A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area
Abstract
1. Introduction
2. Results
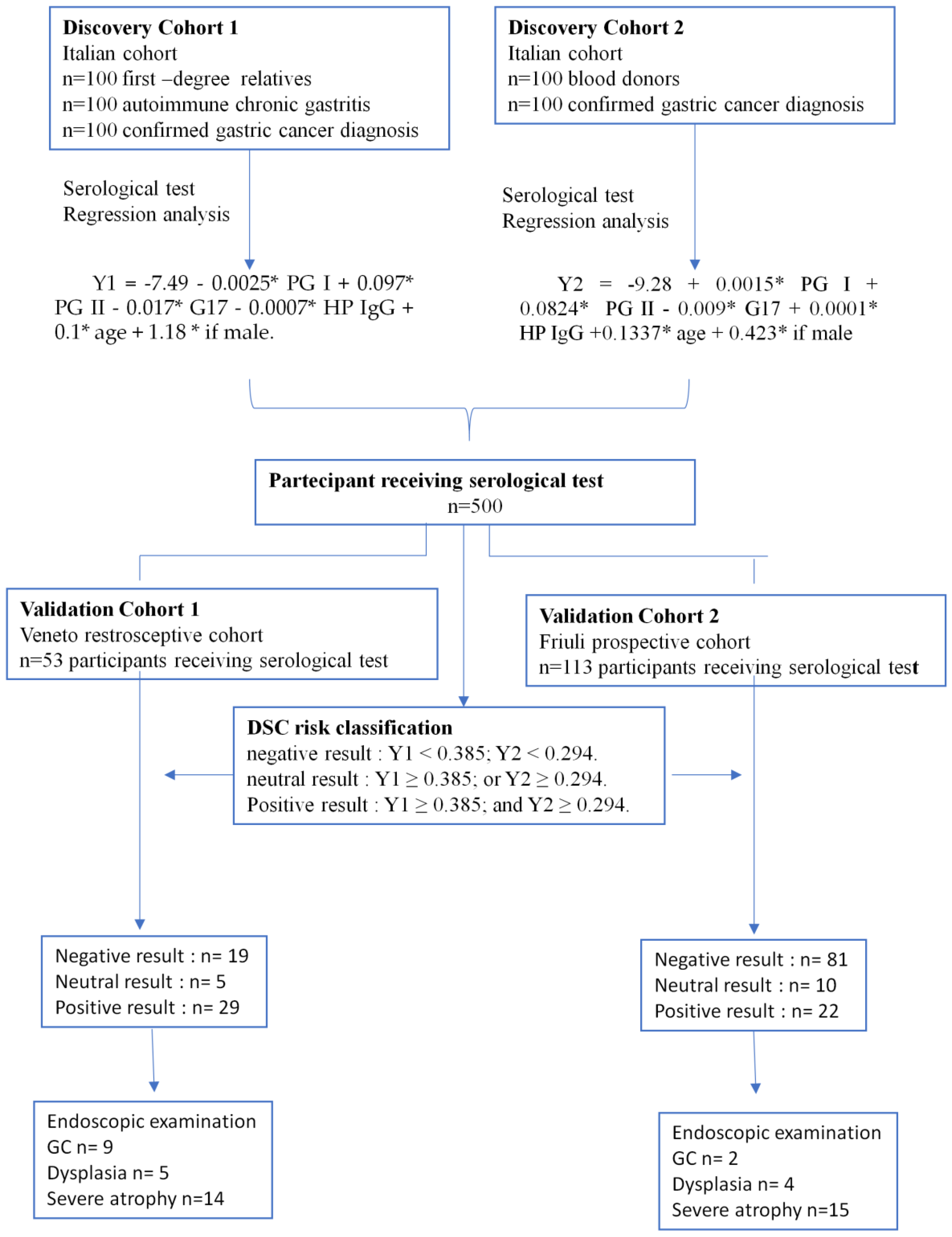
2.1. Study Design
2.2. DSC Classifications for GC Risk
2.3. Gastroscopy and Histopathological Diagnosis
2.4. DSC Classification Accuracy
2.5. Reproducibility of the DSC Method
2.6. Comparison of the Overall Validation Process (n = 166 Cases) Using the DSC Test and the Standardized Pepsinogen Test
3. Discussion
4. Materials and Methods
4.1. Study Cohorts
4.2. Construction of the DSC Model
4.3. DSC Model Assessment in the Validation Cohorts
4.4. Serological Data
4.5. Diagnosis
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vradelis, S.; Maynard, N.; Warren, B.F.; Keshav, S.; Travis, S.P.L. Quality Control in Upper Gastrointestinal Endoscopy: Detection Rates of Gastric Cancer in Oxford 2005–2008. Postgrad. Med. J. 2011, 87, 335–339. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Bosetti, C.; Lucchini, F.; Bertuccio, P.; Negri, E.; Boyle, P.; Levi, F. Cancer Mortality in Europe, 2000–2004, and an Overview of Trends since 1975. Ann. Oncol. 2010, 21, 1323–1360. [Google Scholar] [CrossRef]
- Jun, J.K.; Choi, K.S.; Lee, H.-Y.; Suh, M.; Park, B.; Song, S.H.; Jung, K.W.; Lee, C.W.; Choi, I.J.; Park, E.-C.; et al. Effectiveness of the Korean National Cancer Screening Program in Reducing Gastric Cancer Mortality. Gastroenterology 2017, 152, 1319–1328.e7. [Google Scholar] [CrossRef] [PubMed]
- Dolcetti, R.; De Re, V.; Canzonieri, V. Immunotherapy for Gastric Cancer: Time for a Personalized Approach? Int. J. Mol. Sci. 2018, 19, 1602. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yang, D.-H.; Kim, J.W.; Kim, J.-H.; Kim, J.H.; Min, Y.W.; Lee, S.H.; Bae, J.H.; Chung, H.; Choi, K.D.; et al. Clinical Practice Guideline for Endoscopic Resection of Early Gastrointestinal Cancer. Intest. Res. 2021, 19, 127–157. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The Global, Regional, and National Burden of Stomach Cancer in 195 Countries, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Registro Tumori Friuli Venezia Giulia 2018. Available online: http://Www.Cro.Sanita.FVG (accessed on 1 April 2020).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Areia, M.; Spaander, M.C.; Kuipers, E.J.; Dinis-Ribeiro, M. Endoscopic Screening for Gastric Cancer: A Cost-Utility Analysis for Countries with an Intermediate Gastric Cancer Risk. United Eur. Gastroenterol. J. 2018, 6, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Bersani, G.; Buri, L.; Zullo, A.; Anti, M.; Bianco, M.A.; Di Giulio, E.; Ficano, L.; Morini, S.; Di Matteo, G.; et al. Appropriateness of Upper-GI Endoscopy: An Italian Survey on Behalf of the Italian Society of Digestive Endoscopy. Gastrointest. Endosc. 2007, 65, 767–774. [Google Scholar] [CrossRef]
