P188 Therapy in In Vitro Models of Traumatic Brain Injury
Abstract
1. Introduction
1.1. In Vitro Models of Traumatic Brain Injury
1.2. Current Treatments for Traumatic Brain Injury
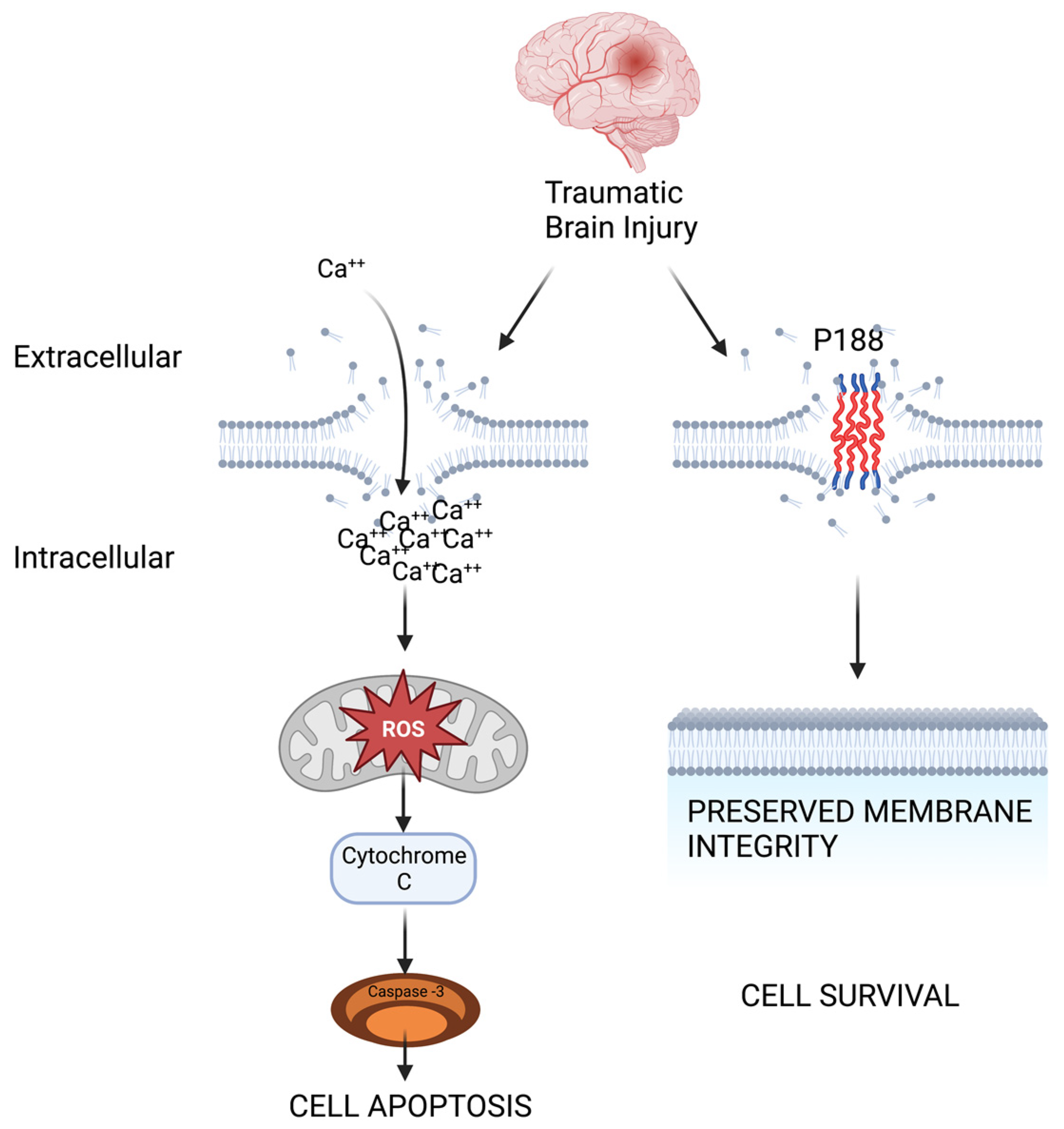
1.3. Therapeutic Potential of Poloxamer 188 as a Membrane Resealant
2. Astrocyte, Endothelial Cell, and Neuronal In Vitro Models of Traumatic Brain Injury Treated with P188
2.1. Astrocytes
2.2. Endothelial Cells
2.3. Neuronal Cells and Ischemia Reperfusion-Based Models
2.4. Neuronal Cells and Mechanical Injury Based Models
3. Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Get the Facts about TBI|Concussion|Traumatic Brain Injury|CDC Injury Center. Published 21 March 2022. Available online: https://www.cdc.gov/traumaticbraininjury/get_the_facts.html (accessed on 25 November 2022).
