation. Mater. Sci. Eng. C 2019, 104, 109937. [Google Scholar] [CrossRef]
- Qu, S.; Bai, Y.; Liu, X.; Fu, R.; Duan, K.; Weng, J. Study on in vitro release and cell response to alendronate sodium-loaded ultrahigh molecular weight polyethylene loaded with alendronate sodium wear particles to treat the particles-induced osteolysis. J. Biomed. Mater. Res. Part A 2013, 101A, 394–403. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, F.; Gong, K.; Liu, Y.; Zhi, W.; Weng, J.; Qu, S. Study on critical-sized ultra-high molecular weight polyethylene wear particles loaded with alendronate sodium: In vitro release and cell response. J. Mater. Sci. Mater. Med. 2017, 28, 56. [Google Scholar] [CrossRef]
- Nich, C.; Takakubo, Y.; Pajarinen, J.; Ainola, M.; Salem, A.; Sillat, T.; Rao, A.J.; Raska, M.; Tamaki, Y.; Takagi, M.; et al. Macrophages—Key cells in the response to wear debris from joint replacements. J. Biomed. Mater. Res. Part A 2013, 101, 3033–3045. [Google Scholar] [CrossRef]
- Ormsby, R.T.; Solomon, L.B.; Yang, D.; Crotti, T.N.; Haynes, D.R.; Findlay, D.M.; Atkins, G.J. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta Biomater. 2019, 87, 296–306. [Google Scholar] [CrossRef]
- Matsumae, G.; Kida, H.; Takahashi, D.; Shimizu, T.; Ebata, T.; Yokota, S.; Alhasan, H.; Aly, M.K.; Yutani, T.; Uetsuki, K.; et al. Determination of optimal concentration of vitamin E in polyethylene liners for producing minimal biological response to prosthetic wear debris. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1587–1593. [Google Scholar] [CrossRef]
- Fang, H.-W.; Sung, Y.-T.; Su, C.-Y.; Chen, C.-C. Electrostatic field may regulate proliferation and immune responses of macrophages induced by polyethylene wear particles. J. Taiwan Inst. Chem. Eng. 2017, 77, 21–29. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, F.; Bo, L.; Zhi, W.; Weng, J.; Qu, S. A novel alginate-encapsulated system to study biological response to critical-sized wear particles of UHMWPE loaded with alendronate sodium. Mater. Sci. Eng. C 2017, 79, 679–686. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Hu, Y.; Jing, Y.; Geng, Z.; Su, J. Bone Repair Biomaterials: A Perspective from Immunomodulation. Adv. Funct. Mater. 2022, 32, 2208639. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Li, J.; Zhou, S.; Wang, Y.; Li, L.; Zhao, F. Photoluminescent carbon dots (PCDs) from sour apple: A biocompatible nanomaterial for preventing UHMWPE wear-particle induced osteolysis via modulating Chemerin/ChemR23 and SIRT1 signaling pathway and its bioimaging application. J. Nanobiotechnol. 2022, 20, 301. [Google Scholar] [CrossRef]
- Boche, M.; Pokharkar, V. Positive effect of alendronate on bone turnover in ovariectomised rats’ osteoporosis: Comparison of transdermal lipid-based delivery with conventional oral administration. Drug Deliv. Transl. Res. 2018, 8, 1078–1089. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Ruiz, C.; García-Martínez, O. Bisphosphonate Modulation of the Gene Expression of Different Markers Involved in Osteoblast Physiology: Possible Implications in Bisphosphonate-Related Osteonecrosis of the Jaw. Int. J. Med. Sci. 2018, 15, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Jerez, H.E.; Altube, M.J.; Gándola, Y.B.; González, L.; González, M.C.; Morilla, M.J.; Romero, E.L. Macrophage apoptosis using alendronate in targeted nanoarchaeosomes. Eur. J. Pharm. Biopharm. 2021, 160, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Maeno, T.; Nishimura, S.; Ogata, F.; Masubuchi, H.; Hara, K.; Yamaguchi, K.; Aoki, F.; Suga, T.; Nagai, R.; et al. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages. Nat. Commun. 2015, 6, 6332. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.F.; Guillet, C.; Massin, P.; Chevalier, S.; Gascan, H.; Baslé, M.F.; Chappard, D. Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem. Pharmacol. 2007, 73, 718–723. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gibon, E.; Pajarinen, J.; Lin, T.-H.; Keeney, M.; Ren, P.-G.; Nich, C.; Yao, Z.; Egashira, K.; Yang, F.; et al. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J. R. Soc. Interface 2014, 11, 20130962. [Google Scholar] [CrossRef]
- Hodges, N.A.; Sussman, E.M.; Stegemann, J.P. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials 2021, 278, 121127. [Google Scholar] [CrossRef]