- Buri, L.; Bersani, G.; Hassan, C.; Anti, M.; Bianco, M.A.; Cipolletta, L.; Di Giulio, E.; Di Matteo, G.; Familiari, L.; Ficano, L.; et al. How to Predict a High Rate of Inappropriateness for Upper Endoscopy in an Endoscopic Centre? Dig. Liver Dis. 2010, 42, 624–628. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Zhang, Y.; Yin, X.; Man, J.; Yang, X.; Lu, M. Global Changing Trends in Incidence and Mortality of Gastric Cancer by Age and Sex, 1990-2019: Findings from Global Burden of Disease Study. J. Cancer 2021, 12, 6695–6705. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M.; Rugge, M. Assessing Risks for Gastric Cancer: New Tools for Pathologists. World J. Gastroenterol. 2006, 12, 5622–5627. [Google Scholar] [CrossRef]
- Rugge, M.; de Boni, M.; Pennelli, G.; de Bona, M.; Giacomelli, L.; Fassan, M.; Basso, D.; Plebani, M.; Graham, D.Y. Gastritis OLGA-Staging and Gastric Cancer Risk: A Twelve-Year Clinico-Pathological Follow-up Study. Aliment. Pharmacol. Ther. 2010, 31, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Terasawa, T.; Nishida, H.; Kato, K.; Miyashiro, I.; Yoshikawa, T.; Takaku, R.; Hamashima, C. Prediction of Gastric Cancer Development by Serum Pepsinogen Test and Helicobacter Pylori Seropositivity in Eastern Asians: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e109783. [Google Scholar] [CrossRef] [PubMed]
- Yanaoka, K.; Oka, M.; Mukoubayashi, C.; Yoshimura, N.; Enomoto, S.; Iguchi, M.; Magari, H.; Utsunomiya, H.; Tamai, H.; Arii, K.; et al. Cancer High-Risk Subjects Identified by Serum Pepsinogen Tests: Outcomes after 10-Year Follow-up in Asymptomatic Middle-Aged Males. Cancer Epidemiol. Biomark. Prev. 2008, 17, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C. Forthcoming Step in Gastric Cancer Prevention: How Can Risk Stratification Be Combined with Endoscopic Screening for Gastric Cancer? Gut Liver 2022, 16, 811–824. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter Pylori Infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Samloff, I.M. Pepsinogens, Pepsins, and Pepsin Inhibitors. Gastroenterology 1971, 60, 586–604. [Google Scholar] [CrossRef]
- Copps, J.; Murphy, R.F.; Lovas, S. The Production and Role of Gastrin-17 and Gastrin-17-Gly in Gastrointestinal Cancers. Protein Pept. Lett. 2009, 16, 1504–1518. [Google Scholar] [CrossRef]
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global Burden of Cancers Attributable to Infections in 2008: A Review and Synthetic Analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, C.; Yuan, Y.; Feng, Q.; Feng, Y.; Hao, Y.; Li, J.; Zhang, K.; Ye, G.; Ye, L.; et al. Development and Validation of a Prediction Rule for Estimating Gastric Cancer Risk in the Chinese High-Risk Population: A Nationwide Multicentre Study. Gut 2019, 68, 1576–1587. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; Yuan, Y. Analysis of Serum Gastrin-17 and Helicobacter Pylori Antibody in Healthy Chinese Population. J. Clin. Lab. Anal. 2020, 34, e23518. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Orzes, E.; Canzonieri, V.; Maiero, S.; Fornasarig, M.; Alessandrini, L.; Cervo, S.; Steffan, A.; Zanette, G.; Mazzon, C.; et al. Pepsinogens to Distinguish Patients With Gastric Intestinal Metaplasia and Helicobacter Pylori Infection Among Populations at Risk for Gastric Cancer. Clin. Transl. Gastroenterol. 2016, 7, e183. [Google Scholar] [CrossRef]
- Miceli, E.; Vanoli, A.; Lenti, M.V.; Klersy, C.; Di Stefano, M.; Luinetti, O.; Dominioni, C.C.; Pisati, M.; Staiani, M.; Gentile, A.; et al. Natural History of Autoimmune Atrophic Gastritis: A Prospective, Single Centre, Long-Term Experience. Aliment. Pharmacol. Ther. 2019, 50, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, S.; Manterola, C. Morphology and Diagnostic Determinants of Gastric Precancerous Conditions. Int. J. Morphol. 2019, 37, 917–927. [Google Scholar] [CrossRef]
- Rugge, M.; Savarino, E.; Sbaraglia, M.; Bricca, L.; Malfertheiner, P. Gastritis: The Clinico-Pathological Spectrum. Dig. Liver Dis. 2021, 53, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Koc, D.O.; Bektas, S. Serum Pepsinogen Levels and OLGA/OLGIM Staging in the Assessment of Atrophic Gastritis Types. Postgrad. Med. J. 2022, 98, 441–445. [Google Scholar] [CrossRef]
- Rugge, M.; Meggio, A.; Pennelli, G.; Piscioli, F.; Giacomelli, L.; De Pretis, G.; Graham, D.Y. Gastritis Staging in Clinical Practice: The OLGA Staging System. Gut 2007, 56, 631–636. [Google Scholar] [CrossRef]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric Cancer: Classification, Histology and Application of Molecular Pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.T.; Kuipers, E.J. The Staging of Gastritis with the OLGA System by Using Intestinal Metaplasia as an Accurate Alternative for Atrophic Gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M. Staging and Grading of Chronic Gastritis. Hum. Pathol. 2005, 36, 228–233. [Google Scholar] [CrossRef]
- Yue, H.; Shan, L.; Bin, L. The Significance of OLGA and OLGIM Staging Systems in the Risk Assessment of Gastric Cancer: A Systematic Review and Meta-Analysis. Gastric. Cancer 2018, 21, 579–587. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of Precancerous Conditions and Lesions in the Stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, Y.; Pan, J.; Zhou, L.; Lin, J. Application of a Novel Scoring System for Gastric Cancer Opportunistic Screening in Hospital Visits. BMC Gastroenterol. 2022, 22, 223. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, A.; Brady, D.A.; Schaal, S.E.; Samloff, I.M.; Dedon, J.; Ruhl, C.E. Gastric Acidity in Older Adults. JAMA 1997, 278, 659–662. [Google Scholar] [CrossRef]
