Effects of Fractionated Radiation Exposure on Vimentin Expression in Cervical Cancers: Analysis of Association with Cancer Stem Cell Response and Short-Term Prognosis
Abstract
1. Introduction
2. Results
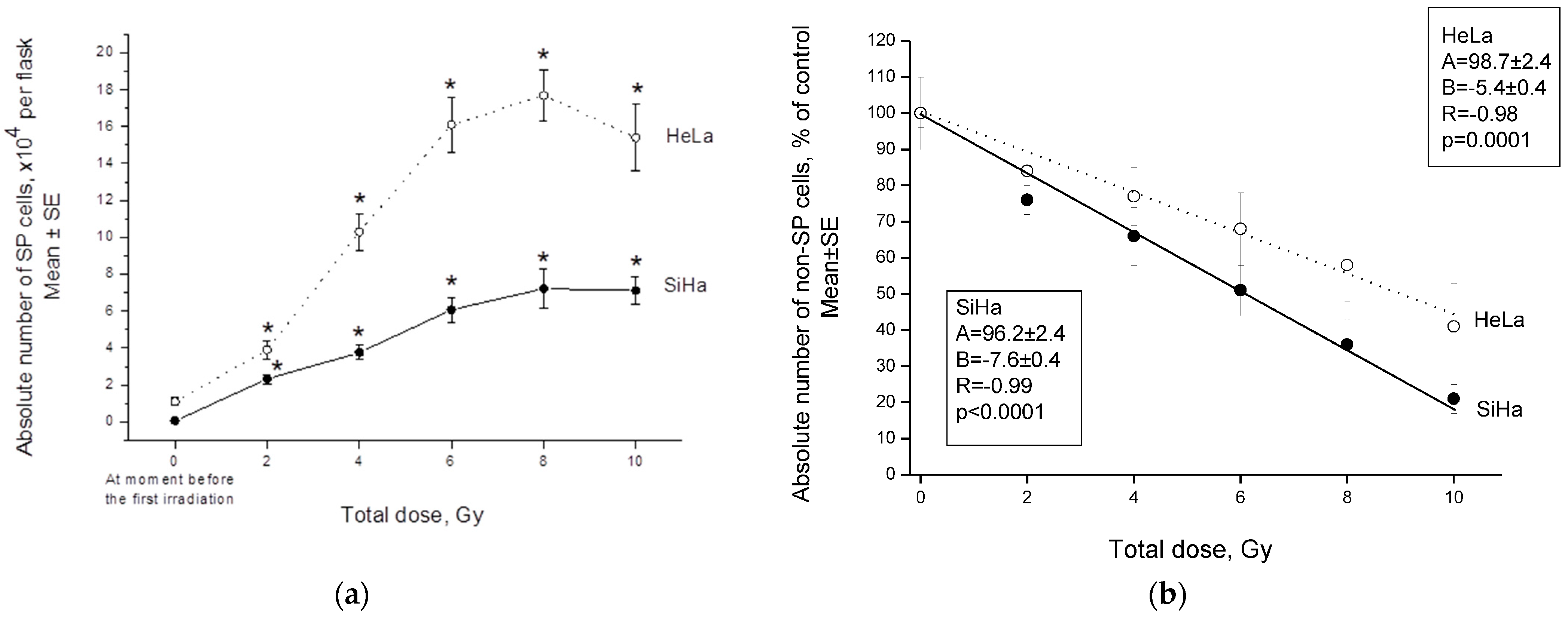
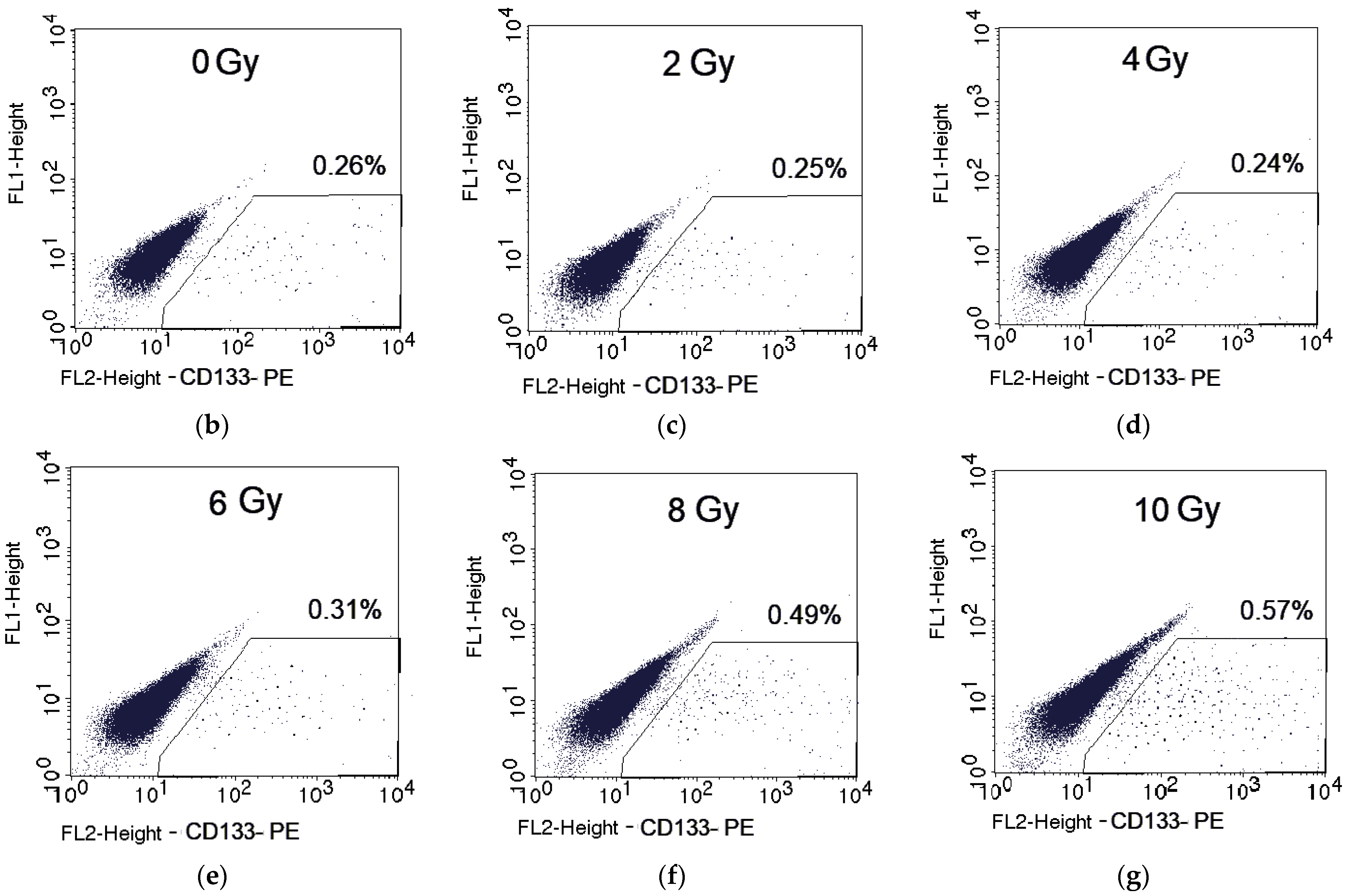
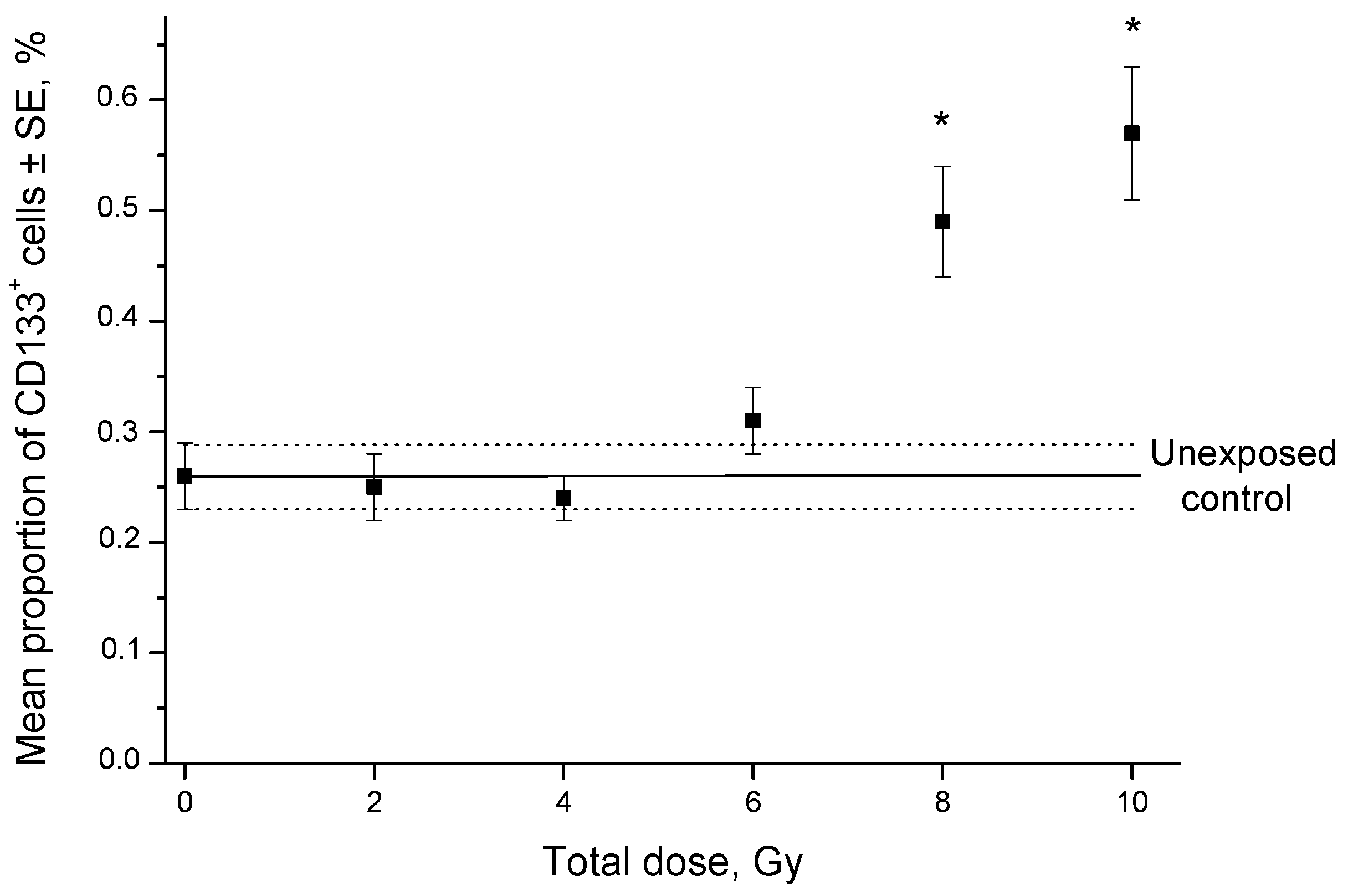
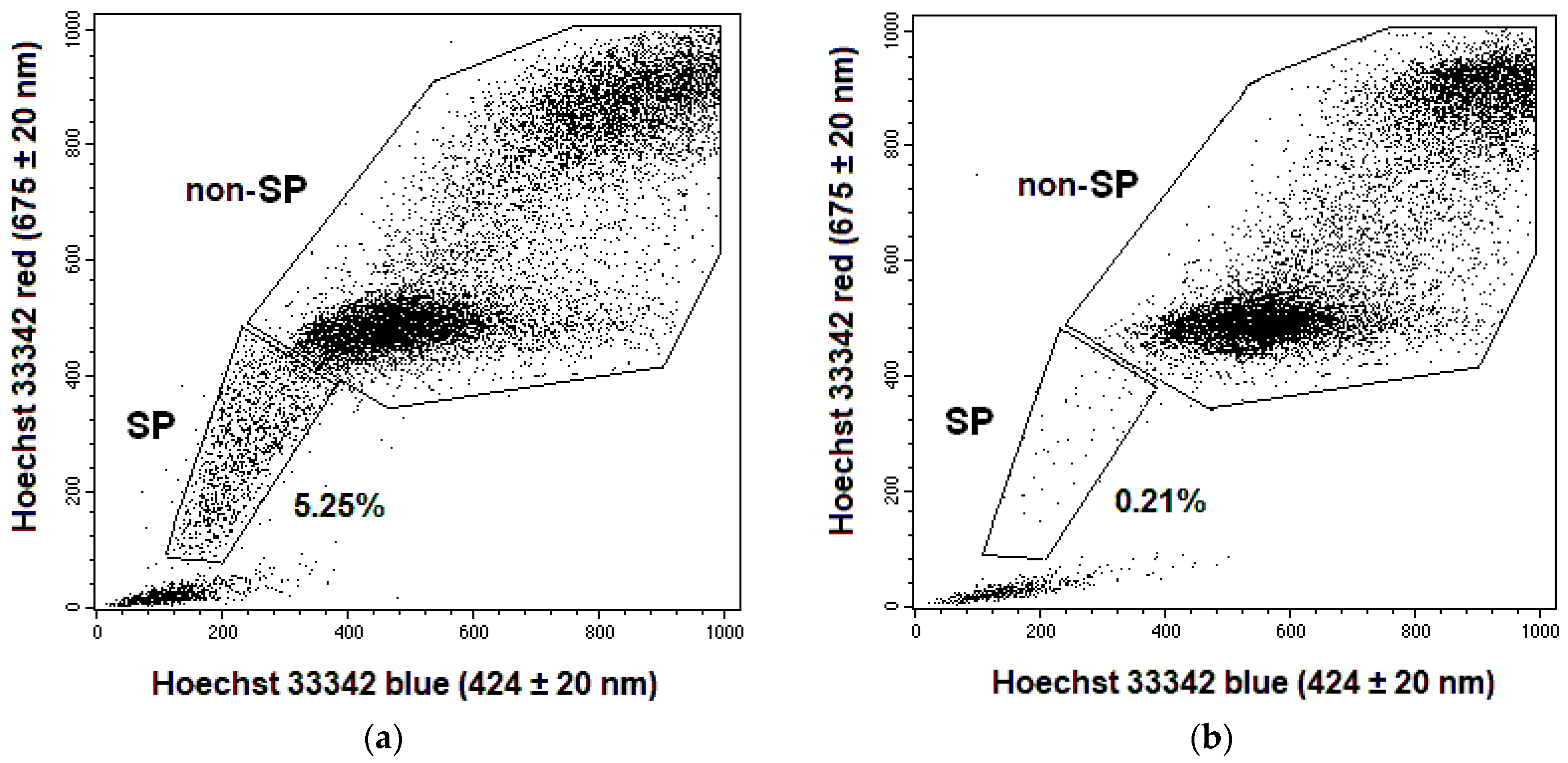
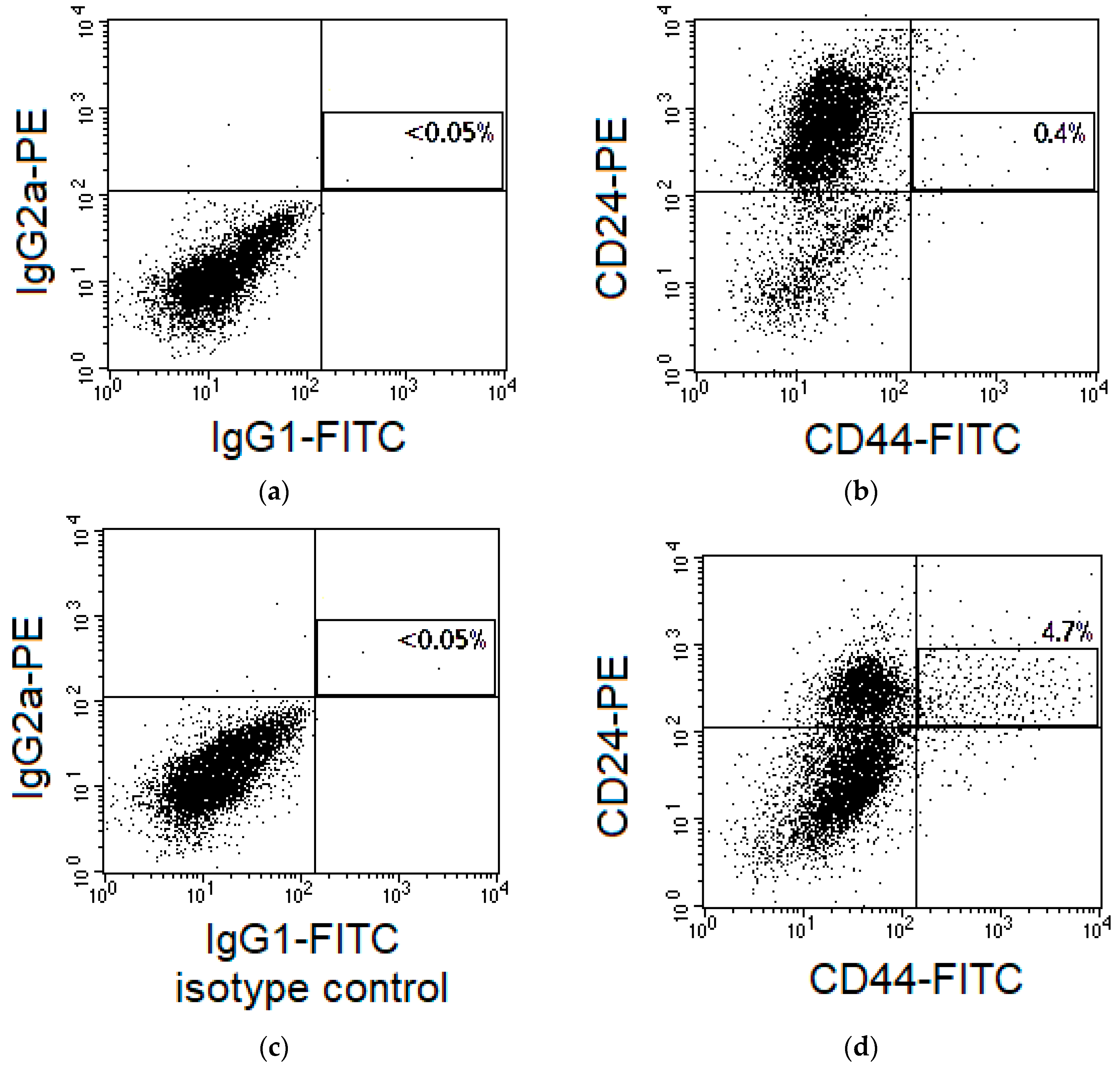
2.1. Changes in the CSC Number after Fractionated Radiation Exposure
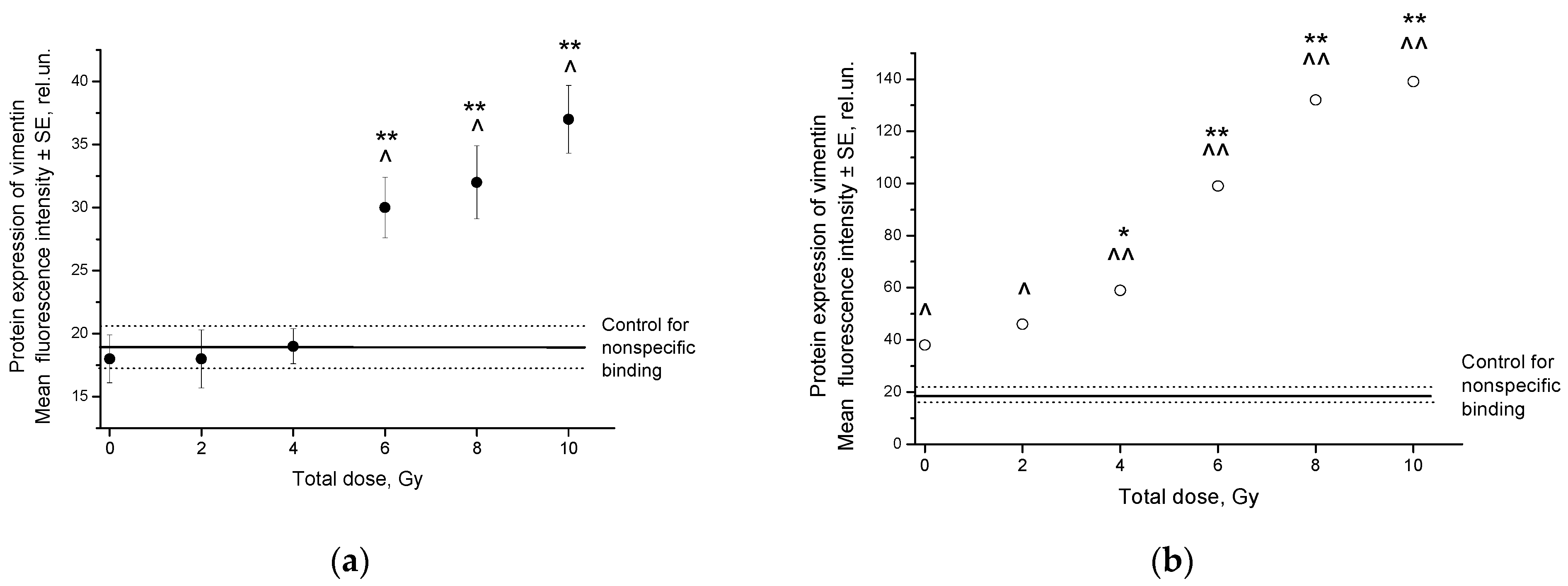
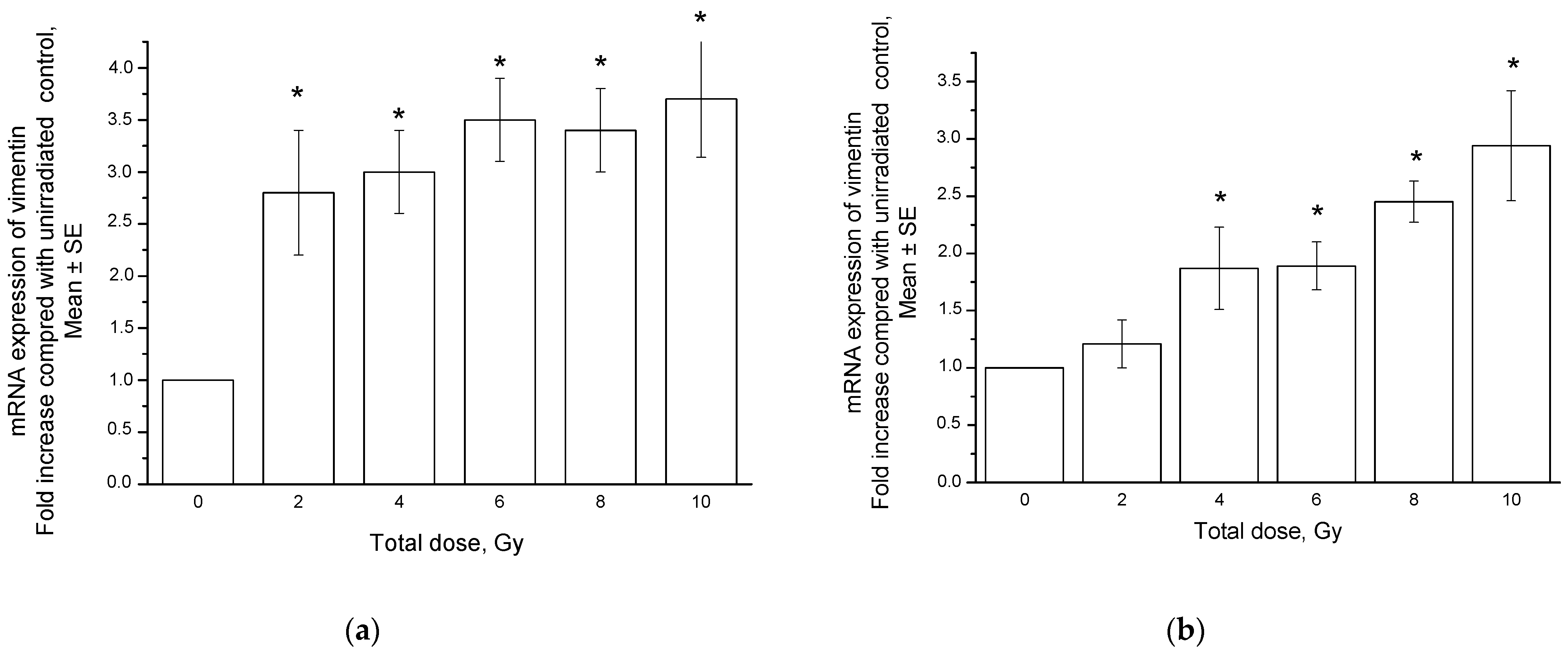
2.2. Radiation-Induced Changes in Vimentin Expression
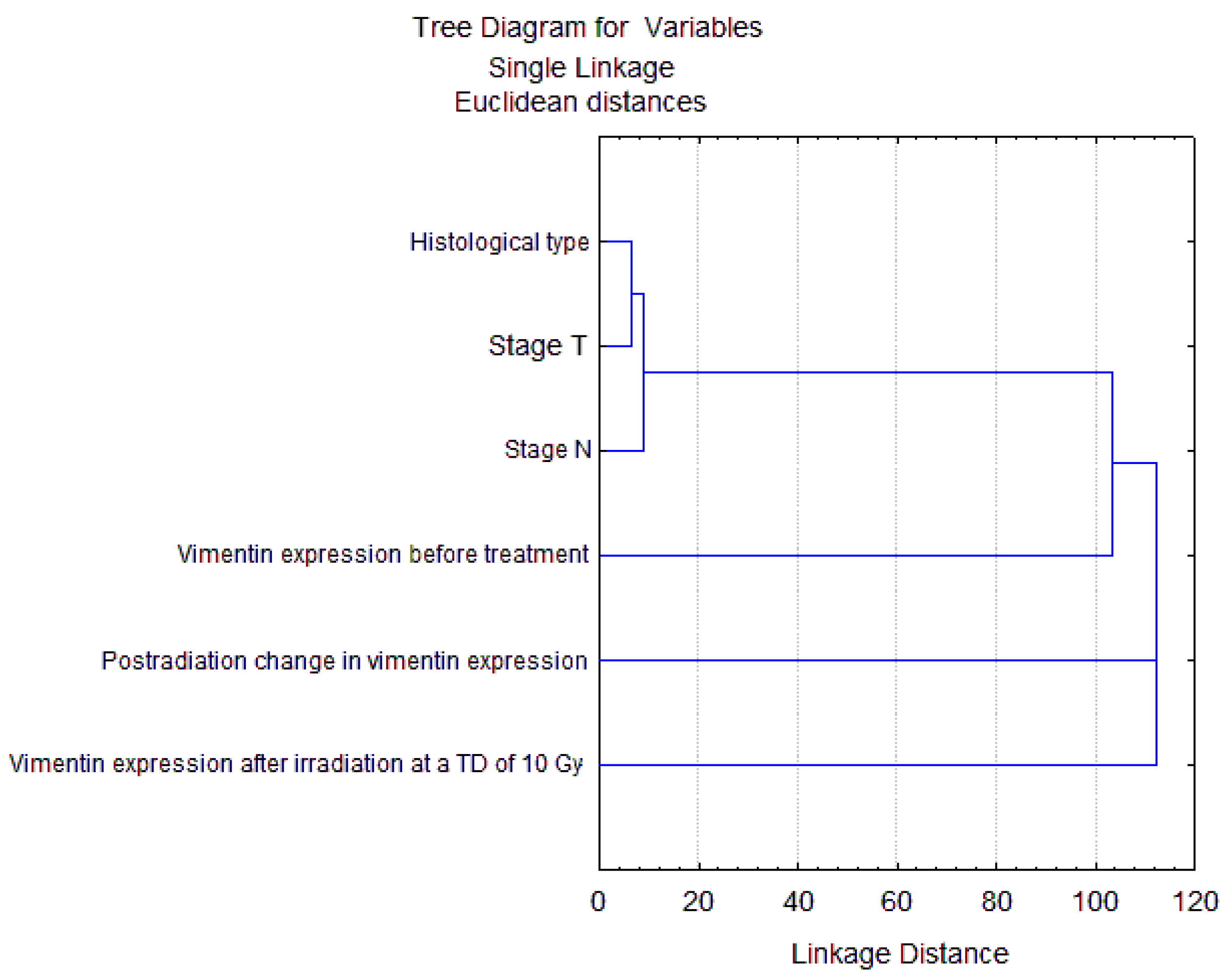
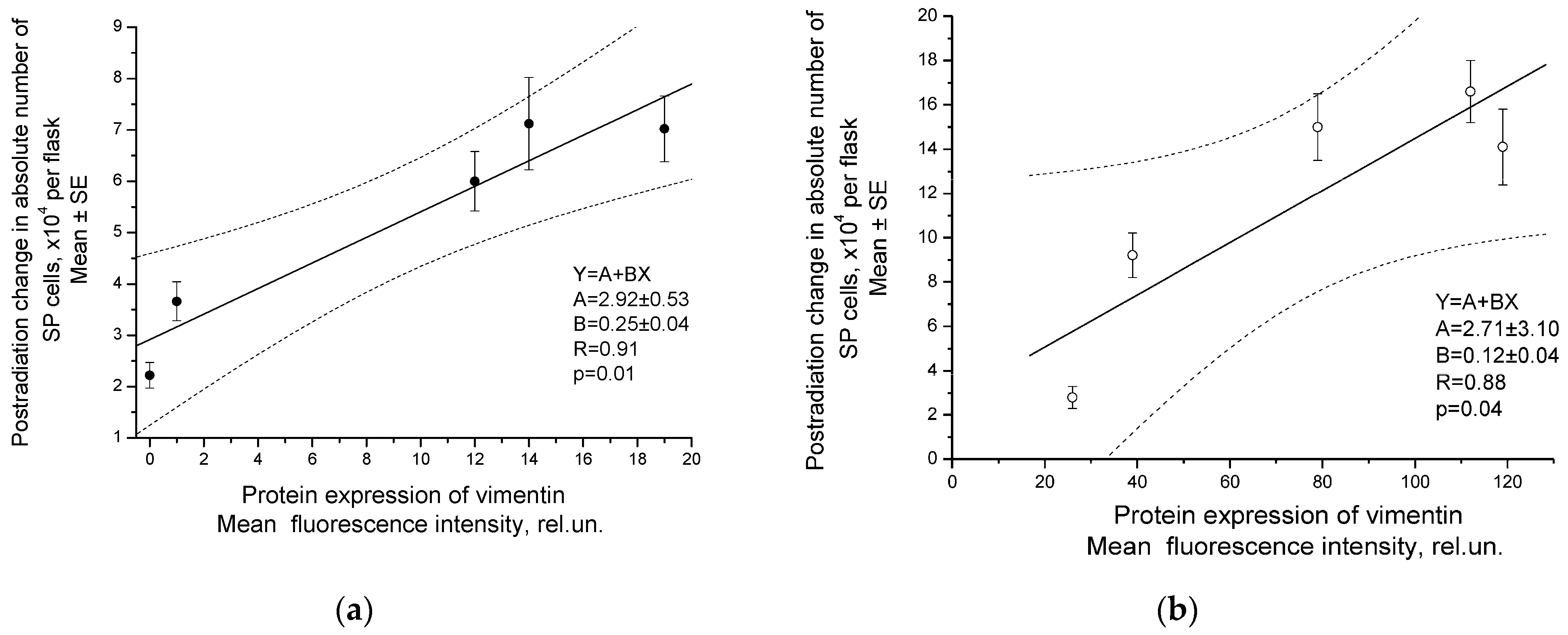
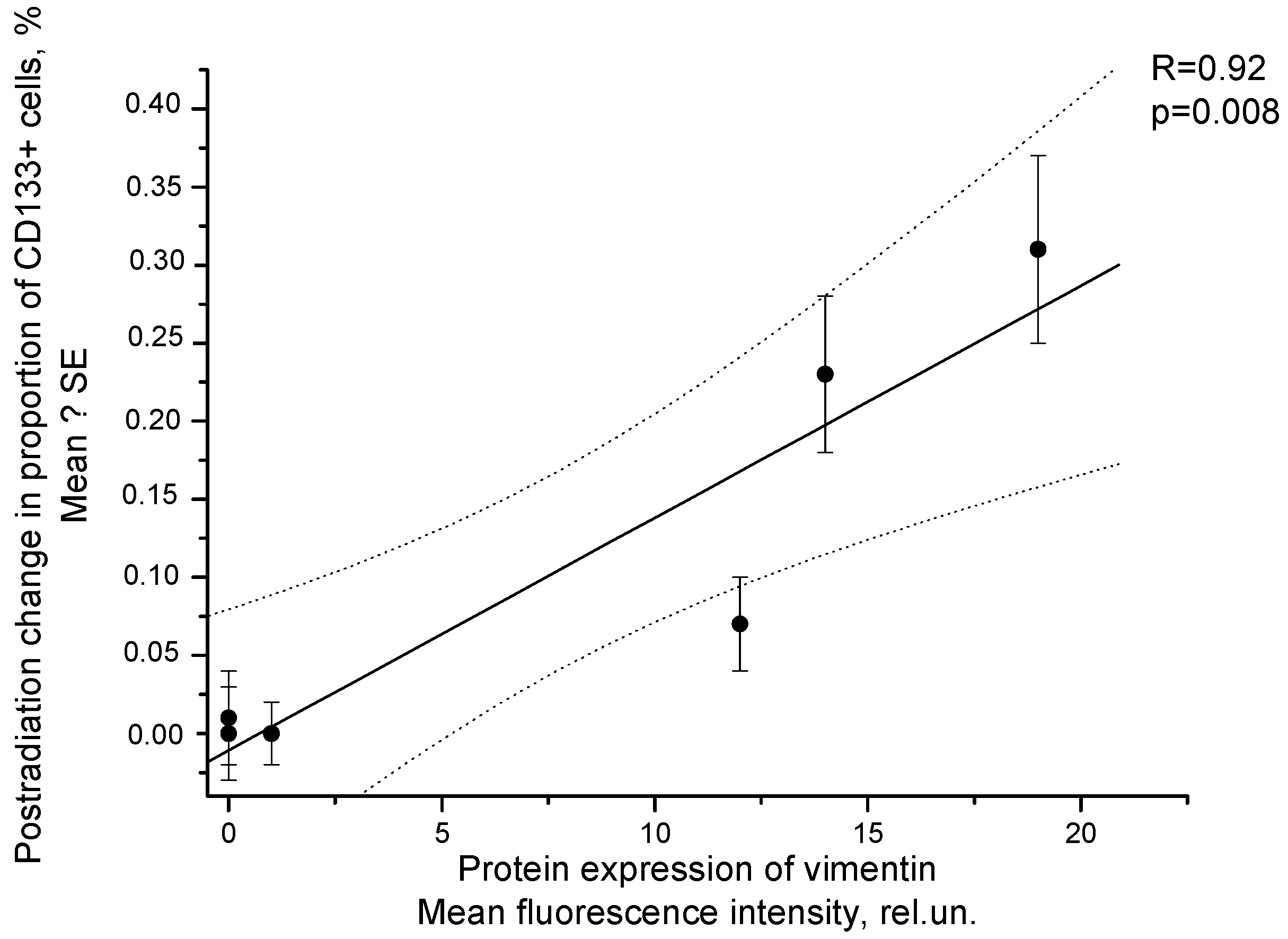
2.3. Correlation of Radiation Response of Cervical CSCs with Postradiation Increase in Expression of Vimentin under Experimental and Clinical Conditions
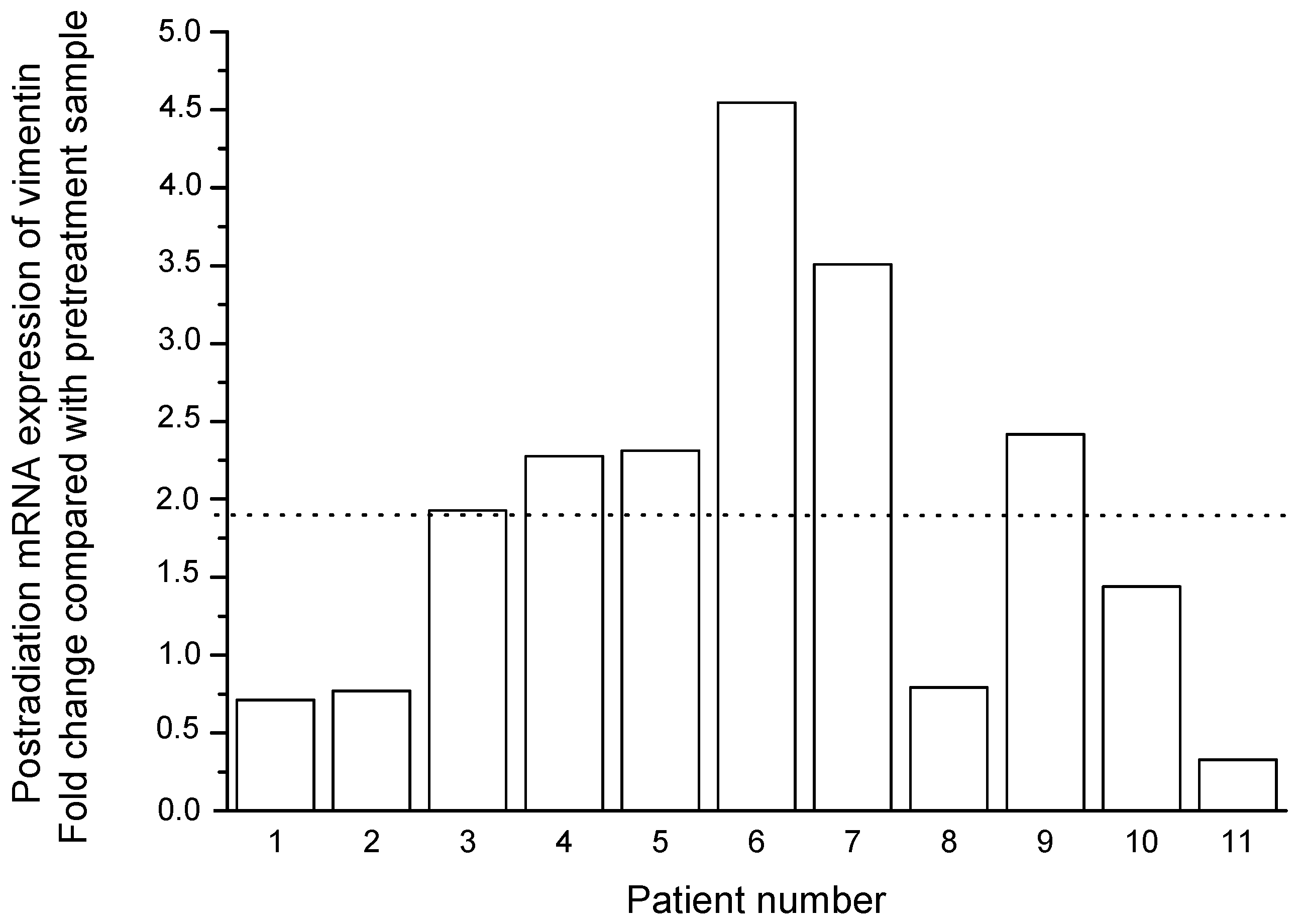
2.4. Prognostic Significance Estimation of Vimentin Expression in Cervical Scraping of CC Patients
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Irradiation
4.2. Patients and Treatment
4.3. CSC Identification in Cell Cultures and Cervical Scrapings from CC Patients
4.4. Determination of Vimentin Expression at Protein Level in Cell Cultures and Cervical Scrapings from CC Patients by Flow Cytometry, Fluorescent Microscopy, and Laser Scanning Confocal Microscopy
4.5. Polymerase Chain Reaction for Detection of mRNA Expression of Vimentin and Stemness-Related Genes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkatas, J.; Singh, M. Cervical cancer: A meta-analysis, therapy and future of nanomedicine. Ecancermedicalscience 2020, 14, 1111. [Google Scholar] [CrossRef] [PubMed]
- Olusola, P.; Banerjee, H.N.; Philley, J.V.; Dasgupta, S. Human Papilloma Virus-Associated Cervical Cancer and Health Disparities. Cells 2019, 8, 622. [Google Scholar] [CrossRef]
