Development of a CRISPRi Human Retinal Pigmented Epithelium Model for Functional Study of Age-Related Macular Degeneration Genes
Abstract
1. Introduction
2. Results
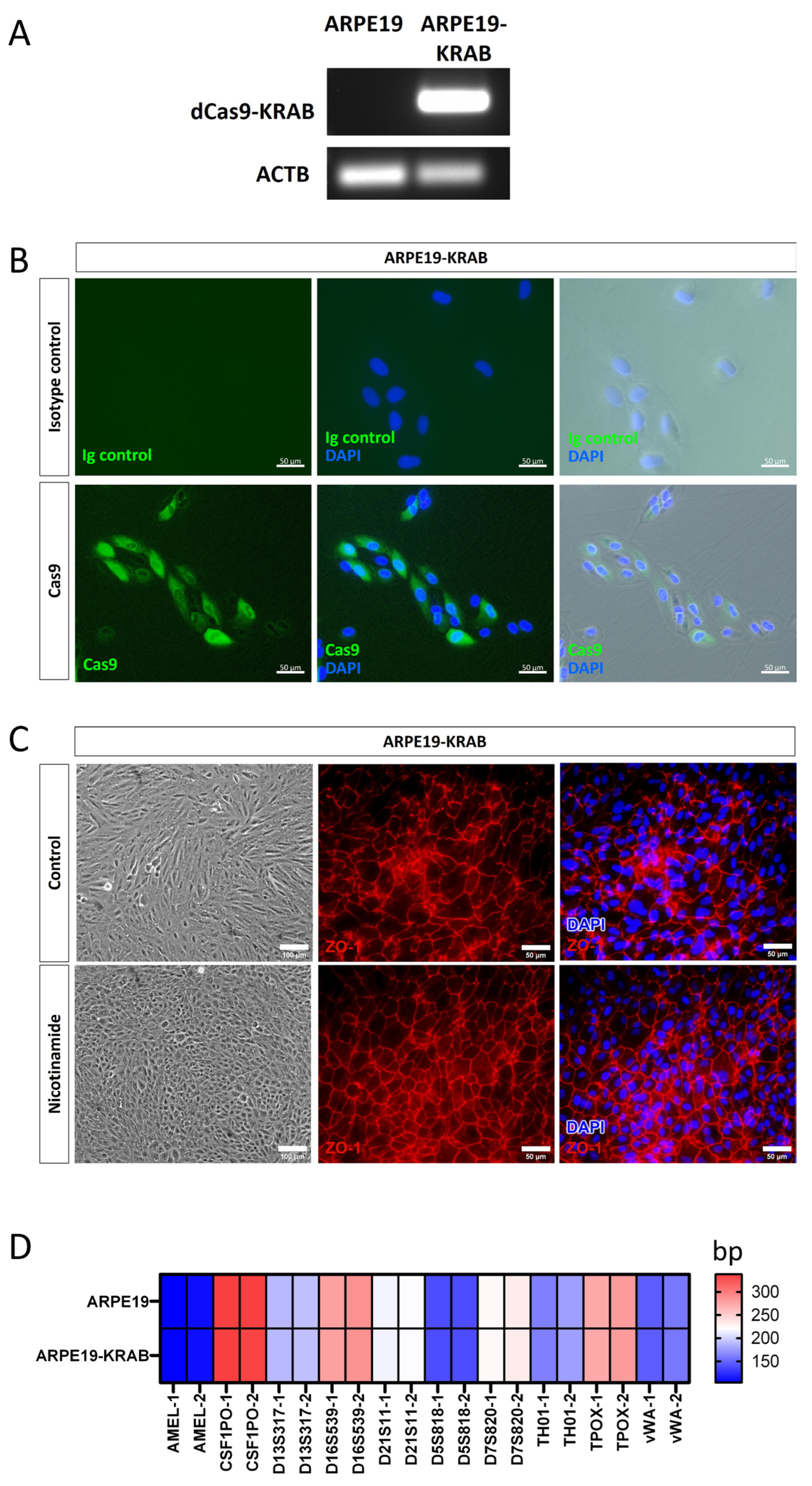
2.1. Generation of a Stable ARPE19 Cell Line for CRISPRi
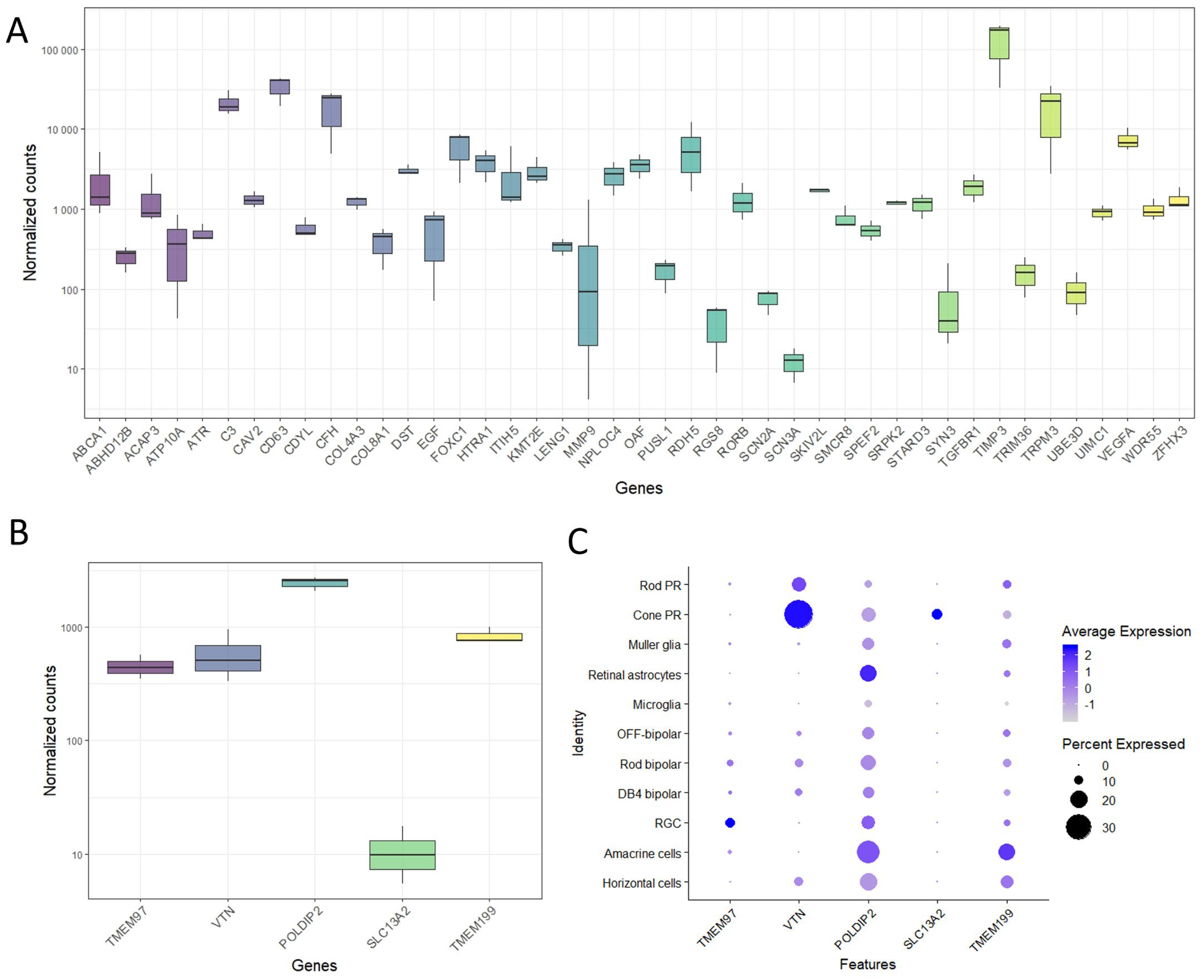
2.2. Gene Expression of TMEM97/VTN Locus in Human Retina
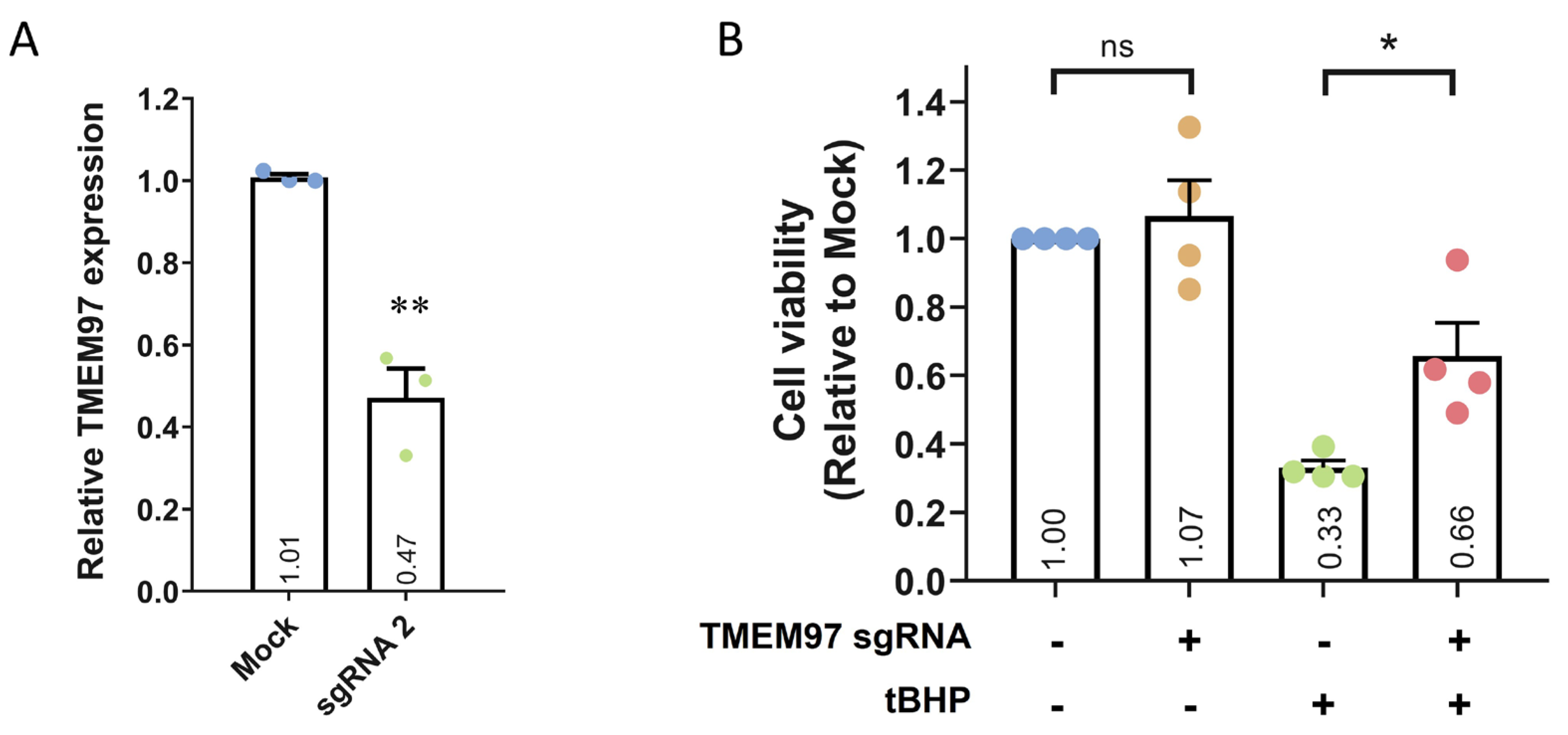
2.3. Loss-of-Function Study of TMEM97 Using CRISPRi
3. Discussion
4. Methods
4.1. Donor Retina Collection
4.2. Transcriptome Analysis Using RNAseq
4.3. Cell Culture
4.4. Lentivirus Generation
4.5. Generation of ARPE19-KRAB
4.6. CRISPR Interference (CRISPRi)-Mediated Knockdown of Gene Expression
4.7. PCR and qPCR Analysis
4.8. Immunoblotting
4.9. Immunocytochemistry
4.10. Cell Viability Assay
4.11. Reactive Oxygen Species (ROS) Analysis
4.12. Short Tandem Repeat Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.-Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A Large Genome-Wide Association Study of Age-Related Macular Degeneration Highlights Contributions of Rare and Common Variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Neale, B.M.; Fagerness, J.; Reynolds, R.; Sobrin, L.; Parker, M.; Raychaudhuri, S.; Tan, P.L.; Oh, E.C.; Merriam, J.E.; Souied, E.; et al. Genome-Wide Association Study of Advanced Age-Related Macular Degeneration Identifies a Role of the Hepatic Lipase Gene (LIPC). Proc. Natl. Acad. Sci. USA 2010, 107, 7395–7400. [Google Scholar] [CrossRef] [PubMed]
- Logue, M.W.; Schu, M.; Vardarajan, B.N.; Farrell, J.; Lunetta, K.L.; Jun, G.; Baldwin, C.T.; Deangelis, M.M.; Farrer, L.A. Search for Age-Related Macular Degeneration Risk Variants in Alzheimer Disease Genes and Pathways. Neurobiol. Aging 2014, 35, 1510.e7–1510.e18. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bhangale, T.R.; Fagerness, J.; Ripke, S.; Thorleifsson, G.; Tan, P.L.; Souied, E.H.; Richardson, A.J.; Merriam, J.E.; Buitendijk, G.H.S.; et al. Common Variants near FRK/COL10A1 and VEGFA Are Associated with Advanced Age-Related Macular Degeneration. Hum. Mol. Genet. 2011, 20, 3699–3709. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Yamashiro, K.; Chen, L.J.; Ahn, J.; Huang, L.; Huang, L.; Cheung, C.M.G.; Miyake, M.; Cackett, P.D.; Yeo, I.Y.; et al. New Loci and Coding Variants Confer Risk for Age-Related Macular Degeneration in East Asians. Nat. Commun. 2015, 6, 6063. [Google Scholar] [CrossRef]
- Nguyen, T.; Urrutia-Cabrera, D.; Liou, R.H.-C.; Luu, C.D.; Guymer, R.; Wong, R.C.-B. New Technologies to Study Functional Genomics of Age-Related Macular Degeneration. Front. Cell Dev. Biol. 2020, 8, 604220. [Google Scholar] [CrossRef]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the Gene That Codes for the σ2 Receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef]
- Oyer, H.M.; Sanders, C.M.; Kim, F.J. Small-Molecule Modulators of Sigma1 and Sigma2/TMEM97 in the Context of Cancer: Foundational Concepts and Emerging Themes. Front. Pharmacol. 2019, 10, 1141. [Google Scholar] [CrossRef]
- Liu, C.-C.; Yu, C.-F.; Wang, S.-C.; Li, H.-Y.; Lin, C.-M.; Wang, H.-H.; Abate, C.; Chiang, C.-S. Sigma-2 receptor/TMEM97 Agonist PB221 as an Alternative Drug for Brain Tumor. BMC Cancer 2019, 19, 473. [Google Scholar] [CrossRef]
- Nicholson, H.E.; Alsharif, W.F.; Comeau, A.B.; Mesangeau, C.; Intagliata, S.; Mottinelli, M.; McCurdy, C.R.; Bowen, W.D. Divergent Cytotoxic and Metabolically Stimulative Functions of Sigma-2 Receptors: Structure-Activity Relationships of 6-Acetyl-3-(4-(4-(4-Fluorophenyl)piperazin-1-Yl)butyl)benzod]oxazol-2(3H)-One (SN79) Derivatives. J. Pharmacol. Exp. Ther 2019, 368, 272–281. [Google Scholar] [CrossRef]