- Malpass, K. Read all about it! Why TBI is big news. Nat. Rev. Neurol. 2013, 9, 179. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Iverson, G.L. ACRM Mild TBI Definition Expert Consensus Group and the ACRM Brain Injury Special Interest Group Mild TBI Task Force. Expert Panel Survey to Update the American Congress of Rehabilitation Medicine Definition of Mild Traumatic Brain Injury. Arch. Phys. Med. Rehabil. 2021, 102, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.D.; Ayaz, S.I.; Lewis, L.M.; Unden, J.; Chen, J.Y.; Mika, V.H.; Saville, B.; Tyndall, J.A.; Nash, M.; Buki, A.; et al. Ability of Serum Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase-L1, and S100B To Differentiate Normal and Abnormal Head Computed Tomography Findings in Patients with Suspected Mild or Moderate Traumatic Brain Injury. J. Neurotrauma 2016, 33, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Crupi, R.; Calabrese, V.; Graziano, A.; Milone, P.; Pennisi, G.; Radak, Z.; Calabrese, E.J.; Cuzzocrea, S. Traumatic Brain Injury: Oxidative Stress and Neuroprotection. Antioxid. Redox Signal. 2013, 19, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Cordaro, M.; Cuzzocrea, S.; Impellizzeri, D. Management of Traumatic Brain Injury: From Present to Future. Antioxidants 2020, 9, 297. [Google Scholar] [CrossRef]
- Jha, R.M.; Kochanek, P.M.; Simard, J.M. Pathophysiology and Treatment of Cerebral Edema in Traumatic Brain Injury. Neuropharmacology 2019, 145 Pt B, 230–246. [Google Scholar] [CrossRef]
- Sandor, B.; Marin, M.; Lapoumeroulie, C.; Rabaï, M.; Lefevre, S.D.; Lemonne, N.; El Nemer, W.; Mozar, A.; Français, O.; Le Pioufle, B.; et al. Effects of Poloxamer 188 on red blood cell membrane properties in sickle cell anaemia. Br. J. Haematol. 2016, 173, 145–149. [Google Scholar] [CrossRef]
- Yasuda, S.; Townsend, D.; Michele, D.E.; Favre, E.G.; Day, S.M.; Metzger, J.M. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature 2005, 436, 1025–1029. [Google Scholar] [CrossRef]
- Salzman, M.M.; Bartos, J.A.; Yannopoulos, D.; Riess, M.L. Poloxamer 188 Protects Isolated Adult Mouse Cardiomyocytes from Reoxygenation Injury. Pharmacol. Res. Perspect. 2020, 8, e00639. [Google Scholar] [CrossRef]
- Salzman, M.; Cheng, Q.; Matsuura, T.; Yannopoulos, D.; Riess, M. Cardioprotection by Poloxamer 188 is Mediated by Nitric Oxide Synthase. FASEB J. 2015, 29 (Suppl. S1), 1026.5. [Google Scholar] [CrossRef]
- Eskaf, J.; Cleveland, W.J.; Riess, M.L. No Direct Postconditioning Effect of Poloxamer 188 on Mitochondrial Function after Ischemia Reperfusion Injury in Rat Isolated Hearts. Int. J. Mol. Sci. 2021, 22, 4879. [Google Scholar] [CrossRef] [PubMed]
- Bartos, J.; Matsuura, T.R.; Tsangaris, A.; Olson, M.; McKnite, S.H.; Rees, J.N.; Haman, K.; Shekar, K.C.; Riess, M.L.; Bates, F.S.; et al. Intracoronary Poloxamer 188 Prevents Reperfusion Injury in a Porcine Model of ST-Segment Elevation Myocardial Infarction. JACC Basic Transl. Sci. 2016, 1, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, J.; Chen, B.; Xiao, S.; Cho, M. Reparative Effects of Poloxamer P188 in Astrocytes Exposed to Controlled Microcavitation. Ann. Biomed. Eng. 2018, 46, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tjahja, J.; Malla, S.; Liebman, C.; Cho, M. Astrocyte Viability and Functionality in Spatially Confined Microcavitation Zone. ACS Appl. Mater. Interfaces 2019, 11, 4889–4899. [Google Scholar] [CrossRef] [PubMed]