- Krause, M.; Dubrovska, A.; Linge, A.; Baumann, M. Cancer stem cells: Radioresistance, prediction of radio-therapy outcome and specific targets for combined treatments. Adv. Drug Deliv. Rev. 2017, 109, 63–73. [Google Scholar] [CrossRef]
- Lytle, N.K.; Barber, A.G.; Reya, T. Stem cells fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer 2018, 18, 669–680. [Google Scholar] [CrossRef]
- Huang, R.; Rofstad, E.K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget 2017, 8, 35351–35367. [Google Scholar] [CrossRef]
- Zamulaeva, I.A.; Selivanova, E.I.; Matchuk, O.N.; Krikunova, L.I.; Mkrtchyan, L.S.; Kulieva, G.Z.; Kaprin, A.D. Quantitative changes in the population of cancer stem cells after radiation exposure in a dose of 10 Gy as a prognostic marker of immediate results of the treatment of squamous cell cervical cancer. Bull. Exp. Biol. Med. 2019, 168, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, R.; Arora, H.; Rizvi, M.A. EMT in cervical cancer: Its role in tumour progression and response to therapy. Cancer Lett. 2015, 356, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.-C.; Tsai, C.-Y.; Tsai, M.-M.; Yeh, C.-T.; Lin, K.-H. Roles of Long Noncoding RNAs in Recurrence and Metastasis of Radiotherapy-Resistant Cancer Stem Cells. Int. J. Mol. Sci. 2017, 18, 1903. [Google Scholar] [CrossRef]
- Gao, X.; Sishc, B.J.; Nelson, C.B.; Hahnfeldt, P.; Bailey, S.; Hlatky, L. Radiation-Induced Reprogramming of Pre-Senescent Mammary Epithelial Cells Enriches Putative CD44(+)/CD24(-/low) Stem Cell Phenotype. Front. Oncol. 2016, 6, 138. [Google Scholar] [CrossRef]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Dekmezian, C.; Pajonk, F. Radiation-Induced Reprogramming of Breast Cancer Cells. Stem Cells 2012, 30, 833–844. [Google Scholar] [CrossRef]