- Vázquez-Rosa, E.; Watson, M.R.; Sahn, J.J.; Hodges, T.R.; Schroeder, R.E.; Cintrón-Pérez, C.J.; Shin, M.-K.; Yin, T.C.; Emery, J.L.; Martin, S.F.; et al. Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury. ACS Chem. Neurosci. 2019, 10, 1595–1602. [Google Scholar] [CrossRef]
- Izzo, N.J.; Colom-Cadena, M.; Riad, A.A.; Xu, J.; Singh, M.; Abate, C.; Cahill, M.A.; Spires-Jones, T.L.; Bowen, W.D.; Mach, R.H.; et al. Proceedings from the Fourth International Symposium on σ-2 Receptors: Role in Health and Disease. eNeuro 2020, 7, ENEURO.0317-20.2020. [Google Scholar] [CrossRef]
- Terada, K.; Migita, K.; Matsushima, Y.; Kamei, C. Sigma-2 Receptor as a Potential Therapeutic Target for Treating Central Nervous System Disorders. Neural Regen. Res. 2019, 14, 1893–1894. [Google Scholar] [CrossRef]
- Hung, S.S.C.; McCaughey, T.; Swann, O.; Pébay, A.; Hewitt, A.W. Genome Engineering in Ophthalmology: Application of CRISPR/Cas to the Treatment of Eye Disease. Prog. Retin. Eye Res. 2016, 53, 1–20. [Google Scholar] [CrossRef]
- Dominguez, A.A.; Lim, W.A.; Qi, L.S. Beyond Editing: Repurposing CRISPR-Cas9 for Precision Genome Regulation and Interrogation. Nat. Rev. Mol. Cell Biol. 2016, 17, 5–15. [Google Scholar] [CrossRef]
- Housden, B.E.; Perrimon, N. Comparing CRISPR and RNAi-Based Screening Technologies. Nat. Biotechnol. 2016, 34, 621–623. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef]
- Sanson, K.R.; Hanna, R.E.; Hegde, M.; Donovan, K.F.; Strand, C.; Sullender, M.E.; Vaimberg, E.W.; Goodale, A.; Root, D.E.; Piccioni, F.; et al. Optimized Libraries for CRISPR-Cas9 Genetic Screens with Multiple Modalities. Nat. Commun. 2018, 9, 5416. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S. Rapid Differentiation of the Human RPE Cell Line, ARPE-19, Induced by Nicotinamide. Exp. Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, S.W.; Lo, C.Y.; Sharov, A.A.; Nguyen, Q.; Fang, L.; Hung, S.S.; Zhu, L.; Zhang, T.; Grünert, U.; Nguyen, T.; et al. A Single-Cell Transcriptome Atlas of the Adult Human Retina. EMBO J. 2019, 38, e100811. [Google Scholar] [CrossRef] [PubMed]
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA Design to Maximize Activity and Minimize off-Target Effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Hung, S.S.C.; Yek, J.; El Wazan, L.; Nguyen, T.; Khan, S.; Lim, S.Y.; Hewitt, A.W.; Wong, R.C.B. A Simple Cloning-Free Method to Efficiently Induce Gene Expression Using CRISPR/Cas9. Mol. Ther. Nucleic Acids 2019, 14, 184–191.24. [Google Scholar] [CrossRef]
- Bae, S.; Park, J.; Kim, J.-S. Cas-OFFinder: A Fast and Versatile Algorithm That Searches for Potential off-Target Sites of Cas9 RNA-Guided Endonucleases. Bioinformatics 2014, 30, 1473–1475. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The Impact of Oxidative Stress and Inflammation on RPE Degeneration in Non-Neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Bringmann, A.; Syrbe, S.; Görner, K.; Kacza, J.; Francke, M.; Wiedemann, P.; Reichenbach, A. The Primate Fovea: Structure, Function and Development. Prog. Retin. Eye Res. 2018, 66, 49–84. [Google Scholar] [CrossRef]
- Radzisheuskaya, A.; Shlyueva, D.; Müller, I.; Helin, K. Optimizing sgRNA Position Markedly Improves the Efficiency of CRISPR/dCas9-Mediated Transcriptional Repression. Nucleic Acids Res. 2016, 44, e141. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Strunz, T.; Lauwen, S.; Kiel, C.; International AMD Genomics Consortium (IAMDGC); den Hollander, A.; Weber, B.H.F. A Transcriptome-Wide Association Study Based on 27 Tissues Identifies 106 Genes Potentially Relevant for Disease Pathology in Age-Related Macular Degeneration. Sci. Rep. 2020, 10, 1584. [Google Scholar] [CrossRef]