- Lotze, F.P.; Riess, M.L. Poloxamer 188 Exerts Direct Protective Effects on Mouse Brain Microvascular Endothelial Cells in an In Vitro Traumatic Brain Injury Model. Biomedicines 2021, 9, 1043. [Google Scholar] [CrossRef]
- Inyang, E.; Abhyankar, V.; Chen, B.; Cho, M. Modulation of in vitro Brain Endothelium by Mechanical Trauma: Structural and Functional Restoration by Poloxamer 188. Sci. Rep. 2020, 10, 3054. [Google Scholar] [CrossRef]
- Meyer, L.J.; Riess, M.L. Evaluation of In Vitro Neuronal Protection by Postconditioning with Poloxamer 188 Following Simulated Traumatic Brain Injury. Life 2021, 11, 316. [Google Scholar] [CrossRef]
- Luo, C.; Li, Q.; Gao, Y.; Shen, X.; Ma, L.; Wu, Q.; Wang, Z.; Zhang, M.; Zhao, Z.; Chen, X.; et al. Poloxamer 188 Attenuates Cerebral Hypoxia/Ischemia Injury in Parallel with Preventing Mitochondrial Membrane Permeabilization and Autophagic Activation. J. Mol. Neurosci. 2015, 56, 988–998. [Google Scholar] [CrossRef]
- Gu, J.-H.; Ge, J.-B.; Li, M.; Xu, H.-D.; Wu, F.; Qin, Z.-H. Poloxamer 188 Protects Neurons against Ischemia/Reperfusion Injury through Preserving Integrity of Cell Membranes and Blood Brain Barrier. PLoS ONE 2013, 8, e61641. [Google Scholar] [CrossRef]
- Serbest, G.; Horwitz, J.; Barbee, K. The Effect of Poloxamer-188 on Neuronal Cell Recovery from Mechanical Injury. J. Neurotrauma 2005, 22, 119–132. [Google Scholar] [CrossRef]
- Luo, C.-L.; Chen, X.-P.; Li, L.-L.; Li, Q.-Q.; Li, B.-X.; Xue, A.-M.; Xu, H.-F.; Dai, D.-K.; Shen, Y.-W.; Tao, L.-Y.; et al. Poloxamer 188 attenuates in vitro traumatic brain injury-induced mitochondrial and lysosomal membrane permeabilization damage in cultured primary neurons. J. Neurotrauma 2013, 30, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, D.; Gallo, G.; Barbee, K.A. Mechanically-induced membrane poration causes axonal beading and localized cytoskeletal damage. Exp. Neurol. 2008, 212, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, T.; Eylen, A.; Lule, S.; Erdener, S.E.; Vural, A.; Karatas, H.; Ozveren, M.F.; Dalkara, T.; Gursoy-Ozdemir, Y. Poloxamer-188 and citicoline provide neuronal membrane integrity and protect membrane stability in cortical spreading depression. Int. J. Neurosci. 2015, 125, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Pille, J.A.; Riess, M.L. Potential Effects of Poloxamer 188 on Rat Isolated Brain Mitochondria after Oxidative Stress In Vivo and In Vitro. Brain Sci. 2021, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.D.; Pan, C.; Bushell, T.; Cromie, W.; Lee, R.C. Amphiphilic, tri-block copolymers provide potent, membrane-targeted neuroprotection. FASEB J. 2001, 15, 1107–1109. [Google Scholar] [CrossRef]
- Bao, H.; Yang, X.; Zhuang, Y.; Huang, Y.; Wang, T.; Zhang, M.; Dai, D.; Wang, S.; Xiao, H.; Huang, G.; et al. The effects of poloxamer 188 on the autophagy induced by traumatic brain injury. Neurosci. Lett. 2016, 634, 7–12. [Google Scholar] [CrossRef]