- López, J.; Poitevin, A.; Mendoza-Martínez, V.; Pérez-Plasencia, C.; García-Carrancá, A. Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer 2012, 12, 48. [Google Scholar] [CrossRef]
- Thankamony, A.P.; Saxena, K.; Murali, R.; Jolly, M.K.; Nair, R. Cancer Stem Cell Plasticity—A Deadly Deal. Front. Mol. Biosci. 2020, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct Target Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef] [PubMed]
- Fedele, M.; Sgarra, R.; Battista, S.; Cerchia, L.; Manfioletti, G. The Epithelial-Mesenchymal Transition at the Crossroads between Metabolism and Tumor Progression. Int. J. Mol. Sci. 2022, 23, 800. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A multi-tool for tumor progression. EMBO J. 2021, 40, 108647. [Google Scholar] [CrossRef] [PubMed]
- Matchuk, O.N.; Zamulaeva, I.A.; Selivanova, E.I.; Mkrtchyan, L.S.; Krikunova, L.I.; Saburov, V.O.; Lychagin, A.A.; Kuliyeva, G.Z.; Yakimova, A.O.; Khokhlova, A.V.; et al. Effect of Fractionated Low-LET Radiation Exposure on Cervical Cancer Stem Cells under Experimental and Clinical Conditions. Biol. Bull. 2020, 47, 1471–1479. [Google Scholar] [CrossRef]
- Wang, K.; Zeng, J.; Luo, L.; Yang, J.; Chen, J.; Li, B.; Shen, K. Identification of a cancer stem cell-like side population in the HeLa human cervical carcinoma cell line. Oncol. Lett. 2013, 6, 1673–1680. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, C.; Zhao, L.; Liu, N.; Li, X.; Yu, W.; Wei, L. Sorting and identification of side population cells in the human cervical cancer cell line HeLa. Cancer Cell. Int. 2014, 14, 3. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The response of CD24-/low/CD44+ breast cancer-initiating cells to radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, C.; Vlashi, E.; Della Donna, L.; Meng, Y.; Dekmezian, C.; Kim, K.; Pajonk, F. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010, 12, R13. [Google Scholar] [CrossRef]