- Ambati, J.; Fowler, B.J. Mechanisms of Age-Related Macular Degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef]
- Finnemann, S.C.; Bonilha, V.L.; Marmorstein, A.D.; Rodriguez-Boulan, E. Phagocytosis of Rod Outer Segments by Retinal Pigment Epithelial Cells Requires alpha(v)beta5 Integrin for Binding but Not for Internalization. Proc. Natl. Acad. Sci. USA 1997, 94, 12932–12937. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Dahrouj, M.; Tang, P.H.; Liu, Y.; Sambamurti, K.; Marmorstein, A.D.; Crosson, C.E. Human Retinal Pigment Epithelium Cells as Functional Models for the RPE in Vivo. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8614–8620. [Google Scholar] [CrossRef]
- Luo, Y.; Zhuo, Y.; Fukuhara, M.; Rizzolo, L.J. Effects of Culture Conditions on Heterogeneity and the Apical Junctional Complex of the ARPE-19 Cell Line. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3644–3655. [Google Scholar] [CrossRef]
- Schnichels, S.; Paquet-Durand, F.; Löscher, M.; Tsai, T.; Hurst, J.; Joachim, S.C.; Klettner, A. Retina in a Dish: Cell Cultures, Retinal Explants and Animal Models for Common Diseases of the Retina. Prog. Retin. Eye Res. 2021, 81, 100880. [Google Scholar] [CrossRef]
- Alfonsetti, M.; Castelli, V.; d’Angelo, M.; Benedetti, E.; Allegretti, M.; Barboni, B.; Cimini, A. Looking for In Vitro Models for Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 10334. [Google Scholar] [CrossRef]
- Rastoin, O.; Pagès, G.; Dufies, M. Experimental Models in Neovascular Age Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4627. [Google Scholar] [CrossRef]