- Morrison, B.; Elkin, B.S.; Dollé, J.P.; Yarmush, M.L. In Vitro Models of Traumatic Brain Injury. Annu. Rev. Biomed. Eng. 2011, 13, 91–126. [Google Scholar] [CrossRef]
- Kumaria, A. In vitro models as a platform to investigate traumatic brain injury. Altern. Lab. Anim. 2017, 45, 201–211. [Google Scholar] [CrossRef]
- Omelchenko, A.; Singh, N.K.; Firestein, B.L. Current advances in in vitro models of central nervous system trauma. Curr. Opin. Biomed. Eng. 2020, 14, 34–41. [Google Scholar] [CrossRef]
- Patterson, L.H.C.; Walker, J.L.; Rodriguez-Mesa, E.; Shields, K.; Foster, J.S.; Valentine, M.T.; Doyle, A.M.; Foster, K.L. Investigating Cellular Response to Impact With a Microfluidic MEMS Device. J. Microelectromech. Syst. 2020, 29, 14–24. [Google Scholar] [CrossRef]
- Cernak, I. Understanding blast-induced neurotrauma: How far have we come? Concussion 2017, 2, CNC42. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.E.; Pfister, B.J. Advancements in in vitro models of traumatic brain injury. Curr. Opin. Biomed. Eng. 2022, 25, 100430. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.; Bauer, B. Matrix metalloproteinases in the brain and blood–brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhang, Y.; Jiang, W.; He, L.; Qu, H. Modulation of autophagy in traumatic brain injury. J. Cell. Physiol. 2020, 235, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.M.; Long, M.; Conte, A.A.; Santhakumar, V.; Pfister, B.J. High Ca2+ Influx During Traumatic Brain Injury Leads to Caspase-1-Dependent Neuroinflammation and Cell Death. Mol. Neurobiol. 2017, 54, 3964–3975. [Google Scholar] [CrossRef] [PubMed]
- Lerouet, D.; Marchand-Leroux, C.; Besson, V.C. Neuropharmacology in traumatic brain injury: From preclinical to clinical neuroprotection? Fundam. Clin. Pharmacol. 2021, 35, 524–538. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Rosset, S.; Lee, T.-R.; Dragunow, M.; Park, T.; Shim, V. In Vitro Models of Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2021, 38, 2336–2372. [Google Scholar] [CrossRef]
- Liu, M.; Wang, A.J.; Chen, Y.; Zhao, G.; Jiang, Z.; Wang, X.; Shi, D.; Zhang, T.; Sun, B.; He, H.; et al. Efficacy and safety of erythropoietin for traumatic brain injury. BMC Neurol. 2020, 20, 399. [Google Scholar] [CrossRef]
- Sultan, W.; Sapkota, A.; Khurshid, H.; Qureshi, I.A.; Jahan, N.; Went, T.R.; Dominic, J.L.; Win, M.; Kannan, A.; Tara, A.; et al. Statins’ Effect on Cognitive Outcome After Traumatic Brain Injury: A Systematic Review. Cureus 2021, 13, e16953. [Google Scholar] [CrossRef]
- Stein, D.G.; Howard, R.B.; Sayeed, I. Chapter 1—Why Did the Phase III Clinical Trials for Progesterone in TBI Fail? An Analysis of Three Potentially Critical Factors. In New Therapeutics for Traumatic Brain Injury; Heidenreich, K.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–18. [Google Scholar] [CrossRef]
- Ghiam, M.K.; Patel, S.D.; Hoffer, A.; Selman, W.R.; Hoffer, B.J.; Hoffer, M.E. Drug Repurposing in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2021, 15, 635483. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2021.635483 (accessed on 25 November 2022).