- Gao, X.; McDonald, J.T.; Hlatky, L.; Enderling, H. Acute and fractionated irradiation differentially modulate glioma stem cell division kinetics. Cancer Res. 2013, 73, 1481–1490. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, R.K.; Yoon, C.H.; An, S.; Hwang, S.G.; Suh, Y.; Park, M.J.; Chung, H.Y.; Kim, I.G.; Lee, S.J. Importance of PKCδ signaling in fractionated-radiation-induced expansion of glioma-initiating cells and resistance to cancer treatment. J. Cell. Sci. 2011, 124, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Cojoc, M.; Peitzsch, C.; Kurth, I.; Trautmann, F.; Kunz-Schughart, L.A.; Telegeev, G.D.; Stakhovsky, E.A.; Walker, J.R.; Simin, K.; Lyle, S.; et al. Aldehyde Dehydrogenase Is Regulated by β-Catenin / TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Res. 2015, 75, 1482–1494. [Google Scholar] [CrossRef]
- Cho, K.J.; Park, E.J.; Kim, M.S.; Joo, Y.H. Characterization of FaDu-R, a radioresistant head and neck cancer cell line, and cancer stem cells. Auris Nasus. Larynx 2018, 45, 566–573. [Google Scholar] [CrossRef]
- Skvortsov, S.; Skvortsova, I.I.; Tang, D.G.; Dubrovska, A. Prostate cancer stem cells: Current understanding. Stem Cells 2018, 36, 1457–1474. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.J.; Bian, L.; Fang, Z.H.; Zhang, Q.Y.; Cheng, J.X. CD44+/CD24+ cervical cancer cells resist radiotherapy and exhibit properties of cancer stem cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1745–1754. [Google Scholar]