- Wright, J.D.; Bialostosky, K.; Gunter, E.W.; Carroll, M.D.; Najjar, M.F.; Bowman, B.A.; Johnson, C.L. Blood Folate and Vitamin B12: United States, 1988-94. In Vital and Health Statistics. Series 11, Data from the National Health Survey; National Center for Health Statistics: Washington, DC, USA, 1998; pp. 1–78. [Google Scholar]
- Bates, C.J.; Schneede, J.; Mishra, G.; Prentice, A.; Mansoor, M.A. Relationship between Methylmalonic Acid, Homocysteine, Vitamin B12 Intake and Status and Socio-Economic Indices, in a Subset of Participants in the British National Diet and Nutrition Survey of People Aged 65 y and Over. Eur. J. Clin. Nutr. 2003, 57, 349–357. [Google Scholar] [CrossRef]
- Lin, Z.; Bian, H.; Chen, C.; Chen, W.; Li, Q. Application of Serum Pepsinogen and Carbohydrate Antigen 72-4 (CA72-4) Combined with Gastrin-17 (G-17) Detection in the Screening, Diagnosis, and Evaluation of Early Gastric Cancer. J. Gastrointest. Oncol. 2021, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mo, T.-M.; Tian, L.; Chen, J.-Q. Gastrin-17 Combined with CEA, CA12-5 and CA19-9 Improves the Sensitivity for the Diagnosis of Gastric Cancer. Int. J. Gen. Med. 2021, 14, 8087–8095. [Google Scholar] [CrossRef]
- Corona, G.; Cannizzaro, R.; Miolo, G.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Steffan, A.; De Re, V. Use of Metabolomics as a Complementary Omic Approach to Implement Risk Criteria for First-Degree Relatives of Gastric Cancer Patients. Int. J. Mol. Sci. 2018, 19, 750. [Google Scholar] [CrossRef]
- Jelski, W.; Chrostek, L.; Laszewicz, W.; Szmitkowski, M. Alcohol Dehydrogenase (ADH) Isoenzyme Activity in the Sera of Patients with Helicobacter Pylori Infection. Dig. Dis. Sci. 2007, 52, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, N.; Osmola, M.; Martin, J.; Blin, J.; Leroy, M.; Jirka, I.; Moussata, D.; Lamarque, D.; Olivier, R.; Tougeron, D.; et al. Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics 2022, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, J.; Hu, R.; Yu, X.; Chen, X.; Zheng, S.; Qin, Y.; Zhou, X.; Wang, Y.; Zheng, L.; et al. Clinical Value of Combined Detection of Serum STim-3 and Pepsinogen for Gastric Cancer Diagnosis. Cancer Manag. Res. 2021, 13, 7759–7769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, M.; Zhang, Y.; Xiao, S.; Lai, X.; Tan, A.; Du, S.; Li, S. Dissecting the Single-Cell Transcriptome Network Underlying Gastric Premalignant Lesions and Early Gastric Cancer. Cell Rep. 2019, 27, 1934–1947.e5. [Google Scholar] [CrossRef]

| Discovery Cohort 1 | Discovery Cohort 2 | Validation Cohort 1 | Validation Cohort 2 n = 113 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQ: 25–75%) | Median (IQ: 25–75%) | Median (IQ: 25–75%) | Median (IQ: 25–75%) | Median (IQ: 25–75%) | Median (IQ: 25–75%) | Median (IQ: 25–75%) | Median (IQ: 25–75%) | ||