- Tobinick, E.; Rodriguez-Romanacce, H.; Kinssies, R.; Kim, N.M. Chapter 7—Perispinal Etanercept for Traumatic Brain Injury|Elsevier Enhanced Reader. In New Therapeutics for Traumatic Brain Injury; Academic Press/Elsevier: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Besson, V.C.; Chen, X.R.; Plotkine, M.; Marchand-Verrecchia, C. Fenofibrate, a peroxisome proliferator-activated receptor α agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci. Lett. 2005, 388, 7–12. [Google Scholar] [CrossRef]
- Lu, Q.; Xiong, J.; Yuan, Y.; Ruan, Z.; Zhang, Y.; Chai, B.; Li, L.; Cai, S.; Xiao, J.; Wu, Y.; et al. Minocycline improves the functional recovery after traumatic brain injury via inhibition of aquaporin-4. Int. J. Biol. Sci. 2022, 18, 441–458. [Google Scholar] [CrossRef]
- Chen, W.N.; Shaikh, M.F.; Bhuvanendran, S.; Date, A.; Ansari, M.T.; Radhakrishnan, A.K.; Othman, I. Poloxamer 188 (P188), A Potential Polymeric Protective Agent for Central Nervous System Disorders: A Systematic Review. Curr. Neuropharmacol. 2022, 20, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, S.A.; Hannig, J.; Lee, R.C.; Lee, K.Y.C. Direct Observation of Poloxamer 188 Insertion into Lipid Monolayers. Biophys. J. 2002, 82, 1453–1459. [Google Scholar] [CrossRef]
- Curry, D.J.; Wright, D.A.; Lee, R.C.; Kang, U.J.; Frim, D.M. Poloxamer 188 Volumetrically Decreases Neuronal Loss in the Rat in a Time-dependent Manner. Neurosurgery 2004, 55, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Moloughney, J.G.; Weisleder, N. Poloxamer 188 (P188) as a Membrane Resealing Reagent in Biomedical Applications. Recent Pat. Biotechnol. 2012, 6, 200–211. [Google Scholar]
- Houang, E.M.; Sham, Y.Y.; Bates, F.S.; Metzger, J.M. Muscle membrane integrity in Duchenne muscular dystrophy: Recent advances in copolymer-based muscle membrane stabilizers. Skelet. Muscle 2018, 8, 31. [Google Scholar] [CrossRef]
- Spurney, C.F.; Guerron, A.D.; Yu, Q.; Sali, A.; Van Der Meulen, J.H.; Hoffman, E.P.; Nagaraju, K. Membrane Sealant Poloxamer P188 Protects Against Isoproterenol Induced Cardiomyopathy in Dystrophin Deficient Mice. BMC Cardiovasc. Disord. 2011, 11, 20. [Google Scholar] [CrossRef]
- Bartos, J.; Matsuura, T.R.; Sarraf, M.; Youngquist, S.T.; McKnite, S.H.; Rees, J.N.; Sloper, D.T.; Bates, F.; Segal, N.; Debaty, G.; et al. Bundled Postconditioning Therapies Improve Hemodynamics and Neurologic Recovery after 17 Minutes of Untreated Cardiac Arrest. Resuscitation 2015, 87, 7–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Emanuele, M.; Zhang, L.; Zhang, Z.G.; Lu, M.; Zhang, T.; Mahmood, A.; Xiong, Y. Treatment of Traumatic Brain Injury with Vepoloxamer (Purified Poloxamer 188). J. Neurotrauma 2018, 35, 661–670. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Bernstein, A.M.; Sofroniew, M.V. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016, 275, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.J.; Lotze, F.P.; Riess, M.L. Simulated Traumatic Brain Injury in in-vitro Mouse Neuronal and Brain Endothelial Cell Culture Models. J. Pharmacol. Toxicol. Methods 2022. [Google Scholar] [CrossRef]