- Matchuk, O.N.; Orlova, N.V.; Zamulaeva, I.A. Changes in the Relative Number of SP Cells of Melanoma Line B16 after Radiation Exposure in vivo. Radiats. Biol. Radioecol. 2016, 56, 487–493. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Kamble, D.; Mahajan, M.; Dhat, R.; Sitasawad, S. Keap1-Nrf2 Pathway Regulates ALDH and Contributes to Radioresistance in Breast Cancer Stem Cells. Cells 2021, 10, 83. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Y.; Yang, Q.; Li, X.; Kong, X.; Zhang, N.; Yuan, C.; Yang, N.; Kong, B. Low-dose radiation-induced epithelial-mesenchymal transition through NF-κB in cervical cancer cells. Int. J. Oncol. 2013, 42, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.-H.; Lee, C.-E. SOCS1 Represses Fractionated Ionizing Radiation-Induced EMT Signaling Pathways through the Counter-Regulation of ROS-Scavenging and ROS-Generating Systems. Cell Physiol. Biochem. 2020, 54, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Meredith, S.L.; Bryant, J.L.; Babur, M.; Riddell, P.W.; Behrouzi, R.; Williams, K.J.; White, A. Irradiation Decreases the Neuroendocrine Biomarker Pro-Opiomelanocortin in Small Cell Lung Cancer Cells In Vitro and In Vivo. PLoS ONE 2016, 11, e0148404. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, H.; Jiang, Z.; Yue, J.; Hou, Q.; Xie, R.; Wu, S. Fractionated irradiation-induced EMT-like phenotype conferred radioresistance in esophageal squamous cell carcinoma. J. Radiat. Res. 2016, 57, 370–380. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, Y.; Ding, X.; Wang, L.; Zhao, Y.; Fei, Y.; Zhu, Y.; Shen, X.; Tan, C.; Liang, Z. Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC. J. Exp. Clin. Cancer Res. 2019, 38, 61. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Shuang, Z.-Y.; Wu, W.-C.; Xu, J.; Lin, G.; Liu, Y.-C.; Lao, X.-M.; Zheng, L.; Li, S. Transforming growth factor-β1-induced epithelial-mesenchymal transition generates ALDH-positive cells with stem cell properties in cholangiocarcinoma. Cancer Lett. 2014, 354, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef]