| Biomarkers | Sex | Age ≤ 65 | Age > 65 | Age ≤ 65 | Age > 65 | Age ≤ 65 | Age > 65 | Age ≤ 65 | Age > 65 |
| G17 | F | 13.9 (3.5–50.3) | 19.5 (3.4–61.8) | 4.0 (1.9–16.3) | 17.6 (2.7–27.9) | 7.3 (3.9–58.6) | 5.3 (3.4–12.4) | 5.1 (2.2–52.2) | 11.1 (2.7–53.1) |
| M | 9.5 (3.3–32.9) | 15.2 (5.9–38.2) | 2.9 (0.5–8.4) | 17.1 (7.1–38.3) | 8.0 (4.8–36.1) | 16.3 (7.9–32.7) | 5.0 (2.7–13.9) | 31.8 (10.5–119.0) | |
| PGI | F | 72 (25.8–119.3) | 87.5 (23.7–141.4) | 63.6 (49.4–173.0) | 127.7 (87.5–210.3) | 78.6 (74.5–139.5) | 102.9 (56.0–251.2) | 60.3 (39.5–105.0) | 78.7 (30.8–144.4) |
| M | 103.2 (70.4–182.9) | 76.4 (38.0–150.8) | 82.3 (50.5–131.2) | 83.7 (38.0–212.8) | 119.5 (101.4–198.1) | 137.2 (53.2–304.5) | 69.7 (35.0–108.7) | 73.5 (16.9–263.7) | |
| PGII | F | 10.1 (7.1–16.9) | 14.0 (8.0–18.1) | 9.0 (4.8–19.8) | 19.3 (16.0–27.1) | 9.9 (7.1–11.2) | 13.0 (9.2–20.5) | 10.9 (6.2–13.7) | 12.7 (10.0–14.8) |
| M | 13.9 (8.7–21.7) | 12.6 (7.5–16.8) | 9.0 (6.2–17.0) | 13.0 (9.2–20.4) | 11.5 (9.6–14.5) | 12.9 (9.7–19.7) | 9.6 (7.8–12.2) | 11.7 (7.9–19.6) | |
| PGI/PGII | F | 6.7 (2.3–11.6) | 6.6 (3.0–9.9) | 8.3 (6.4–10.7) | 6.0 (4.9–7.8) | 10.1 (7.9–11.9) | 9.2 (7.1–10.5) | 7.2 (4.3–9.9) | 6.8 (3.0–8.4) |
| M | 8.43 (5.5–12.2) | 6.3 (3.0–12.0) | 9.2 (6.3–11.6) | 6.3 (3.0–12.0) | 10.7 (10.3–13.4) | 9.1 (7.4–12.5) | 7.6 (3.4–8.7) | 6.7 (1.8–11.1) | |
| H. pylori IgG | F | 14.4 (5.8–72.9) | 38.1 (11.5–60.8) | 27.0 (4.4–76.4) | 47.0 (16.8–71.9) | 5.4 (3.6–21.6) | 14.5 (3.4–41.2) | 5.9 (2.6–16.8) | 10.8 (3.7–32.1) |
| M | 20.6 (6.0–85.6) | 61.3 (11.5–111.2) | 15.0 (3.85–80.38) | 79.4 (21.7–114.1) | 3.8 (2.6–12.1) | 3.1 (2.6–10.6) | 6.4 (3.7–9.2) | 15.6 (6.3–35.4) | |
| DSC | Cohorts | |||||
|---|---|---|---|---|---|---|
| GC Risk Classification | Validation 1 | Validation 2 | Overall Validation Process | |||
| n. | % | n. | % | n. | % | |
| Negative | 19 | 35.8 | 81 | 71.7 | 100 | 60.2 |
| Neutral | 5 | 9.4 | 10 | 8.8 | 15 | 9.0 |
| Positive | 29 | 54.7 | 22 | 19.5 | 51 | 30.7 |
| Total | 53 | 113 | 166 | |||
| Diagnosis | Cohorts | |||
|---|---|---|---|---|
| Validation Cohort 1 N = 53 | Validation Cohort 2 N = 113 | |||
| n. Patients | % | n. Patients | % | |
| Atrophy (OLGA stages 0–II) | 25 | 47.2 | 92 | 81.4 |
| Severe atrophy (OLGA stages III–IV) | 14 | 26.4 | 15 | 13.3 |
| Dysplasia/preneoplastic lesion | 5 | 9.4 | 4 | 3.5 |
| Gastric cancer | 9 | 17.0 | 2 | 1.8 |
| Validation 1 53 Selected Retrospective Cases | Validation 2 113 Consecutive Prospective Cases | Overall Validation Process 166 Cases | ||||
|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Sensitivity | 71.429% | 41.896% to 91.611% | 66.667% | 22.278% to 95.673% | 70.000% | 45.721% to 88.107% |
| Specificity | 51.282% | 34.780% to 67.582% | 83.178% | 74.723% to 89.714% | 74.658% | 66.800% to 81.486% |