- Cadichon, S.B.; Le, H.M.; Wright, D.A.; Curry, D.J.; Kang, U.; Frim, D.M. Neuroprotective effect of the surfactant poloxamer 188 in a model of intracranial hemorrhage in rats. J. Neurosurg. Pediatr. 2007, 106, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Serbest, G.; Horwitz, J.; Jost, M.; Barbee, K.A. Mechanisms of cell death and neuroprotection by poloxamer 188 after mechanical trauma. FASEB J. 2006, 20, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Kilinc, D.; Gallo, G.; Barbee, K.A. Mechanical membrane injury induces axonal beading through localized activation of calpain. Exp. Neurol. 2009, 219, 553–561. [Google Scholar] [CrossRef]
- Wang, J.C.; Bindokas, V.P.; Skinner, M.; Emrick, T.; Marks, J.D. Mitochondrial mechanisms of neuronal rescue by F-68, a hydrophilic Pluronic block co-polymer, following acute substrate deprivation. Neurochem. Int. 2017, 109, 126–140. [Google Scholar] [CrossRef]
- Wolf, M.S.; Bayır, H.; Kochanek, P.M.; Clark, R.S.B. The role of autophagy in acute brain injury: A state of flux? Neurobiol. Dis. 2019, 122, 9–15. [Google Scholar] [CrossRef]
- Li, Z.; Gupta, M.K.; Barajas, M.B.; Duvall, C.L.; Riess, M.L. Abstract 115: Comparison Of Di-block Copolymer-based Cell Membrane Stabilizers To The Tri-block Poloxamer 188 In Protecting Coronary Artery Endothelial Cells Against Hypoxia-Reoxygenation Injury. Circulation 2022, 146 (Suppl. S1), A115. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, G.; Ye, Y.; Kang, E.; Chen, H.; Guo, Z.; He, X. Niche Cells Crosstalk In Neuroinflammation After Traumatic Brain Injury. Int. J. Biol. Sci. 2021, 17, 368–378. [Google Scholar] [CrossRef]
- Li, Z.; Hampton, M.J.W.; Barajas, M.B.; Riess, M.L. Development of a Cell Co-Culture Model to Mimic Cardiac Ischemia/Reperfusion In Vitro. J. Vis. Exp. Jove 2021, 176, e62913. [Google Scholar] [CrossRef]
- Huang, L.; Nakamura, Y.; Lo, E.H.; Hayakawa, K. Astrocyte Signaling in the Neurovascular Unit After Central Nervous System Injury. Int. J. Mol. Sci. 2019, 20, 282. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Vaibhav, K.; Saad, N.M.; Fatima, S.; Vender, J.R.; Baban, B.; Hoda, N.; Dhandapani, K.M. White matter damage after traumatic brain injury: A role for damage associated molecular patterns. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863 Pt B, 2614–2626. [Google Scholar] [CrossRef] [PubMed]
| Author | Disease Model | Cell Type | P188 Protective Mechanism |
|---|---|---|---|
| Non-TBI models treated with P188 | |||
| Sandor et al. [8] | Vaso-occlusive crisis due to sickle cell anemia | Normoxic and hypoxic RBCs | Blood viscosity, RBC aggregation, endothelial cell adhesion |
| Yasuda et al. [9] | Duchenne Muscular Dystrophy | Dystrophic cardiomyocytes | stretch-mediated calcium overload, cardiac passive tension |
| Salzman et al. [10] | Ischemia-reperfusion injury | Adult mouse cardiomyocytes | cell membrane repair, calcium influx |
| Salzman et al. [11] | Ischemia-reperfusion injury | Rat cardiomyocytes | nitric oxide synthase, infarct size |
| Eskaf et al. [12] | Ischemia-reperfusion injury | Rat cardiomyocytes | mitochondrial function (ATP synthesis) |
| Bartos et al. [13] | ST-elevation myocardial infarction | Pig cardiomyocytes | mitochondrial viability, oxidation, infarct size, troponin leak |
| TBI models treated with P188: Astrocytes | |||