- Li, F.; Zhou, K.; Gao, L.; Zhang, B.; Li, W.; Yan, W.; Song, X.; Yu, H.; Wang, S.; Yu, N.; et al. Radiation induces the generation of cancer stem cells: A novel mechanism for cancer radioresistance. Oncol. Lett. 2016, 12, 3059–3065. [Google Scholar] [CrossRef]
- Da, C.; Wu, L.; Liu, Y.; Wang, R.; Li, R. Effects of irradiation on radioresistance, HOTAIR and epithelial-mesenchymal transition/cancer stem cell marker expression in esophageal squamous cell carcinoma. Oncol. Lett. 2017, 13, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Lu, J.; Wang, C.; Xue, X. The prognostic values of the expression of Vimentin, TP53, and Podoplanin in patients with cervical cancer. Cancer Cell Int. 2017, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhou, Y.; Jiang, W.; Yang, X.; Zhu, J.; Feng, D.; Wei, Y.; Li, M.; Yao, F.; Hu, W.; et al. Significance of E-cadherin, β-catenin, and vimentin expression as postoperative prognosis indicators in cervical squamous cell carcinoma. Hum. Pathol. 2012, 43, 1213–1220. [Google Scholar] [CrossRef]
- Oka, K.; Nakano, T.; Arai, T. Adenocarcinoma of the cervix treated with radiation alone: Prognostic significance of S-100 protein and vimentin immunostaining. Obstet Gynecol. 1992, 79, 347–350. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, P.; Niu, Q.; Hu, X. Combined Snail and E-cadherin Predicts Overall Survival of Cervical Carcinoma Patients: Comparison Among Various Epithelial-Mesenchymal Transition Proteins. Front. Mol. Biosci. 2020, 7, 22. [Google Scholar] [CrossRef]
- Chen, R.; Sheng, C.; Ma, R.; Zhang, L.; Yang, L.; Chen, Y. PLAC1 is an independent predictor of poor survival, and promotes cell proliferation and invasion in cervical cancer. Mol. Med. Rep. 2021, 24, 800. [Google Scholar] [CrossRef]