| AUC | 0.614 | 0.470 to 0.744 | 0.749 | 0.659 to 0.826 | 0.723 | 0.649 to 0.790 |
| Positive LR | 1.466 | 0.924 to 2.327 | 3.963 | 1.957 to 8.024 | 2.762 | 1.852 to 4.120 |
| Negative LR | 0.557 | 0.230 to 1.347 | 0.401 | 0.129 to 1.247 | 0.402 | 0.204 to 0.790 |
| Disease prevalence | 0.010% | |||||
| Positive PV | 0.028% | 0.019% to 0.041% | ||||
| Negative PV | 99.996% | 99.992% to 99.998% | ||||
| Accuracy | 74.657% | 67.333% to 81.079% | ||||
| DSC Classification | FIRST TEST | At Median Follow-Up (15.5 Months) | Diagnosis | First Test | At Median Follow-Up (15.5 Months) |
|---|---|---|---|---|---|
| n. (%) | n. (%) | n. (%) | n. (%) | ||
| negative | 17 (65.4%) | 15 (57.7%) | OLGA 0–II | 22 (22.3%) | 22 (22.3%) |
| neutral | 3 (11.5%) | 5 (19.2%) | OLGA III–IV | 3 (11.5%) | 3 (11.5%) |
| positive | 6 (23.1%) | 6 (23.1%) | GC | 1 (3.8%) | 1 (3.8%) |
| DSC Test | PG Test | |||
|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | |
| Sensitivity | 70.00% | 45.721% to 88.107% | 15.00% | 3.207% to 37.893% |
| Specificity | 74.66% | 66.800% to 81.486% | 78.77% | 71.236% to 85.094% |
| AUC | 0.723 | 0.649 to 0.790 | 0.470 | 0.391 to 0.548 |
| Positive LR | 2.762 | 1.852 to 4.120 | 0.71 | 0.238 to 2.099 |
| Negative LR | 0.402 | 0.204 to 0.790 | 1.08 | 0.881 to 1.321 |
| Disease prevalence | 0.01% | 0.01% | ||
| Positive PV | 0.03% | 0.019% to 0.041% | 0.01% | 0.002% to 0.021% |
| Negative PV | 99.996% | 99.992% to 99.998% | 99.989% | 99.987% to 99.991% |
| Accuracy | 74.66% | 67.333% to 81.079% | 78.76% | 71.748% to 84.717% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Re, V.; Realdon, S.; Vettori, R.; Zaramella, A.; Maiero, S.; Repetto, O.; Canzonieri, V.; Steffan, A.; Cannizzaro, R. A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area. Int. J. Mol. Sci. 2023, 24, 3290. https://doi.org/10.3390/ijms24043290
De Re V, Realdon S, Vettori R, Zaramella A, Maiero S, Repetto O, Canzonieri V, Steffan A, Cannizzaro R. A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area. International Journal of Molecular Sciences. 2023; 24(4):3290. https://doi.org/10.3390/ijms24043290
Chicago/Turabian StyleDe Re, Valli, Stefano Realdon, Roberto Vettori, Alice Zaramella, Stefania Maiero, Ombretta Repetto, Vincenzo Canzonieri, Agostino Steffan, and Renato Cannizzaro. 2023. "A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area" International Journal of Molecular Sciences 24, no. 4: 3290. https://doi.org/10.3390/ijms24043290
APA StyleDe Re, V., Realdon, S., Vettori, R., Zaramella, A., Maiero, S., Repetto, O., Canzonieri, V., Steffan, A., & Cannizzaro, R. (2023). A DSC Test for the Early Detection of Neoplastic Gastric Lesions in a Medium-Risk Gastric Cancer Area. International Journal of Molecular Sciences, 24(4), 3290. https://doi.org/10.3390/ijms24043290