| Kanagaraj et al. [14] | Blast-induced TBI | Mouse C8-D1A Astrocytes | cell viability, calcium handling, ROS production |
| Chen et al. [15] | Blast-induced TBI | Mouse C8-D1A Astrocytes | reseal N-type calcium channels, preserve calcium spiking |
| TBI models treated with P188: Endothelial Cells | |||
| Lotze et al. [16] | Ischemia-reperfusion & compression TBI | Mouse brain microvascular endothelial cells | cell viability, metabolic activity, nitric oxide, membrane damage |
| Inyang et al. [17] | Blast-induced TBI | Mouse brain microvascular endothelial cells | MMP-2 & 9, ZO-1, restore tight junctions |
| TBI models treated with P188: Neurons (Ischemia-reperfusion models) | |||
| Meyer et al. [18] | Ischemia-reperfusion & compression TBI | Mouse primary cortical neurons | cell viability, mitochondrial viability, membrane damage, caspase-3 activity |
| Luo et al. [19] | Ischemic injury and glucose deprivation | Mouse primary cortical neurons | mitochondrial cytochrome c, caspase-3, LC3-II, Beclin-1 |
| Gu et al. [20] | Ischemic injury and glucose deprivation Middle cerebral artery occlusion | Mouse hippocampal HT22 neurons Mouse whole brain | membrane resealing MMP-9 |
| TBI models treated with P188: Neurons (Mechanical Injury based models) | |||
| Serbest et al. [21] | Cell-shearing device model | PC2 derived neuronal cells | cell survival, p38 MAPK |
| Luo et al. [22] | Cell-shearing device model | Cultured primary neurons | mitochondrial cytochrome c release, lysosomal cathepsin B release |
| Kilinc et al. [23] | Fluid-shear stress model | Embryonic chick forebrain neurons | intracellular calcium, calpain activity, apoptosis |
| Yildirim et al. [24] | Cortical Spreading Depression | Mouse brain cortex and hippocampal dentate gyrus neurons | megachannel opening |
| Pille et al. [25] | In vivo asphyxia cardiac arrest and in vitro hydrogen peroxide exposure | Rat forebrain neurons | mitochondrial viability |
| Marks et al. [26] | Excitotoxic and oxidative injury | Rat hippocampal and cerebellar neurons | lipid peroxidation, intracellular content loss |
| Bao et al. [27] | Scratch TBI Left-hemispheric drop-weight TBI | Rat PC-12 cells Mouse CD1 cortex and hippocampus | wound healing rate Beclin-1/Bcl-2, LC3II/LC31 ratios, p62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zargari, M.; Meyer, L.J.; Riess, M.L.; Li, Z.; Barajas, M.B. P188 Therapy in In Vitro Models of Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 3334. https://doi.org/10.3390/ijms24043334
Zargari M, Meyer LJ, Riess ML, Li Z, Barajas MB. P188 Therapy in In Vitro Models of Traumatic Brain Injury. International Journal of Molecular Sciences. 2023; 24(4):3334. https://doi.org/10.3390/ijms24043334
Chicago/Turabian StyleZargari, Michael, Luise J. Meyer, Matthias L. Riess, Zhu Li, and Matthew B. Barajas. 2023. "P188 Therapy in In Vitro Models of Traumatic Brain Injury" International Journal of Molecular Sciences 24, no. 4: 3334. https://doi.org/10.3390/ijms24043334
APA StyleZargari, M., Meyer, L. J., Riess, M. L., Li, Z., & Barajas, M. B. (2023). P188 Therapy in In Vitro Models of Traumatic Brain Injury. International Journal of Molecular Sciences, 24(4), 3334. https://doi.org/10.3390/ijms24043334