- Li, G.; Wu, Q.; Gong, L.; Xu, X.; Cai, J.; Xu, L.; Zeng, Y.; He, X.; Wang, Z. FABP4 is an independent risk factor for lymph node metastasis and poor prognosis in patients with cervical cancer. Cancer Cell Int. 2021, 21, 568. [Google Scholar] [CrossRef]
- Xing, F.; Song, Z.; Cheng, Z. High expression of PDIA4 promotes malignant cell behavior and predicts reduced survival in cervical cancer. Oncol Rep. 2022, 48, 184. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Kabekkodu, S.P.; Adiga, D.; Fernandes, R.; Shukla, V.; Bhandari, P.; Pandey, D.; Sharan, K.; Satyamoorthy, K. ZNF471 modulates EMT and functions as methylation regulated tumor suppressor with diagnostic and prognostic significance in cervical cancer. Cell Biol. Toxicol. 2021, 37, 731–749. [Google Scholar] [CrossRef]
- Tian, P.; Zhang, C.; Ma, C.; Ding, L.; Tao, N.; Ning, L.; Wang, Y.; Yong, X.; Yan, Q.; Lin, X.; et al. Decreased chromobox homologue 7 expression is associated with epithelial-mesenchymal transition and poor prognosis in cervical cancer. Open Med. (Wars.) 2021, 16, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tu-mours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Peng, C.; Li, C.; Zhou, Y.; Li, M.; Ling, B.; Wei, H.; Tian, Z. Identification and characterization of cancer stem-like cells from primary carcinoma of the cervix uteri. Oncol. Rep. 2009, 22, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Yeo, E.; McMillan, N.; Yu, C. Silencing oncogene expression in cervical cancer stem-like cells inhibits their cell growth and self-renewal ability. Cancer Gene Ther. 2011, 18, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Primer-BLAST. A Tool for Finding Specific Primers. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi (accessed on 27 November 2022).
- RefFinder. Available online: https://heartcure.com.au/reffinder (accessed on 27 December 2022).