- McCarty, N.S.; Graham, A.E.; Studená, L.; Ledesma-Amaro, R. Multiplexed CRISPR Technologies for Gene Editing and Transcriptional Regulation. Nat. Commun. 2020, 11, 1281. [Google Scholar] [CrossRef]
- Grundman, M.; Morgan, R.; Lickliter, J.D.; Schneider, L.S.; DeKosky, S.; Izzo, N.J.; Guttendorf, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; et al. A Phase 1 Clinical Trial of the Sigma-2 Receptor Complex Allosteric Antagonist CT1812, a Novel Therapeutic Candidate for Alzheimer’s Disease. Alzheimers. Dement. 2019, 5, 20–26. [Google Scholar] [CrossRef]
- Davidson, M.; Saoud, J.; Staner, C.; Noel, N.; Luthringer, E.; Werner, S.; Reilly, J.; Schaffhauser, J.-Y.; Rabinowitz, J.; Weiser, M.; et al. Efficacy and Safety of MIN-101: A 12-Week Randomized, Double-Blind, Placebo-Controlled Trial of a New Drug in Development for the Treatment of Negative Symptoms in Schizophrenia. Am. J. Psychiatry 2017, 174, 1195–1202. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd Ed.). Meas.: Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Hung, S.S.C.; Van Bergen, N.J.; Jackson, S.; Liang, H.; Mackey, D.A.; Hernández, D.; Lim, S.Y.; Hewitt, A.W.; Trounce, I.; Pébay, A.; et al. Study of Mitochondrial Respiratory Defects on Reprogramming to Human Induced Pluripotent Stem Cells. Aging 2016, 8, 945–957. [Google Scholar] [CrossRef]
- Crombie, D.E.; Curl, C.L.; Raaijmakers, A.J.; Sivakumaran, P.; Kulkarni, T.; Wong, R.C.; Minami, I.; Evans-Galea, M.V.; Lim, S.Y.; Delbridge, L.; et al. Friedreich’s Ataxia Induced Pluripotent Stem Cell-Derived Cardiomyocytes Display Electrophysiological Abnormalities and Calcium Handling Deficiency. Aging 2017, 9, 1440–1452. [Google Scholar] [CrossRef]