- Liu, G.; Liu, N.; Xu, Y.; Ti, Y.; Chen, J.; Chen, J.; Zhang, J.; Zhao, J. Endoplasmic reticulum stress-mediated inflammatory signaling pathways within the osteolytic periosteum and interface membrane in particle-induced osteolysis. Cell Tissue Res. 2016, 363, 427–447. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, B.; Cheng, M.; Wang, J.; Zhang, X. Curcumin Attenuation of Wear Particle-Induced Osteolysis via RANKL Signaling Pathway Suppression in Mouse Calvarial Model. Mediat. Inflamm. 2017, 2017, 5784374. [Google Scholar] [CrossRef]
- Hou, J.; Su, H.; Kuang, X.; Qin, W.; Liu, K.; Pan, K.; Zhang, B.; Yang, S.; Yang, S.; Peng, X.; et al. Knowledge Domains and Emerging Trends of Osteoblasts-Osteoclasts in Bone Disease From 2002 to 2021: A Bibliometrics Analysis and Visualization Study. Front. Endocrinol. 2022, 13, 922070. [Google Scholar] [CrossRef]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key Triggers of Osteoclast-Related Diseases and Available Strategies for Targeted Therapies: A Review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, J.; Lei, L.; Sun, W.; Wu, Y.; Ding, P.; Chen, L. The positive effects of secreting cytokines IL-17 and IFN-γ on the early-stage differentiation and negative effects on the calcification of primary osteoblasts in vitro. Int. Immunopharmacol. 2018, 57, 1–10. [Google Scholar] [CrossRef]
- Ban, Y.; Wu, Y.-y.; Yu, T.; Geng, N.; Wang, Y.-y.; Liu, X.-g.; Gong, P. Response of osteoblasts to low fluid shear stress is time dependent. Tissue Cell 2011, 43, 311–317. [Google Scholar] [CrossRef]
- Wang, H.; Bi, H.; Gao, T.; Zhao, B.; Ni, W.; Liu, J. A homogalacturonan from Hippophae rhamnoides L. Berries enhance immunomodulatory activity through TLR4/MyD88 pathway mediated activation of macrophages. Int. J. Biol. Macromol. 2018, 107, 1039–1045. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, M.; Ren, Y.; Lou, R.; Xie, H.; Yu, W.; Liu, X.; Ma, X. Controlling Gel Structure to Modulate Cell Adhesion and Spreading on the Surface of Microcapsules. ACS Appl. Mater. Interfaces 2016, 8, 19333–19342. [Google Scholar] [CrossRef]
- Jablonski, H.; Kauther, M.D.; Bachmann, H.S.; Jäger, M.; Wedemeyer, C. Calcitonin Gene-Related Peptide Modulates the Production of Pro-Inflammatory Cytokines Associated with Periprosthetic Osteolysis by THP-1 Macrophage-Like Cells. Neuroimmunomodulation 2015, 22, 152–165. [Google Scholar] [CrossRef]
- Mughal, B.B.; Leemans, M.; Spirhanzlova, P.; Demeneix, B.; Fini, J.B. Reference gene identification and validation for quantitative real-time PCR studies in developing Xenopus laevis. Sci. Rep. 2018, 8, 496. [Google Scholar] [CrossRef]
- Zeng, Q.; Han, Y.; Li, H.; Chang, J. Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 42–51. [Google Scholar] [CrossRef]
| Gene | Direction | Sequence(5′-3′) |
|---|---|---|
| TNF-α | Forward | AAGTTCCCAAATGGCCTCCC |
| Reverse | TGGTTTGCTACGACGTGGG | |
| IL-6 | Forward | TAGTCCTTCCTACCCCAATTTCC |
| Reverse | TTGGTCCTTAGCCACTCCTTC | |
| IL-1β | Forward | GCCACCTTTTGACAGTGATGA |
| Reverse | ATGTGCTGCTGCGAGATTTG | |
| RANK | Forward | CCAGGAGAGGCATTATGAGCA |
| Reverse | ACTGTCGGAGGTAGGAGTGC | |
| GAPDH | Forward | GGACCAGGTTGTCTCCTGTG |
| Reverse | CATTGAGAGCAATGCCAGCC |
| Gene | Direction | Sequence(5′-3′) |
|---|---|---|
| ALP | Forward | GGAACGGATCTCGGGGTACA |
| Reverse | ATGAGTTGGTAAGGCAGGGT | |
| RANKL | Forward | GGGGGAGCACTAAGAACTGG |
| Reverse | GTTGGACACCTGGACGCTAA | |
| OPG | Forward | CAGTGTGCAACGGCATATCG |
| Reverse | CCAGGCAAGCTCTCCATCAA | |
| GAPDH | Forward | GGACCAGGTTGTCTCCTGTG |
| Reverse | CATTGAGAGCAATGCCAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shi, F.; Qu, S. Insight into the Molecule Impact of Critical-Sized UHMWPE-ALN Wear Particles on Cells by the Alginate-Encapsulated Cell Reactor. Int. J. Mol. Sci. 2023, 24, 3510. https://doi.org/10.3390/ijms24043510
Liu Y, Shi F, Qu S. Insight into the Molecule Impact of Critical-Sized UHMWPE-ALN Wear Particles on Cells by the Alginate-Encapsulated Cell Reactor. International Journal of Molecular Sciences. 2023; 24(4):3510. https://doi.org/10.3390/ijms24043510
Chicago/Turabian StyleLiu, Yumei, Feng Shi, and Shuxin Qu. 2023. "Insight into the Molecule Impact of Critical-Sized UHMWPE-ALN Wear Particles on Cells by the Alginate-Encapsulated Cell Reactor" International Journal of Molecular Sciences 24, no. 4: 3510. https://doi.org/10.3390/ijms24043510
APA StyleLiu, Y., Shi, F., & Qu, S. (2023). Insight into the Molecule Impact of Critical-Sized UHMWPE-ALN Wear Particles on Cells by the Alginate-Encapsulated Cell Reactor. International Journal of Molecular Sciences, 24(4), 3510. https://doi.org/10.3390/ijms24043510