| Clinical, Morphological Parameters and Treatment | Number of Patients | Proportion of Vimentin-Positive Cells, % Average Value ± SE | |||
|---|---|---|---|---|---|
| Before Treatment | After Radiation Exposure at a TD of 10 Gy | Postradiation Changes | |||
| FIGO stage | I | 6 | 17.4 ± 6.4 | 38.6 ± 9.4 | 20.2 ± 6.0 |
| II | 18 | 14.6 ± 3.0 | 24.2 ± 3.3 | 9.6 ± 3.1 | |
| III | 11 | 13.5 ± 3.3 | 27.4 ± 4.9 | 13.9 ± 6.6 | |
| Status of lymph node involvement | N0 | 23 | 14.8 ± 2.4 | 29.0 ± 3.7 | 14.2 ± 3.8 |
| N+ | 12 | 14.5 ± 4.2 | 24.4 ± 4.0 | 9.9 ± 3.9 | |
| Histological type of squamous cell CC | Keratinizing | 7 | 12.2 ± 3.7 | 19.5 ± 5.1 | 7.3 ± 5.8 |
| Nonkeratinizing | 28 | 15.5 ± 2.5 | 29.8 ± 3.1 | 14.3 ± 3.2 | |
| Treatment | Radiotherapy | 3 | 27.2 ± 9.5 | 37.6 ± 10.3 | 10.4 ± 9.3 |
| Chemoradiotherapy | 32 | 13.6 ± 2.0 | 25.9 ± 2.7 | 12.3 ± 3.0 | |
| Patient Number | Total Dose, Gy | Gene | ||||||
|---|---|---|---|---|---|---|---|---|
| CHOP | NANOG | OCT4 | SNAIL | SOX2 | TWIST | VIM | ||
| 1 | 0 | 0.26 | 7.74 | 64.97 | 1.16 | 0.03 | 0.03 | 110.80 |
| 10 | 0.04 | 8.01 | 72.63 | 0.18 | 0.02 | 0.02 | 78.93 | |
| 2 | 0 | 0.50 | 35.86 | 105.02 | 6.80 | 0.03 | 0.16 | 316.16 |
| 10 | 0.56 | 5.26 | 83.64 | 9.82 | 0.01 | 0.05 | 243.21 | |
| 3 | 0 | 0.48 | 3.85 | 46.35 | 2.76 | 0.01 | 0.16 | 88.32 |
| 10 | 0.54 | 38.65 | 70.63 | 7.37 | 0.27 | 0.72 | 170.34 | |
| 4 | 0 | 0.02 | 9.18 | 2.79 | 0.14 | 0.24 | 0.50 | 66.16 |
| 10 | 0.03 | 8.31 | 10.23 | 0.83 | 0.30 | 0.89 | 150.55 | |
| 5 | 0 | 0.03 | 7.68 | 4.93 | 0.51 | 0.64 | 0.13 | 9.85 |
| 10 | 0.42 | 10.48 | 62.68 | 1.66 | 0.08 | 0.48 | 22.78 | |
| 6 | 0 | 0.02 | 8.85 | 4.81 | 0.16 | 0.30 | 0.24 | 26.45 |
| 10 | 0.23 | 12.29 | 143.00 | 3.12 | 0.02 | 0.54 | 120.25 | |
| 7 | 0 | 0.02 | 9.08 | 3.17 | 0.02 | 0.13 | 0.25 | 13.95 |
| 10 | 0.05 | 14.76 | 7.28 | 0.14 | 0.12 | 0.18 | 48.96 | |
| 8 | 0 | 0.04 | 12.94 | 4.12 | 0.14 | 0.04 | 0.72 | 74.75 |
| 10 | 0.14 | 17.11 | 7.10 | 0.12 | 0.19 | 0.43 | 59.17 | |
| 9 | 0 | 0.10 | 5.97 | 1.49 | 0.20 | 1.00 | 0.03 | 19.00 |
| 10 | 0.14 | 7.12 | 1.95 | 0.53 | 0.56 | 0.09 | 45.92 | |
| 10 | 0 | 0.03 | 3.78 | 4.56 | 0.47 | 0.08 | 0.26 | 19.29 |
| 10 | 0.04 | 9.28 | 9.81 | 0.36 | 0.05 | 0.17 | 27.74 | |
| 11 | 0 | 0.30 | 4.46 | 8.50 | 1.07 | 0.12 | 0.47 | 30.42 |
| 10 | 0.09 | 12.65 | 5.98 | 1.30 | 0.06 | 0.15 | 9.99 | |
| Indicator (Predictor) | Beta 1 | p-Level for Predictor | R | p-Level for Model in the Whole |
|---|---|---|---|---|
| Disease stage (FIGO) | 0.38 | 0.03 | 0.44 | 0.049 |
| The change in the proportion of vimentin-positive cells after irradiation at a TD of 10 Gy | 0.18 | 0.17 |
| Gene | 5′→3′ Sequence (F–Forward, R–Revers) | PCR Product Size, bp |
|---|---|---|
| ALAS1 | F: TGCTGCAAAGATCTGACCCCTC | 113 |
| R: AAACTCATGGGCCACATCACAC | ||
| GAPDH | F: CCTCTGACTTCAACAGCGACA | 101 |
| R: GTTGTCATACCAGGAAATGAGCTTG | ||
| GUSB | F: GCGTAGGGACAAGAACCACC | 120 |
| R: TCCAAGGATTTGGTGTGAGCG | ||
| IPO8 | F: GCAGAGTGTCATGCAGCTAAAC | 120 |
| R: GACCCCTCGAGTTAATCTCTCCA | ||
| YWHAZ | F: TGGTGATGACAAGAAAGGGATTGT | 119 |
| R: AGTTAAGGGCCAGACCCAGT | ||
| VIM | F: GAGATGCTTCAGAGAGAGGAAGCC | 112 |
| R: CTTGCAAAGATTCCACTTTGCGT | ||
| NANOG | F: GATGCCTCACACGGAGACTG | 108 |
| R: GCAGAAGTGGGTTGTTTGCC | ||
| OCT4 | F: AGGAGAAGCTGGAGCAAAACC | 81 |
| R: GGAGCTTGGCAAATTGCTCG | ||
| SOX2 | F: CATGAAGGAGCACCCGGATT | 124 |
| R: CCCGCTCGCCATGCTATT | ||
| SNAIL | F: AGCGAGCTGCAGGACTCTAA | 125 |
| R: CAGATGAGCATTGGCAGCGA | ||
| TWIST | F: GGCCGGAGACCTAGATGTCATT | 150 |
| R: CACGCCCTGTTTCTTTGAATTTGG | ||
| CHOP | F: ACCTCCTGGAAATGAAGAGGAAGA | 111 |
| R: GAGGTGCTTGTGACCTCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamulaeva, I.; Matchuk, O.; Selivanova, E.; Mkrtchian, L.; Yakimova, A.; Gusarova, V.; Lipunov, N.; Krikunova, L.; Ivanov, S.; Kaprin, A. Effects of Fractionated Radiation Exposure on Vimentin Expression in Cervical Cancers: Analysis of Association with Cancer Stem Cell Response and Short-Term Prognosis. Int. J. Mol. Sci. 2023, 24, 3271. https://doi.org/10.3390/ijms24043271
Zamulaeva I, Matchuk O, Selivanova E, Mkrtchian L, Yakimova A, Gusarova V, Lipunov N, Krikunova L, Ivanov S, Kaprin A. Effects of Fractionated Radiation Exposure on Vimentin Expression in Cervical Cancers: Analysis of Association with Cancer Stem Cell Response and Short-Term Prognosis. International Journal of Molecular Sciences. 2023; 24(4):3271. https://doi.org/10.3390/ijms24043271
Chicago/Turabian StyleZamulaeva, Irina, Olga Matchuk, Elena Selivanova, Liana Mkrtchian, Anna Yakimova, Victoria Gusarova, Nikita Lipunov, Liudmila Krikunova, Sergey Ivanov, and Andrey Kaprin. 2023. "Effects of Fractionated Radiation Exposure on Vimentin Expression in Cervical Cancers: Analysis of Association with Cancer Stem Cell Response and Short-Term Prognosis" International Journal of Molecular Sciences 24, no. 4: 3271. https://doi.org/10.3390/ijms24043271
APA StyleZamulaeva, I., Matchuk, O., Selivanova, E., Mkrtchian, L., Yakimova, A., Gusarova, V., Lipunov, N., Krikunova, L., Ivanov, S., & Kaprin, A. (2023). Effects of Fractionated Radiation Exposure on Vimentin Expression in Cervical Cancers: Analysis of Association with Cancer Stem Cell Response and Short-Term Prognosis. International Journal of Molecular Sciences, 24(4), 3271. https://doi.org/10.3390/ijms24043271