- Hung, S.S.C.; Wong, R.C.B.; Sharov, A.A.; Nakatake, Y.; Yu, H.; Ko, M.S.H. Repression of Global Protein Synthesis by Eif1a-like Genes That Are Expressed Specifically in the Two-Cell Embryos and the Transient Zscan4-Positive State of Embryonic Stem Cells. DNA Res. 2013, 20, 391–402. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-H.; Urrutia-Cabrera, D.; Lees, J.G.; Mesa Mora, S.; Nguyen, T.; Hung, S.S.C.; Hewitt, A.W.; Lim, S.Y.; Edwards, T.L.; Wong, R.C.B. Development of a CRISPRi Human Retinal Pigmented Epithelium Model for Functional Study of Age-Related Macular Degeneration Genes. Int. J. Mol. Sci. 2023, 24, 3417. https://doi.org/10.3390/ijms24043417
Wang J-H, Urrutia-Cabrera D, Lees JG, Mesa Mora S, Nguyen T, Hung SSC, Hewitt AW, Lim SY, Edwards TL, Wong RCB. Development of a CRISPRi Human Retinal Pigmented Epithelium Model for Functional Study of Age-Related Macular Degeneration Genes. International Journal of Molecular Sciences. 2023; 24(4):3417. https://doi.org/10.3390/ijms24043417
Chicago/Turabian StyleWang, Jiang-Hui, Daniel Urrutia-Cabrera, Jarmon G. Lees, Santiago Mesa Mora, Tu Nguyen, Sandy S. C. Hung, Alex W. Hewitt, Shiang Y. Lim, Thomas L. Edwards, and Raymond C. B. Wong. 2023. "Development of a CRISPRi Human Retinal Pigmented Epithelium Model for Functional Study of Age-Related Macular Degeneration Genes" International Journal of Molecular Sciences 24, no. 4: 3417. https://doi.org/10.3390/ijms24043417
APA StyleWang, J.-H., Urrutia-Cabrera, D., Lees, J. G., Mesa Mora, S., Nguyen, T., Hung, S. S. C., Hewitt, A. W., Lim, S. Y., Edwards, T. L., & Wong, R. C. B. (2023). Development of a CRISPRi Human Retinal Pigmented Epithelium Model for Functional Study of Age-Related Macular Degeneration Genes. International Journal of Molecular Sciences, 24(4), 3417. https://doi.org/10.3390/ijms24043417






